Abstract
The opioid buprenorphine has been shown to provide adequate postoperative analgesia in both companion and laboratory animals. However, its use is still hindered by the need for multiple parenteral injections to achieve continuous analgesia. The purpose of the current study was to conduct a pharmacokinetic analysis of 2 new long-acting formulations of buprenorphine—an injectable sustained-release buprenorphine (SRB) and a transdermal buprenorphine (TDB) patch—in healthy Göttingen minipigs by using liquid chromatography–electrospray ionization–tandem mass spectrometry. Administration of 0.18 mg/kg SC SRB and 30 μg/h TDB achieved AUC0-Tlast of 221.6 ± 26.8 and 25.2 ± 3.9 ng × h/mL, respectively, compared with 9.7 ± 1.4 ng*h/mL for 0.02 mg/kg IV buprenorphine. By using a hypothesized therapeutic plasma buprenorphine concentration threshold of 0.1 ng/mL, therapeutic concentrations were achieved at the first study time point (5 to 30 min) and lasted an average of 8.0 ± 1.3 h for intravenous buprenorphine and 264.0 ± 32.2 h for SRB. TDB achieved therapeutic concentrations in 12 to 24 h after patch application, which lasted until the patch was removed at 72 h. The results of this study suggest that SRB and TDB are long-acting alternatives for pain management, and their use could decrease animal handling and stress, thereby simplifying pain management and improving welfare in laboratory swine.
Abbreviation: AUC0-Tlast, AUC to last quantifiable plasma concentration; Cmax, peak plasma concentration; SRB, sustained-release buprenorphine; TDB, transdermal buprenorphine; Tmax, time of peak plasma concentration; VAP, vascular access port
Buprenorphine is a semisynthetic, partial µ-opioid receptor agonist used for analgesia in many companion and laboratory animal species.7,29 Its popularity is due to a lower risk of respiratory depression and prolonged analgesia in comparison to pure µ-opioid agonists, such as fentanyl and hydromorphone.7,29 Buprenorphine is often the analgesic of choice in swine because a single 0.01- to 0.1-mg/kg dose can provide analgesia for as long as 8 to 12 h with minimal adverse effects.7,33
Swine are valuable animal models for cardiovascular, digestive, urinary, and integumentary research.32,33 As a result, laboratory swine often undergo major surgeries resulting in considerable postoperative pain. To provide analgesia of adequate efficacy and duration throughout the postoperative period, multiple injections of buprenorphine are necessary. Repeated injections and the associated handling and momentary pain can become increasingly stressful to swine with each subsequent injection. In addition, the recurrent peak and trough plasma concentrations associated with multiple parenteral injections potentially result in periods of inadequate pain relief at trough levels. A buprenorphine formulation that can be administered less frequently or in a noninjectable formulation but that can deliver a controlled and constant amount of drug over time potentially would eliminate the disadvantages of repeated dosing.
Recently 2 new formulations of buprenorphine have become available: an injectable sustained-release buprenorphine (SRB) and a transdermal buprenorphine (TDB) patch. Both formulations are designed to provide a consistent, controlled release of buprenorphine over the course of several days after a single administration. Recent studies in mice,4 rats,8 cats,5 and dogs24 support the ability of SRB to provide an extended duration of analgesia compared with that of the standard buprenorphine formulation. In addition, therapeutic plasma concentrations have been shown to last as long as 72 h in rats8 and 5 d in both macaques23 and dogs.24 Although SRB has been studied in several animal species, it has yet to be evaluated in swine.
TDB has been extensively used in human medicine however, unlike SRB, there are relatively few studies evaluating the efficacy of TDB in animals. High-dose (35, 52.5, and 70 µg/h) and low-dose (5, 7.5, 10, 15, and 20 µg/h) TDB patches, lasting 3 and 7 d, respectively, have been used effectively in humans to treat moderate to severe, chronic pain such as lower back pain and that due to osteoarthritis or cancer.6,9,14,20,26,30,31,35 Currently, TDB studies in animals have only been performed by using high-dose patches and have been limited to dogs1,21,25 and cats.22 In dogs, detectable plasma buprenorphine concentrations have been shown to last 72 h after the application of a single 52.5-µg/h TDB patch25 and 108 h with a 70-µg/h patch.1 In addition, a 70-µg/h TDB patch has been reported to provide equal postoperative analgesia in dogs that underwent an ovariohysterectomy when compared with 0.2 mg/kg of the standard formulation of buprenorphine administered subcutaneously every 6 h during the postoperative period. 21 To date, TDB has not been evaluated in swine.
Because of the success seen with both SRB and TDB in other species, further investigation into the pharmacokinetics and clinical efficacy of SRB and TDB in swine is warranted. These buprenorphine formulations have the potential for extended drug delivery with a single administration and thus the potential to positively affect animal welfare by minimizing animal stress. The purpose of this study was to evaluate the pharmacokinetics of these 2 new formulations of buprenorphine compared with a standard dose of intravenous buprenorphine in Göttingen minipigs. We hypothesized that both SRB and TDB would achieve quantifiable plasma buprenorphine concentrations above an estimated therapeutic threshold of 0.1 ng/mL for a longer duration than that of a standard dose of intravenous buprenorphine, thus supporting the use of these new formulations as long-acting analgesics for pain management in swine.
Materials and Methods
Animals.
Female Göttingen minipigs (Sus scrofa domestica; n = 11) were obtained from Marshall Bioresources (North Rose, NY), where they were maintained in full-barrier facilities and monitored for pathogens according to the guidelines by the Federation of European Laboratory Animal Science Associations. According to the vendor's heath monitoring report, all animals were free of major swine bacterial, viral, and parasitic pathogens. Minipigs were housed in accordance with the Guide for the Care and Use of Laboratory Animals13 with adherence to The Animal Welfare Act at either the University of Illinois at Chicago or AbbVie (North Chicago, IL). Both of the facilities are accredited by AAALAC. All work was conducted as described in animal care protocols approved by the corresponding facility's IACUC. Minipigs were housed in pairs in solid-floor pens with wood shavings (Teklad Laboratory Pine Shavings 7088, Harlan, Madison, WI) or slatted-floor pens with a boxed area of wood shavings (Teklad Shredded Aspen 7093, Harlan). Environmental conditions were maintained at 68 to 76 °F (20.0 to 24.4 °C) and 30% to 70% humidity with a 12:12-h light:dark cycle at both facilities. Water was provided ad libitum, and minipigs were fed an age-appropriate ration once or twice daily (Teklad Miniswine Diet 8753, Harlan). Environmental enrichment was provided in the form of sanitized toys, food treats, and social interaction with humans. All minipigs were determined to be healthy through a physical examination by a veterinarian and hematologic and biochemical analyses prior to study.
Vascular access port (VAP) surgery.
At 16 to 17 wk of age, minipigs underwent surgery to implant a VAP. Animals were anesthetized with ketamine (20 mg/kg IM; Boehringer Ingelheim, St Joseph, MO) and xylazine (2 mg/kg IM; Lloyd, Shenandoah, IA). After intubation, minipigs were maintained on 1% to 3% isoflurane or 3% to 5% sevoflurane, and lactated Ringer solution was administered intravenously at a rate of 10 mL/kg/h via an ear-vein catheter. Buprenorphine (0.02 mg/kg IM; Reckitt Benckiser Pharmaceuticals, Richmond, VA), meloxicam (0.4 mg/kg SC or IV; Boehringer Ingelheim or Norbrook, Newry, North Ireland), and a local incisional block of bupivacaine (1 mg/kg; Hospira, Lake Forest, IL) were administered preoperatively for analgesia. Cefazolin (20 mg/kg IV; Sandoz, Princeton, NJ) was administered for prophylactic antibiotic therapy. The skin of the 2 surgical sites, ventral neck and dorsal back, was aseptically prepared prior to VAP implantation.
The VAP consisted of a titanium port (Gridlock CP2 Port, Access Technologies, Skokie, IL) with an attachable 7-French rounded tip hydrocoat catheter (Access Technologies). Implantation into the right external jugular vein was performed by using a previously described method.34 Briefly, an approximately 4-cm incision was made over the right jugular furrow on the ventral aspect of the neck. Subcutaneous tissue was bluntly dissected to isolate the external jugular vein, and a 2-mm incision was made into the vessel. The catheter was introduced into the vessel lumen, advanced just cranial to the heart, and subsequently anchored into place with nonabsorbable suture (3-0 Sofsilk, Covidien, Mansfield, MA). An approximately 5-cm curvilinear incision was made caudal to the right scapula, and the subcutaneous tissue was bluntly dissected to make a pocket to accommodate the port. The catheter then was passed through a tunneling rod from the neck, above the scapula, and coupled to the port. Patency of the catheter was checked by withdrawing blood from the port, followed by flushing the catheter with 6 mL 0.9% saline and 2.5 mL taurolidine–citrate catheter solution (Access Technologies, Skokie, IL) to lock the catheter. The port was anchored to the underlying musculature by using nonabsorbable suture (3-0 Prolene, Ethicon, San Lorenzo, PR), and both incisions were closed in anatomic layers. Aseptic technique was used throughout the surgical procedure.
Postoperative analgesia was continued with administration of buprenorphine (0.02 to 0.03 mg/kg IM) twice daily for 2 d and meloxicam (0.4 mg/kg PO; Boehringer Ingelheim) once daily for 2 to 4 d. Cephalexin (20 to 25 mg/kg PO; Teva, Sellersville, PA) was administered twice daily for 5 to 7 d for prophylactic antibiotic treatment. After surgery, minipigs were singly housed for at least 5 d to allow the surgical incisions to heal. No bandaging or jackets were needed to protect the incisions. Most minipigs had at least 14 d to recover from surgery before the initiation of pharmacokinetic studies with intravenous buprenorphine and SRB. Two minipigs received SRB instead of buprenorphine preoperatively and thus were enrolled in the pharmacokinetic study at time of surgery.
Blood collection and VAP maintenance.
To facilitate a low-stress environment during sample collection, minipigs were acclimated to a Panepinto sling or gentle restraint in the arms of a technician. This goal was accomplished through twice-daily training sessions and positive reinforcement. The restraint time was gradually increased each session until the animal was comfortable being restrained for 5 to 10 min. Training sessions began after the arrival of minipigs and continued until initiation of the pharmacokinetic study, for a total training duration of approximately 3 wk. In addition, to minimize discomfort of blood collection from the VAP, 2% lidocaine ointment (Akorn, Lake Forest, IL) was applied topically to the skin overlying the VAP approximately 45 min prior to sampling.
All blood samples were collected from the VAP by using aseptic technique consisting of 3 applications of povidone–iodine solution, sterile surgical gloves, sterile syringes, and sterile 22-gauge Huber needles or right-angle 22-gauge Huber infusion sets (Access Technologies) to access the port. Approximately 2.5 mL taurolidine–citrate catheter locking solution was removed from the catheter and discarded prior to collection of a 2-mL blood sample. The blood sample was transferred to an EDTA blood collection tube and placed immediately on ice. After collection, the catheter was flushed with 6 mL 0.9% saline and locked with 2.5 mL taurolidine–citrate solution. To maintain catheter patency, VAP were accessed for blood collection or flushed with saline and replaced with locking solution at least once weekly throughout the study.
Blood samples were centrifuged at 1000 × g for 10 min within 15 min of collection. Plasma aliquots were collected and stored at −80 °C until shipped on dry ice to AbbVie for analysis.
Pharmacokinetics.
Five naïve female Göttingen minipigs (weight, 10.8 to 17.3 kg; age, 18 to 20 wk) were used to evaluate the pharmacokinetics of intravenous buprenorphine at the University of Illinois at Chicago. Fourteen days after VAP implantation, minipigs were restrained gently by using techniques stated previously and lightly anesthetized with 3% isoflurane mixed with 100% oxygen via mask for approximately 2 to 3 min. The injection site was prepared with alcohol, and 0.02 mg/kg IV buprenorphine was administered into the lateral saphenous vein. Isoflurane was discontinued immediately after buprenorphine administration, and minipigs were provided 100% oxygen until fully recovered. This procedure allowed for accurate intravenous injection with rapid recovery. Blood samples were collected immediately prior to buprenorphine administration and at 5, 15, 30, and 60 min and 2, 4, 8, 12, and 24 h after administration.
Five naïve female Göttingen minipigs (weight, 12.8 to 16.7 kg; age, 22 to 25 wk) were used to evaluate the pharmacokinetics of SRB at AbbVie. Animals were gently restrained and lightly anesthetized with 7% sevoflurane mixed with 100% oxygen via mask for approximately 2 to 3 min prior to administration of 0.18 mg/kg SRB SC (ZooPharm, Laramie, WY) in the flank skinfold. Sevoflurane was discontinued immediately after SRB injection, and animals were provided 100% oxygen until fully recovered. This procedure allowed for accurate subcutaneous injection of SRB with rapid recovery from anesthesia. Blood was collected immediately prior to SRB administration; at 0.5, 1, 2, 4, 6, 8, 12, 24, 48, 72, 96, 120, 168, and 240 h after administration for all 5 minipigs; and at 336 h in 3 of the 5 minipigs.
Five female Göttingen minipigs (weight, 12.6 to 27.0 kg; age, 20 to 35 wk old) were used to evaluate the pharmacokinetics of TDB. Four of the 5 minipigs used for the intravenous buprenorphine study also were used for the TDB study after a washout period of at least 10 d between studies. The VAP of the remaining minipig became nonfunctional during the washout period and therefore was substituted with a naïve minipig to complete the TDB study. This minipig was used in the TDB study directly after the 14-d surgical recovery period. The hair of the dorsal trunk was shaved and the skin cleaned with saline approximately 1 d prior to application of TDB. A dose of 30 µg/h was administered by applying 2 patches (1 each of 20 µg/h and 10 µg/h) to the shaved area of the dorsal trunk of each minipig. The patches were placed approximately 1 to 2 cm to the right and left of midline between the 12th thoracic and 2nd lumbar vertebrae. Both patches were covered with a single clear occlusive bandage (Bioclusive, Systagenix, Quincy, MA) and a flexible bandage (VetFlex, Butler Schein Animal Health, Dublin, OH) was placed around the abdomen of the minipig. Blood was collected prior to TDB application and at 1, 2, 4, 8, 12, 24, 36, 48, 60, and 72 h after application.
Animal health and adverse effects.
Animal health was monitored throughout the duration of blood collection for all pharmacokinetic studies. Minipigs were evaluated visually at least twice daily by cageside observation to monitor food consumption, fecal consistency, level of sedation, activity level, evidence of respiratory depression, and presence of vomit. Weight was monitored twice each week during SRB and TDB pharmacokinetic studies to ensure that animals maintained body weight. For the intravenous study, minipigs were weighed on the day of buprenorphine administration but not thereafter since a long-term effect on appetite was not expected. In addition, the administration sites for SRB and TDB were evaluated twice daily for the duration of study for evidence of skin reactions such as erythema, swelling, and pruritus. In addition, all TDB patches were checked twice daily to ensure sufficient skin adherence throughout the study.
Sample analysis.
Plasma sample analysis for buprenorphine concentration was completed at AbbVie according to an adapted liquid chromatography–electrospray ionization–tandem mass spectrometry method.12 Briefly, an automated liquid handling workstation (MICROLAB Star, Hamilton Robotics, Reno, NV) placed 400 µL aliquots of plasma into each well of a 2-mL, 96-well plate and extracted the plasma with 1 mL ethyl acetate:hexane at a ratio of 9:1. The samples were vortexed for 5 min, centrifuged for 10 min at 410 × g, and then returned to the workstation. A 800-µL sample of supernatant was transferred to each well of a clean 96-well plate and evaporated at room temperature under a stream of nitrogen. Samples were reconstituted in 30 µL acetonitrile, vortexed briefly, mixed with 70 µL 0.1% formic acid in water containing 0.025% trifluoroacetic acid, and vortexed again. Each sample was injected (Eksigent 200, AB Sciex, Framingham, MA) onto a 50 × 0.5-mm, 3 µm column (Chrom XP C18-EP-120, Eksigent, Dublin, CA) for mass spectrometric analysis (Triple Quad 5500, Eksigent 200, AB Sciex). Buprenorphine concentration in each sample was determined by using calibration curves with known peak:area ratios. The lower limit of quantitation for a 400-µL plasma sample was 0.03 ng/mL.
Pharmacokinetic analysis.
The plasma buprenorphine concentration obtained after drug administration was compared with time data for the intravenous buprenorphine, TDB, and SRB groups. Maximal observed plasma buprenorphine concentration (Cmax) and the time at which Cmax occurred (Tmax) were determined directly from the individual observed concentration-versus-time data. Pharmacokinetic parameters were derived by using a model-independent approach (noncompartmental analysis) according to a uniform weighting scheme. Half-life (t1/2) was calculated by using the harmonic mean. The AUC to last quantifiable plasma concentration (AUC0-Tlast) was calculated by using the linear trapezoidal rule for 24 h after intravenous injection of buprenorphine (AUC0–24), 336 h after injection of SRB (AUC0–336), and 72 h after application of TDB (AUC0–72). All pharmacokinetic calculations were performed by using WinNonlin (version 5.2, Pharsight, Cary, NC). Values from minipigs in each group are presented as mean ± SEM.
Results
Pharmacokinetics.
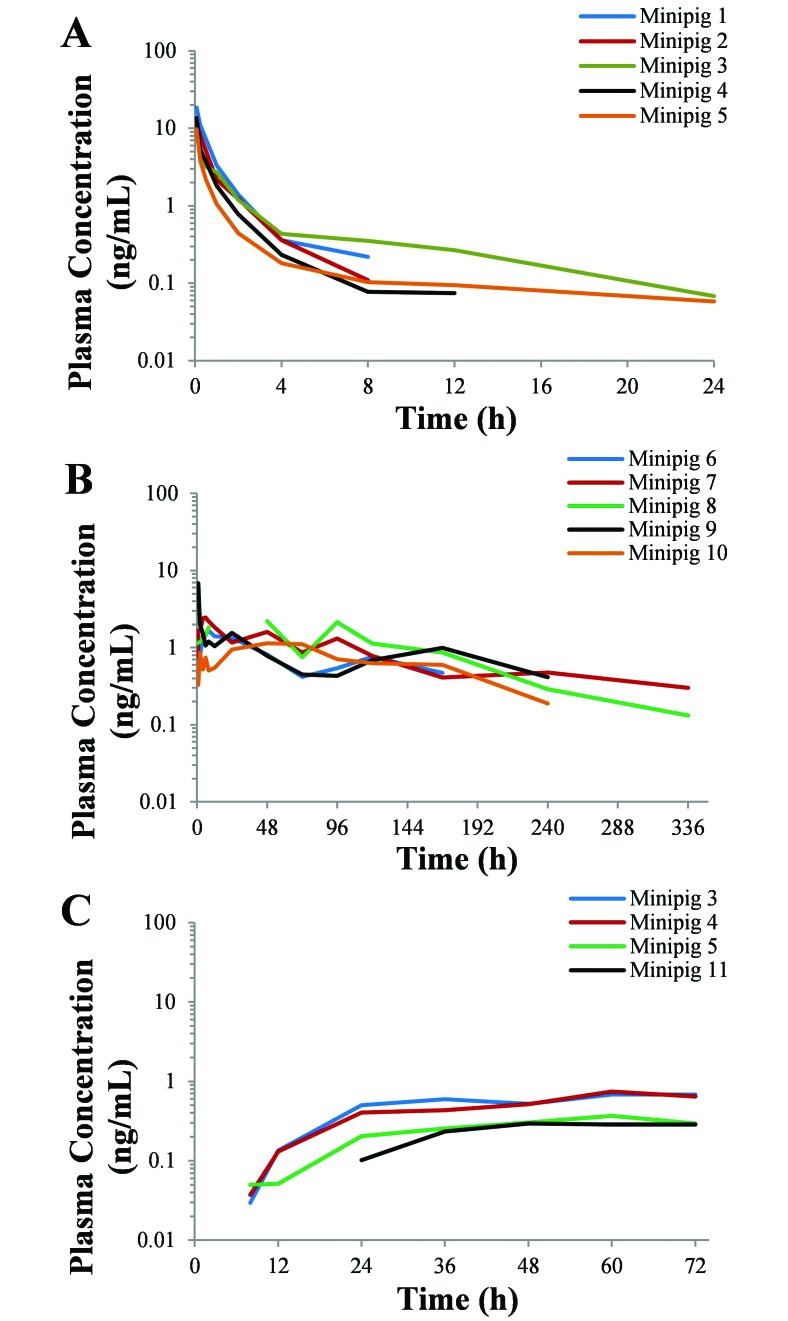
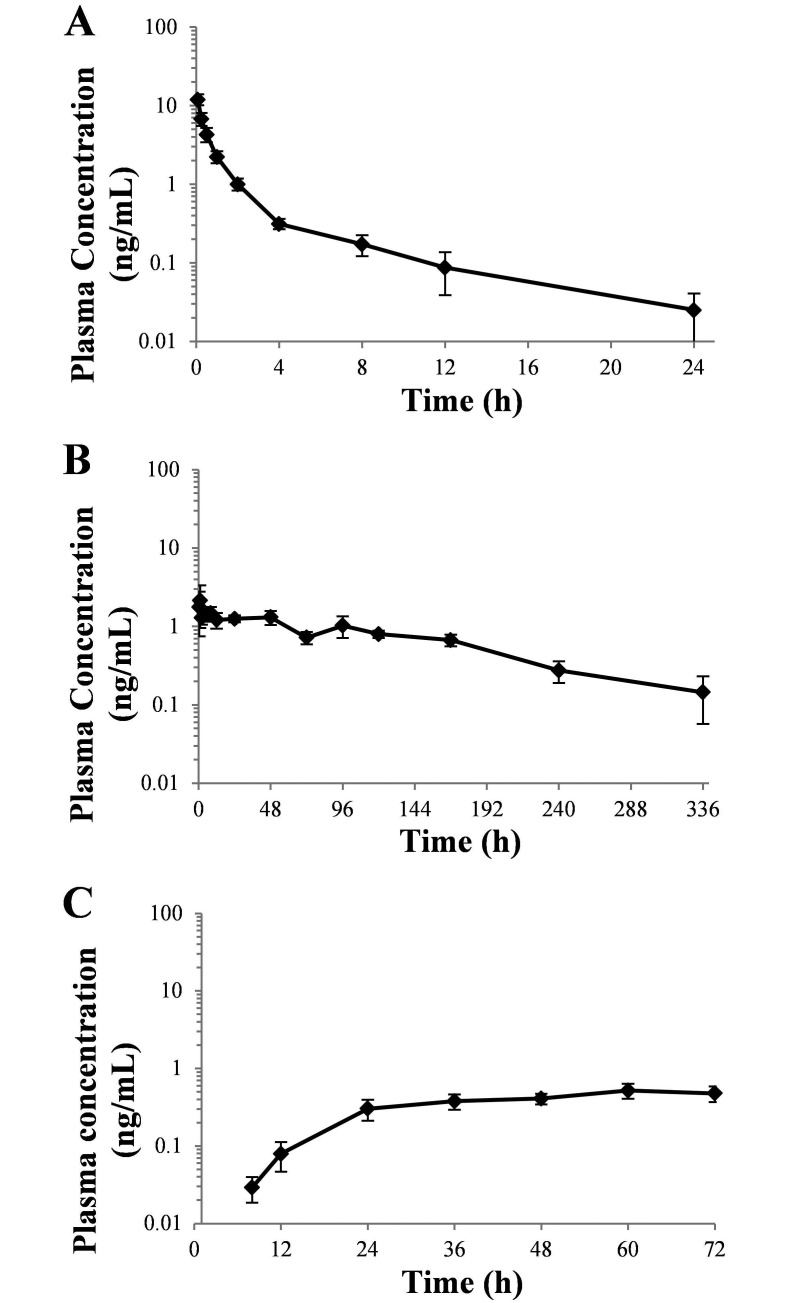
Plasma buprenorphine concentration compared with time curves for all minipigs are reported in Figure 1. Pharmacokinetic parameters for all 3 formulations of buprenorphine are summarized in Table 1. Buprenorphine was detected in the plasma after intravenous administration in all 5 minipigs for at least 8 h and had a t1/2 of 2.4 ± 0.9 h and AUC0–24 of 9.7 ± 1.4 ng × h/mL. Two minipigs still had low, but quantifiable plasma buprenorphine concentrations (0.07 and 0.06 ng/mL) at the last study time point, which was at 24 h after injection (Figure 1 A). The mean plasma buprenorphine concentration exceeded 0.1 ng/mL for 8.0 ± 1.26 h (Figure 2 A).
Figure 1.
Duration of plasma buprenorphine concentrations after a single administration of (A) 0.02 mg/kg IV buprenorphine, (B) 0.18 mg/kg SC SRB, and (C) 30 µg/h topical TDB in female Göttingen minipigs. The last time point of each curve represents the last buprenorphine plasma concentration for that animal.
Table 1.
Buprenorphine pharmacokinetics (mean ± SEM) for IV buprenorphine, SRB, and TDB
| IV | SRB | TDB | |
| C0 or Cmax (ng/mL) | 17.7 ± 3.2 | 2.9 ± 1.0 | 0.6 ± 0.1 |
| Tmax (h) | not done | 22.2 ± 10.6 | 63 ± 3.0 |
| Tlast (h) | 15.2 ± 3.7 | 264.0 ± 32.2a | 72b |
| AUC0-Tlast (ng × h/mL) | 9.7 ± 1.4 | 221.6 ± 26.8 | 25.2 ± 3.9 |
| AUC0-∞ (ng ×h/mL) | 10.5 ± 1.6 | 261.5 ± 26.8 | not quantifiable |
| Elimination rate constant (1/h) | 0.4 ± 0.1 | 0.007 ± 0.001 | not quantifiable |
| t1/2(h) (harmonic mean) | 2.4 ± 0.9 | 102.5 ± 14.3 | not quantifiable |
| Clearance | 2.1 ± 0.4 | not quantifiable | not quantifiable |
| Mean residence time (h) | 2.2 ± 0.9 | not quantifiable | not quantifiable |
| Volume of distribution at steady state (L/kg) | 4.2 ± 1.4 | not quantifiable | not quantifiable |
Minipigs received a single administration of buprenorphine (0.02 mg/kg IV; n = 5), SRB (0.18 mg/kg SC; n = 5), or TDB (30 µg/h topical, n = 4).
Figure 2.
Plasma buprenorphine concentration (mean ± SEM) compared with time after a single administration of (A) 0.02 mg/kg IV buprenorphine (n = 5), (B) 0.18 mg/kg SC SRB (n = 5 for time points 0–224 h, n = 2 for 336 h), and (C) 30 µg/h topical TDB (n = 4) in female Göttingen minipigs.
The plasma buprenorphine concentrations for SRB were highly variable between animals over the evaluation period, with peak plasma concentrations ranging between 1.1 and 6.9 ng/mL. The t1/2 and AUC0–336 were calculated as 102.5 ± 14.3 h and 221.6 ± 26.9 ng × h/mL, respectively. Four of the 5 minipigs still had detectable plasma concentrations at the 240-h (10-d) time point, and 2 of 3 minipigs at the 336-h (14-d) time point (Figure 1 B). Plasma buprenorphine concentrations reached an average Cmax of 2.9 ± 1.0 ng/mL at a Tmax of 22.2 ± 10.6 h. The mean plasma buprenorphine concentration remained above 0.1 ng/mL for 264.0 ± 32.2 h (Figure 2 B).
Plasma buprenorphine concentration for TDB was first detectable for 3 of 5 minipigs at the 8-h time point and at 24 h in 1 minipig (Figure 1 C). The remaining minipig failed to have detectable plasma buprenorphine concentrations at any time point throughout the evaluation period and was excluded from further analysis. The AUC0–72 was calculated as 25.2 ± 3.9 ng × h/mL. The AUC0-∞ and t1/2 were unable to be calculated. Plasma buprenorphine concentrations reached a Cmax of 0.6 ± 0.1 ng/mL at a Tmax of 63.0 ± 3 h. The mean plasma buprenorphine concentration was still above 0.1 ng/mL at the last time point (72 h; Figure 2 C).
Animal health and adverse effects.
Throughout the evaluation period, minipigs exhibited no change in appetite, activity level, or fecal consistency, and there was no evidence of sedation, respiratory depression, or vomit. Adverse effects were limited to skin reactions.
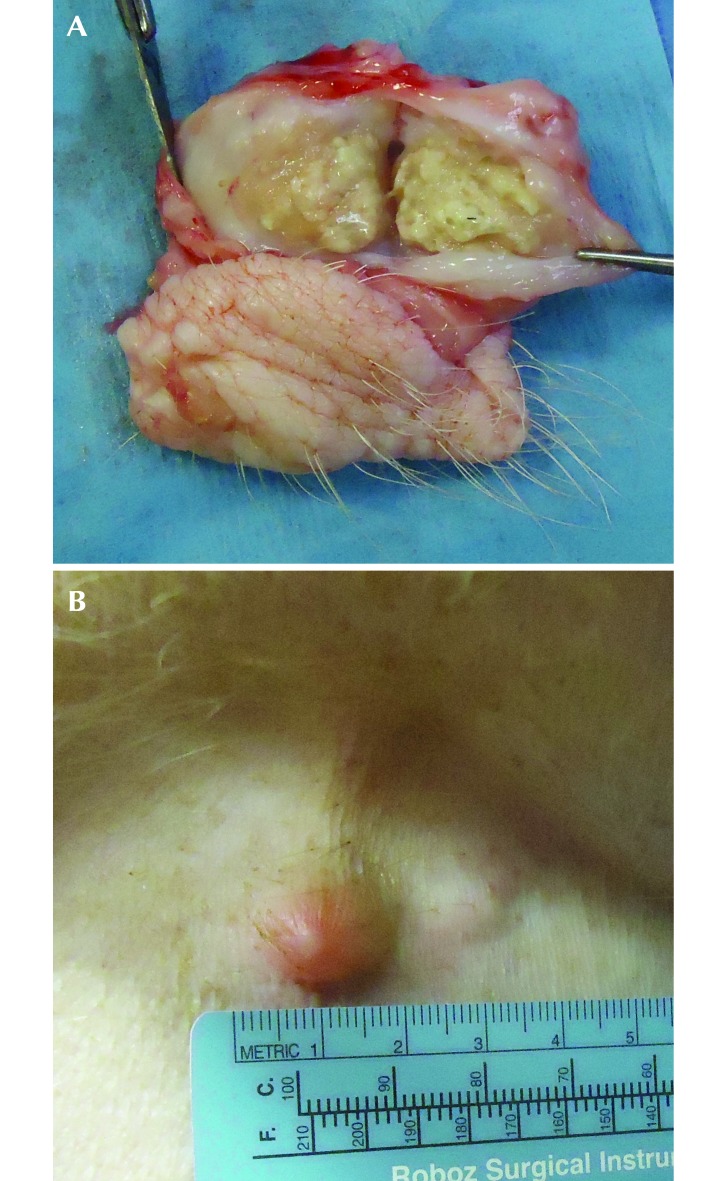
A subcutaneous injection-site reaction was noted in 4 of 5 minipigs that received SRB (Figure 3 A and B). The reactions were first detected at 1 wk after injection as small, firm subcutaneous swellings that grew over time to 5 mm to 2 cm in diameter. The reaction spontaneously resolved within 4 mo in 2 minipigs, drained to the skin surface in 4 mo in 1 minipig, and was biopsied at 2 mo in 1 minipig. The lesion was identified as a pyogranuloma, with central clear vacuoles containing sparse gray material, consistent with an injection site reaction. There was no erythema of the skin associated with the lesion, no evidence of pain or pruritus was present, and no veterinary intervention was required in any of the affected minipigs.
Figure 3.
Injection site reactions associated with SRB in the minipig. (A) Gross image of an injection site reaction of the flank skin fold 2 mo after SRB administration. (B) Gross image of the injection site reaction in the flank skin fold 4 mo after SRB administration. This reaction ruptured and drained within 1 wk.
All TDB patches remained intact for the duration of the study. One minipig developed an application-site reaction. The reaction was recognized on removal of the patches as a very mild, diffuse erythema, with a small number of papules on the skin underlying the drug-delivery portion of the patches. There was no visible skin reaction underlying the nondrug-delivery portion of the patch or the transparent occlusive bandage. The papules and erythema resolved within 4 d and did not require treatment. No evidence of pruritus or pain in the affected minipig was noted during the study evaluation period or after patch removal. No other TDB application-site reactions were noted in any of the other minipigs.
Discussion
Buprenorphine is used frequently in swine because of its relatively long half-life, yet injections still need to be administered 2 to 3 times a day to provide adequate postoperative analgesia.7,33 Repeated administration of injectable drugs can result in increased animal stress and possible periods of inadequate analgesia when plasma concentrations are at their lowest prior to the next injection. The current study evaluates the pharmacokinetics of 2 extended-release formulations of buprenorphine, SRB and TDB, in female Göttingen minipigs. At a hypothesized plasma buprenorphine therapeutic threshold of 0.1 ng/mL, therapeutic plasma buprenorphine concentrations lasted 8.0 ± 1.3 h after a single intravenous injection of 0.02 mg/kg buprenorphine, 264.0 ± 32.2 h after a single subcutaneous injection of 0.18 mg/kg SRB, and 72 h after application of 30 µg/h TDB. These findings support the use of SRB and TDB as long-acting formulations of buprenorphine in Göttingen minipigs. Furthermore, the ease of administration of these products can simplify postoperative pain management in laboratory swine and improve animal welfare.
Two approaches are commonly used to evaluate analgesic agents: (1) behavioral assessments of analgesiometric tests or postsurgical pain and (2) pharmacokinetic studies. Research on buprenorphine in swine has largely been focused on analgesiometric tests11,28 and postoperative assessments10,19,28 rather than pharmacokinetics and supports the administration of 0.01 mg/kg or higher to provide adequate postoperative analgesia.7,28 Only a single study has collected plasma buprenorphine concentrations in swine. These were reported to be 1.0 to 12.7 ng/mL 12 h after a single 0.1-mg/kg intramuscular buprenorphine injection, but this time point was the only one reported, and no other pharmacokinetic parameters were evaluated.19 The current study is the first to report comprehensive pharmacokinetic parameters of a single dose of buprenorphine in swine.
Ideally, analgesic dosages are determined by correlating plasma concentrations with adequate analgesia in the face of a painful stimulus to determine the therapeutic plasma concentration threshold. The therapeutic threshold of buprenorphine in swine has not yet been identified; however, it has been identified in dogs and humans and is fairly uniform between those species. Through correlating pharmacokinetic data and clinical pain assessments, a therapeutic plasma buprenorphine concentration threshold has been identified as 0.1 to 0.5 ng/mL in humans6,30 and 0.1 to 0.6 ng/mL in dogs.16,24 Thus, the putative target for a therapeutic plasma buprenorphine concentration threshold for minipigs in this study was 0.1 ng/mL. Future studies could aim to verify whether this plasma concentration correlates with adequate analgesia in swine.
Plasma buprenorphine concentrations after the administration of 0.02 mg/kg IV buprenorphine remained above the putative therapeutic threshold of 0.1 ng/mL for 4 h in 1 minipig, 8 h in 3 minipigs, and 12 h in 1 minipig. This finding correlated well with previous studies of buprenorphine in Yorkshire and Yorkshire–Landrace swine.10,28 In one study, use of the same dose and route of buprenorphine (0.02 mg/kg IV) increased latency to a skin-twitch test for as long as 10 h.28 Another study reported that pigs required rescue analgesia for post-thoracotomy pain at 5 to 8 h after the administration of 0.1 mg/kg IM buprenorphine.10 The results of these previous studies combined with the plasma buprenorphine concentrations in the current study, which remained above the therapeutic threshold for 8.0 ± 1.3 h, suggest that a dosing interval of 12 h for parenteral buprenorphine may not provide consistent adequate analgesia for all swine.
The pharmacokinetics of SRB were unique in Göttingen minipigs compared with other species. A single subcutaneous injection of 0.18 mg/kg SRB achieved a Cmax of 2.9 ± 1.0 ng/mL. This peak plasma concentration was lower than that reported in nonhuman primates (15.3 ng/mL)23 and dogs (5.6 ng/mL)24 but was comparable to that in rats (2.7 ng/mL).8 Nevertheless, this lower peak concentration in rats still provided adequate analgesia during thermal stimulus testing and after the creation of a unicortical tibial defect.8 Even though minipigs had a low peak plasma concentration, the duration of continuous therapeutic concentrations (264.0 ± 32.2 h) was considerably longer than the reported 72 h in rats8 and 5 d in nonhuman primates23 and dogs.24 Minipigs also had a notably higher AUC0-Tlast of 221.6 ± 26.8 ng × h/mL compared with these other species.23,24 The dose of 0.18 mg/kg SRB chosen for the current study was based on equivalent dosing of standard buprenorphine at 0.02 mg/kg every 8 h for 3 d and was comparable to that used in nonhuman primate and dog studies.23,24 Still, additional studies should be performed to determine whether the administration of a smaller SRB dose can still produce therapeutic plasma buprenorphine concentrations but remain detectable in the plasma for less time after injection.
The pharmacokinetics of TDB in Göttingen minipigs in the current study were comparable to those of low-dose TDB in humans.15,26 The administration of 30 μg/h TDB in minipigs resulted in a Cmax of 0.6 ± 0.1 ng/mL, whereas a single application of 10- or 20-μg/h TDB patches in adult humans resulted in a Cmax of 0.226 and 0.471 ng/mL, respectively.15,26 In contrast, a higher peak plasma concentration of 2.01 ng/mL was observed after the administration of a 52.5-μg/h TDB patch in 9.6 to 15.6 kg dogs.25 The current study used a combination of 2 low-dose patches, one at 10 μg/h and the other at 20 μg/h, to deliver 30 μg/h TDB to 12.6- to 27.0-kg minipigs. This dose fell within the recommended intravenous buprenorphine infusion rate of 0.5 to 10 μg/kg/h.33 Although this minipig dose was lower than that reported in dogs, peak plasma concentrations ranged between 0.37 and 0.75 ng/mL and still exceeded the hypothesized therapeutic plasma buprenorphine concentration threshold for minipigs.
Intravenous buprenorphine and SRB achieved therapeutic concentrations quickly, as indicated by plasma buprenorphine concentrations that exceeded 0.1 ng/mL by the first evaluation time point (that is, 5 or 30 min). In contrast, TDB did not achieve therapeutic concentrations until 12 to 24 h after application. These findings are consistent with those after TDB administration in both dogs25 and humans6,15 and after other transdermal analgesics in swine, such as fentanyl.18,36 The delay in onset was a result of the diffusion kinetics of buprenorphine down the concentration gradient across the skin.2,3 Consequently, to achieve therapeutic plasma buprenorphine concentrations postoperatively, TDB must be applied 12 to 24 h prior to surgery. After the delayed onset, plasma concentrations from TDB reached Tmax (63 ± 3.0 h) later than with either intravenous buprenorphine or SRB but continued to exceed the therapeutic threshold throughout the study (last time point at 72 h). Therefore, the duration of therapeutic concentrations due to TDB application in minipigs could not be calculated, and prolonged evaluation beyond 72 h should be considered.
In addition to prolonged therapeutic plasma buprenorphine concentrations, SRB and TDB have other advantages and disadvantages that merit consideration. For instance, therapeutic plasma concentrations are achieved within minutes after the injection of SRB rather than in 12 to 24 h as with TDB. In addition, a single injection of SRB eliminates the chance of premature discontinuation of analgesia, which might occur if a transdermal patch falls off. In contrast, TDB does not require injection, and the patches can easily be removed to discontinue drug administration if any adverse reactions occur.
Both SRB and TDB resulted in localized skin reactions, which were more frequent with SRB than TDB. Of the 5 minipigs given SRB, 4 developed a self-limiting, injection site reaction. Similar reactions have occurred in mice,4 rats,8 dogs,24 cats5 and nonhuman primates.23 The skin reactions in rats have been suggested to result from SRB seeping from the injection site and onto the skin.8 As a result, care was taken in the current study to ensure that injection was complete prior to withdrawal of the needle, and the skin of the injection site was pinched for approximately 15 s to prevent any drug from coming in contact with the skin. Despite the careful technique, localized skin reactions still occurred at the injection site. The reaction in minipigs was consistent with a foreign-body reaction and could be due to either the copolymer or solvent used in the sustained-release formulation. Even though these reactions resolved without intervention, the frequency of occurrence remains a concern. Moreover, SRB use in conjunction with specific research studies, such as dermatologic studies and those involving immunosuppression, should be considered carefully, given that stimulation of the inflammatory process could confound results.
The TDB application-site reaction seen in one minipig is the first report of a skin reaction due to TDB in any animal, although the incidence of erythema or irritation of the skin in humans is 20% to 25%.9,14 The occurrence of an application-site reaction in minipigs but not other species is not surprising given the similarities between human and swine skin. The thickness, follicle structure and density, blood supply, and permeability of Gottingen skin have all been reported to be more comparable to those of human skin than of the skin of densely haired animals.17,27 The skin reaction in our minipig did not appear to be associated with any pain or pruritus, resolved without complication after removal of the patch, and does not contraindicate the use of TDB in swine.
One obstacle of transdermal analgesics in animals is maintaining appropriate skin adhesion of the patches throughout the postoperative period. For example, a recent study of TDB in cats reported the loss of 4 of 6 patches within the first 2 d after application.22 However, no bandaging or other measures were used to protect the patches in the previous study,22 illustrating the necessity for bandaging with transdermal drugs in animals. One minipig in the current study did not achieve detectable plasma concentrations, even though the patches remained affixed to the skin and the bandaging over the patch remained intact. Similar complications have been reported in dogs treated with TDB.25 It is plausible that even if the patch remains adhered to the skin and the bandage remains intact, regrowth of hair underneath the patch might prevent a sufficient skin-to-patch interface for effective drug diffusion. Indeed, visible regrowth of hair was noted under the patches that fell off in the aforementioned cat TDB study.22 Hair regrowth underneath the transdermal patches may be a particularly valid concern in Gottingen minipigs, given that they have remarkably stiff hair in comparison to other domestic swine and animal species.27 An alternative explanation may be that some minipigs have a decreased skin permeability to buprenorphine, preventing detectable plasma concentration from being attained. Even so, these results indicate that, despite the species, a small population of subjects may be unable to achieve therapeutic plasma buprenorphine concentrations with TDB, highlighting the importance of postoperative pain assessments to ensure that animals receive adequate analgesia.
One limitation of the current study is the lack of a postoperative pain model or an analgesiometric test to assess clinical efficacy at the putative therapeutic plasma concentration threshold of 0.1 ng/mL in minipigs. Pharmacokinetic studies alone cannot determine the physiologic effects of a drug, and obtaining detectable plasma drug concentrations does not always ensure adequate analgesia. For example, cats achieve detectable plasma buprenorphine concentrations with peak concentrations ranging between 5.37 to 13.7 ng/mL after application of a 35-µg/h TDB patch, yet they exhibit no significant change in thermal latencies over baseline.22 This finding demonstrates the importance of correlating plasma buprenorphine concentrations obtained from pharmacokinetic studies with clinical efficacy.
The current study is the first to evaluate the pharmacokinetics of intravenous buprenorphine, SRB, and TDB in laboratory swine. Given a therapeutic plasma buprenorphine concentration threshold of 0.1 ng/mL, a single subcutaneous injection of 0.18 mg/kg SRB will last for 264 ± 32.2 h, and a single application of 30 µg/h TDB will last for 72 h. In comparison, 0.02 mg/kg IV buprenorphine achieves therapeutic concentrations for only 8 h. Localized administration reactions can be seen with both extended-release buprenorphine formulations, but they were self-limiting, were not distressful, and should not preclude the use of these analgesics in swine. Although additional studies are necessary to verify clinical efficacy, the results from our study support the use of SRB and TDB as long-acting formulations of buprenorphine in female Göttingen minipigs.
Acknowledgments
We thank Ron Barthelemy, Benji Cuyugan, and Trish Galassi for their assistance with sample collection; Kuldip Mirakhur for surgical VAP assistance; and Josh Decker for histopathology.
References
- 1.Andaluz A, Moll X, Ventura R, Abellan R, Fresno L, Garcia F. 2009. Plasma buprenorphine concentrations after the application of a 70-µg/h transdermal patch in dogs. Preliminary report. J Vet Pharmacol Ther 32:503–505. [DOI] [PubMed] [Google Scholar]
- 2.Bajaj S, Whiteman A, Brander B. 2011. Transdermal drug delivery in pain management. CEACCP 11:39–43. [Google Scholar]
- 3.Cachia E, Ahmedzai SH. 2011. Transdermal opioids for cancer pain. Curr Opin Support Palliat Care 5:15–19. [DOI] [PubMed] [Google Scholar]
- 4.Carbone ET, Lindstrom KE, Diep S, Carbone L. 2012. Duration of action of sustained-release buprenorphine in 2 strains of mice. J Am Assoc Lab Anim Sci 51:815–819. [PMC free article] [PubMed] [Google Scholar]
- 5.Catbagan DL, Quimby JM, Mama KR, Rychel JK, Mich PM. 2011. Comparison of the efficacy and adverse effects of sustained-release buprenorphine hydrochloride following subcutaneous administration and buprenorphine hydrochloride following oral transmucosal administration in cats undergoing ovariohysterectomy. Am J Vet Res 72:461–466. [DOI] [PubMed] [Google Scholar]
- 6.Evans HC, Easthope SE. 2003. Transdermal buprenorphine. Drugs 63:1999–2010. [DOI] [PubMed] [Google Scholar]
- 7.Fish RE, Brown MJ, Danneman PJ, Karas AZ. 2008. Anesthesia and analgesia in laboratory animals, 2nd ed. San Diego (CA): Academic Press. [Google Scholar]
- 8.Foley PL, Liang H, Crichlow AR. 2011. Evaluation of a sustained-release formulation of buprenorphine for analgesia in rats. J Am Assoc Lab Anim Sci 50:198–204. [PMC free article] [PubMed] [Google Scholar]
- 9.Hans G, Robert D. 2009. Transdermal buprenorphine – a critical appraisal of its role in pain management. J Pain Res 2:117–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harvey-Clark CJ, Gilespie K, Riggs KW. 2000. Transdermal fentanyl compared with parenteral buprenorphine in postsurgical pain in swine: a case study. Lab Anim 34:386–398. [DOI] [PubMed] [Google Scholar]
- 11.Hermansen K, Pederson LE, Olsen HO. 2009. The analgesic effect of buprenorphine, etorphine, and pethidine in the pig: a randomized, double blind cross-over study. Acta Pharmacol Toxicol (Copenh) 59:27–35. [DOI] [PubMed] [Google Scholar]
- 12.Huang W, Moody DE, McCance-Katz EF. 2006. The in vivo glucuronidation of buprenorphine and norbuprenorphine determined by liquid chromatography–electrospray ionization–tandem mass spectrometry. Ther Drug Monit 28:245–251. [DOI] [PubMed] [Google Scholar]
- 13.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 14.James IGV, O'Brien CM, McDonald CJ. 2010. A randomized, double-blind, double-dummy comparison of the efficacy and tolerability of low-dose transdermal buprenorphine (BuTrans 7-day patches) with buprenorphine sublingual tablets (Temegesic) in patients with osteoarthritis pain. J Pain Symptom Manage 40:266–278. [DOI] [PubMed] [Google Scholar]
- 15.Kapil RP, Cipriano A, Friedman K, Michels G, Shet MS, Colucci SV, Apseloff G, Kitzmiller J, Harris SC. 2013. Once-weekly transdermal buprenorphine application results in sustained and consistent steady-state plasma levels. J Pain Symptom Manage 46:65–75. [DOI] [PubMed] [Google Scholar]
- 16.Ko JC, Freeman LJ, Barletta M, Weil AB, Payton ME, Johnson BM, Inoue T. 2011. Efficacy of oral transmucosal and intravenous administration of buprenorphine before surgery for postoperative analgesia in dogs undergoing ovariohysterectomy. J Am Vet Med Assoc 238:318–328. [DOI] [PubMed] [Google Scholar]
- 17.Mahl JA, Vogel BE, Court M, Kolopp M, Roman D, Nogues V. 2006. The minipig in dermatotoxicology: methods and challenges. Exp Toxicol Pathol 57:341–345. [DOI] [PubMed] [Google Scholar]
- 18.Malavasi LM, Augustsson H, Jensen-Waern M, Nyman G. 2005. The effect of transdermal delivery of fentanyl on activity in growing pigs. Acta Vet Scand 46:149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malavasi LM, Jensen-Waern M, Augustsson H, Lindberg JE, Nyman G. 2006. Effects of preoperative epidural morphine and intramuscular buprenorphine in pigs subjected to abdominal surgery: a pilot study. Scientia Agraria Paranaensis 5:21–30. [Google Scholar]
- 20.Mitra F, Chowdhury S, Shelley M, Williams G. 2013. A feasibility study of transdermal buprenorphine versus transdermal fentanyl in the long-term management of persistent noncancer pain. Pain Med 14:75–83. [DOI] [PubMed] [Google Scholar]
- 21.Moll X, Fresno L, Garcia F, Prandi D, Andaluz A. 2011. Comparison of subcutaneous and transdermal administration of buprenorphine for preemptive analgesia in dogs undergoing elective ovariohysterectomy. Vet J 187:124–128. [DOI] [PubMed] [Google Scholar]
- 22.Murrell JC, Robertson A, Taylor PM, McCown JL, Bloomfield M, Sear JW. 2007. Use of a transdermal matrix patch of buprenorphine in cats: preliminary pharmacokinetic and pharmacodynamics data. Vet Rec 160:578–583. [DOI] [PubMed] [Google Scholar]
- 23.Nunamaker EA, Halliday LC, Moody DE, Fang WB, Lindeblad M, Fortman JD. 2013. Pharmacokinetics of 2 formulations of buprenorphine in macaques (Macaca mulatta and Macaca fascicularis). J Am Assoc Lab Anim Sci 52:48–56. [PMC free article] [PubMed] [Google Scholar]
- 24.Nunamaker EA, Stolarik DF, Ma J, Wilsey A, Jenkins GJ, Medina CL. 2014. Clinical efficacy of sustained-release buprenorphine with meloxicam for postoperative analgesia in beagle dogs undergoing ovariohysterectomy. J Am Assoc Lab Anim Sci 53:494–501. [PMC free article] [PubMed] [Google Scholar]
- 25.Pieper K, Schuster T, Levionnois O, Matis U, Bergadano A. 2011. Antinociceptive efficacy and plasma concentrations of transdermal buprenorphine in dogs. Vet J 187:335–341. [DOI] [PubMed] [Google Scholar]
- 26.Plosker GL. 2011. Buprenorphine 5-, 10-, and 20-μg/h transdermal patch: a review of its use in the management of chronic nonmalignant pain. Drugs 71:2491–2509. [DOI] [PubMed] [Google Scholar]
- 27.Qvist MH, Hoeck U, Kreilgaard B, Madsen F, Frokjaer S. 2000. Evaluation of Göttingen minipig skin for transdermal in vitro permeation studies. Eur J Pharm Sci 11:59–68. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez NA, Cooper DM, Risdahl JM. 2001. Antinociceptive activity of and clinical experience with buprenorphine in swine. Contemp Top Lab Anim Sci 40:17–20. [PubMed] [Google Scholar]
- 29.Roughan JV, Flecknell PA. 2002. Buprenorphine: a reappraisal of its antinociceptive effects and therapeutic use in alleviating postoperative pain in animals. Lab Anim 36:322–343. [DOI] [PubMed] [Google Scholar]
- 30.Sittl R, Griessinger N, Likar R. 2003. Analgesic efficacy and tolerability of transdermal buprenorphine in patients with inadequately controlled chronic pain related to cancer and other disorders: a multicenter, randomized, double-blind, placebo-controlled trial. Clin Ther 25:150–168. [DOI] [PubMed] [Google Scholar]
- 31.Sittl R, Likar R, Nautrup BP. 2005. Equipotent doses of transdermal fentanyl and transdermal buprenorphine in patients with cancer and noncancer pain: results of a retrospective cohort study. Clin Ther 27:225–237. [DOI] [PubMed] [Google Scholar]
- 32.Smith AC, Swindle MM. 2006. Preparation of swine for the laboratory. ILAR J 47:358–363. [DOI] [PubMed] [Google Scholar]
- 33.Swindle MM. 2007. Swine in the laboratory, 2nd ed. Boca Raton (FL): Taylor and Francis Group. [Google Scholar]
- 34.Swindle MM, Nolan T, Jacobson A, Wolf P, Dalton MJ, Smith AC. 2005. Vascular access port (VAP) usage in large animal species. Contemp Top Lab Anim Sci 44:7–17. [PubMed] [Google Scholar]
- 35.Uberall MA, Muller-Schwefe GHH. 2012. Low-dose 7-day transdermal buprenorphine in daily clinical practice–perceptions of elderly patients with moderate nonmalignant chronic pain. Curr Med Res Opin 28:1585–1595. [DOI] [PubMed] [Google Scholar]
- 36.Wilkinson AC, Thomas ML, Morse BC. 2001. Evaluation of a transdermal fentanyl system in Yucatan miniature pigs. Contemp Top Lab Anim Sci 40:12–16. [PubMed] [Google Scholar]