Abstract
Rodent pinworms persist in many institutions, suggesting deficiencies in eradication and diagnostic processes. When pinworms are detected, treatment success is common, but false-negative test results during health surveillance or after treatment likely contribute to the continued presence of this parasite. PCR testing is not always practical, and increased information regarding the life cycle and general epidemiology of pinworm infestations could improve the sensitivity of traditional nonPCR detection methods and improve eradication efforts. We therefore investigated a pinworm (Syphacia muris) infestation in Sprague–Dawley rats (Rattus norvegicus) to develop a more accurate testing strategy. In addition, we sought to determine the duration of egg viability by using an in vitro hatching protocol to assess environmental persistence. Finally, we tested the ovicidal efficacy of a disinfectant used at our institution. Eggs were shed in higher numbers in the midafternoon as compared with other times of the day, and the sex of the host had no consistent effect on egg shedding. Egg shedding showed periodicity over time, with shedding decreasing to 0 at 2- to 3-wk intervals. Neither cecal examination nor tape tests alone reliably predicted pinworm infestation, and results of the 2 tests did not necessarily coincide. Eggs aged for as long as 7 mo remained viable, indicating a potential for recontamination from the environment. Finally, gaseous chlorine dioxide was an effective ovicidal agent, with a kill rate of 99.7%. These results suggest that strategies for S. muris eradication can be optimized to increase detection and elimination.
Rodent pinworms are oxyurid nematodes of the genera Syphacia and Aspiculuris. These parasites are detected routinely within rodent facilities, and their persistence despite control measures indicates deficiencies in the diagnostic and eradication processes. Many institutions never truly eradicate pinworms, presumably due to a failure to identify the presence of the organism or ineffective treatments once detected. However, a number of treatments are available and have proven to be efficacious,6,16,31 implying that other factors may be responsible for pinworm persistence. Although expense of detection, pervasiveness of eggs in the environment,23 longevity of egg viability,11,14 and ineffectiveness of traditional methods of sanitation14 are all potential factors, recent findings that PCR-based testing can discover parasites where traditional methods fail15,29 suggests that failure of detection is the primary reason for the persistence of these organisms.
The presence of pinworm infestations is undesirable due to potential negative effects on research efforts. For example, altered growth of certain strains of rats and mice has been linked to Syphacia muris infestation,27,39 and impaired electrolyte transport in spontaneously hypertensive S. muris-infested Wister–Kyoto rats was reported in another study.22 In addition, pinworms can affect behavior, as suggested by a study finding decreased exploratory behavior in mice with S. obvelata.25 Although most of these studies were retrospective in nature and results have not been duplicated, pinworms may be a true confounder in other areas of research.
More recent work has demonstrated that rodent pinworms modulate immune function. Syphacia infections can alter a host's humoral response to nonparasitic antigenic stimuli,34 elicit a Th2-type immune response with elevated cytokine production,26 and induce a Th2-mediated autoimmunity.1 In addition, rodent pinworms can increase myelopoiesis and erythropoiesis and alter bone marrow reactivity to interleukin 179 as well as manipulate signal transduction in the bone marrow cells of the host.18 Furthermore, pinworms may be a negative confounder in other models: nonobese diabetic mice with another helminth infection show a reduced incidence of insulin-dependent diabetes mellitus.12 The effects of pinworms can indeed be far-reaching, and efforts to control this parasite are indeed indicated.
S. muris is the most prevalent pinworm of rats. The life cycle of S. muris is direct and completed in 7 to 8 d,21 making this particular pinworm ideal for epidemiologic study. Adult worms of S. muris inhabit the cecum and colon, and female worms migrate to the anus and deposit all their eggs on the perianal region of the host before dying. The eggs embryonate within a few hours, at which point they are considered infective.35 Infection is believed to occur via 3 modes: 1) direct ingestion of the eggs; 2) ingestion of food or water contaminated with the eggs; and 3) retroinfection,10 although this mode has been debated. Ingestion of eggs is considered to be the primary mode of infection, and the eggs are reported to remain viable within the environment for as long as 4 wk.11,14 Antemortem diagnosis traditionally is made by identification of these eggs on a perianal cellophane tape, given the ease of collection and interpretation, although direct examination of the cecum postmortem is considered the most dependable method for S. muris diagnosis.4
Several nuances of the pinworm life cycle and even the test sample may reduce the sensitivity of diagnostic testing. Female S. muris are reported to deposit their eggs primarily in the afternoon,37 which may lead to false-negative test results if collection occurs in the morning. In addition, male mice are believed to be more susceptible to Aspiculuris tetraptera pinworm infection than are female mice,7 suggesting that testing of female mice could misrepresent infestation status. Furthermore, adult mice develop resistance to A. tetraptera infestation, as evidenced by the decreased numbers of larvae and adult worms in adult compared with weanling and juvenile mice.8 Selecting older animals for testing therefore may yield a false portrayal of infestation status. Finally, colony infestations may persist due to recontamination from the environment, given that these eggs are viable for at least 4 wk after deposition.14
In the present study, we investigated the time of maximal egg deposition, changes in infestation status with increasing age, and sex-associated differences in egg shedding. We also attempted to characterize the course of infestation, and we compared the ability of several traditional diagnostic tests to detect a known S. muris infestation. Finally, we investigated whether egg viability changes with time from deposition and whether our customary biosafety cabinet disinfectant, chlorine dioxide both in liquid and gaseous forms, affected S. muris eggs. The results of these investigations were used to recommend methods to increase the sensitivity of traditional detection methods for S. muris infestations in laboratory rats.
Materials and Methods
Rats and husbandry.
Five Sprague–Dawley rats (Crl:SD; 4 male, 1 female; age, birth to 6 mo) were donated from an inhouse colony after detection of a spontaneous infestation with S. muris during routine colony health surveillance. These rats subsequently were bred with additional Sprague–Dawley rats (Crl:SD) that were free of pinworms to perpetuate the infestation. All animals used in this study were either the 5 initial positive animals or their offspring. Sentinel testing indicated that all rats were negative for pneumonia virus of mice, reovirus, Sendai virus, lymphocytic choriomeningitis virus, rat coronavirus, sialodacryoadenitis virus, rat parvovirus, Kilham rat virus, Toolan H1 parvovirus, rat theilovirus, cilia-associated respiratory bacillus, and Mycoplasma pulmonis. Rats were also free of ectoparasites and endoparasites other than Syphacia muris on inhouse screening tests. Animals on study were maintained in an isolated quarantine area apart from the main colonies. Rats were pair-housed in individually ventilated cages (Allentown Caging Equipment, Allentown, PA) on a dedicated rack and provided ad libitum access to autoclaved rodent chow (2018SX, Harlan Teklad, Indianapolis, IN) and filtered reverse-osmosis–treated water via an automated in-cage watering system (Rees Scientific, Trenton, NJ). The room was maintained on a 14:10-h light:dark cycle. Room temperature was 22.8 ± 2 °C, and average humidity was between 30% and 70% with a target of 40% during the experimental period. The animal care and use program is accredited by AAALAC, all experimental procedures were reviewed and approved by the Johns Hopkins University Animal Care and Use Committee, and animals were maintained in accordance with the Guide for the Care and Use of Laboratory Animals.19
Perianal cellophane tape testing.
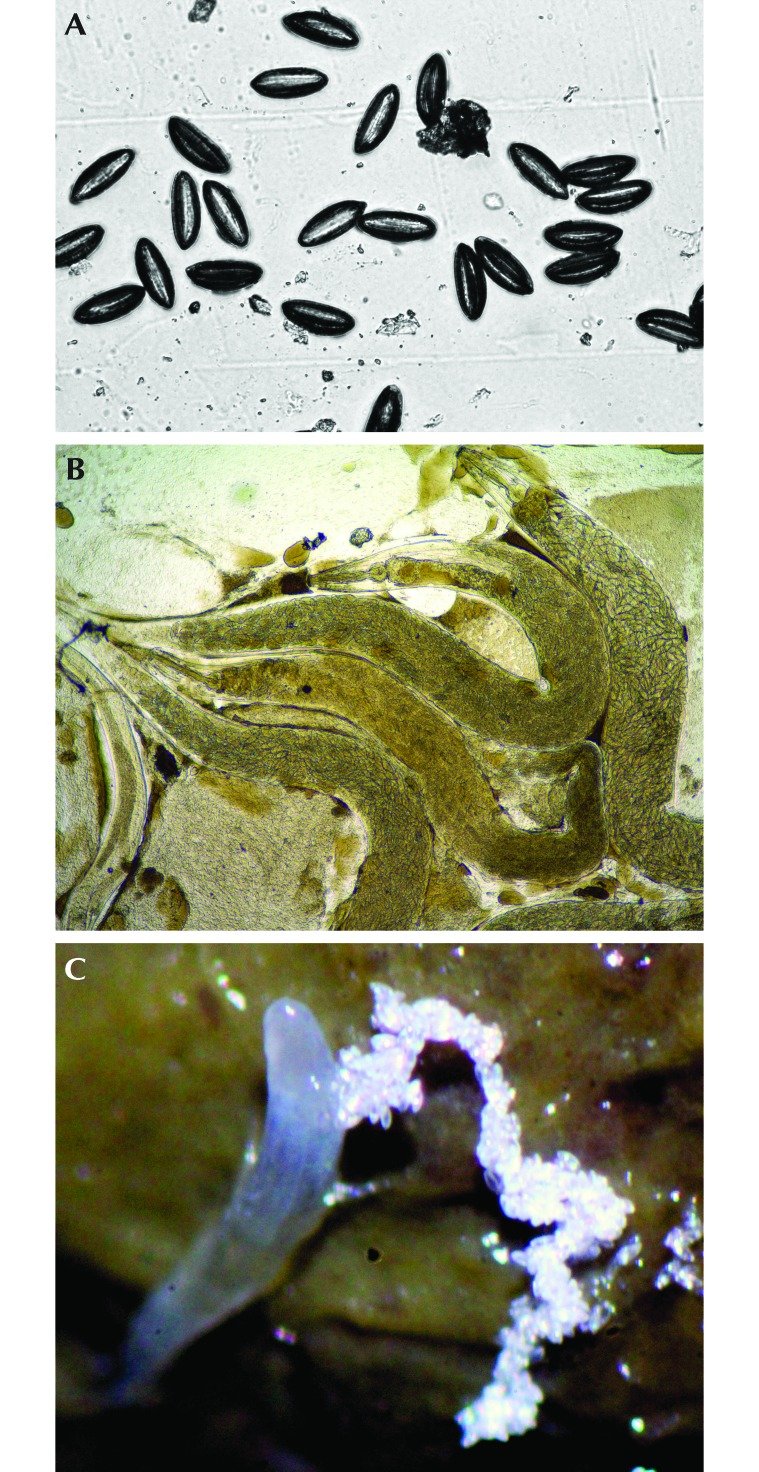
For each rat, a single piece of cellophane tape was firmly applied to the anal region 3 consecutive times at each time point. Tape length varied from 25 mm to 30 mm. Tapes were attached to standard 25 mm × 75 mm microscope slides, and eggs were counted by using a 4× microscope objective (Figure 1 A and B).
Figure 1.
Findings on perianal tape tests collected at 1400 in the afternoon include both Syphacia muris (A) eggs and (B) pinworms (b). Slides are unstained. (C) S. muris pinworms shedding eggs within the colon at necropsy.
Collection of daytime samples to determine peak egg shedding by time and by sex.
Ten rats confirmed positive by tape test (5 male, 5 female; age, 30 to 180 d) were used to determine the time of peak daytime egg shedding. Tape tests were performed at 5 time points (0800, 1000, 1200, 1400, and 1600) on 5 separate days over a period of 4 wk. Slides were randomized and blinded by an unbiased participant. One of the authors (TM) counted the number of eggs on each slide.
Collection of samples to characterize infestation.
Two separate litters from infected parents were followed to an age of 124 d. The 2 litters comprised 12 female and 12 male rats for a total of 24 animals. The 2 litters were asynchronous in time. At 21 d, animals were weaned into single-sex pair-housed cages, and the first perianal tape test was performed. Samples then were collected at 7-d intervals until and including day 124. All sampling occurred at 1400, according to preliminary data from the previous study component. These samples were read and the numbers of eggs recorded.
Necropsy.
We euthanized 24 rats via carbon dioxide asphyxiation at day 124. Tape tests were collected as described earlier. The gastrointestinal tract was exposed, and the cecum and colon were placed in a culture dish. These components were opened under a dissecting microscope and the contents examined by using saline to facilitate worm identification. The numbers of worms in the cecum and colon were counted and recorded for each rat (Figure 1 C). Necropsies occurred primarily during the afternoon and early-evening hours.
Hatching solution.
A variation of a previously published hatching solution14 was used. A solution of 1.6 g sodium phosphate dibasic heptahydrate (NA2HPO4 · 7H2O) and 95 mL deionized water was heated gently and subsequently cooled after the compound had dissolved. This solution was added to a solution of 0.07 g dibasic potassium phosphate (KH2PO4), 1.0 g porcine trypsin, and 5 mL deionized water. Two additional solutions were made and added: one composed of 0.26 g ox bile and 3 mL deionized water and the second composed of 0.2 g L-cysteine hydrochloride monohydrate (DL-C3H7NO2S) and 2.5 mL 1 N HCl. Once all solutions were mixed in a chemical fume hood, the resulting hatching medium was titrated to a pH of approximately 7.2. This pH reflected both our success rates in pilot studies and ideal values reported in the literature.3,38
Hatching protocol.
Hatching solution that had been prepared in the previous 24 h was stored in a flask and brought to a temperature of 37 °C by placement in a water bath. This temperature had previously been used successfully in egg hatching.14 Once the temperature was established, slides with eggs were immersed in the hatching solution within glass slide-staining receptacles, which were subsequently placed within the water bath. Cellophane tape collected for hatching experiments was inverted on a glass slide with the sticky surface placed outward to facilitate exposure of the eggs to the hatching solution. Slides were bathed in approximately 100 mL of the medium within these receptacles and allowed to sit overnight for 18 h. Slides were removed, rinsed with de-ionized water, allowed to dry, and then read under a 10× microscope objective. The numbers of hatched and unhatched eggs were counted separately. Hatching was considered to have occurred when any portion of the egg cuticle was disrupted. Most often, a worm-like appendage was seen to extrude from the egg itself.
Egg viability.
Eggs were collected on reverse cellophane tape and stored at room temperature (20 ± 2 °C) in a sealed slide box for 4, 8, 12, 16, 20, 22, 24, 26, 28, and 30 wk after collection. These slides then were tested as described in the hatching protocol with control slides containing pinworm eggs collected during the previous 24 h. Attempts were made to include 100 eggs per slide, but this was not always feasible due to the variability of shedding at the established collection time points.
Disinfectant efficacy.
Embryonated eggs collected within the previous week were prepared as described for the hatching protocol. These eggs were exposed to 1 of 3 disinfectants: chlorine dioxide liquid (Vimoba tablets, Quip Laboratories, Wilmington, DE) at concentrations of 100 or 200 mg/L chlorine dioxide gas at 400 mg/L (MB10 tablets, Quip Laboratories) by using a proprietary apparatus (Quip Laboratories) to generate gas. Exposure to the given agent followed manufacturer instructions: slides were submerged in liquid for 10 min or exposed to the gas form for 2 h. The chlorine dioxide generator was placed within a rodent cage-changing station that had been wrapped with multiple layers of thin, clinging plastic wrap, which effectively created an airtight space within the station. There were no other animals or activities in the room during the experiment. Temperature was maintained at 20 °C, and humidity was 38% within the wrapped station. Biologic indicators (Bacillus atrophaeus) were included in the treatment run of the gaseous form to confirm efficacy. Gas levels within the room outside of the change station were monitored by using a portable analyzer (4000 Series Compact Portable Analyzer, Interscan, Chatsworth, CA) to ensure personnel safety. Quip Laboratories provided the custom apparatus and ran the gas exposure. They however had no influence on collection and analysis or reporting of data. After exposure, sample slides were run through the aforementioned hatching protocol with a control, untreated slide. Hatched and unhatched eggs were counted.
Statistical analysis.
ANOVA and post hoc Tukey comparison tests were used to determine differences in shedding by both time and sex; t-tests were used to compare shedding at weaning age compared with day 124 and sex-associated differences in shedding at both weaning and day 124. One-tailed t tests were used to compare hatching rates in eggs exposed to chlorine dioxide formulations with those of controls. Correlations were used to compare egg counts with worm counts at necropsy. Percentage detection rate, that is, the ability of a test to detect a known infestation, was calculated as the number of rats that tested positive for pinworms during a specific test divided by the total number of known positive samples multiplied by 100%. Rats were considered to be pinworm-positive if they had at least one positive tape test over the course of the study. This percentage was calculated for the perianal tape test, cecal–colonic direct examination, and for both tests combined at necropsy. χ2 tests were used to compare the proportions of hatched eggs in experimental compared with control samples for viability and disinfectant experiments. P values of less than 0.05 were considered significant. Data were analyzed by using a commercial statistical software package (PRISM version 6.0, GraphPad Software, San Diego, CA).
Results
Peak shedding of S. muris eggs.
Peak daytime egg counts occurred at 1400 daily. The number of eggs shed varied significantly throughout the day (F4, 425 = 4.625, P < 0.01). The number of eggs increased from 0800 through 1400, with a decrease in eggs at 1600. The number of eggs collected at 1400 was significantly higher than the number of eggs collected during morning hours (0800: q245 = 5.296, P < 0.05; 1000: q245 = 4.146, P < 0.05).
Time course of egg shedding.
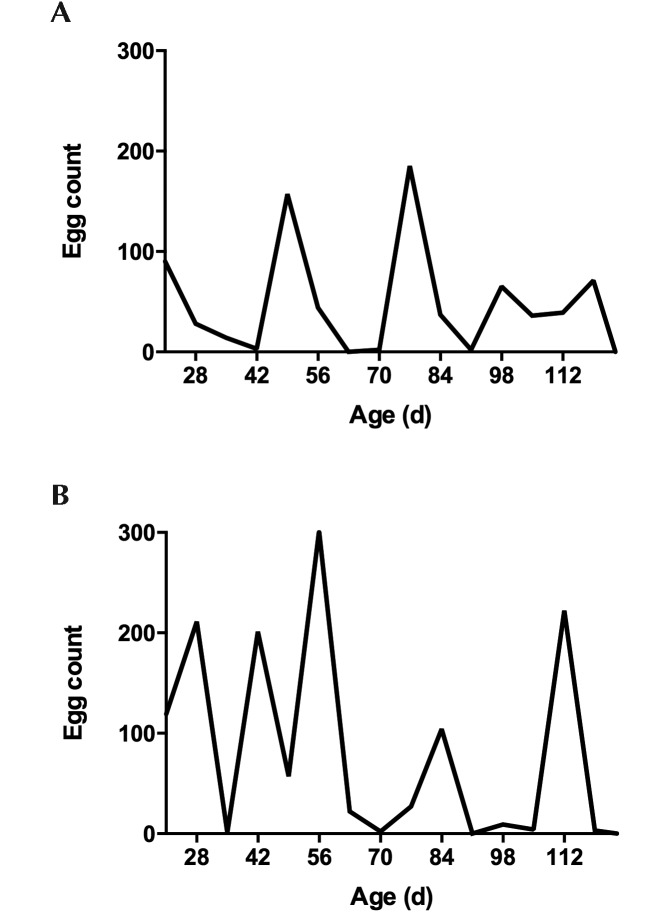
We expected that rats would have a patent infection at weaning and would therefore be shedding at 21 d. This situation occurred in half of the study population: one litter had high egg counts at time of weaning (day 21), whereas egg shedding was not evident until 1 wk after weaning in the other litter. Egg shedding showed a periodicity, with shedding declining to 0 at 2- to 3-wk intervals (Figure 2).
Figure 2.
Syphacia muris egg counts through 124 d of age in representative (A) female and (B) male Sprague–Dawley rats. Note that egg counts return to 0 repeatedly at 2- to 3-wk intervals throughout the infestation.
Incidence of egg shedding with increasing age.
Egg shedding at weaning (day 21) was not significantly different from that at 124 d of age, nor was it significantly different before and after 9 wk of age (days 21 through 63 compared with days 70 through 124), which is the time to development of resistance to oxyuriasis in mice.28
Sex-associated effect on egg shedding.
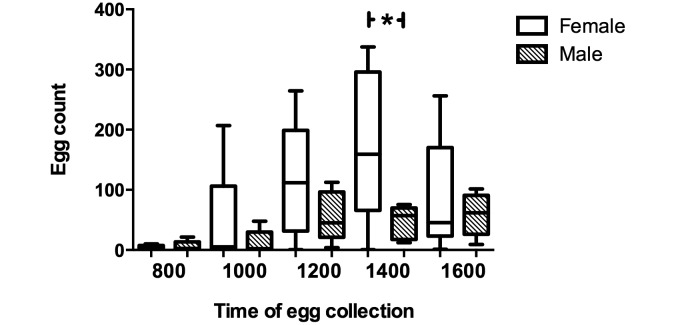
Our data regarding sex-associated effects were inconsistent. When comparing subjects of mixed ages at different times of day, female rats typically shed more eggs than did male rats (F9, 240 = 3.743, P < 0.01; Figure 3), although this difference was only significant at the peak shedding time of 1400 (P < 0.01). However, comparing weekly samples from subjects of the same age over the course of a 124-d infestation showed no difference in egg burden between sexes. In addition, there were no consistent differences in egg shedding when age-matched rats of different sexes were compared at specific time points, primarily weaning (day 21: P = 0.3196) and day 124 (P > 0.05). The highly variable nature of the data from age-matched rats precluded significance despite a trend toward increased egg shedding in female rats.
Figure 3.
Sex-associated differences of Sprague–Dawley rats in the shedding of Syphacia muris eggs at 5 times throughout the day (n = 10; 5 female and 5 male). Female rats shed significantly (P < 0.05) more eggs at 1400 than do male rats.
Correlation of worm burden at necropsy and perimortem egg count.
Table 1 reports the numbers of eggs and worms found at necropsy. Egg count on perianal tape test did not correlate with either cecal (r = 0.39) or colonic (r = 0.42) pinworm counts at necropsy. Cecal counts and colonic counts also varied significantly by subject (P < 0.05). Furthermore, among 24 rats, 3 were negative for infestation on all tests, another 3 rats tested negative by tape test but had worms in the cecum or colon, and 6 more rats were positive by tape test but lacked worms in the cecum or colon (Table 1). Perianal tape test detected 75% of the S. muris-infected rats, whereas direct examination of the cecum and colon detected only 62.5%. Using a combination of both tests produced a detection rate of 91.7%.
Table 1.
Syphacia murisegg counts on tape tests and worm counts from the cecum and colon at time of necropsy
| Litter | Sex | Tape | Cecum | Colon |
| A | F | 0 | 0 | 0 |
| F | 7 | 21 | 2 | |
| F | 7 | 7 | 0 | |
| F | 166 | 20 | 8 | |
| F | 207 | 20 | 8 | |
| F | 0 | 2 | 8 | |
| F | 113 | 3 | 0 | |
| F | 92 | 5 | 0 | |
| M | 4 | >30 | 2 | |
| M | 8 | 8 | 4 | |
| M | 88 | 2 | 3 | |
| M | 53 | 4 | 0 | |
| B | F | 9 | 2 | 1 |
| F | 5 | 0 | 0 | |
| F | 0 | 6 | 0 | |
| F | 6 | 6 | 2 | |
| M | 6 | 0 | 0 | |
| M | 0 | 0 | 0 | |
| M | 1 | 0 | 0 | |
| M | 0 | 5 | 9 | |
| M | 10 | 0 | 0 | |
| M | 0 | 0 | 0 | |
| M | 1 | 0 | 0 | |
| M | 2 | 8 | 2 |
In general, worm burdens were difficult to quantify.
Viability of pinworm eggs in the environment.
Eggs held 4, 8, 12, and 16 wk from deposition hatched at rates that were not significantly different from those of control samples (Table 2). There were significant reductions in hatching compared with that of controls for the remaining samples (that is, those held for 20, 22, 24, 26, 28, and 30 wk after deposition), suggesting decreased viability after 20 wk (P < 0.05; Table 2). Nevertheless, even at 30 wk after the time of collection, 69.2% of S. muris eggs still hatched, indicating continued viability for the majority of the sample.
Table 2.
Viability of Syphacia muriseggs in the environment
| No. of eggs counted |
Hatching rate (%) |
||||
| Week | Control | Experimental | Control | Experimental | P (χ2 test) |
| 4 | 35 | 208 | 85.7 | 77.4 | nonsignficant |
| 8 | 211 | 94 | 86.7 | 84.0 | nonsignficant |
| 12 | 3 | 137 | 100.0 | 58.4 | nonsignficant |
| 16 | 135 | 54 | 94.1 | 88.9 | nonsignficant |
| 20 | 67 | 34 | 82.1 | 61.8 | < 0.05 |
| 22 | 260 | 68 | 85.0 | 76.5 | < 0.05 |
| 24 | 66 | 261 | 81.8 | 46.7 | < 0.01 |
| 26 | 207 | 54 | 90.8 | 81.5 | < 0.05 |
| 28 | 17 | 318 | 82.4 | 30.5 | < 0.01 |
| 30 | 90 | 91 | 81.1 | 69.2 | < 0.05 |
S. muris eggs can remain viable in the environment for at least 30 wk from time of collection. Control eggs were collected during the previous 24 h, and experimental eggs were aged 4 to 30 wk prior to testing in the hatching protocol.
Susceptibility of S. muris eggs to chlorine dioxide.
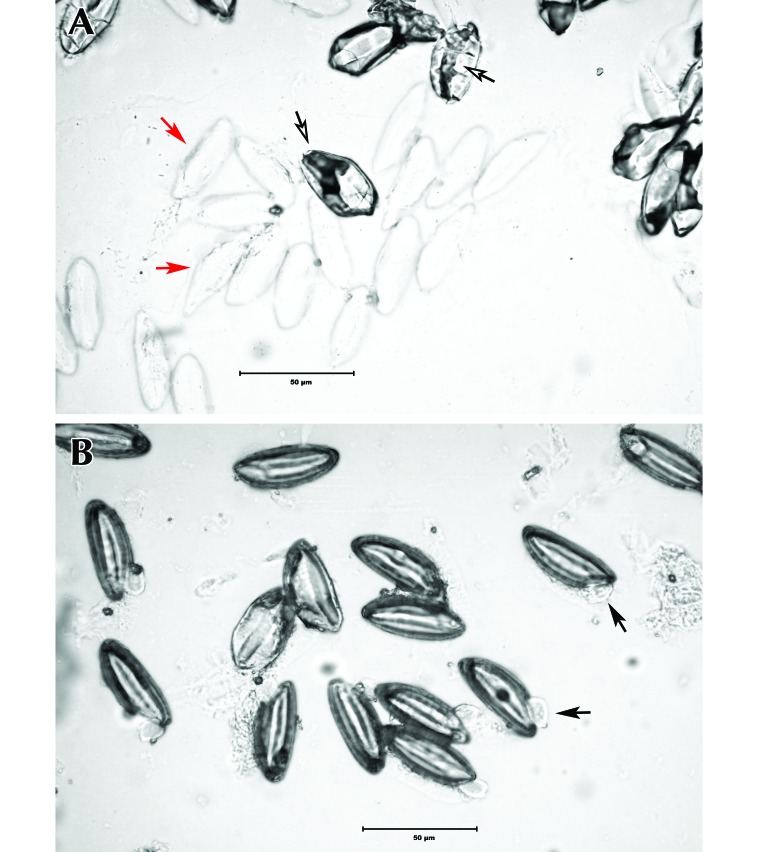
Egg hatching after treatment with chlorine dioxide was significantly reduced as compared with that of unexposed control eggs (P < 0.01; Table 3). Eggs exposed to 400 mg/L chlorine dioxide gas hatched at a rate of 0.3%. Biologic indicators supported efficacy of the gaseous treatment. Furthermore, these eggs showed morphologic differences in the appearance of the capsule, as compared with control eggs (Figure 4). Liquid forms were significantly (P < 0.01) less effective at preventing hatching than was the gaseous form of chlorine dioxide.
Table 3.
Hatching rates of Syphacia muriseggs exposed to 3 forms of chlorine dioxide
| No. of eggs counted |
Hatching rate (%) |
||||
| Disinfectant | Control | Treated | Control | Treated | P (χ2 test) |
| 100 mg/L chlorine dioxide liquid | 269 | 124 | 84.8 | 61.2 | < 0.01 |
| 200 mg/L chlorine dioxide liquid | 100 | 263 | 96.0 | 32.7 | < 0.01 |
| 400 mg/L chlorine dioxide gas | 401 | 599 | 85.0 | 0.3 | < 0.01 |
Controls consisted of eggs collected at the same time but not exposed to the testing agent.
Figure 4.
(A) Syphacia muris eggs exposed to 400 mg/L chlorine dioxide gas compared with (B) control eggs. Hatched eggs are considered viable and capable of infection. The cuticle of hatched eggs appears compromised and may sometimes show a worm-like appendage (black arrow) protruding from the egg itself. Eggs exposed to the experimental condition have distorted capsules and irregular contents (open arrow) or appear indistinct (red arrow).
Eggs exposed to 200 mg/L chlorine dioxide liquid hatched at a rate of 32.7%, whereas those exposed to 100 mg/L chlorine dioxide liquid hatched at a rate of 61.2%.
Discussion
Information regarding the epidemiology of Syphacia muris, the most frequent cause of pinworm infestation in rats, can greatly inform diagnostic and treatment procedures. In this study, we characterized the course of S. muris in immunocompetent rats over several months. We found that our findings support generally accepted knowledge regarding rat pinworm epidemiology in some cases but not in others.
In accordance with previous reports,37 our study demonstrated a daytime periodicity in egg laying, with the highest numbers of eggs being collected at 1400. Similar patterns of circadian egg release are found in other rodent endoparasites.24,30 The exact evolutionary mechanism for a circadian rhythm of egg release varies for each parasite and can be understood in terms of either the ability to increase local transmission or the ability to promote widespread dispersal of infective forms.20 Circadian patterns are thought to increase the probability of successful transmission, especially when synchronized with host mobility and fecal production.20 For example, peak shedding of the trematode Echinostoma caproni is correlated with both peak fecal production and peak activity levels in mice, suggesting a strategy to maximize transmission.30 Likewise, female S. muris deposit eggs in the afternoon,5 which coincides with periods of low activity in laboratory rats.33 This strategy promotes retention of eggs within the immediate environment until embryonation several hours later, thereby increasing the likelihood of autoinfection or transmission to other conspecifics. Although we cannot say that this periodicity is a true circadian rhythm given that we did not collect eggs at all hours of the day, these data still represent a distinct pattern that could affect diagnostic sampling, which is typically conducted during daytime hours. On the basis of our data, we recommend that perianal tape testing should occur as close as possible to the peak egg shedding time of 1400, to maximize the sensitivity of this particular diagnostic test.
In addition, periodicity extends to the life cycle of the oxyurid. We found that egg shedding during the first 4 mo of life reliably cycled over 2- to 3-wk periods, with shedding decreasing to 0 between cycles. This cycle was apparent in rats followed for 4 mo and may not be as obvious in larger colonies that are enzootically infested. Nevertheless, this pattern suggests that these animals were reinfecting themselves on a regular basis. The following sequence of events likely explains this phenomenon: growth and development of pinworms in the gastrointestinal tract is followed by egg shedding and associated death of the female pinworms. A period during which eggs cannot be detected ensues before the infection cycle is restarted via ingestion of eggs from the perianal area or the environment. Because egg counts in subjects dropped to 0 for several weeks before infestation was reestablished, false-negative diagnoses would occur if samples were collected in these interim periods. Diagnosis based on necropsy would similarly be affected if procedures occur between active infestations, as demonstrated in several animals in this study. We therefore recommend additional testing at 1 to 2 wk after negative results from a suspected positive colony.
Our results differed from those of colleagues who found a 100% sensitivity in tape tests performed at various times of the day to detect S. muris infestation.17 Furthermore, they found complete concurrence between tape tests and presence of worms at necropsy and no evidence of cycling of infections.17 Possible explanations for these disparate findings include differences in environmental factors or genetic susceptibility to pinworms.
Given the 7- to 8-d life cycle of S. muris, we expected that rats would be infested and shedding eggs at weaning. However, we found eggs at weaning in only 1 of 2 litters, with eggs detected in the second litter a week later. Because eggs are persistent in the environment, the timing of infestation may coincide with the onset of eating solid food and increased environmental exposure. The incisors of rats erupt between 6 and 8 d of age, and the first molars erupt around 16 d of age.40 Given these time points, infection could manifest between 13 and 24 d of age, suggesting that egg shedding at weaning age is more than possible. However, infection could be delayed if shedding by the mother drops to 0 within that time period. Therefore, negative results at weaning in a litter suspected to be positive for S. muris should be confirmed by a second test at least a week later to avoid a false-negative diagnosis.
Previous reports involving other species have shown sex-associated differences regarding
S. muris, but we did not see any consistent differences between male and female rats in egg shedding or in worm burden. Previous literature states that wild male mice have higher Aspiculuris burdens than do female mice.7,13 Similar observations have been noted in male hamsters, which have increased numbers of Syphacia and are infected more often than are female hamsters.36 These sex-associated differences are believed to result from the role of testosterone in parasite-host burdens and its negative influence on the immune system.2 Our variable results in a small group did not confirm this difference. In fact, we only saw sex-associated differences in the experiment to determine peak shedding time, and in that case, female rats shed more eggs at 1400 than did male rats. Because this pattern of egg shedding has direct bearing on diagnostic testing, we recommend afternoon tape testing of female rather than male rats.
Egg shedding at weaning did not differ from that at 124 d of age, suggesting that rats in our study did not become resistant to infection during the first 4 mo of life. In contrast, mice typically develop resistance to A. tetraptera infection between 4 and 9 wk of age, causing levels of infestation to decrease.28 Older literature similarly has reported that rats infected with S. muris either become refractory to infestation or become susceptible after an initial period of resistance.32,36 Our findings suggest samples from rats between weaning and 4 mo of age are equally likely to detect S. muris infestation within a mixed-age colony.
Although viability decreased with time, 69.2% of S. muris eggs hatched at 30 wk after deposition, indicating continued viability at this time point (compared with the 4-wk time point previously reported14). A much longer period of follow up is necessary to determine the true ‘expiration date’ of S. muris eggs. Regardless, persistently viable eggs within the environment have long been implicated as one means by which rodent pinworm infection is maintained,11 and our results support this possibility. We did not investigate the circumstances under which viable eggs could actually reinfest a host, but this experiment seems like the logical next step in understanding the significance of environmental contamination.
Given the demonstrated persistence of S. muris eggs in the environment, we evaluated the ovicidal capacity of a chlorine dioxide disinfectant used in our rodent facilities. Other colleagues tested a number of agents by using the same hatching protocol and reported a 3.6% hatching rate for a 2-h exposure to 75 mg/L liquid chlorine dioxide.14 In contrast, we used the recommended exposure time of 10 min to a more concentrated solution, a practice that seems more appropriate for routine disinfection of animal-handling surfaces. We therefore were not able to reproduce previous results. At best, our hatching rate (with 200 mg/L liquid chlorine dioxide) was still 32.7%. Higher concentrations may have been more effective, but 200 mg/L is typically the highest concentration recommended for routine disinfection. In contrast to results with chlorine dioxide liquid, we found that chlorine dioxide gas killed very nearly 100% of S. muris eggs. Compared with those of other compounds,14 this is a markedly high ovicidal rate. Furthermore, the eggs subjected to the gaseous treatment differed in appearance to control eggs (Figure 4), suggesting that the gas may have caused degenerative changes to the capsule thereby affecting viability. To our knowledge, this study constitutes the first report of the use of chlorine dioxide gas with S. muris eggs and suggests that this compound could truly aid in eradication efforts if used for environmental cleaning.
On the basis of our findings, we have the following suggestions regarding diagnostic testing for S. muris. First, diagnostic tape testing should be performed midafternoon to coincide with peak egg shedding. Furthermore, if testing occurs at 1400, female rats are more likely to test positive than are male rats. Second, rats from a suspected positive colony that test negative for S. muris should be retested in 1 or 2 wk to prevent false negatives due to delayed egg shedding at weaning or the absence of egg shedding between infestation cycles. Third, multiple rats should be tested, to maximize the incidence of testing during the egg shedding phase. Fourth, at necropsy, combining tape tests with examination of the cecum or colon for pinworms will maximize the ability to find true positives. We also conclude that S. muris reinfection from the environment remains a possibility for at least 30 wk and that chlorine dioxide gas is effective for environmental decontamination.
Acknowledgments
This research was supported by Research Animal Resources at the Johns Hopkins University School of Medicine. Student support was provided by NIH grant R25 OD 010913. We acknowledge the help provided by Kelly Metcalf Pate, Eric Hutchinson, and Lauren Albacarys throughout the course of the study. We also acknowledge Donna Monroe and Tim Hidell for providing consultation and assistance with the disinfection portion of the study.
References
- 1.Agersborg SS, Garza KM, Tung KSK. 2001. Intestinal parasitism terminates self-tolerance and enhances neonatal induction of autoimmune disease and memory. Eur J Immunol 31:851–859. [DOI] [PubMed] [Google Scholar]
- 2.Alexander J, Stimson WH. 1988. Sex hormones and the course of parasitic infection. Parasitol Today 4:189–193. [Google Scholar]
- 3.Anya AO. 1966. Experimental studies on the physiology of hatching of eggs of Aspiculuris tetraptera Schulz (Oxyuridea: Nematoda). Parasitology 56:733–744. [DOI] [PubMed] [Google Scholar]
- 4.Anya AO. 1966. Studies on the biology of some oxyurid nematodes. II. The hatching of eggs and development of Aspiculuris tetraptera Schulz within the host. J Helminthol 40:261–268. [DOI] [PubMed] [Google Scholar]
- 5.Baker DG. 2007. Flynn's parasites of laboratory animals. Oxford (UK): Blackwell Publishing. [Google Scholar]
- 6.Barlow SC, Brown MM, Price HV. 2005. Eradication of Syphacia muris from food-restricted rats without environmental decontamination. Contemp Top Lab Anim Sci 44:23–25. [PubMed] [Google Scholar]
- 7.Behnke JM. 1975. Aspiculuris tetraptera in wild Mus musculus. The prevalence of infection in male and female mice. J Helminthol 49:85–90. [DOI] [PubMed] [Google Scholar]
- 8.Behnke JM. 1976. Aspiculuris tetraptera in wild Mus musculus. Age resistance and acquired immunity. J Helminthol 50:197–202. [PubMed] [Google Scholar]
- 9.Bugarski D, Jovcic G, Katic-Radivojevic S, Petakov M, Krstic A, Stojanovic N, Milenkovic P. 2006. Hematopoietic changes and altered reactivity to IL17 in Syphacia obvelata-infected mice. Parasitol Int 55:91–97. [DOI] [PubMed] [Google Scholar]
- 10.Chan KF. 1952. Life-cycle studies on the nematode Syphacia obvelata. Am J Hyg 56:14–21. [DOI] [PubMed] [Google Scholar]
- 11.Clifford CB, Watson J. 2008. Old enemies: still with us after all these years. ILAR J 49:291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooke A, Tonks P, Jones FM, O'Shea H, Hutchings P, Fulford AJC, Dunne DW. 1999. Infection with Schistosoma mansoni prevents insulin-dependent diabetes mellitus in nonobese diabetic mice. Parasite Immunol 21:169–176. [DOI] [PubMed] [Google Scholar]
- 13.Derothe JM, Loubes C, Orth A, Renaud F, Moulia C. 1997. Comparison between patterns of pinworm infection (Aspiculuris tetraptera) in wild and laboratory strains of mice, Mus musculus. Int J Parasitol 27:645–651. [DOI] [PubMed] [Google Scholar]
- 14.Dix J, Astill J, Whelan G. 2004. Assessment of methods of destruction of Syphacia muris eggs. Lab Anim 38:11–16. [DOI] [PubMed] [Google Scholar]
- 15.Feldman SH, Bowman SG. 2007. Molecular phylogeny of the pinworms of mice, rats, and rabbits and its use to develop molecular beacon assays for the detection of pinworms in mice. Lab Anim (NY) 36:43–50. [DOI] [PubMed] [Google Scholar]
- 16.Hickman D, Swan M, Hartman GP. 2008. A cost-effective and efficacious method of pinworm treatment for large colonies of mice. Lab Anim (NY) 37:308–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hill WA, Randolph MM, Mandrell TD. 2009. Sensitivity of perianal tape impressions to diagnose pinworm (Syphacia spp.) infections in rats (Rattus norvegicus) and mice (Mus musculus). J Am Assoc Lab Anim Sci 48:378–380. [PMC free article] [PubMed] [Google Scholar]
- 18.Ilic V, Krstic A, Katic-Radivojevic S, Jovcic G, Milenkovic P, Bugarski D. 2010. Syphacia obvelata modifies mitogen-activated protein kinases and nitric oxide synthases expression in murine bone marrow cells. Parasitol Int 59:82–88. [DOI] [PubMed] [Google Scholar]
- 19.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press. [Google Scholar]
- 20.Kennedy CR. 1976. Reproduction and dispersal. In: Kennedy CR. Ecological aspects of parasitology. Amsterdam (The Netherlands): North-Holland Publishing. [Google Scholar]
- 21.Lewis JW, D'Silva J. 1986. The life-cycle of Syphacia muris Yamaguti (Nematoda: Oxyuroidea) in the laboratory rat. J Helminthol 60:39–46. [DOI] [PubMed] [Google Scholar]
- 22.Lubcke R, Hutcheson FA, Barbezat GO. 1992. Impaired intestinal electrolyte transport in rats infested with the common parasite Syphacia muris. Dig Dis Sci 37:60–64. [DOI] [PubMed] [Google Scholar]
- 23.Lytvynets A, Langrova I, Lachout J, Vadlejch J. 2013. Detection of pinworm eggs in the dust of laboratory animals breeding facility, in the cages, and on the hands of the technicians. Lab Anim 47:71–73. [DOI] [PubMed] [Google Scholar]
- 24.McMahon JE. 1976. Circadian rhythm in Schistosoma haematobium egg excretion. Int J Parasitol 6:373–377. [DOI] [PubMed] [Google Scholar]
- 25.McNair DM, Timmons EH. 1977. Effects of Aspiculuris tetraptera and Syphacia obvelata on exploratory behavior of an inbred mouse strain. Lab Anim Sci 27:38–42. [PubMed] [Google Scholar]
- 26.Michels C, Goyal P, Nieuwenhuizen N, Brombacher F. 2006. Infection with Syphacia obvelata (pinworm) induces protective Th2 immune responses and influences ovalbumin-induced allergic reactions. Infect Immun 74:5926–5932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohn G, Philipp EM. 1981. Effects of Syphacia muris and the anthelmintic fenbendazole on the microsomal monooxygenase system in mouse liver. Lab Anim 15:89–95. [DOI] [PubMed] [Google Scholar]
- 28.Panter HC. 1969. Studies on host–parasite relationships. Syphacia obvelata in the mouse. J Parasitol 55:74–78. [PubMed] [Google Scholar]
- 29.Parel JD, Galula JU, Ooi HK. 2008. Characterization of rDNA sequences from Syphacia obvelata, Syphacia muris, and Aspiculuris tetraptera and development of a PCR-based method for identification. Vet Parasitol 153:379–383. [DOI] [PubMed] [Google Scholar]
- 30.Platt TR, Hussey GL, Zelmer DA. 2013. Circadian egg production by Echinostoma caproni (Digenea: Echinostomatidae) in ICR mice. J Parasitol 99:179–182. [DOI] [PubMed] [Google Scholar]
- 31.Pritchett KR, Johnston NA. 2002. A review of treatments for the eradication of pinworm infections from laboratory rodent colonies. Contemp Top Lab Anim Sci 41:36–46. [PubMed] [Google Scholar]
- 32.Roman E. 1969. Modalites d'infestation et resistance au parasitisme dans l'oxyurose du rat a Syphacia muris. Bull Assoc Diplomes Microbiol Fac Pharm Nancy 114:1–7. [Google Scholar]
- 33.Saibaba P, Sales GD, Stodulski G, Hau J. 1996. Behaviour of rats in their home cages: daytime variations and effects of routine husbandry procedures analysed by time sampling techniques. Lab Anim 30:13–21. [DOI] [PubMed] [Google Scholar]
- 34.Sato Y, Ooi HK, Nonaka N, Oku Y, Kamiya M. 1995. Antibody production in Syphacia obvelata-infected mice. J Parasitol 81:559–562. [PubMed] [Google Scholar]
- 35.Stahl W. 1963. Studies on the life cycle of Syphacia muris, the rat pinworm. Keio J Med 12:55–60. [DOI] [PubMed] [Google Scholar]
- 36.Taffs LF. 1976. Pinworm infections in laboratory rodents: a review. Lab Anim 10:1–13. [DOI] [PubMed] [Google Scholar]
- 37.van der Gulden WJ. 1967. Diurnal rhythm in egg production by Syphacia muris. Exp Parasitol 21:344–347. [DOI] [PubMed] [Google Scholar]
- 38.van der Gulden WJ, van Aspert-van Erp AJ. 1976. Syphacia muris: response to environmental stimuli when hatching in vitro. Exp Parasitol 39:45–50. [DOI] [PubMed] [Google Scholar]
- 39.Wagner M. 1988. The effect of infection with the pinworm (Syphacia muris) on rat growth. Lab Anim Sci 38:476–478. [PubMed] [Google Scholar]
- 40.Weisse I. 1993. Aging and ocular changes. In: Mohr U, Dungworth LD, Capen CC. Pathobiology of the aging rat. Washington (DC): International Life Sciences Institute Press. [Google Scholar]