Abstract
Neuromodulators play a key role in adjusting animal behavior based on environmental cues and internal needs. Here, we review the regulation of Drosophila feeding behavior to illustrate how neuromodulators achieve behavioral plasticity. Recent studies have made rapid progress in determining molecular and cellular mechanisms that translate the metabolic needs of the fly into changes in neuroendocrine and neuromodulatory states. These neuromodulators in turn promote or inhibit discrete feeding behavioral subprograms. This review highlights the links between physiological needs, neuromodulatory states, and feeding decisions.
Keywords: neuromodulation, feeding, Drosophila melanogaster, behavioral plasticity
Introduction
Animals have evolved diverse behavioral repertoires to facilitate survival. Many of these behaviors are plastic and subject to modulation by environmental cues and internal needs. Neuromodulators, such as biogenic amines and neuropeptides, play a critical role in achieving behavioral plasticity. Work in invertebrates, particularly in the crustacean stomatogastric ganglion and C. elegans, has provided detailed insight into how neuromodulators alter biophysical properties of individual neurons and reconfigure circuits [1,2]. Here, we will examine how these lessons apply to establishing flexibility in a complex innate behavior by reviewing recent findings on neuromodulation in the fruit fly feeding circuit.
The basic challenge in food intake regulation is to maintain energetic homeostasis by balancing food consumption with energy expenditure. Recent studies of Drosophila feeding illustrate several principles of how neuromodulatory systems link physiological needs to flexible expression of adaptive behaviors. This review focuses on how metabolic changes are translated into neuroendocrine and neuromodulatory states and how these in turn impinge on central circuits to regulate feeding decisions.
Structure and plasticity in Drosophila feeding behavior
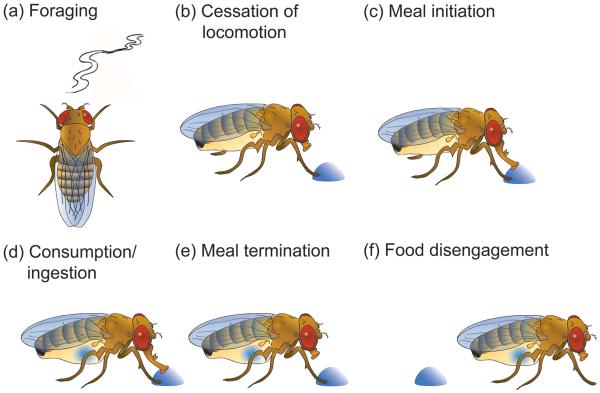
Drosophila feeding behavior is composed of a series of behavioral modules or subprograms (Figure 1). In a food-deprived state, an adult fruit fly will forage for potential food sources (a, panel a, Figure 1) [3,4]. Chemosensory detection of a palatable food leads to cessation of locomotion (b), meal initiation (c), and consumption (d) [5,6]. Post-ingestive signals generated during consumption cause meal termination (e) and disengagement from the food source (f) by reactivating locomotion. Flexibility in this behavioral ensemble depends on the molecular and cellular mechanisms that couple internal states to altered physiology of the nervous system.
Figure 1. Modules in the feeding behavioral repertoire in Drosophila melanogaster.
(a) Flies use odor cues during foraging to find food. (b)Activation of chemosensory bristles on tarsi leads to cessation of locomotion and (c) meal initiation. (d) Activation of sensory bristles on the proboscis leads to food ingestion. (e) Post-ingestive signals lead to meal termination and (f) food disengagement.
Nutritional status is converted into neuromodulatory states
The central nervous system monitors systemic energy balance and alters feeding probability based on internal nutritional state. Remarkably, recent studies reveal that a small number of specialized central brain neurons directly sense specific circulating macronutrients and modify feeding. Behavioral evidence that flies have internal nutrient sensors came from studies showing that flies can distinguish sugars based solely on caloric content in the absence of sweet taste detection [7•-10]. One internal nutrient sensor is Gr43a, a gustatory receptor that is expressed in taste neurons in the periphery and a few central neurons in the superior protocerebrum [11••]. These central neurons directly sense circulating fructose levels and promote feeding in nutrient-deprived flies. A second internal sensor has been identified that selectively reports amino acid availability [12••]. In Drosophila larvae and mammals, vacant tRNAs generated by amino acid depletion activate a conserved kinase GCN2 that leads to a behavioral change preventing feeding on food with amino acid imbalances [13]. In Drosophila, GCN2 is activated in three central dopaminergic neurons to trigger an intracellular signaling cascade that leads to reduced sensitivity to GABAergic input, increased neuronal activity and dopamine release, resulting in food rejection. These studies thus identify central brain mechanisms that sense availability of specific nutrients, convert it to a change in neuromodulator output, and promote or inhibit feeding. Importantly, comparable central mechanisms are likely to exist for other classes of macronutrients and in other animals. For example, there is accumulating evidence that central sensors directly detect the levels of circulating carbohydrates and amino acids in mammals [13-15].
Peripheral tissues also report changes in physiological state by producing systemic endocrine signals. For example, leptin and insulin are adiposity signals secreted in proportion to available energy reserves that reduce food intake in mammals [16]. Similarly, the fat body and the corpus cardiacum are two endocrine organs in Drosophila that coordinate metabolism and behavior with nutritional state by secreting systemic signals [17]. The fat body combines the functions of mammalian adipose tissue and liver [18]. It monitors available carbohydrate, amino acid and fat levels and secretes humoral signals such as the leptin ortholog Unpaired2 (Upd2) to modify physiology [19,20•]. Interestingly, Upd2 controls insulin release in medial neurosecretory cells (mNSCs) that regulate food intake [21,22]. Similarly, the corpus cardiacum plays an important role in glucose homeostasis by secreting the adipokinetic hormone (AKH) – a functional homolog of mammalian glucagon. Corpus cardiacum cells depolarize and secrete AKH under conditions of energy deprivation. AKH mobilizes stored energy reserves by triggering lipolysis, glycogenolysis and trehalose release in the fat body [23]. AKH signaling also adjusts selective aspects of feeding behavior [3,24,25].
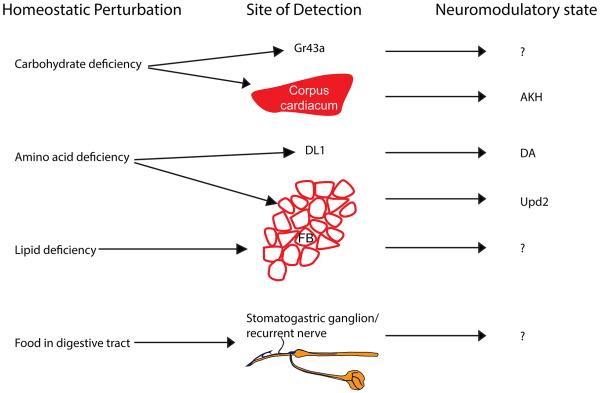
In most vertebrate and invertebrate species, feeding thresholds are rapidly modulated during the course of a meal before systemic homeostasis is restored [6,26]. This indicates that feeding thresholds are at least in part established by signals emanating from the digestive tract such as cholecystokinin and other gut hormones that suppress food intake in mammals [27]. Although the role of post-ingestive signals has not been studied in Drosophila, work in the blowfly demonstrated that the recurrent nerve connecting the visceral nervous system to the CNS is important to inhibit consumption [28]. In summary, evidence from both vertebrates as well as insects suggests that animals have evolved multiple feedback mechanisms to monitor the physiological status of the organism [29]. Imbalances in macronutrient homeostasis are translated into neuromodulator and endocrine states that are read out by downstream circuits (Figure 2).
Figure 2. Conversion of homeostatic perturbations into neuromodulatory states.
Metabolic needs are detected by designated peripheral organs, or directly by central neurons, that convert the physiological perturbation into an endocrine or neuromodulatory signal. AKH – adipokinetic hormone, DA – dopamine, DL1 – cluster of dopaminergic neurons, FB – fat body, Gr43a – protocerebral neurons expressing the fructose receptor Gr43a, Upd2 – unpaired 2, ‘?’ – unknown neuromodulator.
Converting neuromodulatory states into feeding decisions
A dozen neuromodulatory systems in Drosophila have been implicated to date in food intake regulation. Many of the signaling systems appear to be functionally conserved throughout evolution, including orthologs for mammalian peptidergic signals tachykinin, cholecystokinin, neuropeptide Y, Neuromedin U and insulin [21,30•-33]. Although our understanding of how neuromodulators sway feeding decisions is far from complete, recent studies serve to illustrate conserved functions of neuromodulators (please see [1,2] for general reviews).
Antagonistic actions of neuromodulators
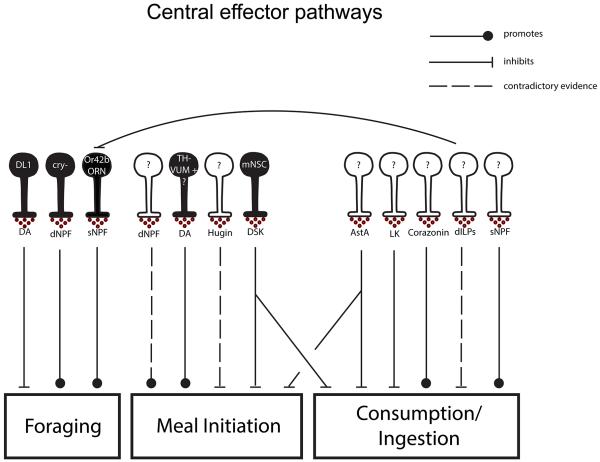
Multiple central effector pathways have been identified that report hunger and satiety states and antagonistically regulate feeding (Figure 3). For example, both small Neuropeptide F (sNPF) and corazonin are peptides that promote consumption. Overexpression of sNPF promotes short-term consumption in adults [34] and activation of corazonin neurons causes increased sucrose consumption in starved flies [35]. Whether these cues independently and coordinately promote intake is unknown.
Figure 3. Central neuromodulatory systems that regulate individual feeding modules.
Neuromodulatory systems that have been implicated to date in the regulation of one or more feeding subprograms. DA – dopamine, LK-leucokinin, DSK – drosulfakinin, ‘cry-’ – cryptochrome negative NPF neurons. Filled neurons – identified causal cells releasing respective neuromodulator, unfilled neurons – causal cells releasing respective neuromodulators unknown.
Different sets of neuropeptides inhibit feeding. Drosulfakinin (DSK) is the fly ortholog of cholecystokinin in mammals, which is a canonical satiety signal released from the gut. Reducing DSK expression solely in a small subset of medial neurosecretory cells (mNSCs) increases food intake by 40% in hungry flies and increases the probability of consuming food containing deterrents [21]. Interestingly, these cells also co-express Drosophila insulin-like peptides (dILPS), suggesting that multiple satiety signals may be co-released. Another anorexigenic insect neuropeptide is allatostatin A (AstA), which suppresses both meal initiation and ingestion of appetitive substances [35]. The targets for AstA neurons, DSK and dILPs are not resolved. The complexity of neuromodulatory regulation demonstrates that feeding and termination of feeding are antagonistic actions influenced by different sets of neuromodulators in response to different internal states.
Neuromodulator release site dictates function
A single neuromodulator can have multiple and sometimes conflicting roles in a single behavior. Recent studies demonstrate that dopamine influences feeding by multiple mechanisms. Using an elegant molecular genetic approach to label neurons that respond to dopamine, Inagaki and colleagues showed that sugar-sensing gustatory neurons are targets for dopamine in hungry flies. Dopamine is detected by the DopEcR receptor in sensory neurons, leading to increased taste-induced responses and reduced feeding thresholds after a period of starvation [36••]. A second dopaminergic mechanism that promotes feeding initiation requires the D2R receptor [37••]. Activation of a single dopamine neuron, TH-VUM, in the subesophageal zone triggers proboscis extension in a D2R-dependent manner. TH-VUM activity increases with food deprivation, suggesting that it links changes in nutritional state to changes in dopamine release, adjusting the probability of meal initiation to nutrients. Contrary to the orexigenic effects of DA, the same neuromodulator represses food intake in larvae in response to amino acid deprivation [12]. Therefore, neurons expressing the same neuromodulator are often subdivided into independent functional units that have different and sometimes antagonistic effects on behavior.
The observation that subsets of cells harboring the same neuromodulator can impact behavior in often divergent ways underscores the importance of localizing the precise cellular substrate giving rise to the modulatory signal. This is apparent for dopamine signaling, where a single dopaminergic neuron in the SOG promotes proboscis extension, a cluster of 6 cells in posteriolateral protocerebrum suppress the expression of appetitive memory and a different group of 3 cells in the DL1 cell cluster promotes food aversion [12,37,38]. These findings argue that small clusters or even a single cell can comprise functionally important neuromodulatory units that have profound and specific consequences on behavior.
Neuromodulators regulate the expression of behavioral subprograms
As is evident from the previous examples, neuromodulatory systems do not always act as global coordinators of behavior but often have more nuanced local effects, adjusting the expression of a single or a few feeding subprograms. For example, leucokinin, an ortholog of the mammalian anorexigenic neuropeptide tachykinin, selectively affects meal termination [30]. Mutations in the leucokinin or leucokinin receptor (LKR) genes or ablation of neurons expressing these genes causes an increase in meal size. Interestingly, however, overall food intake does not change due to compensatory changes in meal frequency. This demonstrates that some neuromodulatory systems can selectively influence the expression of a single subprogram of a behavior, thus changing the pattern of behavioral modules without a clear effect on long-term caloric intake.
Analyzing neuromodulator function with regard to particular feeding subprograms provides a way to reconcile a number of conflicting findings in the field. For example, Drosophila insulin-like peptides (dILPS) play an important yet complicated role in regulating feeding behaviors that is confounded by their role in carbohydrate metabolism. This is mirrored by the complex role insulin plays in mammalian energy homeostasis. Although mammalian insulin acts as an adiposity signal in the brain where it acts to suppress food intake, perturbing insulin function does not lead to obesity despite hyperphagic symptoms in type 1 diabetes, as insulin is required peripherally for macronutrient uptake in tissues [39]. Drosophila melanogaster has eight different insulin like peptides and one described insulin receptor (dInR) [33,40]. In larvae, pan-neuronal overexpression of dILP2 and dILP4 but not dILP3 led to a reduction in feeding rate [22]. Increased insulin signaling in adults results in reduced foraging behavior [41••]. In contrast to these reports, a series of studies have reported that insulin signaling has no discernable effects on meal initiation or overall food consumption in adult Drosophila [37,42,43]. This seems to suggest that insulin alters feeding by fine-tuning the expression of only a single or subset of feeding behavioral modules as opposed to acting as a global satiety signal suppressing all aspects of feeding. A more careful dissection of the role of different insulin peptides and their targets is warranted to clarify its role in food intake.
Single behavioral subprograms are fine-tuned by multiple modulator systems
Studies of starvation-dependent foraging illustrate that single behavioral subprograms are controlled by multiple neuromodulatory systems. For example, a recent study demonstrates that olfactory-driven foraging is upregulated by sNPF signaling and suppressed by insulin signaling [41]. Root and colleagues showed that olfactory receptor neurons (ORNs) expressing the Or42b receptor are required for attraction to apple cider vinegar under food-deprived conditions. The enhanced attraction is mediated by sNPF autocrine signaling in Or42b neurons that acts to increase presynaptic release. Under fed states, high insulin downregulates sNPFR expression in ORNs, decreasing sensitivity. Thus a global change in insulin levels may be translated into a local change in sNPFR signaling, altering olfactory sensitivity. Beyond the first order relay, foraging is also regulated by dNPF – an ortholog of the mammalian orexigenic peptide NPY. In starved states, four central dNPF neurons are required for foraging behavior and mediate attraction to a wide variety of appetitive odors [4]. Thus, multiple signals likely acting at different sites can regulate foraging probability.
Identifying the targets of neuromodulation
For the majority of neuromodulators with significant effects on feeding, including allatostatin, hugin, corazonin, and others, the target neuronal populations have not been identified [32,35•]. Molecular genetic tools, such as TANGO-map, that enable visualization of neurons activated in response to specific neuromodulatory signals, will be indispensable to bridge this gap [36]. Previous work in crustaceans suggests that almost every neuron and synapse is subject to neuromodulation by one or more modulatory systems [44]. Regulation of the Drosophila feeding circuit is likely to be equally complex. Peptidergic and aminergic modulators regulate the gain of appetitive sensory stimuli already at the axonal terminals of first-order sensory neurons [36,41]. Although some of the plasticity in feeding behavior can be ascribed to regulation of sensory neurons, this is unlikely to account for all observed behavioral adaptability [6]. A better understanding of the neural circuitry for feeding will provide a basis for examining modulatory mechanisms that promote or inhibit feeding-associated behaviors.
Conclusions
Recent work in Drosophila and other model systems has revealed how neuromodulators respond to changes in nutrient availability to adjust discrete aspects of feeding. Systemic signals from peripheral tissues and circulating nutrients report nutritional status to the brain. Central effector pathways release neuromodulators to independently promote or inhibit discrete feeding behavioral subprograms. Recent studies have made rapid progress by identifying and characterizing key signals that regulate feeding. Future efforts to determine the hierarchy or independence of neuromodulatory interactions, the neural circuit changes associated with neuromodulation, and the temporal dynamics of regulation will be important to examine how feeding is dynamically regulated over the course of a meal, a day or a lifetime.
Highlights.
Signals from peripheral tissues and circulating nutrients report nutritional status
Multiple cues signal hunger and satiety
Subprograms of feeding behavior are under independent modulatory control
Acknowledgements
K.S. is supported by a grant from the NIH (DK098747) and an HHMI Early Career Scientist award.
A-H.P. was supported by a predoctoral grant from Boehringer-Ingelheim Fonds. We would like to thank Dr. Christoph Scheper for help with Figure 1 and Dr. Brendan Mullaney for comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
Nothing declared.
References
- 1.Bargmann CI. Beyond the connectome: how neuromodulators shape neural circuits. Bioessays. 2012;34:458–65. doi: 10.1002/bies.201100185. [DOI] [PubMed] [Google Scholar]
- 2.Marder E. Neuromodulation of neuronal circuits: back to the future. Neuron. 2012;76:1–11. doi: 10.1016/j.neuron.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee G, Park JH. Hemolymph sugar homeostasis and starvation-induced hyperactivity affected by genetic manipulations of the adipokinetic hormone-encoding gene in Drosophila melanogaster. Genetics. 2004;167:311–323. doi: 10.1534/genetics.167.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beshel J, Zhong Y. Graded encoding of food odor value in the Drosophila brain. J. Neurosci. 2013;33:15693–704. doi: 10.1523/JNEUROSCI.2605-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edgecomb RS, Harth CE, Schneiderman AM. Regulation of feeding behavior in adult Drosophila melanogaster varies with feeding regime and nutritional state. J Exp Biol. 1994;197:215–235. doi: 10.1242/jeb.197.1.215. [DOI] [PubMed] [Google Scholar]
- 6.Dethier VG. The Hungry Fly. Harvard University Press; 1976. [Google Scholar]
- 7•.Dus M, Min S, Keene AC, Lee GY, Suh GSB. Taste-independent detection of the caloric content of sugar in Drosophila. Proc. Natl. Acad. Sci. U. S. A. 2011;108:11644–9. doi: 10.1073/pnas.1017096108. The authors show that Drosophila can form a food preference based on the caloric content of the food in the absence of sweet sensing taste receptors or functional gustatory receptor neurons.
- 8.Fujita M, Tanimura T. Drosophila evaluates and learns the nutritional value of sugars. Curr. Biol. 2011;21:751–755. doi: 10.1016/j.cub.2011.03.058. [DOI] [PubMed] [Google Scholar]
- 9•.Stafford JW, Lynd KM, Jung AY, Gordon MD. Integration of taste and calorie sensing in Drosophila. J. Neurosci. 2012;32:14767–14774. doi: 10.1523/JNEUROSCI.1887-12.2012. The authors show that Drosophila uses both taste and calorie sensing to execute feeding decisions and that the relative contribution of each may change in time. These two systems enable flies to distinguish non-nutritive sugars from nutritive ones and adjust feeding preferences under conditions where sweet taste does not faithfully reflect caloric content.
- 10.Burke CJ, Waddell S. Remembering nutrient quality of sugar in Drosophila. Curr. Biol. 2011;21:746–750. doi: 10.1016/j.cub.2011.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11••.Miyamoto T, Slone J, Song X, Amrein H. A fructose receptor functions as a nutrient sensor in the Drosophila brain. Cell. 2012;151:1113–1125. doi: 10.1016/j.cell.2012.10.024. The study identifies a cluster of central neurons expressing the fructose receptor Gr43A that links food intake to circulating levels of carbohydrates.
- 12••.Bjordal M, Arquier N, Kniazeff J, Pin JP, Léopold P. Sensing of amino acids in a dopaminergic circuitry promotes rejection of an incomplete diet in Drosophila. Cell. 2014;156:510–521. doi: 10.1016/j.cell.2013.12.024. The study identifies 3 dopaminergic neurons that link amino acid availability to foraging behavior in Drosophila larvae. The authors show that lack of essential amino acids leads to increased DA output form the DL1 cluster of DA neurons in a GCN2 kinase dependent manner that in turn facilitates leaving amino acid poor food sources.
- 13.Hao S, Sharp JW, Ross-Inta CM, McDaniel BJ, Anthony TG, Wek RC, Cavener DR, McGrath BC, Rudell JB, Koehnle TJ, et al. Uncharged tRNA and sensing of amino acid deficiency in mammalian piriform cortex. Science. 2005;307:1776–1778. doi: 10.1126/science.1104882. [DOI] [PubMed] [Google Scholar]
- 14.Domingos AI, Sordillo A, Dietrich MO, Liu Z-W, Tellez L a, Vaynshteyn J, Ferreira JG, Ekstrand MI, Horvath TL, de Araujo IE, et al. Hypothalamic melanin concentrating hormone neurons communicate the nutrient value of sugar. Elife. 2013;2:e01462. doi: 10.7554/eLife.01462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.González JA, Jensen LT, Fugger L, Burdakov D. Metabolism-independent sugar sensing in central orexin neurons. Diabetes. 2008;57:2569–2576. doi: 10.2337/db08-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 17.Leopold P, Perrimon N. Drosophila and the genetics of the internal milieu. Nature. 2007;450:186–188. doi: 10.1038/nature06286. [DOI] [PubMed] [Google Scholar]
- 18.Buch S, Pankratz MJ. Making metabolic decisions in Drosophila. Fly (Austin) 2009;3:74–77. doi: 10.4161/fly.3.1.7795. [DOI] [PubMed] [Google Scholar]
- 19.Geminard C, Rulifson EJ, Leopold P. Remote control of insulin secretion by fat cells in Drosophila. Cell Metab. 2009;10:199–207. doi: 10.1016/j.cmet.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 20•.Rajan A, Perrimon N. Drosophila cytokine unpaired 2 regulates physiological homeostasis by remotely controlling insulin secretion. Cell. 2012;151:123–137. doi: 10.1016/j.cell.2012.08.019. The study identifies Unpaired 2 (Upd2) as the homolog of the mammalian adiposity signal leptin. Upd2 is secreted from the fat body in fed states and regulates activity in insulin secreting cells in the brain that regulate growth and energy metabolism.
- 21.Söderberg J a E, Carlsson M a, Nässel DR. Insulin-producing cells in the Drosophila brain also Express satiety-inducing cholecystokinin-like peptide, drosulfakinin. Front. Endocrinol. (Lausanne) 2012;3:109. doi: 10.3389/fendo.2012.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Q, Zhang Y, Xu J, Shen P. Regulation of hunger-driven behaviors by neural ribosomal S6 kinase in Drosophila. Proc Natl Acad Sci U S A. 2005;102:13289–13294. doi: 10.1073/pnas.0501914102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim SK, Rulifson EJ. Conserved mechanisms of glucose sensing and regulation by Drosophila corpora cardiaca cells. Nature. 2004;431:316–320. doi: 10.1038/nature02897. [DOI] [PubMed] [Google Scholar]
- 24.Gruber F, Knapek S, Fujita M, Matsuo K, Bräcker L, Shinzato N, Siwanowicz I, Tanimura T, Tanimoto H. Suppression of conditioned odor approach by feeding is independent of taste and nutritional value in Drosophila. Curr. Biol. 2013 doi: 10.1016/j.cub.2013.02.010. doi:10.1016/j.cub.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 25.Bharucha KN, Tarr P, Zipursky SL. A glucagon-like endocrine pathway in Drosophila modulates both lipid and carbohydrate homeostasis. J Exp Biol. 2008;211:3103–3110. doi: 10.1242/jeb.016451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woods SC, Seeley RJ, Porte D, Jr., Schwartz MW. Signals that regulate food intake and energy homeostasis. Science. 1998;280:1378–1383. doi: 10.1126/science.280.5368.1378. [DOI] [PubMed] [Google Scholar]
- 27.Murphy KG, Bloom SR. Gut hormones and the regulation of energy homeostasis. Nature. 2006;444:854–859. doi: 10.1038/nature05484. [DOI] [PubMed] [Google Scholar]
- 28.Dethier VG, Bodenstein D. Hunger in the blowfly. Z. Tierpsychol. 1958;15:129–140. [Google Scholar]
- 29.Miyamoto T, Wright G, Amrein H. Nutrient sensors. Curr. Biol. 2013;23:R369–373. doi: 10.1016/j.cub.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30•.Al-Anzi B, Armand E, Nagamei P, Olszewski M, Sapin V, Waters C, Zinn K, Wyman RJ, Benzer S. The leucokinin pathway and its neurons regulate meal size in Drosophila. Curr Biol. 2010;20:969–978. doi: 10.1016/j.cub.2010.04.039. The authors identify leucokinin as a neuropeptide that selectively regulates meal size.
- 31.Wu Q, Wen T, Lee G, Park JH, Cai HN, Shen P. Developmental control of foraging and social behavior by the Drosophila neuropeptide Y-like system. Neuron. 2003;39:147–161. doi: 10.1016/s0896-6273(03)00396-9. [DOI] [PubMed] [Google Scholar]
- 32.Melcher C, Pankratz MJ. Candidate gustatory interneurons modulating feeding behavior in the Drosophila brain. PLoS Biol. 2005;3:e305. doi: 10.1371/journal.pbio.0030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baker KD, Thummel CS. Diabetic larvae and obese flies-emerging studies of metabolism in Drosophila. Cell Metab. 2007;6:257–266. doi: 10.1016/j.cmet.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee KS, You KH, Choo JK, Han YM, Yu K. Drosophila short neuropeptide F regulates food intake and body size. J Biol Chem. 2004;279:50781–50789. doi: 10.1074/jbc.M407842200. [DOI] [PubMed] [Google Scholar]
- 35•.Hergarden AC, Tayler TD, Anderson DJ. Allatostatin-A neurons inhibit feeding behavior in adult Drosophila. Proc. Natl. Acad. Sci. 2012;109:3967–3972. doi: 10.1073/pnas.1200778109. The study identifies allatostatin-A as a potent food intake suppressing neuromodulator that regulates multiple feeding subprograms.
- 36••.Inagaki HK, Jagadish S, Barnea G, Ishimoto H, Ben-Tabou de-Leon S, Wong AM, Kitamoto T, Axel R, Anderson DJ. Visualizing neuromodulation in vivo: TANGO-mapping of dopamine signaling reveals appetite control of sugar sensing. Cell. 2012;148:583–595. doi: 10.1016/j.cell.2011.12.022. The authors show that dopamine adjusts the gain of the taste system at the level of the gustatory sensory neuron. They demonstrate that hunger increases DA signaling in the SOG to enhance the sensory response to sugars. Furthermore, the authors adapt the TANGO-map system to Drosophila as a strategy to detect a change in a neuromodulatory signal.
- 37••.Marella S, Mann K, Scott K. Dopaminergic modulation of sucrose acceptance – behavior in Drosophila. Neuron. 2012;73:941–950. doi: 10.1016/j.neuron.2011.12.032. This study identifies a single dopaminergic neuron, TH-VUM, that regulates meal initiation. Tonic activity in this neuron reports food deprivation time and acute activation directly triggers meal initiation.
- 38.Krashes MJ, DasGupta S, Vreede A, White B, Armstrong JD, Waddell S. A neural circuit mechanism integrating motivational state with memory expression in Drosophila. Cell. 2009;139:416–427. doi: 10.1016/j.cell.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwartz MW, Woods SC, Porte D, Jr., Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 40.Colombani J, Andersen DS, Leopold P. Secreted peptide Dilp8 coordinates Drosophila tissue growth with developmental timing. Science. 2012;336:582–585. doi: 10.1126/science.1216689. [DOI] [PubMed] [Google Scholar]
- 41••.Root CM, Ko KI, Jafari A, Wang JW. Presynaptic facilitation by neuropeptide signaling mediates odor-driven food search. Cell. 2011;145:133–144. doi: 10.1016/j.cell.2011.02.008. The authors show that starvation-dependent foraging is regulated by a global insulin signal that regulates an autocrine sNPF signal in Or42b positive ORNs. sNPFR epxression in Or42b ORNs is upregulated in starvation states and is required for starvation-induced foraging. This is another example where behavior is regulated at the level of sensory neurons.
- 42.Broughton SJ, Slack C, Alic N, Metaxakis A, Bass TM, Driege Y, Partridge L. DILP-producing median neurosecretory cells in the Drosophila brain mediate the response of lifespan to nutrition. Aging Cell. 2010;9:336–346. doi: 10.1111/j.1474-9726.2010.00558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wong R, Piper MD, Wertheim B, Partridge L. Quantification of food intake in Drosophila. PLoS One. 2009;4:e6063. doi: 10.1371/journal.pone.0006063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harris-Warrick RM, Johnson BR. Checks and balances in neuromodulation. Front. Behav. Neurosci. 2010;4 doi: 10.3389/fnbeh.2010.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]