Abstract
Background
Sex hormones are known to have significant effects on pathophysiology of cardiovascular disease.
Objective
To study the association between sex hormone levels and sudden cardiac arrest (SCA).
Methods
In the ongoing Oregon Sudden Unexpected Death Study (catchment population approximately 1 million), cases of SCA were compared with matched controls. Testosterone and estradiol levels were measured from blood samples drawn at the time of the SCA event in cases and during a routine visit in controls.
Results
Among cases (n=149; 64.1 ± 11.7 years; 73.2% male), compared to controls (n=149; 64.2 ±11.6 years; 72.5% male), median testosterone levels were significantly lower in males (4.4 vs. 5.4 ng/ml; p=0.01). Median estradiol levels were higher in male (68 vs. 52 pg/ml; p<0.001) and female cases (54 vs. 36 pg/ml; p<0.001). In multivariate analysis, higher testosterone levels were associated with lower SCA odds only in males (OR 0.75; 95% CI 0.58–0.96; p=0.02). Higher estradiol levels were associated with higher SCA odds in both males (OR 2.0; 95% CI 1.5–2.6; p<0.001) and females (OR 3.5; 95% CI 1.9–6.4; p<0.001). A higher testosterone/estrogen ratio was associated with lower SCA odds in males only (OR 0.5; 95% CI 0.4–0.7; p<0.001). In a canine model of SCA, plasma testosterone levels were not significantly altered by the cardiac arrest event.
Conclusions
We observed significant differences in sex hormone levels in patients who suffered SCA, with potential mechanistic implications. The role of sex hormones in the genesis of fatal ventricular arrhythmias warrants further exploration.
Keywords: Sudden cardiac arrest, testosterone, estradiol
Introduction
The last two decades have witnessed a sharp rise in the global burden of cardiovascular disease1. At least 50% of cardiovascular mortality is comprised of sudden cardiac arrest (SCA), a devastating condition due to its unexpected nature and the very low rates of survival following resuscitation2. Coronary artery disease (CAD) is the most commonly associated cardiac condition in the majority of SCA cases; however SCA may be the first manifestation of heart disease in many cases3. Though left ventricular (LV) systolic dysfunction is an important risk factor for SCA in a subgroup of patients, there is now broad acknowledgement of the fact that there are likely to be multiple other mechanisms of ventricular arrhythmogenesis and these need to be uncovered, so that more meaningful risk prediction, prevention and management strategies can be developed4.
It is also well recognized that there are important sex differences in manifestation of SCA, with women having an overall lower incidence, less structural heart disease and different presenting rhythms5–7. However, the exact role of the endogenous sex hormones in cardiovascular disease, and SCA in particular, is not well understood. Though female sex hormones were traditionally considered to be protective against cardiovascular disease8, the hormone replacement therapy (HRT) trials have challenged this assumption9, 10. Studies have also pointed towards the role of declining androgen levels with age as a risk factor for cardiac disease in men11. Whether sex hormones have a specific role in the pathophysiology of SCA has not been well studied before. We evaluated the association between sex hormone levels and SCA in a community-based case-control study, utilizing SCA cases and controls with coronary artery disease (CAD) in the same population. We also studied the effects of the ventricular fibrillation (VF) event as well as the process of cardio-pulmonary resuscitation (CPR) on testosterone levels in a canine model of SCA.
Methods
Study Population
The Oregon Sudden Unexpected Death Study (Oregon SUDS) is a prospective community based study of SCA in the Portland, Oregon metropolitan area (population approximately 1 million), ongoing since 2002. The detailed methods for this study have been published earlier12, 13. Briefly, cases of SCA occurring in the community are identified using multiple sources, including first responders, local hospitals and the county medical examiner’s office. Detailed medical records, circumstances of arrest and autopsy reports (if any) are collected for all cases of suspected sudden death. SCA is defined as a sudden, unexpected loss of the pulse due to a likely cardiac etiology (when witnessed) or a sudden death occurring within 24 hours of last being seen in the usual state of health (if unwitnessed). Non-cardiac etiologies of cardiac arrest such as drug overdose, trauma/violence and chronic terminal illnesses are excluded. SCA is diagnosed using an in-house adjudication process involving three physicians. During the same time period, controls with documented CAD but no history of ventricular arrhythmia were recruited from the same geographical area from several sources which included clinics of participating hospitals, patients receiving a coronary angiogram, subjects transported by emergency medical services for acute coronary ischemia, and from among members of a local health maintenance organization. CAD was defined as stenosis of ≥ 50% in any of the major coronary arteries on angiography or a history of prior myocardial infarction or coronary revascularization. Patients with CAD were chosen as controls to eliminate potential confounding by factors related to CAD and to be able to identify factors unique to SCA, since the vast majority of SCA cases will also have underlying CAD14. All available clinical variables were obtained from medical records for cases (prior to arrest) and controls. For the present analysis, white SCA cases age ≥ 18 years, with documented CAD and ventricular fibrillation (VF) as the presenting rhythm were included. An equal number of controls matched for age, gender and race served as the comparison group. The study was approved by the institutional review boards of all participating hospitals. The canine model for assessing potential influence of the cardiac arrest event on testosterone levels was approved by the institutional animal ethics committee.
Measurement of Sex Hormone Levels
Sex hormone assays were performed on 149 SCA cases and 149 age and gender matched CAD controls. For cases, the levels were measured in plasma processed from blood collected at the time of the SCA event and subsequently stored at −80 degrees Celsius. Blood samples were collected by emergency medical service (EMS) first responders during the resuscitation process in the field. The sample was usually collected early in the resuscitation process when blood is withdrawn for confirmation of successful intravenous access. For controls, levels were measured at a routine visit. Testosterone and Estradiol (17β – estradiol, E-2) levels were measured using the Access 2 Immunoassay system (Beckman Coulter, Inc., California). This is a paramagnetic particle, chemiluminescent immunoassay for the quantitative determination of levels in human plasma, using an antibody capture technique.
Influence of the cardiac arrest event on sex hormone levels in a canine model
We evaluated the influence of cardiac arrest and CPR on testosterone levels in a canine model of cardiac arrest. This study was performed in fifteen female hound dogs, between the ages of 12 and 18 months. The animals were placed under general anesthesia and blood was drawn at baseline for measurement of testosterone levels. Under general anesthesia, VF was induced by T wave shock and CPR performed according to standard protocol for 5 minutes. In order to closely correspond with the human SCA conditions for measurement, blood for testosterone levels was drawn once again following VF arrest and resuscitation following the standard advanced cardiac life support protocol. Plasma obtained from the blood was stored at minus 80 degrees Celsius and testosterone levels were measured.
Analysis
Sex hormone levels were expressed as median and inter-quartile range (IQR). Non-parametric tests were used to compare levels between cases and controls to account for the non-normal distribution of hormone values. Other continuous variables were compared using t tests and categorical variables were compared using the Pearson’s Chi square test. Multiple logistic regression was used to calculate odds ratios for SCA after adjusting for covariates. For multivariable analysis, sex hormone levels were divided into quartiles based on the distribution in controls and modeled as an ordinal variable to estimate odds per quartile increase in level. A p-value of ≤ 0.05 was considered statistically significant. For the canine experiment, paired t-tests were used to determine whether there was a significant change in levels before and after cardiac arrest and resuscitation. All analysis was performed using the Statistical Package for the Social Scientist (SPSS) version 21.0 (SPSS, IBM Corporation, New York).
Results
Subject Characteristics
Table 1 shows the demographic and clinical characteristics of the cases and controls. Over 70% were male and the female subjects (40 cases and 41 controls) had a mean age of about 68 years (68.3 ± 11.8 for cases vs. 68.4 ± 11.4 for controls). The prevalence of diabetes (31.2% vs. 32.9%; p=0.76), obesity (38.8% vs. 42.3%; p=0.6) and smoking (current smoker 44.6% vs. 38.7%; p=0.4) were not significantly different between cases and controls, and mean cholesterol levels (186 ± 52 vs. 171 ± 53 mg/dl; p=0.1) were similar. The mean and median times to EMS arrival by the patient’s side were 7.3 ± 4.2 minutes and 6.7 minutes respectively. 36 out of 149 cases (24.1%) were definitively shown to have acute MI associated with SCD by ECG, coronary angiography or autopsy showing fresh coronary thrombus. However this is likely to be an underestimate since many acute coronary syndromes may be missed in those who did not survive to have an evaluation.
Table 1.
Clinical and Demographic Characteristics of Cases and Controls
| Cases (n=149) | Controls (n=149) | P value* | |
|---|---|---|---|
| Age | 64.1 ± 11.7 | 64.2 ± 11.6 | -- |
| Male | 109 (73.2) | 108 (72.5) | -- |
| Body Mass Index† | 29.5 ± 6.4 | 29.8 ± 6.2 | 0.8 |
| Obesity† | 33 (38.8) | 63 (42.3) | 0.6 |
| Diabetes‡ | 44 (31.2) | 49 (32.9) | 0.76 |
| Current Smoker§ | 37 (44.6) | 36 (38.7) | 0.4 |
| Cholesterol (mg/dL)¶ | 186 ± 52 | 171 ± 53 | 0.1 |
T test for continuous variables and Chi-square test for categorical variables
Data available for 85 cases and all controls (Obesity defined as BMI ≥ 30)
Data available for 83 cases and 93 controls
Data available for 42 cases and 116 controls
Defined as either a medical chart diagnosis of diabetes or prescription of anti-diabetic medications
Testosterone Levels
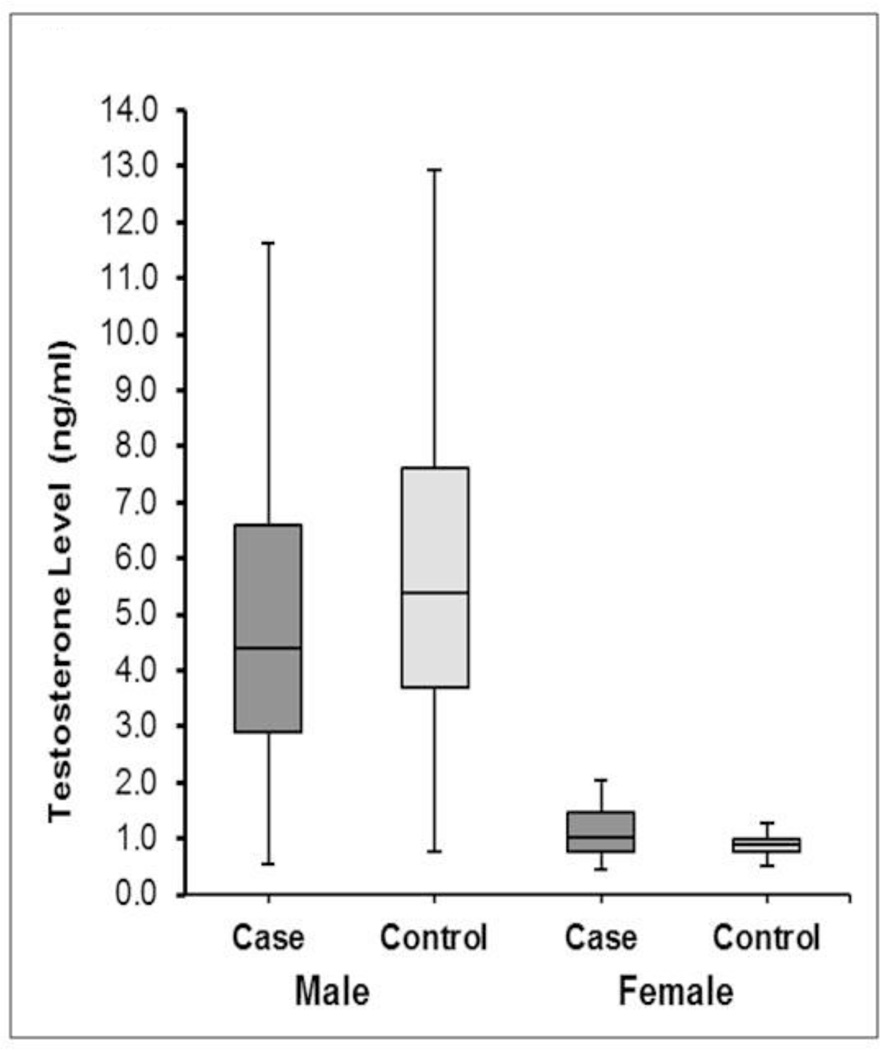
The median testosterone levels were significantly lower among male SCA cases compared to controls (4.4 vs. 5.4 ng/mL; p=0.01) (Table 2, Figure 1). A higher proportion of male cases had testosterone levels in the lowest quartile compared to controls (40% vs. 25%; p=0.02). Among females, testosterone levels were lower compared to males. However, average testosterone levels were higher in female cases compared to controls (1.03 vs. 0.90 ng/mL; p=0.02). A testosterone level of >0.90 ng/mL was more likely to be observed in female cases than controls (67.5% vs. 48.8%; p=0.09). In multivariable models, adjusted for age and diabetes, higher testosterone levels were associated with lower odds for SCA in males (OR 0.75; 95% CI 0.58–0.96; p=0.02; Table 3). Every quartile increase in testosterone lowered SCA odds by 25%. There was no significant association between testosterone levels and SCA among females in multivariable analysis (p=0.1; Table 3).
Table 2.
Sex Hormone Levels in SCA Cases and Controls
| Sex Hormone | Cases (n=149) [median (IQ Range)] |
Controls (n=149) [median (IQ Range)] |
P value* |
|---|---|---|---|
| Testosterone (males)(ng/mL) | 4.4 (2.9–6.6) | 5.4 (3.7–7.6) | 0.01 |
| Testosterone (females)(ng/mL) | 1.03 (0.78–1.47) | 0.90 (0.78–0.99) | 0.02 |
| Estradiol (males)(pg/mL) | 68.0 (54.5–82.0) | 52.0 (43.2–64.0) | <0.001 |
| Estradiol (females)(pg/mL) | 54.5 (41.0–80.7) | 36.0 (28.0–45.0) | <0.001 |
| T/E Ratio (males) | 0.067 (0.04–0.09) | 0.105 (0.07–0.15) | <0.001 |
| T/E Ratio (females) | 0.019 (0.01–0.03) | 0.025 (0.01–0.03) | 0.06 |
Independent samples Mann-Whitney U test
T/E- Testosterone/Estradiol
IQ Range- Inter Quartile Range
Figure 1.
Box Plot showing the Distribution of Testosterone Levels in Cases and Controls
Table 3.
Multivariable Odds Ratios for SCA per Quartile Increase in Sex Hormone Level
| Odds Ratio (95% CI) | P value | |
|---|---|---|
| Testosterone (males) | 0.75 (0.58–0.96) | 0.02 |
| Testosterone (females) | 1.4 (0.9–2.0) | 0.1 |
| Estradiol (males) | 2.0 (1.5–2.6) | <0.001 |
| Estradiol (females) | 3.5 (1.9–6.4) | <0.001 |
| T/E Ratio (males) | 0.5 (0.4–0.7) | <0.001 |
| T/E Ratio (females) | 0.8 (0.5–1.1) | 0.2 |
Estradiol Levels
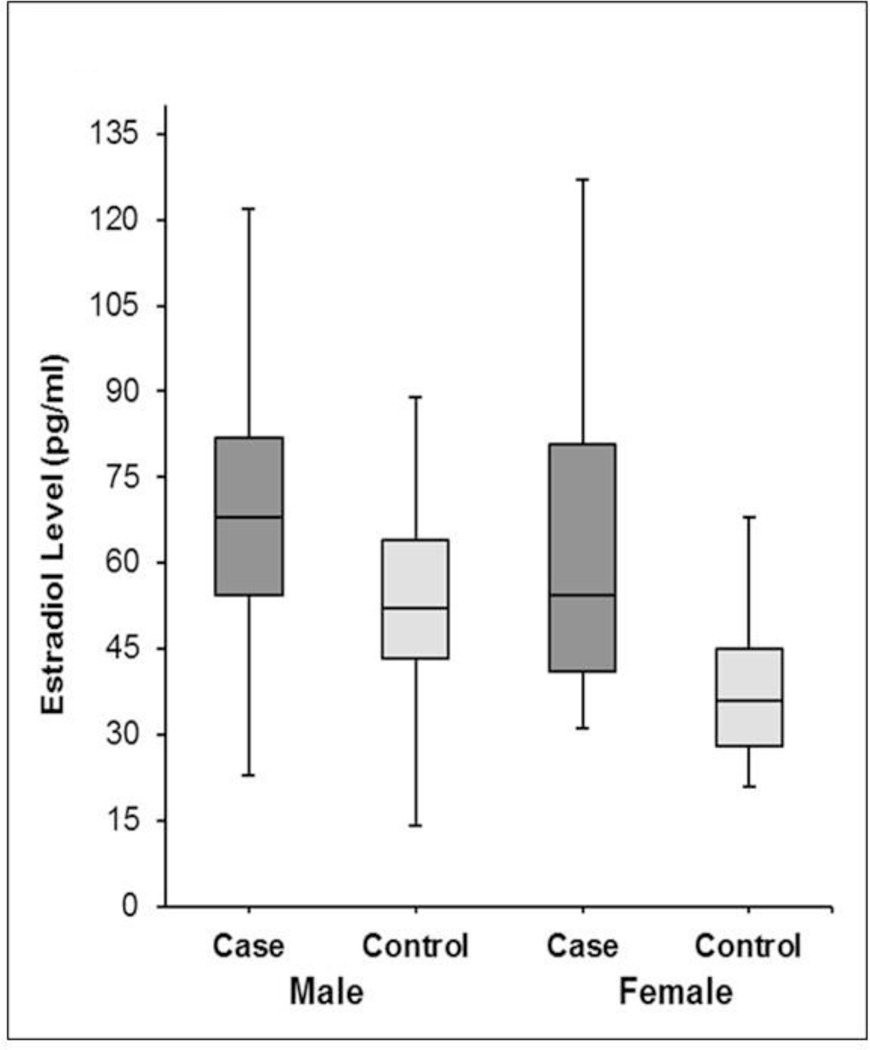
The median estradiol levels were significantly higher in both male (68 vs. 52 pg/ml; p<0.001) and female cases (54.5 vs. 36.0 pg/ml; p<0.001) compared to controls (Table 2, Figure 2). While estradiol levels above the median cut-off were seen in a significantly greater proportion of male SCA cases (79% vs. 47%; p<0.001), the difference was even more pronounced among female cases (92% vs 49%; p<0.001). In multivariable analysis, adjusted for age and diabetes, there was a strong association between estradiol levels and SCA. In males, the SCA odds doubled with one quartile increase in estradiol (OR 2.0; 95% CI 1.5–2.6; p<0.001). In females, the odds for SCA more than tripled with every quartile increase in estradiol levels (OR 3.5; 95% CI 1.9–6.4; p<0.001) (Table 3).
Figure 2.
Box Plot showing the Distribution of Estradiol Levels in Cases and Controls
Testosterone/Estrogen (T/E) Ratio
The T/E ratio was significantly lower in male cases (Table 2). While there was a trend towards a lower value in female cases, it did not reach statistical significance. In multivariable analysis, a higher T/E ratio was associated with lower odds for SCA in males (OR 0.5; 95% CI 0.4–0.7), but not in females (p=0.2; Table 3).
Canine Model
VF was successfully induced in all fifteen dogs. Pre and post SCA testosterone levels were measurable with adequate quality control from the obtained plasma in all cases. In the 15 animals, median plasma testosterone level was similar before and after cardiac arrest and resuscitation (before: 0.72 ng/mL, IQR 0.67 – 0.93; after: 0.74 ng/mL, IQR 0.68 – 0.81). The mean change in testosterone levels was a 0.04 ng/mL increase, which did not meet statistical significance (p=0.20).
Discussion
To the best of our knowledge, this is the first study to assess the relationship between sex hormone levels and occurrence of SCA in the community. There are several points of interest that emerge from this study. Firstly, higher levels of testosterone appear to be associated with lower odds of SCA in men. Secondly, higher levels of estradiol were strongly related to increased SCA odds in both sexes. Thirdly, higher T/E ratio was associated with lower SCA odds among males, but not females, though there was a trend in the same direction. Taken together, these results appear to indicate that low testosterone levels and high estradiol levels are particularly associated with SCA. Most cases of SCA in the community have significant underlying CAD; however in order to identify novel mechanisms of ventricular arrhythmias, high risk subgroups within the population with CAD need to be defined. While there is no evidence for a causal relationship between SCA and sex hormones in this study, it opens the door for further research into potential mechanisms.
We found no significant change in testosterone levels due to the cardiac arrest and resuscitation process in a canine model of VF arrest. These findings suggest that the observed differences in sex hormone levels are likely to have preceded the cardiac arrest event.
There are limited studies addressing the association between sex hormones in SCA. Wehr et al, in a study of 2078 men referred for coronary angiography, reported an association of low testosterone levels with increased mortality from heart failure, but did not find a specific association with SCA15. They also reported an association between low testosterone and overall cardiovascular mortality in post-menopausal diabetic women16. Epidemiological studies have linked lower androgen levels with higher risk of cardiac disease; however whether there is a causal role remains unclear17. Lower levels of testosterone are associated with a greater incidence of diabetes18, while higher levels are associated with better lipid values19 and a lower prevalence of atherosclerosis in the general population20. Studies have also suggested that testosterone supplementation may improve LV function, with obvious implications for SCA risk21. Higher testosterone levels being associated with lower SCA odds in this study is in concordance with this body of literature. One plausible mechanism may be the reported association between higher testosterone levels and shorter QT interval22, since a prolonged QTc has been shown to be a risk factor for SCA23. On the other hand, a recent study demonstrated an increased prevalence of the early repolarization (ER) pattern which has been linked to SCA, in association with higher testosterone levels. However, the association was mainly observed for the “benign” variants of ER with a weaker association with the “malignant” ER pattern24. Clearly, a better understanding of the influence of testosterone on electrocardiographic parameters will help clarify its role in SCA mechanisms.
With regard to the female sex hormones, a recent analysis of SCA in a large cohort of post-menopausal women showed no clear relationship between HRT and the incidence of SCA25. However, given the low numbers of SCA overall, the authors concluded that an effect in either direction could not be conclusively ruled out. Female sex hormones were considered to play an important role in reducing the incidence of cardiac disease in pre-menopausal women. However, the large HRT trials showed no benefit of combined estrogen/progesterone supplementation and possible harm with a greater risk of cardiac disease9, 10. This raises the question of whether either of these hormones have potentially deleterious effects. In a study of transgenic LQT2 rabbits, estradiol increased the incidence of SCA, whereas both progesterone and dihydrotestosterone appeared to reduce events.26 It has been suggested that the ratio of progesterone to estrogen may be more important than individual levels27. The current study suggests that higher estradiol levels could adversely impact arrhythmia risk. While the pathophysiologic basis of such an association is uncertain, the presence of sex steroid receptors in the myocardium has been recognized and several studies have suggested that sex hormones modulate the function of both calcium and potassium channels in the heart. Further, they may also alter the density of ion channels through influence on genomic pathways28. However, translation of this body of largely experimental evidence into the clinical realm needs further efforts.
Interestingly, we observed the highest median levels of estradiol among male SCA cases. Potential mechanisms remain speculative and one possibility is increased conversion of testosterone to estradiol in peripheral fat cells. Visceral obesity and metabolic syndrome, which are also cardiovascular risk factors, are thought to play an important role in the peripheral conversion of testosterone to estradiol by aromatase29. While there was no difference in body mass index between cases and controls, we were not able to assess differences in peripheral fat.
Lastly, the dynamic inter-relation between testosterone and estradiol merits consideration. The finding from the present study of a higher T/E ratio being related to lower SCA odds suggests that the relative levels of each hormone could modulate risk. Since SCA is more common in males compared to females, it may seem paradoxical that lower testosterone levels are associated with increased SCA odds. However, considering the fact that male SCD victims in the community are mainly middle-aged or older, a greater age-related relative decline in testosterone levels and altered T/E balance secondary to increased peripheral conversion of testosterone in association with obesity/other metabolic risk factors may be possible explanations. A more comprehensive understanding of these interactions is necessary to fully elucidate the role of sex steroids in the pathophysiology of SCA.
Limitations
A study of this nature is subject to several limitations. The measurement of sex hormone levels was done only in a subset of the study population and larger studies in more diverse populations are needed to validate these findings. Hormone levels in cases were measured during the SCA event and though we demonstrated that levels were not affected by cardiac arrest in an animal model, some confounding related to the event itself cannot be conclusively ruled out. Plasma hormone levels are strongly influenced by metabolic factors such as obesity and diabetes, which are also factors independently associated with SCA. Owing to the community based nature of the study we did not have comprehensive information on the metabolic profile for all subjects in a uniform manner; however BMI and frequency of diabetes were similar between cases and controls with information available in a good proportion of subjects. Due to limited numbers, adjustment for a more comprehensive list of covariates could not be carried out. The role, especially of the QTc in potential mechanisms linking sex hormones and SCA warrants further exploration in larger studies. The influence of other important parameters such as LV ejection fraction could not be evaluated as data on these variables were available only for a subset. Though these results need to be interpreted with caution and no causal relationship can be inferred, they serve as the first evidence for a potential association between sex hormone levels and occurrence of SCA in the community. Larger, prospective studies, as well as fundamental investigation targeted at a better understanding of the effects of sex steroids on arrhythmogenesis at a molecular level will pave the way for new insights in this area.
Conclusion
In this community based study, higher testosterone levels were associated with lower SCA odds only in males, while higher estradiol levels were strongly associated with higher SCA odds in both sexes. Higher T/E ratio was associated with lower SCA odds only among males. The specific role of sex hormones in SCA mechanisms and their potential interaction with other cardiovascular risk factors warrants further evaluation.
Clinical Perspectives.
It is well recognized that sex hormones play an important role in cardiovascular pathophysiology and that there are important sex differences in cardiovascular risk. However an association between sex hormone levels and sudden cardiac arrest (SCA) in the community has not been previously explored. We measured levels of sex hormones in patients who suffered SCA due to ventricular fibrillation (VF) and made comparisons with age & sex matched controls. We found significant differences between the two groups. Testosterone levels were lower among male SCA cases and slightly higher in female cases, compared to control subjects. Estradiol (E2) levels were higher in both male and female cases compared to controls. In adjusted analyses, higher testosterone levels were associated with lower likelihood of SCA in males but not females. Higher estradiol levels were strongly associated with greater chances of SCA in both males and females. This is the first report of an association between sex hormone levels and SCA in the community. While these findings need to be confirmed in additional larger investigations, they suggest that higher testosterone levels are protective against SCA in males; and lower estrogen levels are protective in both sexes.
Acknowledgments
The authors would like to acknowledge the significant contribution of American Medical Response, Portland/Gresham fire departments, and the Oregon State Medical Examiner’s office.
Funding Sources: Funded in part, by National Heart, Lung, and Blood Institute grants R01HL088416 and HL105170 to Dr Chugh. Dr. Chugh holds the Pauline and Harold Price Chair in Cardiac Electrophysiology Research at the Heart Institute, Cedars-Sinai Medical Center, Los Angeles, CA.
List of Abbreviations
- SCA
Sudden Cardiac Arrest
- T/E ratio
Testosterone/Estradiol ratio
- LV
Left Ventricular
- CAD
Coronary artery disease
- HRT
Hormone Replacement Therapy
- PEA
Pulseless Electrical Activity
- LQT
Long QT
- QTc
Corrected QT interval
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures: None
REFERENCES
- 1.Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: A systematic analysis for the global burden of disease study 2010. Lancet. 2013;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 2.Nichol G, Thomas E, Callaway CW, et al. Resuscitation Outcomes Consortium I. Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA. 2008;300:1423–1431. doi: 10.1001/jama.300.12.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Vreede-Swagemakers JJ, Gorgels AP, Dubois-Arbouw WI, van Ree JW, Daemen MJ, Houben LG, Wellens HJ. Out-of-hospital cardiac arrest in the 1990's: A population-based study in the maastricht area on incidence, characteristics and survival. J Am Coll Cardiol. 1997;30:1500–1505. doi: 10.1016/s0735-1097(97)00355-0. [DOI] [PubMed] [Google Scholar]
- 4.Chugh SS. Early identification of risk factors for sudden cardiac death. Nature reviews. 2010;7:318–326. doi: 10.1038/nrcardio.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kannel WB, Wilson PW, D'Agostino RB, Cobb J. Sudden coronary death in women. Am Heart J. 1998;136:205–212. doi: 10.1053/hj.1998.v136.90226. [DOI] [PubMed] [Google Scholar]
- 6.Albert CM, Chae CU, Grodstein F, Rose LM, Rexrode KM, Ruskin JN, Stampfer MJ, Manson JE. Prospective study of sudden cardiac death among women in the united states. Circulation. 2003;107:2096–2101. doi: 10.1161/01.CIR.0000065223.21530.11. [DOI] [PubMed] [Google Scholar]
- 7.Chugh SS, Uy-Evanado A, Teodorescu C, Reinier K, Mariani R, Gunson K, Jui J. Women have a lower prevalence of structural heart disease as a precursor to sudden cardiac arrest: The ore-suds (Oregon Sudden Unexpected Death Study) J Am Coll Cardiol. 2009;54:2006–2011. doi: 10.1016/j.jacc.2009.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grodstein F, Stampfer M. The epidemiology of coronary heart disease and estrogen replacement in postmenopausal women. Prog Cardiovasc Dis. 1995;38:199–210. doi: 10.1016/s0033-0620(95)80012-3. [DOI] [PubMed] [Google Scholar]
- 9.Manson JE, Hsia J, Johnson KC, et al. Women's Health Initiative I. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003;349:523–534. doi: 10.1056/NEJMoa030808. [DOI] [PubMed] [Google Scholar]
- 10.Grady D, Herrington D, Bittner V, et al. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and estrogen/progestin replacement study follow-up (HERS II) JAMA. 2002;288:49–57. doi: 10.1001/jama.288.1.49. [DOI] [PubMed] [Google Scholar]
- 11.Feldman HA, Johannes CB, McKinlay JB, Longcope C. Low dehydroepiandrosterone sulfate and heart disease in middle-aged men: Cross-sectional results from the massachusetts male aging study. Ann Epidemiol. 1998;8:217–228. doi: 10.1016/s1047-2797(97)00199-3. [DOI] [PubMed] [Google Scholar]
- 12.Chugh SS, Jui J, Gunson K, et al. Current burden of sudden cardiac death: Multiple source surveillance versus retrospective death certificate-based review in a large U.S. Community. J Am Coll Cardiol. 2004;44:1268–1275. doi: 10.1016/j.jacc.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 13.Havmoeller R, Reinier K, Teodorescu C, Uy-Evanado A, Mariani R, Gunson K, Jui J, Chugh SS. Low rate of secondary prevention ICDs in the general population: Multiple-year multiple-source surveillance of sudden cardiac death in the Oregon Sudden Unexpected Death Study. J Cardiovasc Electrophysiol. 2012 doi: 10.1111/j.1540-8167.2012.02407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adabag AS, Peterson G, Apple FS, Titus J, King R, Luepker RV. Etiology of sudden death in the community: Results of anatomical, metabolic, and genetic evaluation. Am Heart J. 2010;159:33–39. doi: 10.1016/j.ahj.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wehr E, Pilz S, Boehm BO, Marz W, Grammer T, Obermayer-Pietsch B. Low free testosterone is associated with heart failure mortality in older men referred for coronary angiography. Eur J Heart Fail. 2011;13:482–488. doi: 10.1093/eurjhf/hfr007. [DOI] [PubMed] [Google Scholar]
- 16.Wehr E, Pilz S, Boehm BO, Grammer TB, Marz W, Obermayer-Pietsch B. Low free testosterone levels are associated with all-cause and cardiovascular mortality in postmenopausal diabetic women. Diabetes Care. 2011;34:1771–1777. doi: 10.2337/dc11-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thijs L, Fagard R, Forette F, Nawrot T, Staessen JA. Are low dehydroepiandrosterone sulphate levels predictive for cardiovascular diseases? A review of prospective and retrospective studies. Acta Cardiol. 2003;58:403–410. doi: 10.2143/AC.58.5.2005304. [DOI] [PubMed] [Google Scholar]
- 18.Selvin E, Feinleib M, Zhang L, Rohrmann S, Rifai N, Nelson WG, Dobs A, Basaria S, Golden SH, Platz EA. Androgens and diabetes in men: Results from the third national health and nutrition examination survey (NHANES III) Diabetes care. 2007;30:234–238. doi: 10.2337/dc06-1579. [DOI] [PubMed] [Google Scholar]
- 19.Gutai J, LaPorte R, Kuller L, Dai W, Falvo-Gerard L, Caggiula A. Plasma testosterone, high density lipoprotein cholesterol and other lipoprotein fractions. Am J Cardiol. 1981;48:897–902. doi: 10.1016/0002-9149(81)90356-8. [DOI] [PubMed] [Google Scholar]
- 20.Hak AE, Witteman JC, de Jong FH, Geerlings MI, Hofman A, Pols HA. Low levels of endogenous androgens increase the risk of atherosclerosis in elderly men: The Rotterdam Study. J Clin Endocrinol Metab. 2002;87:3632–3639. doi: 10.1210/jcem.87.8.8762. [DOI] [PubMed] [Google Scholar]
- 21.Zhang YZ, Xing XW, He B, Wang LX. Effects of testosterone on cytokines and left ventricular remodeling following heart failure. Cell Physiol Biochem. 2007;20:847–852. doi: 10.1159/000110444. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Ouyang P, Post WS, Dalal D, Vaidya D, Blasco-Colmenares E, Soliman EZ, Tomaselli GF, Guallar E. Sex-steroid hormones and electrocardiographic QT-interval duration: Findings from the Third National Health and Nutrition Examination Survey and the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2011;174:403–411. doi: 10.1093/aje/kwr172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chugh SS, Reinier K, Singh T, Uy-Evanado A, Socoteanu C, Peters D, Mariani R, Gunson K, Jui J. Determinants of prolonged QT interval and their contribution to sudden death risk in coronary artery disease: The Oregon Sudden Unexpected Death Study. Circulation. 2009;119:663–670. doi: 10.1161/CIRCULATIONAHA.108.797035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Junttila MJ, Tikkanen JT, Porthan K, Oikarinen L, Jula A, Kentta T, Salomaa V, Huikuri HV. Relationship between testosterone level and early repolarization on 12-lead electrocardiograms in men. J Am Coll Cardiol. 2013;62:1633–1634. doi: 10.1016/j.jacc.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 25.Bertoia ML, Allison MA, Manson JE, Freiberg MS, Kuller LH, Solomon AJ, Limacher MC, Johnson KC, Curb JD, Wassertheil-Smoller S, Eaton CB. Risk factors for sudden cardiac death in post-menopausal women. J Am Coll Cardiol. 2012;60:2674–2682. doi: 10.1016/j.jacc.2012.09.031. [DOI] [PubMed] [Google Scholar]
- 26.Odening KE, Choi BR, Liu GX, Hartmann K, Ziv O, Chaves L, Schofield L, Centracchio J, Zehender M, Peng X, Brunner M, Koren G. Estradiol promotes sudden cardiac death in transgenic long qt type 2 rabbits while progesterone is protective. Heart rhythm. 2012;9:823–832. doi: 10.1016/j.hrthm.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawasaki R, Machado C, Reinoehl J, Fromm B, Baga JJ, Steinman RT, Lehmann MH. Increased propensity of women to develop torsades de pointes during complete heart block. J Cardiovasc Electrophysiol. 1995;6:1032–1038. doi: 10.1111/j.1540-8167.1995.tb00380.x. [DOI] [PubMed] [Google Scholar]
- 28.Pham TV, Rosen MR. Sex, hormones, and repolarization. Cardiovasc. Res. 2002;53:740–751. doi: 10.1016/s0008-6363(01)00429-1. [DOI] [PubMed] [Google Scholar]
- 29.Nawata H, Watanabe T, Yanase T, Nomura M, Ashida K, Min L, Fan W. Sex hormone and neuroendocrine aspects of the metabolic syndrome. Prog Brain Res. 2010;182:175–187. doi: 10.1016/S0079-6123(10)82007-2. [DOI] [PubMed] [Google Scholar]