Abstract
In vitro fertilization (IVF) is used to produce mouse embryos for a variety of reasons. We evaluated the effect of the method of euthanasia on the fertilization rate in 2 different IVF protocols. Oocytes collected from C57BL/6J female mice euthanized by CO2 inhalation or cervical dislocation were used in IVF with fresh sperm from either wild-type or genetically engineered C57BL/6J. Compared with CO2 inhalation, cervical dislocation improved the resulting rate of fertilization by 18% in an IVF method using Cook media and by 13% in an IVF method using methyl-B cyclodextrin and reduced glutathione. The lower fertilization rate due to euthanasia by CO2 inhalation was accompanied by changes in blood pH and body temperature despite efforts to minimize temperature drops. In our hands, euthanasia by cervical dislocation improved fertilization rates and consequently reduced the number of egg-donor mice required.
Abbreviations: CD, cervical dislocation; IVF, in vitro fertilization; GE, genetically engineered
Responsible laboratory animal care and budgetary constraints require that laboratories reduce the number of animals used to fulfill their needs. In vitro fertilization (IVF) is a valuable tool used for efficient assisted reproduction in genetically engineered (GE) mice. IVF is also used for reconstituting mice from cryopreserved sperm, which enables laboratories to archive their valuable genetically engineered strains, thereby reducing animal numbers, rack space, and maintenance breeding.14 Over the last few years, several modifications of sperm cryopreservation and IVF have been published in an effort to improve the fertilization rate.2,13,17,22-24 Many publications do not clearly state how the mice were euthanized, and the effect of the euthanasia method on the fertilization rate has not been examined. This study compares 2 methods of euthanasia, CO2 inhalation and cervical dislocation (CD), and their effects on the rate of fertilization in 2 different IVF methods using C57BL/6J oocytes with fresh sperm from wildtype or GE C57BL/6J mice, to remove the variable of the sperm's ability to survive cryopreservation.
Materials and Methods
Animals.
All methods in the study were approved by the IACUC. Wildtype C57BL/6J male and female mice were obtained from The Jackson Laboratory (Bar Harbor, ME). Additional male mice were obtained from various colonies within our animal facility and are collectively referred to as genetically engineered (GE) male mice hereafter. They were viable, healthy male mice that had no known fertility phenotypes and had been outcrossed onto a predominately C57BL/6J background by investigators at the National Human Genome Research Institute (NIH, Bethesda, MD). The mice were housed in ventilated microisolation cages with 480-cm2 of floor space (Super Mouse 750, Lab Products, Seaford, DE) and containing bedding (NEPCO, Warrensburg, NY) and nesting material (Ancare, Bellmore, NY). All mice were maintained in a AAALAC-accredited animal facility on a 14:10-h light:dark cycle, at 22 ± 4 °C, with an average of 42% humidity and free-choice food (Prolab RMH 1800 Autoclavable, Lab Diet, Brentwood, MO) and water, as set forth in the Guide for Care and Use of Laboratory Animals.9 Mice were free of all known murine pathogens (Assessment Plus, Charles River, Wilmington, MA). Male mice were housed either individually or with no more than 4 littermates and had not been mated for at least 1 wk prior to IVF to optimize the number of sperm present. They ranged in age from 2 to 9 mo. Female mice were age-matched within experimental groups but varied from 6 to 18 wk of age between experiments and were housed at a maximum of 5 mice per cage.
Euthanasia methods. CO2 inhalation.
For mice euthanized by CO2 inhalation, animals were placed in a cage (Super Mouse 750, Lab Products) measuring 30 cm L × 16 cm W × 14 cm H, covered with a Euthanex lid (Euthanex, Allentown, PA). Medical Grade USP Grade A 100% carbon dioxide was administered using a Western Medica CO2 flow meter (Westlake, OH) at a displacement rate of 20%/L/min. After secession of agonal breathing, CD was performed on all mice to ensure death, as required by our IACUC guidelines.
CD.
Unanesthetized mice were euthanized by CD by a trained investigator in accordance with AVMA guidelines.12 The thumb and index finger were placed on either side of the base of the mouse's skull and with a quick pull of its tail, the cervical vertebrae were separated from the skull.
Comparison of euthanasia methods during IVF method A. Sperm collection.
A single wildtype or GE C57BL/6J male mouse was euthanized by CO2 inhalation for each experiment. Sperm collection was based on a modification of the method of Byers and colleagues.3 The vas deferens and cauda epididymis were removed, cleaned of any blood vessel and fat, and placed in 1.0 mL of Research Vitro Fertilization media (K-RVFE-50, Cook Medical, Brisbane, Australia) without an oil overlay in a center-well organ-culture dish (Falcon, 353037, Becton Dickinson, Franklin Lakes, NJ) containing 4 mL of sterile distilled water in the outer ring to control evaporation and that had been equilibrated in a 5% CO2 37 °C incubator (Hera Cell 150, Waltham, MA) overnight to achieve a pH of 7.4. The vas deferentia were emptied and the epididymides sliced 4 or 5 times with a no. 11 sterile scalpel (Cincinnati Surgical, Cincinnati, OH) to release sperm. The dish then was placed in the 5% CO2 incubator maintained at 37 °C and sperm allowed to capacitate for 15 to 30 min before use. After capacitation and immediately after the oocytes were added to each fertilization dish, 10 μL of the sperm suspension was added, without calculation of sperm concentration. The age of the male mice ranged from 2 to 9 mo.
Egg collection and IVF.
The IVF was done according to a modification of the method of Byers and colleagues.3 For each experiment, wildtype C57BL/J6 female mice were superovulated with pregnant mare serum gonadotropin (5 IU IP; National Hormone and Peptide Program), followed by human chorionic gonadotropin (5 IU IP; Novarel, Ferring Pharmaceuticals Parsippany, NJ) 47 h later. Superovulated female mice were euthanized 13 to 14 h after injection of human chorionic gonadotropin injection.
All dishes for egg collection, fertilization, washing, and incubation were made with Research Vitro Fertilization media (K-RVFE-50, Cook Medical) and overlaid with sterile mineral oil (M5310, Sigma, St Louis, MO). The dish for collecting oocytes was a 35-mm dish (430165, Corning, Corning, NY) containing a 150-μL drop of media. A 60-mm nontreated suspension culture dish (430589, Corning) was used to contain a 500-μL fertilization drop, 3 wash drops (150-μL each), and a fourth 150-μL media drop for overnight incubation after fertilization. Prior to use, all dishes were placed in a Billups–Rothenberg modular incubator chamber (Del Mar, CA), flushed with a mixture of 5% CO2, 5% O2, and 90% N2 and incubated at 37 °C overnight.
For female mice euthanized by CO2 inhalation, groups of 3 were euthanized as described earlier. In addition, the euthanasia cage was lined only with a paper towel and placed on a 32 °C slide warmer (model 26020, Labline, Melrose Park, IL), monitored with an infrared thermometer (IR400, Extech, Nashua, NH). For dissection, female mice were immediately placed on a 47 to 50 °C slide warmer. Fallopian tubes (oviducts) were removed and placed in the mineral oil of the warm egg-collection dish. Each cumulus–oocyte complex was removed from the ampulla and pulled by using no. 5 Dumont forceps (Fine Scientific Tools, Foster City, CA) through the oil to the drop of media. Once all cumulus–oocyte complexes had been added to the drop, a 20-μL wide-bore pipette tip (HR-250WS, Rainin, Oakland, CA) was used to add them to the 500-μL fertilization drop in the fertilization dish. The average of the time from beginning CO2 administration until the addition of sperm was 10 min 28 s (n = 17; 1 SD, 1 min 37 s).
For female mice euthanized by CD, groups of 3 mice were euthanized as described previously and immediately placed on the 47 to 50 °C slide warmer. Their oviducts were removed and oocytes treated in the same way as for the CO2 method. The average of the time from CD until the addition of sperm was 5 min 27 s (n = 18; 1 SD, 1 min 19 s).
Each experiment included at least 3 fertilization dishes for each method of euthanasia. Euthanasia alternated between CO2 inhalation and CD to minimize any skewing of results due to the length of time after sperm capacitation or injection of human chorionic gonadotropin.
After the addition of sperm and oocytes, the dishes were incubated either in a bench-top incubator (model BT37, Planer, Middlesex, UK) or Billups–Rothenberg chamber for 4.5 to 5 h under a mixture of 5% CO2, 5% O2, and 90% N2 and maintained at 37 °C. Any fragmented eggs were discarded. By using a pulled glass transfer pipette, the remaining eggs were rinsed in the 3 wash drops and incubated in the fourth drop overnight in the previously mentioned incubators.
The following morning, at approximately 18 h after combining oocytes and sperm in a fertilization drop, the numbers of 2-cell embryos, 1-cell embryos, dead embryos, and fragmented eggs were counted and used to calculate fertilization percentages. In experiments using only wildtype C57BL/6J female and male mice, the 2-cell embryos were cultured to blastocysts in KSOM media plus amino acids (MR-121-D, Specialty Media, EMD Millipore, Billerica, MA) in a 5% CO2 37 °C incubator. In the experiments using C57BL/6J GE male mice, the 2-cell embryos were cryopreserved by the protocol described by Glenister.7
CO2 inhalation compared with CD in IVF method B. IVF and sperm dishes.
All dishes were prepared the morning of the IVF. All chemicals used in the media came from Sigma. Sperm dishes for preincubation were made according to Takeo23,24 and incubated in the benchtop incubator with the 3-gas mixture at 37 °C for 20 min. The IVF dishes were made according to Takeo,23,24 except for minor changes. The egg collection dish was a 30-mm dish with a 150-μL drop of fertilization media comprising high-calcium human tubular fluid media16 supplemented with 1 mM reduced glutathione (G6013, Sigma) overlaid with sterile mineral oil. The fertilization dish was a 60-mm dish with a 200-μL drop of fertilization media and 4 drops (150 μL each) high-calcium human tubular fluid media without reduced glutathione overlaid with sterile mineral oil. The egg collection and fertilization dishes were incubated at 37 °C under a mixture of 5% CO2, 5% O2, and 90% N2 for at least 30 min before use. There was a minimum of 2 dishes for each method of euthanasia per experiment.
Sperm collection.
A single male mouse was euthanized by CO2 inhalation for each experiment. The cauda epididymides were dissected, cleaned of any blood and fat, and placed in the oil of the sperm preincubation dish. After a small cut was made in each epididymis by using a sterile no. 11 scalpel, a small bead of sperm was squeezed out and pulled with sterile no. 5 Dumont forceps through the oil into the media drop. The dish was placed back in the incubator for a minimum of 1 h.
Egg collection and IVF.
The oocytes were harvested in the same manner as for method A, except that while the CO2 was being administered, the cages were not on a slide warmer. The methods of euthanasia, CO2 inhalation and CD, were alternated as previously described. Once euthanasia was complete, the mice were dissected on a 47 to 50 °C slide warmer. The oviducts were removed and placed in the mineral oil of a 37 °C, equilibrated 30-mm dish containing a 150-μL drop of fertilization media. The cumulus–oocyte complexes were transferred to the 60-mm dish containing the 200-μL fertilization drop, as described in method A. By using a 20-μL pipette tip (RT-L10F, Rainin) that had been trimmed to an angle of approximately 45° with a razor blade, preincubated swimming sperm was collected from the outer edge of the sperm preincubation drop and added to the cumulus–oocyte complexes in the fertilization drop. All dishes in a given experimental group received the same volume of sperm. The concentration of the GE mouse sperm was determined by visual subjective judgment based on experience and the shedding of cumulus cells within 20 min of adding sperm. The volume added to the cumulus–oocyte complexes ranged from 3 to 10 μL, with 5 μL being the amount most commonly used.
The remaining steps of the method B IVF procedure were performed as described for method A. Approximately fifty 2-cell embryos from each euthanasia method were cultured to blastocyst stage in KSOM plus amino acids (MR-121-D, END Millipore). All other 2-cell embryos were cryopreserved according to the protocol described by Glenister.7
Measurement of blood pH.
Five C57BL/6J female mice were euthanized by CO2 inhalation as described earlier. As soon as euthanasia was complete, a 1-mL syringe with a 23-gauge needle was used to obtain blood by cardiocentesis. Without exposure to air, approximately 100 μL of the blood was transferred for immediate analysis for pCO2 and pH (iStat 200, Abaxis, Union City, CA) by using CG4+ cartridges (Abbott, Abbott Park, IL). The 5 female mice that were euthanized by CD were sampled in the same way. As a control, 4 female mice were deeply anesthetized with a 1.25% solution of 2,2,2-tribromoethanol (T48402 Sigma) in 2-methyl 2-butyl alcohol (24048-6 Sigma) at a dose of 0.02 mL/g IP, and blood pH and pCO2 were tested in the same manner.
Measurement of body temperature.
Five female mice each were euthanized by CO2 inhalation at room temperature, CO2 inhalation while the cage was on a 32 °C slide warmer, or CD. Immediately after death, temperatures were taken by using a rectal temperature probe (Microtherma 2 with a RET-3 probe, Thermoworks, Alpine, UT), after which the mice were placed on a slide warmer (47 to 50 °C). Temperatures were taken at 1-min intervals for 5 min, to record any changes over time.
Statistical analysis.
All statistical analysis was completed by using Prism 6.0 (GraphPad Software, San Diego, CA). Fertilization rates for methods A and B (Table 1 and 2) were compared by using a Wilcoxon test. Results of 2-cells embryos progressing to blastocysts (Table 3) were assessed with a Fisher test. Blood chemistry results were analyzed by one-way ANOVA with posttests, and P values corrected for multiple testing. P values less than 0.05 are considered significant.
Table 1.
Fertilization rate for CO2compared with CD as type of euthanasia for IVF method A
| CO2 |
CD |
||||
| Experiment | Male strain | No. of eggs | No. (%) of 2-cell embryos | No. of eggs | No. (%) of 2-cell embryos |
| 1 | Wildtype B6 | 127 | 17 (13%) | 121 | 37 (31%) |
| 2 | Wildtype B6 | 184 | 43 (23%) | 171 | 83 (48%) |
| 3 | GE B6 | 271 | 215 (79%) | 296 | 255 (86%) |
| 4 | GE B6 | 267 | 11 (4%) | 184 | 40 (22%) |
| 5 | GE B6 | 294 | 200 (68%) | 336 | 271 (81%) |
| 6 | GE B6 | 292 | 210 (72%) | 339 | 268 (79%) |
| 7 | GE B6 | 290 | 19 (7%) | 298 | 35 (12%) |
| 8 | GE B6 | 255 | 101 (40%) | 243 | 190 (78%) |
| 9 | GE B6 | 394 | 108 (27%) | 300 | 125 (42%) |
| 10 | GE B6 | 286 | 57 (20%) | 211 | 59 (28%) |
| Total | 2660 | 981 (37%) | 2499 | 1363 (55%) | |
For all groups, the fertilization rate (no. of 2-cell embryos) was significantly (P < 0.005; Wilcoxon test) different between the CO2 and CD euthanasia methods.
Table 2.
Fertilization rate for CO2inhalation compared with CD as type of euthanasia for IVF method B
| CO2 inhalation |
CD |
||||
| Experiment | Male strain | No. of eggs | No. (%) of 2-cell embryos | No. of eggs | No. (%) of 2-cell embryos |
| 1 | GE B6 | 181 | 139 (77) | 169 | 136 (80) |
| 2 | GE B6 | 101 | 45 (45) | 85 | 66 (78) |
| 3 | GE B6 | 104 | 72 (69) | 159 | 143 (90) |
| 4 | GE B6 | 97 | 79 (81) | 132 | 125 (95) |
| 5 | GE B6 | 121 | 108 (89) | 107 | 101 (94) |
| 6 | GE B6 | 152 | 120 (79) | 138 | 120 (87) |
| Total | 756 | 563 (74)a | 790 | 691 (87)a | |
The fertilization rate (no. of 2-cell embryos) differed significantly (P < 0.05; Wilcoxon test) between the CO2 and CD methods of euthanasia.
Table 3.
Numbers of C57Bl/6J embryos from method A that progressed to blastocysts in vitro
| Method of euthanasia | No. of Eggs | No. of 2-cell embryos | % Fertilization | No. of blastocysts | % of 2-cell embryos that progressed to blastocysts |
| CO2 inhalation | 311 | 60 | 19a | 37 | 62b |
| CD | 292 | 120 | 41a | 84 | 70b |
Rates of fertilization differed significantly (P < 0.001) between euthanasia methods.
Number of blastocysts from cultured 2-cell embryos did not differ (P = 0.312; Fisher test) between euthanasia methods.
Results
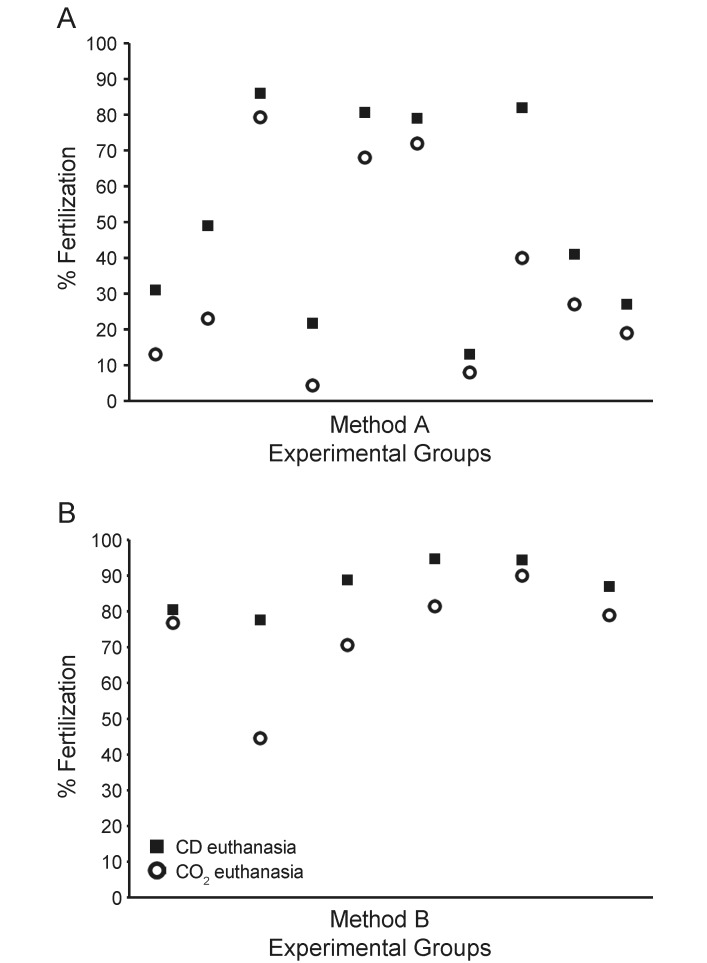
To determine whether the method of euthanasia used for female egg donors affected fertilization rates in IVF, we compared 2 euthanasia techniques, CD alone compared with CO2 inhalation followed by CD, in each of 2 different IVF methods. In the first IVF method (method A), the fertilization rates for oocytes obtained after CD alone averaged 55% compared with 37% for CO2 inhalation followed by CD, an 18% improvement. (Table 1 and Figure 1 A). For IVF method B, fertilization rates for oocytes obtained after CD averaged 87% compared with 74% for CO2 inhalation, a 13% improvement (Table 2 and Figure 1 B).
Figure 1.
Fertilization rates are higher with CD than CO2 in 2 IVF methods. (A) IVF method A. (B) IVF method B. Fertilization rates for each experimental group are shown for female euthanasia by CD (black square) or CO2 (open circle).
Because the fertilization rate varied, we investigated whether the euthanasia method affected the progression to blastocysts after IVF. The percentages of C57BL/6J 2-cell embryos proceeding to blastocysts from the 2 different euthanasia methods are shown in Table 3. In this small sample, twice as many 2-cell embryos were produced by CD IVF than by CO2 inhalation IVF, and of these 2-cell embryos, 70% progressed to blastocysts regardless of euthanasia method.
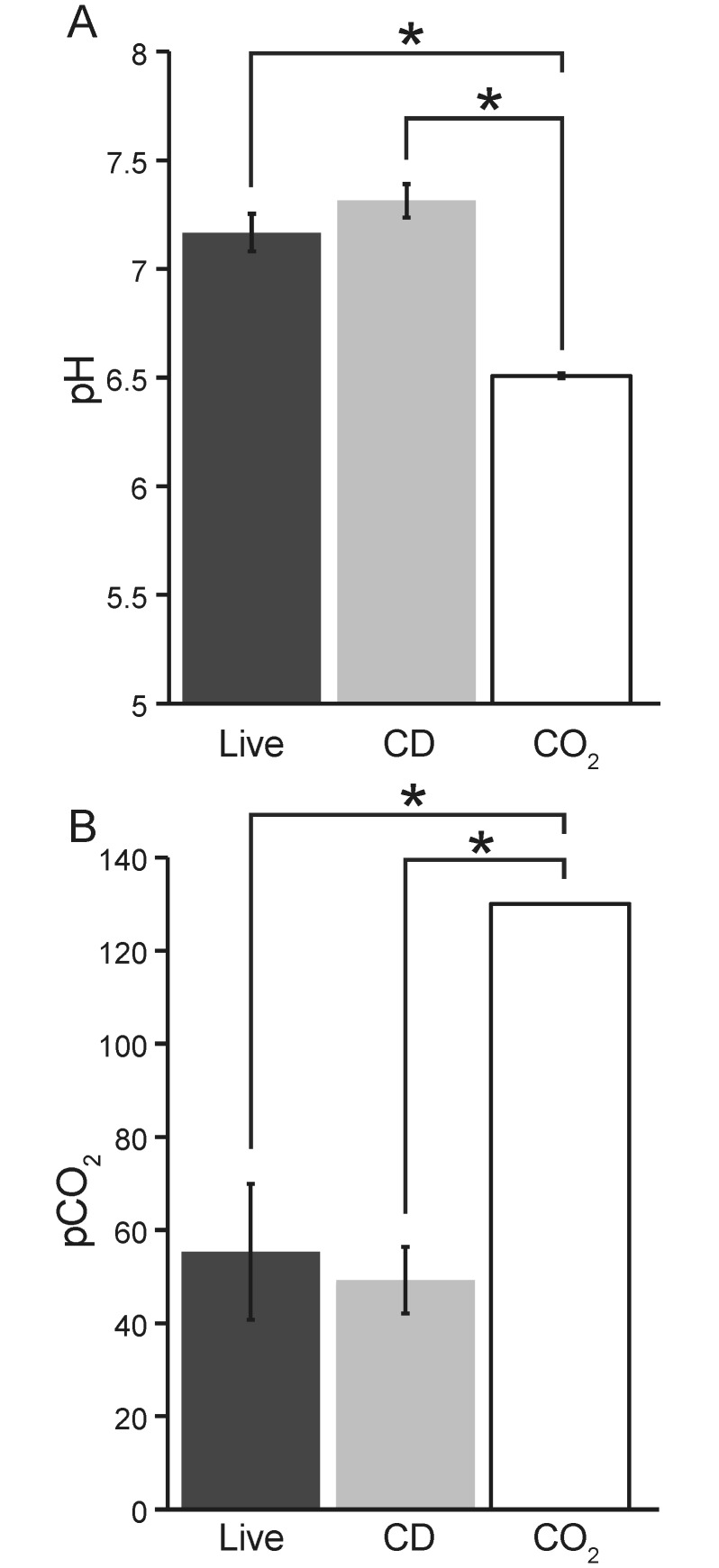
The blood pH of female mice euthanized by CO2 inhalation (6.5) was significantly lower than that of those euthanized by CD (7.31) and of live, tribromoethanol-anesthetized female mice (7.17; Figure 2). The pCO2, which is the measure of the partial pressure of CO2 in whole blood and used to diagnose respiratory-induced acid–base disturbances, was significantly (P < 0.05) higher for the female mice euthanized by CO2 inhalation (130 mm Hg) compared with those euthanized by CD (49.2 mm Hg) or anesthetized with tribromoethanol (55.33 mm Hg; Figure 2). In addition, for the mice euthanized by CO2, the actual pH may be lower and the actual pCO2 may be higher than recorded, because the levels recorded are the limits of measure for the analyzer used.
Figure 2.
Blood pH and pCO2 (mean ± 1 SD) of live (anesthetized; n = 4), CD-euthanized (n = 5), and CO2-euthanized (n = 5) female mice. *, Values significantly (P < 0.0001, one-way ANOVA) different. The testing system had a lower limit of 6.5 for pH and an upper limit of 130 mm Hg CO2.
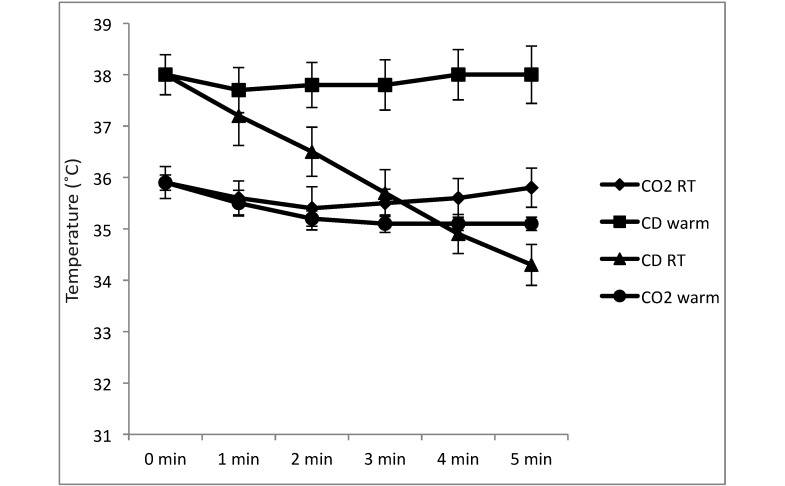
The body temperature at death was lower in the female mice euthanized by CO2 inhalation (35.9 °C) compared with CD (38 °C). The temperature did not vary by more than 1 °C over 5 min when euthanasia methods were combined (or not) with the use a slide warmer. Temperatures from additional female mice euthanized by CD but not put on a slide warmer after death are included for comparison (Figure 3).
Figure 3.
Change in body temperature (mean ± 1 SD) over time, with 0 being at time of death, for 4 groups (n = 5 each) of female mice: CO2 administered at room temperature followed by placement on a slide warmer for dissection (CO2 RT); CO2 administered on a slide warmer followed by placement on a slide warmer for dissection (CO2 warm); CD followed by placement on a slide warmer for dissection (CD warm); and cervical dislocation without subsequent placement on a slide warmer (CD RT).
Discussion
One of the most important principles of animal experimentation is to minimize animal use to achieve the experimental objectives. To this end, we measured the effect of the euthanasia method used during IVF. Because IVF is useful for rescuing a GE line due to a nonbreeding male mouse, for generating embryos for cryopreservation, for reconstituting a GE mouse line from frozen sperm, and for obtaining age-matched embryos, improving the fertilization rate reduces the number of egg donors needed. The current study used C57BL/6J mice, because currently this is the preferred background for many transgenic mouse constructs.8,10
Although whether using CO2 inhalation for euthanasia is humane has been debated,4,11 the AVMA Guidelines12 require the use of CO2 inhalation for euthanasia, unless euthanasia by CD is scientifically justified. A review of studies about the use of CO2 inhalation for euthanasia raised concerns that exposure to CO2 appears to cause pain and distress to the mice.4 In contrast to these sources, the 2013 AVMA Guidelines state that when CO2 is administered correctly, “animals will lose consciousness before CO2 concentrations become painful.”12
The administration of CO2 changes the respiration rate, heart rate, and blood pressure.4 One of the well-known physiologic effects of CO2 inhalation is the acidification of blood.12 A rise in the blood pCO2 concentration from the normal upper limit of 42 mm Hg usually results in a decrease in blood pH.5 The normal blood pH is 7.3 to 7.4.7 The depressant effects on breathing of the tribromoethanol anesthesia are reflected in the blood pH (7.17) and pCO2 (55.33 mm Hg) as compared with those values after CD euthanasia (pH 7.31 and 49.2 mm Hg). It has long been known that pH can affect the fertilization rate of mouse embryos when IVF is performed.15 In a previous study, oocytes were exposed for 1 h before IVF to media ranging in pH from 6.6 to 7.8. Below a pH of 7.2, significantly fewer oocytes progressed to the 2-cell stage.20 Although in our study oocytes from CO2-euthanized mice were exposed to a blood pH of 6.5 for a shorter time, some changes that altered fertilization may have occurred. All dishes, once made and incubated in the 3-gas mixture, were manipulated as little as possible to limit exposure to ambient air and maintain a pH of 7.4 to 7.5.
In a pharmacologic study, compared with CD, euthanasia of mice by CO2 inhalation lowered the blood pH, resulting in the redistribution of the study compounds from tissue and into the blood.1 The same study found that isoflurane overdose as a method of euthanasia did not appear to alter the pH of mouse blood compared with that after CD.1 In our pilot studies comparing isoflurane overdose, CO2 inhalation, and CD euthanasia, isoflurane overdose supported slightly better fertilization rates for IVF than CD (data not presented). Isoflurane has a negative effect on mouse oocytes, increasing the number of unusable oocytes, and CD euthanasia likely is a better method for obtaining oocytes.21 We speculate that our favorable results with isoflurane reflect differences due to strain background (CD1 compared with C56Bl/J6) and hormone dosage (10 IU compared with 5 IU each of pregnant mare serum gonadotropin and human chorionic gonadotropin). Given that most labs use CO2 inhalation for euthanasia4 and in light of concerns about the effects of isoflurane on oocytes, we chose to focus on differences between CO2 inhalation and CD as methods of euthanasia for IVF.
The male mice for our study were euthanized by CO2 inhalation. Ideally, testing the effect of the 2 euthanasia methods on sperm quality would require sperm sources that were identical but subjected to each method, which is not possible. Although using multiple animals for each group of GE mice may have compensated for individual differences within that GE group, we decided to only study the effects of euthanasia by CD and CO2 inhalation on oocytes.
When rodents are anesthetized, the body temperature can drop.5 CO2 euthanasia can initially have anesthetic effects.12 In method A, in an effort to maintain body temperature and prevent the oocytes from cooling, the cages of females to be euthanized by CO2 inhalation were placed on a slide warmer. Because the mice were very agitated when the slide warmer was at 37 °C, we decreased it to 32 °C. However even at the decreased temperature, the mice were still somewhat jumpy as the CO2 was administered, and this jumpiness was not observed when no slide warmer was used. The reason for this agitation is difficult to determine, but the behavior seemed to be an indication of distress. For that reason, and because the temperature of the CO2 gas lowered the temperature inside the cage used for euthanasia by only 0.4 °C, we did not use the slide warmer during CO2 inhalation for method B. For each dish, the oocytes from only 3 female mice were harvested, regardless of the number of oocytes present, so that each fertilization dish was exposed to room temperature for the same amount of time. The experiments were repeated to gather enough data to compensate for variations in the numbers of oocytes. Therefore the difference in fertilization rate between CO2 inhalation and CD might be attributed to the oocytes cooling, because the mice euthanized by CO2 inhalation compared with CD started at a cooler body temperature, regardless of whether the slide warmer was used, before being dissected on the slide warmer.
The time needed to remove the ampulla from 3 females and place it in the warm media is approximately 2 min. The average temperature at 2 min of the mice euthanized by CD and dissected on the warming tray was 37.8 °C, compared with 35.4 °C for those euthanized by CO2 inhalation. Studies have shown that cooling to room temperature can lead to changes in the meiotic spindles of human oocytes18 and mouse oocytes.19 Another study confirmed that cooling human oocytes to 32 °C led to spindle changes, and although warming to 37 °C appeared to allow the spindles to recover, the results suggested that repolymerization of the spindles was incomplete.25 In addition, compared with human oocytes, a greater percentage of mouse oocytes appears able to recover to normalcy.18 Additional study is required to determine whether the more subtle cooling experienced in the current study affected the oocytes. Regardless, we now routinely perform IVF of CD-euthanized female mice without using the warming tray during dissection, with no apparent loss of fertility (data not shown).
The length of time between the beginning of euthanasia and the addition of sperm to the oocytes averaged 10.5 min for CO2 inhalation and 5.5 min for CD. A question not answered in this study was the effect of the additional 5 min within the oviducts on the oocytes from the CO2-euthanized mice. A previous study showed that leaving the oviducts in the euthanized mouse for 30 min negatively affected the number of live ooctyes.6 Because the additional 5 min is required to achieve death when using CO2 inhalation, any possible effect of the delay would be difficult to avoid.
Although the percentages of 2-cell embryos progressing to blastocysts were similar between euthanasia methods (Table 3), fertilization rates differed significantly. This difference implies that once fertilization takes place, there is no advantage or disadvantage to either euthanasia method in regard to embryo development, but because CD euthanasia results in greater numbers of 2-cell embryos because of the increased fertilization rate, its use represents a refinement of the IVF protocol.
As can be seen in the direct comparison of the 2 methods of euthanasia, CD supported a higher rate of fertilization for IVF done by either the Byers method (method A)3 or the Takeo method (method B).23,24 The greatest variable in the fertilization rate was the sperm from different colonies of GE mice. Although the individual male used for each experimental group affected the fertilization rate, the method of euthanasia contributed to the overall rate. Even if a given male's sperm did not fertilize well, the fertilization rate still increased when oocytes were obtained after CD euthanasia compared with CO2 euthanasia. Given the importance of maintaining the correct pH and temperature of the oocytes and in reducing the number of egg donors needed because of the increase in the fertilization, CD for IVF procedures appears to be scientifically justified as the appropriate method of euthanasia and fulfills the goals of reducing animal numbers and refining methodology.
Acknowledgments
This research was supported by the Intramural Research Program of the National Human Genome Research Institute, NIH.
We thank Drs Shelley Hoogstraten-Miller, Pam Schwartzberg, and Leslie Biesecker for their review and comments on this manuscript.
References
- 1.Angus DW, Baker J, Mason R, Martin I. 2008. The potential influence of CO2, as an agent for euthanasia, on the pharmacokinetics of basic compounds in rodents. Drug Metab Dispos 36:375–379. [DOI] [PubMed] [Google Scholar]
- 2.Bath ML. 2010. Inhibition of in vitro fertilizing capacity of cryopreserved mouse sperm by factors released by damaged sperm and stimulation by glutathion. PLoS ONE 5:e9387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Byers SL, Payson S, Taft R. 2005. Performance of 10 inbred mouse strains following assisted reproductive technologies (ARTs). Theriogenology 65:1716–1726. [DOI] [PubMed] [Google Scholar]
- 4.Conlee KM, Stephens ML, Rowan AN, King LA. 2005. Carbon dioxide for euthanasia: concerns regarding pain and distress, with special reference to mice and rats. Lab Anim 39:137–161. [DOI] [PubMed] [Google Scholar]
- 5.Flecknell P. 1996. Laboratory animal anaesthesia, 2nd ed, p 91, 98–100 San Diego (CA): Academic Press. [Google Scholar]
- 6.George MA, Doe BG. 1989. The influence of handling procedures during mouse oocyte and embryos recovery on viability and subsequent development in vitro. J In Vitro Fert Embryo Transfer 6:69–72. [DOI] [PubMed] [Google Scholar]
- 7.Glenister PH, Rall WF. 2000. Cryopreservation and rederivation of embryos and gametes, p 27–59 In: Jackson IJ, Abbott CM. Mouse genetics and transgenics: a practical approach. Oxford (UK): Oxford University Press. [Google Scholar]
- 8.Gondo Y. 2010. Now and future of mouse mutagenesis for human disease models. J Genet Genomics 37:559–572. [DOI] [PubMed] [Google Scholar]
- 9.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 10.International Mouse Knockout Consortium. Collins FS, Rossant J, Wurst W. 2007. A mouse for all reasons. Cell 128:9–13. [DOI] [PubMed] [Google Scholar]
- 11.Leach MC, Bowell V, Allan T, Morton D. 2002. Aversion to gaseous euthanasia agents in rats and mice. Comp Med 52:249–257. [PubMed] [Google Scholar]
- 12.Leary S, Underwood W, Anthony R, Cartner S, Corey D, Grandin T, Greenacre CB, Gwaltney-Bran S, McCrackin MA, Meyer R, Miller D, Shearer J, Yanong R. [Internet]. 2013. AVMA guidelines for the euthanasia of animals: 2013 edition. [Cited March 2014]. Available at: https://www.avma.org/KB/Policies/Documents/euthanasia.pdf [Google Scholar]
- 13.Liu L, Nutter L, Law N, McKerlie C. 2009. Sperm freezing and in vitro fertilization in 3 substrains of C57BL/6 mice. J Am Assoc Lab Anim Sci 48:39–43. [PMC free article] [PubMed] [Google Scholar]
- 14.Marschall S, Huffstadt U, Balling R, deAngelis MH. 1999. Reliable recovery of inbred mouse lines using cryopreserved spermatozoa. Mamm Genome 10:773–776. [DOI] [PubMed] [Google Scholar]
- 15.Miyamoto H, Toyoda Y, Chang MC. 1974. Effect of hydrogen-ion concentration on in vitro fertilization of mouse, golden hamster, and rat eggs. Biol Reprod 10:487–493. [DOI] [PubMed] [Google Scholar]
- 16.Nakagata N. 2011. Cryopreservation of mouse spermatozoa and in vitro fertilization, p 60 In: Hofker MA, van Deursen JM. Transgenic mouse methods and protocols: methods in molecular biology, vol 693 New York (NY): Humana Press. [DOI] [PubMed] [Google Scholar]
- 17.Ostermeier GC, Wiles MV, Farley JS, Taft RA. 2008. Conserving, distributing, and managing genetically modified mouse lines by sperm cryopreservation. PLoS ONE 3:e2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pickering SJ, Braude PR, Johnson MH, Cant A, Currie J. 1990. Transient cooling to room temperature can cause irreversible disruption of the meiotic spindle in the human oocyte. Fertil Steril 54:102–108. [DOI] [PubMed] [Google Scholar]
- 19.Pickering SJ, Johnson M. 1987. The influence of cooling on the organization of the meiotic spindle of the mouse oocyte. Hum Reprod 2:207–216. [DOI] [PubMed] [Google Scholar]
- 20.Puissant F, Degueldre M, Buisson L, Leroy F. 1986. Effects of carbon dioxide acidification of mouse oocytes before in vitro fertilization, culture, and transfer. Gamete Res 13:223–230. [Google Scholar]
- 21.Roustan A, Perrin J, Berthelot-Ricou A, Lopez E, Botta A, Courbiere B. 2012. Evaluating methods of mouse euthanasia on the oocyte quality: cervical dislocation versus isoflurane inhalation. Lab Anim 46:167–169. [DOI] [PubMed] [Google Scholar]
- 22.Taguma K, Nakamura C, Ozaki A, Suzuki C, Hachisu A, Kobayashi K, Mochida K, Ogura A, Kaneda H, Wakana S. 2009. A practical novel method for ensuring stable capacitation of spermatozoa from cryopreserved C57BL/6J sperm suspension. Exp Anim 58:395–401. [DOI] [PubMed] [Google Scholar]
- 23.Takeo T, Nakagata N. 2010. Combination medium of cryoprotective agents containing L-glutamine and methyl-B-cyclodextrin in a preincubation medium yields a high fertilization rate for cryopreserved C57BL/6J mouse sperm. Lab Anim 44:132–137. [DOI] [PubMed] [Google Scholar]
- 24.Takeo T, Nakagata N. 2011. Reduced glutathione enhances fertility of frozen–thawed C57BL/6 mouse sperm after exposure to methyl β-cyclodextrin. Biol Reprod 85:1066–1072. [DOI] [PubMed] [Google Scholar]
- 25.Wang WH, Ming L, Hackett RJ, Odenbourg R, Keefe D. 2001. Limited recovery of meiotic spindles in living human oocytes after cooling–rewarming observed using polarized light microscopy. Hum Reprod 16:2374–2378. [DOI] [PubMed] [Google Scholar]