Abstract
Insulin resistance, a hallmark of impaired glucose tolerance and type 2 diabetes (T2D), arises from dysfunction of insulin action and subsequent glucose uptake by peripheral tissues, predominantly skeletal muscle and fat. Exocytosis of glucose transporter (GLUT4)-containing vesicles facilitated by soluble NSF attachment receptor (SNARE) protein isoforms and Munc18c mediates this glucose uptake. Emerging evidences, including recent human clinical studies, point to pivotal roles for Munc18c in peripheral insulin action in adipose and skeletal muscle. Intriguing new advances are also initiating debates regarding the molecular mechanism(s) controlling Munc18c action. Thus, the objective of this review is to present a balanced perspective of new continuities and controversies surrounding the regulation and requirement for Munc18c in the regulation of peripheral insulin action.
Keywords: SNARE proteins, GLUT4 vesicle exocytosis, glucose uptake, skeletal muscle, adipose
Munc18c and maintenance of peripheral insulin sensitivity
Maintenance of normal glucose homeostasis requires the synchronized orchestration of insulin action at peripheral tissues with insulin secretion from the pancreas. Food intake stimulates secretion of insulin from pancreatic islet beta cells. While virtually all cell types express insulin receptors (IR), only select types, such as skeletal muscle and adipose tissue, elicit IR-mediated insulin-stimulated glucose uptake. Glucose uptake is highly regulated in skeletal muscle and adipose tissue – maintained at very low levels under resting/basal conditions and robustly activated in response to insulin to restore glucose homeostasis or ‘euglycemia’. Skeletal muscle accounts for approximately ~80% of glucose clearance/uptake, while adipose tissue accounts for the remaining 20% [1]. Glucose uptake into muscle and fat cells is mediated by the insulin-responsive glucose transporter, GLUT4 [2]. GLUT4 is a membrane-spanning protein present on intracellular vesicles in these cell types [3]. In response to insulin, GLUT4 vesicles translocate from the cell interior to the PM where they dock and fuse through interactions between vesicle- and plasma membrane (PM)-localized SNARE proteins [4–9]. Upon vesicle fusion and GLUT4 integration into the PM, GLUT4 facilitates uptake of extracellular glucose - GLUT4 has a high affinity for glucose (KM ~4 mM), providing a steep gradient for rapid glucose clearance. In this process, a thorough understanding of how SNARE proteins dock and fuse these incoming GLUT4 vesicles remains elusive. This gap in knowledge presents a prominent health-related challenge, given that recent findings of deficits/defects in SNARE proteins, as well as in additional SNARE-accessory proteins, are now linked to a variety of metabolic disease states [10–12].
To date, six vesicle SNARE (v-SNAREs) isoforms and eleven target-membrane SNARE (t-SNAREs) proteins have been identified in muscle and adipocytes [13], although only the v-SNARE protein VAMP2 and the t-SNARE proteins Syntaxin 4 and SNAP23 are essential for GLUT4 vesicle exocytosis events at the PM [14–16]; the phrase “GLUT4 vesicle translocation/exocytosis” often refers to a grouping of events, including vesicle trafficking to and docking and fusion events at the PM. Canonical SNARE complex assembly, wherein initial formation of a binary complex composed of Syntaxin and SNAP25 binds with VAMP to produce the ternary heterotrimeric SNARE core complex, has been most extensively studied in vitro, using recombinant SNARE isoforms relevant to synaptic vesicle exocytosis [17–19]. The three SNARE proteins (Syntaxin, SNAP25, and VAMP) form a stable four α-helical bundle, with VAMP and Syntaxin each contributing one α-helix and the remaining two α-helices deriving from SNAP25 [20, 21]. While in vitro studies using isoforms pertinent to GLUT4 vesicle recapitulate this SNARE assembly process [22], a new in situ proximity ligation assay demonstrates the existence of Syntaxin 4-VAMP2 binary complexes, which are inhibitory for SNARE complex assembly until overcome by the addition of the SNARE accessory protein Munc18c [23]. Indeed, Munc18c has been appreciated as a relevant factor for GLUT4 vesicle translocation since its discovery and initial characterizations in the mid-1990s [24–26]; however, given that it can exert both negative and positive actions upon GLUT4 vesicle exocytosis events, Munc18c’s role and mechanism of action has remained highly controversial and elusive.
Herein, we will focus our attention specifically on Munc18c. First, the newly discovered role of Munc18c as a substrate for IR will be examined. This finding marked a breakthrough in understanding how the insulin signal coordinately triggered mobilization of intracellular GLUT4 vesicles, while simultaneously preparing SNARE proteins at the PM for vesicle docking and fusion. Next, a variety of recently identified post-translational modifications (PTMs) for Munc18c and their differential impact upon SNARE protein function in skeletal muscle versus adipocytes will be discussed. Finally, we will debate the role and requirement for Munc18c (and how ‘too much of a good thing’ can be bad) in the mechanics of SNARE complex assembly pertaining to maintenance of peripheral insulin sensitivity and glucose uptake.
Munc18c - an atypical insulin receptor substrate
Skeletal muscle and adipocytes express two Munc18 isoforms, Munc18b and Munc18c. Both are members of the Sec1-Munc18 (SM) family of proteins, which broadly spans plants, yeast, worms, flies and mammalian systems. SM proteins are 66–68 kDa soluble proteins with no transmembrane domains. Approximately 50% of the cellular content of Munc18c is localized to the PM, which is attributed to its high affinity for its cognate Syntaxin isoform, Syntaxin 4 [27, 28]. Although Munc18b and Munc18c share ~49% sequence identity, Munc18b is functionally inert in glucose uptake, leaving Munc18c as the sole isoform regulating GLUT4 vesicle docking and fusion [25, 26]. With the focus upon Munc18c, questions regarding its precise role in this exocytotic process were interrogated, principally by comparing it to the neuronal isoform operative in synaptic vesicle exocytosis, Munc18-1 (the most-studied Munc18) [28].
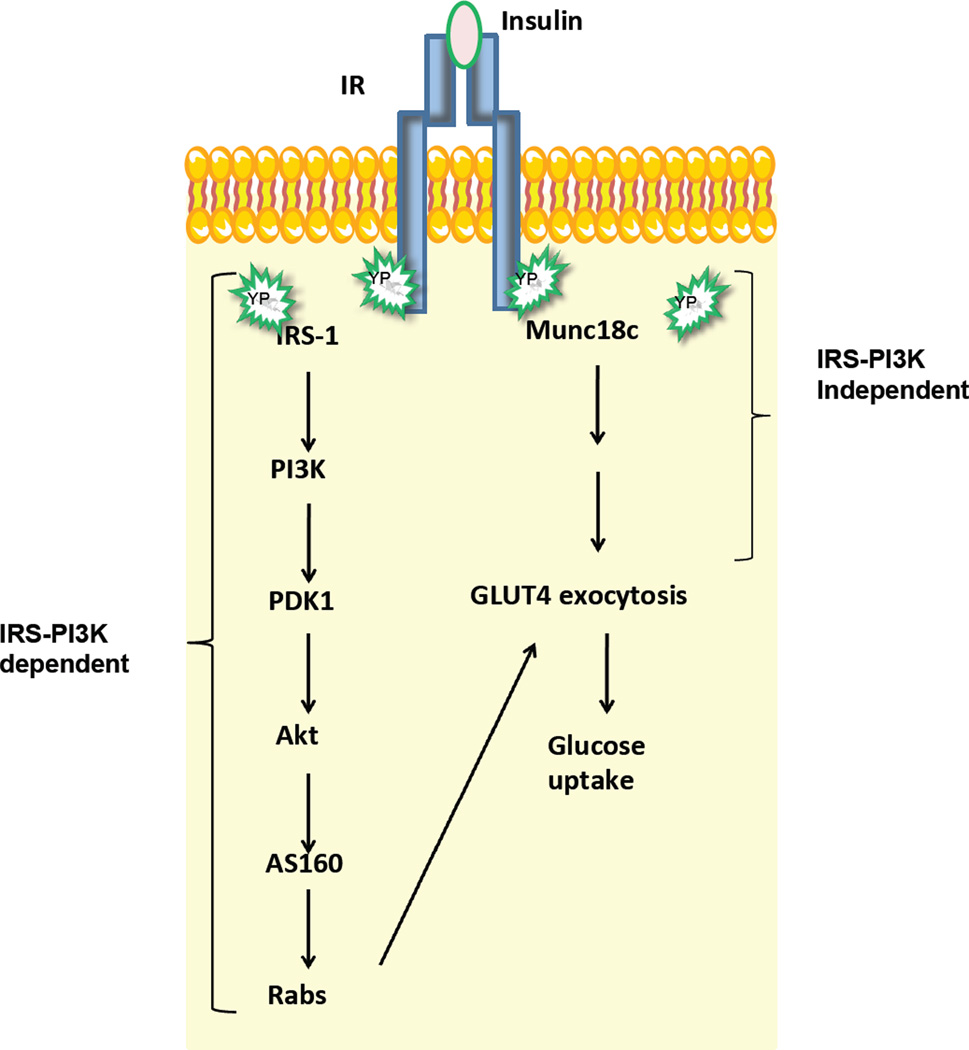
Munc18-1 and its yeast homolog Sec1p interact with Rab GTPases and/or Rab effector proteins [29], so for many years the focus was upon identifying Rab partners for Munc18c in skeletal muscle and fat cells. Rab proteins act at the last step in the insulin signaling cascade, activated just prior to the time at which GLUT4 vesicles dock and fuse [30]. In brief, insulin-mediated IR activation triggers the sequential canonical activation cascade: IR→Insulin Receptor Substrate (IRS-1)→Phosphatidylinositide kinase (PI3-Kinase)→generating Phosphatidylinositol-(3,4,5)-trisphosphate (PIP3) to activate→3-Phosphoinositide-dependent kinase-1 (PDK-1)→AKT/Protein kinase B→Akt substrate of 160 kDa (AS160), a Rab-GAP (GTPase activating protein) to promote recruitment of GLUT4 vesicles to the PM [30– 34]. Although no Rab partners were found, several reports of the insulin-stimulated tyrosine-phosphorylation of Munc18c arose [35, 36]. With the ensuing search for Munc18c kinases and phosphatases, Munc18c was identified as a direct target of IR, becoming rapidly phosphorylated in response to insulin, but in a manner independent of PI3K activation [37, 38]. Hence, this suggested that Munc18c functioned at a proximal step, perhaps in parallel with IRS-1 activation, instead of at a step distal to PI3K and Rab proteins, as modeled in Fig. 1.
Figure 1. Role of Munc18c in the steps of insulin-stimulated GLUT4 exocytosis and glucose uptake in skeletal muscle and fat cells.
Binding of insulin to the insulin receptor (IR) triggers downstream signaling cascades that culminate in GLUT4 exocytosis leading to glucose uptake. IR phosphorylates IRS-1 to trigger a PI3K-dependent pathway to evoke trafficking of GLUT4 storage vesicles to the PM. In parallel, IR phosphorylates Munc18c in an IRS-1 and PI3K-independent pathway, preparing the SNARE proteins at the PM for the incoming GLUT4 vesicles, culminating in GLUT4 deposition onto the cell surface to facilitate glucose uptake into these cell types. PI3K, phosphatidylinositol-3 kinase; PDK1, phosphoinositide dependent kinase 1; Akt/PKB, protein kinase B; AS160, a Rab GTPase-activating protein.
Within a timeframe similar to the tyrosine phosphorylation and activation of IRS-1 by IR in skeletal muscle and adipose cells, Munc18c is rapidly bound by IR and phosphorylated at Tyr521 within 5 min [37, 38]. Although Tyr219 was not detected in proteomic screens [36], it was found to be phosphorylated using a Tyr219-phosphospecific antibody in 3T3L1 adipocytes [38]. Tryptic peptide mapping indicates Tyr219 to localize to the far end of a short tryptic peptide, perhaps accounting for its lack of detection by mass spectrometric detection. Nevertheless, mutation of Tyr219 (Y219F) impairs insulin-stimulated GLUT4 vesicle translocation. However, the Y219F mutation fails to impair IR binding, whereas the Y521F-mutation did block IR binding. Hence, we speculate that Tyr219 may be targeted by kinases beyond IR. Intriguingly, Tyr219 in the Munc18c crystal structure is predicted to be in close proximity to Tyr521, with Tyr521 localizing to a disordered region of Munc18c [13, 38–40]; disordered regions are notable sites of pivotal post-translational modifications and conformational changes [41, 42].
Insulin-stimulated Munc18c phosphorylation occurs concomitantly with its dissociation from Syntaxin 4 at the PM. While in vitro studies show that the phosphorylation of Munc18c by IR occurs independent of its binding to Syntaxin 4 [38], Syntaxin 4 interaction with Munc18c shows dependency upon the phosphorylation status of Munc18c [37]. For example, Syntaxin 4 bound to the Munc18c-Y219F and –Y521F mutants fails to dissociate from either in insulin-stimulated 3T3L1 adipocytes. However, phosphomimetic mutants Munc18c-Y219E and –Y521E exhibit dissociation from Syntaxin 4 both in adipocytes and in vitro [37, 38]. These mutants were used to demonstrate a functional importance for Munc18c phosphorylation at these sites in GLUT4 vesicle exocytosis in 3T3L1 adipocytes [38]. Because IRS-1 failed to bind Munc18c and treatment with a PI3K inhibitor ceased to impact IR-Munc18c binding, IR-induced phosphorylation of Munc18c was concluded to be independent of IR→IRS→PI3K signaling [38]. Taken together, these recent studies suggest a revised model of insulin action in muscle and fat cells, wherein insulin-stimulated IR emits a coordinated signal to evoke SNARE assembly steps in concert with vesicle mobilization to the PM, culminating in SNARE core complex assembly and GLUT4 vesicle fusion.
Very recently, protein tyrosine phosphatase 1B (PTPIB) was identified as the counterpart to IR kinase in insulin-stimulated Munc18c phosphorylation [43]. PTP1B is a non-receptor tyrosine-specific phosphatase, localized to the cytoplasmic face of the endoplasmic reticulum (ER) in adipocytes, and is a physiological regulator of glucose homeostasis [44]. Interestingly, PTP1B is also a known phosphatase for IR [45]. However, how ER-localized PTP1B can facilitate dephosphorylation of PM-localized phospho-IR and phospho-Munc18c remains an open question. It has been suggested that PTP1B might interface with IR during its transit to and from the PM; PTP1B has also been proposed to reside in ‘stretchable’ dynamic regions of the ER membrane that come into contact with its substrates located at the PM [44, 46]. PTP1B may interact with Munc18c at the PM, given that Tyr219-phosphorylated Munc18c is known to localize to the PM of 3T3L1 adipocytes [38], and Tyr219 is a known target for PTP1B [43]. Moreover, Tyr521-phosphorylated Munc18c has been suggested to activate a pool of Syntaxin 4 at the PM, again placing phospho-Munc18c at the PM [23].
Combined with prior work showing that a substantial portion of cellular SM protein content, including that of Munc18c, exists in the cytoplasm [47], it is tempting to speculate that Munc18c might undergo dephosphorylation by PTP1B at the PM and then cycle into the cytosol. Since PTP1B knockout mice exhibit increased insulin sensitivity and resistance to weight gain when fed a high-fat diet [44], the need for understanding Munc18c-PTP1B modifications is of clear relevance. Of additional interest would be to determine if PTP1B and/or IR were also modifiers of Syntaxin 4. Although not yet investigated, Syntaxin 4 was identified in the original proteomic screen as an insulin-induced phosphoprotein in 3T3L1 adipocytes at Tyr115 and Tyr215 [36].
Classifying the role(s) of Munc18c in insulin-stimulated glucose uptake
Over-expression studies designate Munc18c as a negative regulator
Munc18c function in 3T3L1 adipocytes was first interrogated using transiently over-expressing high levels of recombinant Munc18c via adenoviral transduction or electroporation. Since insulin-stimulated GLUT4 vesicle translocation and glucose uptake were inhibited, Munc18c was deemed a negative regulator [25, 26]. Moreover, a genetic model of moderate/low levels of Munc18c over-expression in skeletal muscle and adipose (~3–5 fold over endogenous levels) exhibited peripheral insulin resistance, associated with attenuated skeletal muscle glucose uptake and GLUT4 vesicle exocytosis; surprisingly, adipose tissue glucose uptake was unimpaired [48]. One possible explanation might be that skeletal muscle is more sensitive to alterations in Munc18c levels than adipocytes. A recent human study shows that while Munc18c levels were elevated in skeletal muscle of T2D subjects relative to non-diabetic individuals, they were unaltered in adipose tissue [11] (Table 1). Further, the negative action of Munc18c appears to be linked to Syntaxin 4 abundance. For example, in vivo adenoviral over-expression of Munc18c in mouse skeletal muscle inhibited GLUT4 translocation to the transverse-tubule membranes but not the sarcolemmal membranes [49], coinciding with relatively reduced levels of Syntaxin 4 selectively in the transverse-tubule membrane. This provided early evidence that perhaps a balance between Munc18c and Syntaxin 4 is vital to Munc18c actions.
Table 1.
Munc18c abundances in skeletal muscle and fat under pathophysiological states
| Tissue | Munc18c level | Reference | ||
|---|---|---|---|---|
| Human | Type 2 Diabetes | Skeletal muscle | Increased | [11] |
| Adipose tissue | No change | [11] | ||
| Obesity (BMI>50) | Adipose tissue | Decreased | [68] | |
| Mouse | Obesity (High-fat Diet) | Skeletal muscle | Increased | [67] |
| Adipose tissue | Decreased | [43] |
BMI, body mass index
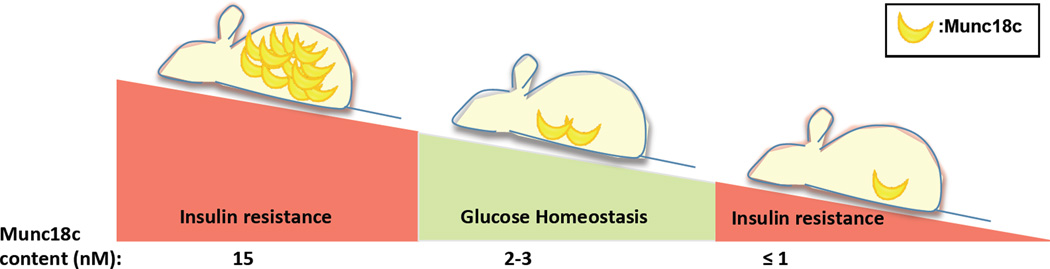
The negative effect of Munc18c in vitro is controversial and may be related to the balance between Munc18c-Syntaxin 4 necessary for appropriate SNARE complex assembly. For example, the addition of excess Munc18c to Syntaxin 4–containing SNARE complexes at ratios of 2:1 or 10:1 were inhibitory for in vitro liposome vesicle fusion [50], and yet at a 5:1 ratio, others observed Munc18c to accelerate SNARE-mediated vesicle fusion [28]. While these differences are likely related to methodological differences between the studies, other reports from human T2D muscle and rodent muscle support the notion that as little as a 3-fold excess of the otherwise endogenous content of ~2–3 nM Munc18c in skeletal muscle is enough to impair to glucose uptake and peripheral insulin sensitivity (Fig. 2) [11, 48].
Figure 2. Delicate balance of Munc18c levels.
Amounts of Munc18c higher or lower than optimum levels correlate with decreased GLUT4 exocytosis and glucose uptake, thus making Munc18c both an inhibitory factor and a requisite factor for insulin action in peripheral tissues. Evidently, only optimum levels, neither less nor more, of Munc18c can effectively maintain glucose homeostasis.
Depletion studies of Munc18c – reclassified as a required regulator
An early indication for the requirement of Munc18c in GLUT4 vesicle exocytosis arose from the finding that the inhibitory action of Munc18c could be offset by concurrent over-expression of its binding partner Syntaxin 4 [51], essentially ‘soaking-up’ excess Munc18c and alleviating the inhibition. Recent uses of RNAi (knockdown) and Munc18c heterozygous knockout mice (homozygous mice die by E7.5) ultimately supported the model in which Munc18c is a required factor for insulin-stimulated GLUT4 translocation and glucose uptake in 3T3L1 adipocytes and skeletal muscle, respectively [38, 52–54] (Fig. 2).
However, one questions whether adipocytes might have a functional tolerance for fluctuations in Munc18c protein levels arises when assessing Munc18c knockout and knockdown studies. For example, one study showed that adipocytes differentiated in vitro from mouse embryo fibroblasts derived from a rare surviving Munc18c null mouse model had no impairments in GLUT4 vesicle translocation; remarkably, they even showed a left-shift in response to insulin [55]. In addition, stable depletion of Munc18c from 3T3L1 adipocytes using lentiviral RNAi was without impact upon GLUT4 vesicle translocation [43]. And yet, multiple studies using transiently depleted Munc18c (siRNA transfection) showed impaired GLUT4 vesicle fusion [38, 53]. This could suggest that long-term stable depletion of Munc18c in adipocytes is accompanied by other, as-of-yet undetected, compensatory mechanisms for the loss of Munc18c. In addition, variability in outcomes in Munc18c depletion studies could be related to insulin dosage: in adipocytes transiently depleted of Munc18c, the inhibition of GLUT4 vesicle exocytosis was evident upon stimulation with 100 nM insulin, but not with 1 nM insulin [53].
These discordant cultured adipocyte data could be reconciled by performing in vivo hyperinsulinemic-euglycemic clamp studies with the viable heterozygous Munc18c knockout line of mice [54] or assaying ex vivo the primary adipocytes therefrom. An added benefit would be the ability to assess similarities/differences between skeletal muscle and fat responsiveness within the same animal. For now, whether these discrepancies are related to differences in the tolerance of fat cells versus muscle cells to variations in Munc18c content or due to additional methodological/gene targeting variations must remain to be determined.
Munc18c in SNARE complex assembly
Controlling Munc18c and SNARE assembly: Doc2b
Early studies in 3T3L1 adipocytes demonstrated that over-expression of the known Munc18-1/Munc18c binding protein Double C2-domain isoform b (Doc2b) enhanced glucose uptake [56]. Furthermore, transgenic mice over-expressing Doc2b in skeletal muscle have heightened peripheral insulin sensitivity, owing to enhanced insulin-stimulated GLUT4 exocytosis in skeletal muscle, concurrent with increased Syntaxin 4-SNAP23-VAMP2 (SNARE complex) assembly and decreased Syntaxin 4-Munc18c binding in muscle lysates [57]. Moreover, Doc2b knockout mice exhibit peripheral insulin resistance and impaired insulin-stimulated GLUT4 accumulation at skeletal muscle cell surfaces [58]. Strikingly, decreased SNARE complex assembly and increased Munc18c-Syntaxin 4 binding are seen within skeletal muscle extracts from Doc2b knockout mice [58], indicating that Doc2b modulates binding of Munc18c to Syntaxin 4, and hence SNARE complex assembly. In vitro, Doc2b can also bind to Syntaxin 4 [59], and this was similarly observed in 3T3L1 adipocytes [56]. Doc2b was also seen to translocate to the PM in adipocytes in a calcium-sensitive manner [56]. In contrast, Doc2b binding to Syntaxin 4 is not observed in skeletal muscle, irrespective of calcium level [58]. While calcium levels are arguably higher in skeletal muscle than cultured adipocytes, which may account for the visible lack of Doc2b translocation in muscle, the lack of binding of Doc2b to Syntaxin 4 in skeletal muscle is unexplained at this time. Regardless of the detailed binding interactions however, all studies unequivocally agree that Doc2b is both required and limiting for insulin-stimulated GLUT4 vesicle exocytosis, via a facilitative role in SNARE assembly. While this may qualify Doc2b as a therapeutic target of interest, comparing/contrasting the mode of Doc2b action in primary adipocytes versus skeletal muscle will be a priority for target optimization.
Regulation of Munc18c binding to Syntaxin 4
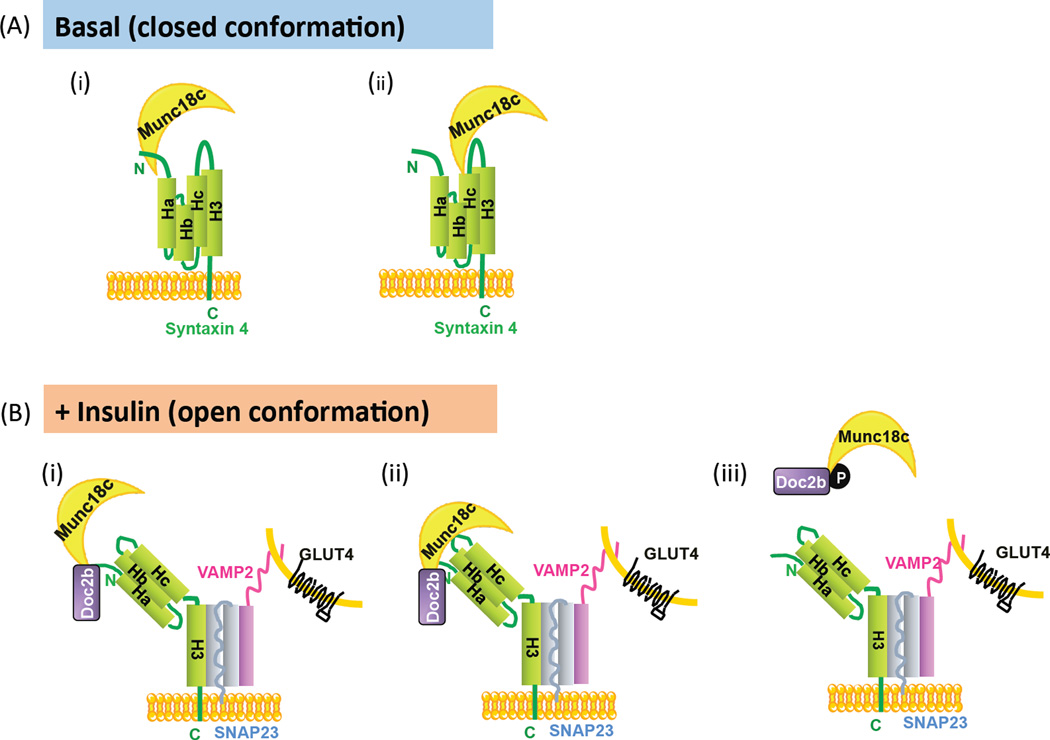
If Doc2b binds to Munc18c or Syntaxin 4 but not both, then Doc2b may have to compete for interaction between Munc18c and Syntaxin 4. Munc18c-Syntaxin 4 interaction is of high affinity, with a KD ~ 30 nM [60, 61]. Structural analyses indicate that Munc18c resembles an arch-shaped clasp and interacts with Syntaxin 4 via multiple binding modes. Syntaxin 4, like other syntaxin family members, has been demonstrated to adopt two conformations – open (accessible for SNARE complex assembly) or closed – in 3T3L1 adipocytes [62] and in vitro [61]. Closed Syntaxin 4 is thought to be bound by Munc18c, keeping Syntaxin 4 inactive in the absence of insulin stimulation. Over the past 8 years, delineation of the regions of Syntaxin 4 protein required for Munc18c association (in the closed conformation) has been contentious. For example, in 2006, evidence arguing the importance of the far N-terminus of Syntaxin 4 in its association with Munc18c was provided [39] (Fig. 3A-i). However, subsequent studies argued this region to be one of several contact sites, providing evidence showing that Munc18c-Syntaxin 4 binding still occurs when the N-terminal Syntaxin 4 peptide is deleted [60–62]. Indeed, other contact sites such as the Hc-linker region of Syntaxin 4 appear to be sufficient in mediating interaction with Munc18c [60] (Fig. 3A-ii). Despite this, it is speculated that upon insulin stimulation, Syntaxin 4 adopts the open conformation, although how its interactions with Munc18c change remains an open question. One possibility is that the Syntaxin 4 N-terminal peptide stays bound to Munc18c (Fig. 3B-i) [40, 63], similar to configurations reported for other Munc18-Syntaxin pairs [63]. An alternative conformation, derived from clonal cell studies, demonstrates interaction of Munc18c with Syntaxin 4 through two contact sites of Munc18c [64] (Fig. 3B-ii). While in vitro data would suggest that Doc2b joins these SNARE complexes through Syntaxin 4, depicted in Fig 3B (i–ii) [56, 59], Munc18c association with the SNARE macromolecular complex is not detectable in lysates prepared from skeletal muscle or adipocytes [11, 58]. Instead, insulin stimulation of these tissue/cell types yields rapid phosphorylation of Munc18c at the PM, coinciding with reductions in Munc18c-Syntaxin 4 binding and concurrent increases in Munc18c-Doc2b binding [38, 58, 60, 65]. This switch occurs simultaneously with increased Syntaxin 4 integration into SNARE complexes (Fig. 3B-iii).
Figure 3. Binding modes of Munc18c in SNARE complex assembly.
(A) Munc18c is presumed to keep Syntaxin 4 in a closed conformation via two distinct configurations, under basal conditions: (A-i) Munc18c is bound the N terminal peptide region of Syntaxin 4 [39], or (A-ii) Munc18c binds to the Hc-linker region of Syntaxin 4 [60]. (B) Under insulin-stimulated conditions, Munc18c participates with the open conformation of Syntaxin 4 in three possible configurations: (B-i) Munc18c binds to the N- terminal peptide of Syntaxin 4, possibly concurrent with Doc2b binding Syntaxin 4, or alternatively, through two contact points [28, 56, 63, 64] (B-ii). Taking into account that insulin stimulates the tyrosine-phosphorylation of Munc18c, a third configuration exists (B-iii) wherein phosphorylated Munc18c dissociates from Syntaxin 4 [38, 43, 65] and concurrently switches its binding to Doc2b [58, 60].
Going forward, we speculate that additional Munc18c PTMs (BOX 1) will further impact these Munc18c-Syntaxin 4 and SNARE assembly configuration models. Using a recent example, Munc18c undergoes palmitoylation in adipose tissue [66]. Given that palmitoylation drives proteins to membranes, it is conceivable that this could impact how Munc18c cycles between the PM and cytoplasm, and hence affect SNARE interactions. Although indirectly related, Doc2b may also undergo stimulus-dependent PTMs that impact its binding interactions with Munc18c to impact SNARE assembly in skeletal muscle and fat cell types.
BOX 1: Additional posttranslational modifications of Munc18c.
Beyond tyrosine phosphorylation, Munc18c undergoes a number of other PTMs, such as serine/threonine phosphorylation, glycosylation, and palmitoylation, in response to normal and aberrant stimuli (Table I). Indeed, protein kinase C phosphorylates Munc18c at threonine residues in pancreatic acinar cells, and correlates with Munc18c dissociation from Syntaxin 4 [79]. Although early studies using over-expression of a Munc18c T569 mutant failed to show defective GLUT4 translocation in adipocytes [80], this should be retested in cells depleted of endogenous Munc18c, a testing platform now preferred for examining the requirement of PTMs. Munc18c can be O-linked glycosylated in adipocytes [81]. Distinct from the phosphorylation events mentioned so far, this O-linked glycosylation occurs under conditions of insulin resistance. Although the site(s) of O-linked glycosylation have yet to be determined, a glycosylation site prediction program projects both Ser6 and Ser87 to have high potential for this modification (ww.cbs.dtu.dk/services). Three additional residues (Thr42, Ser125 and Ser453) carry potential for O-linked glycosylation, with all predicted sites being surface-exposed. Mass spectrometry is required to pinpoint these putative glycosylation events, as reliance upon pharmacological inhibitors and activators is fraught with specificity issues. Caveats with this method include the relatively low abundance of Munc18c in fat/muscle cells and the highly dynamic nature of the glycosylation process. Nevertheless, mapping these putative Munc18c glycosylation sites and testing their importance to glucose uptake will yield potentially landmark advances to our understanding of insulin resistance and prediabetes.
Recent proteomic advances using mass spectrometric analyses have revealed that Munc18c can also be palmitoylated (a lipid modification occurring on cysteine residues), in 3T3L1 adipocytes and murine adipose tissue [66]. Although Munc18c contains 16 cysteines, motifs for farnesylation and myristoylation are not present, indicating palmitoylation to be the primary lipid modification. Since palmitoylation is reversible and drives protein localization to the PM, it can be speculated that palmitoylation provides a mechanistic means for the shuttling of Munc18c from the cytoplasm to the PM (and vice versa). Thus, in lieu of requiring distinct cohorts of SNARE and SM proteins, distinct types of modifications modulate the ubiquitous Munc18c and Syntaxin 4 machinery to suit the stimulus-specific response of these specialized cell types, thereby adding a new layer of complexity to the well-established SNARE hypothesis.
Munc18c and Syntaxin 4 abundances in obesity and diabetes
Clearly, too much or too little of Munc18c is detrimental in vivo for maintenance of peripheral insulin sensitivity (Fig. 2). However, in attempts to resolve how much is physiologically beneficial, the field has endeavored to correlate Munc18c levels with various disease states (Table 1). When compared to obese or lean non-diabetic individuals, humans with T2D have higher-than-normal levels of Munc18c protein in skeletal muscle [11], which correlates with observed elevations of Munc18c in skeletal muscle of high-fat diet-induced obese mice [67]. Thus, Munc18c over-abundance appears to have an inhibitory role, consistent with previously discussed data. In contrast, lower-than-normal levels of Munc18c are observed in adipose tissue from high-fat-diet-induced obese mice [43]. This finding was recapitulated in a recent report of deficient levels of Munc18c in visceral and subcutaneous adipose depots from insulin-resistant morbidly obese human patients (BMI > 50) [68]. Together, these data suggest that Munc18c levels may be differentially controlled by factors acting at the level of the tissue (e.g. adipose versus muscle) to evoke different effects.
Regulation of Munc18c abundance in skeletal muscle and adipose tissues
Identification of factors controlling expression and/or stability of Munc18c is an emerging area of interest. Early studies implicated Syntaxin 4 as a regulator of Munc18c levels: 1) Munc18c levels in Syntaxin 4 heterozygous knockout mice were decreased, even though other SNARE protein abundances remained unchanged [14], and 2) Munc18c protein levels were elevated in Syntaxin 4 over-expressing mice [69]. A recent report implied a potential chaperone-type relationship between Munc18-Syntaxin proteins [70], suggesting that Syntaxin 4 might be required for Munc18c stability. Additional recent evidences suggest that SNARE proteins are subject to proteosomal degradation, as well as miRNA targeting [71, 72], indicating that Munc18c may be subject to similar processes. The first examination of Munc18c gene expression in human adipocytes suggests regulation by insulin through LXRα and SREBP-1c [68]. Moreover, this report includes a prospective study of morbidly obese patients shortly after bariatric surgery, wherein Munc18c expression was found to be the main determinant of improvements in HOMA-IR [68]. Cumulatively, these findings indicate that identification and exploitation of possible mechanisms regulating Munc18c expression would be advantageous for deriving therapeutic strategies for metabolic control.
Concluding remarks and future perspectives
Munc18c has been implicated in insulin action and GLUT4 vesicle exocytosis for nearly 20 years, and although some crucial questions regarding functional and structural aspects have been answered, detailed molecular mechanisms of Munc18c’s actions are incompletely understood. Given new evidences in support of the notion that Munc18c functions as a positive factor in Syntaxin 4-based SNARE complex assembly and insulin-stimulated glucose uptake, a new set of questions pertaining to the targeting of Munc18c to promote these processes has arisen (BOX 2). New clinical data regarding changes in Munc18c transcript and protein levels under various metabolic pathophysiologic conditions further prompt the need to investigate how Munc18c expression is regulated at both transcriptional and post-transcriptional levels. Post-translational modifications of Munc18c are emerging as important factors in SNARE mechanisms, thus future studies should be performed in physiologically relevant systems to gain understanding of Munc18c action(s), particularly under conditions of nutritional or metabolic stresses inherent to obesity, insulin resistance and T2D. Munc18c, Syntaxin 4 and Doc2b are also emerging as crucial factors required for insulin release from human and rodent pancreatic beta cells [58, 73, 74]. This prompts the intriguing possibility that interventions aimed at these factors could simultaneously remediate defects associated with peripheral insulin resistance and dysfunctional insulin release, two of the primary defects associated with T2D. With the availability of new biosensors of SNARE configurations [75, 76] and of actin remodeling [77, 78], as well as genetically-engineered Munc18c mouse models, an exciting foray into Munc18c’s likely broad importance to metabolic health is anticipated.
BOX 2: Outstanding questions.
Would maintaining Munc18c in a perpetually phosphorylated state in vivo promote insulin sensitivity or be detrimental for glucose metabolism?
Is Syntaxin 4 phosphorylation pertinent to Munc18c function, and is this relevant to GLUT4 vesicle exocytosis and glucose uptake? If so, what are the kinases and phosphatases which regulate this PTM?
What is the functional role of cytosolic Munc18c?
How is the Munc18c gene regulated under normal and diabetogenic conditions? Does this occur in a tissue-specific manner?
Are Munc18c, Syntaxin 4 or Doc2b feasible therapeutic targets for prevention and/or remediation of peripheral insulin resistance or diabetes?
BOX 1 Table I.
Post translational modifications of Munc18c in adipocytes
| Modification | Residue | Function | Reference |
| p-Tyr | Y521 | Positively correlates with GLUT4 exocytosis | [38, 43, 65] |
| Y218 | No significant effect | [65] | |
| Y219 | Positively correlates with GLUT4 exocytosis | [38] | |
| p-Ser/Thr | T569 | No significant effect | [80] |
| O-linked Glycosylation | ND | Decreased insulin stimulated GLUT4 translocation | [81] |
| S-acylation (palmitoylation) | ND | ND | [66] |
ND, not determined
Highlights.
Maintenance of glucose homeostasis requires peripheral glucose clearance.
Glucose clearance requires SNARE-mediated GLUT4 vesicle exocytosis.
Munc18c is a crucial yet complex regulator of SNARE complex formations.
Vascillations in Munc18c content or function deregulate homeostatic mechanisms
ACKNOWLEDGEMENTS
We apologize that owing to space limitations we have been unable to cite many impactful studies. This study was supported by grants from the National Institutes of Health (DK067912 to D.C.T.; DK094488 to S.M.Y.), the Juvenile Diabetes Research Foundation (17-2013-454, to D.C.T.), and American Heart Association (12PRE11890042 to L.R).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure summary: LR, SMY, EO, and DCT have nothing to declare.
REFERENCES
- 1.DeFronzo RA, et al. Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin-dependent (Type II) diabetes mellitus. J Clin Invest. 1985;76:149–155. doi: 10.1172/JCI111938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foley K, et al. Endocytosis, recycling, and regulated exocytosis of glucose transporter 4. Biochemistry. 2011;50:3048–3061. doi: 10.1021/bi2000356. [DOI] [PubMed] [Google Scholar]
- 3.Leto D, Saltiel AR. Regulation of glucose transport by insulin: traffic control of GLUT4. Nat Rev. Mol Cell Biol. 2012;13:383–396. doi: 10.1038/nrm3351. [DOI] [PubMed] [Google Scholar]
- 4.Klip A. The many ways to regulate glucose transporter 4. Appl Physiol Nutr Metab. 2009;34:481–487. doi: 10.1139/H09-047. [DOI] [PubMed] [Google Scholar]
- 5.Kandror KV, Pilch PF. The sugar is sIRVed: sorting Glut4 and its fellow travelers. Traffic. 2011;12:665–671. doi: 10.1111/j.1600-0854.2011.01175.x. [DOI] [PubMed] [Google Scholar]
- 6.Stenkula KG, et al. Insulin controls the spatial distribution of GLUT4 on the cell surface through regulation of its postfusion dispersal. Cell Metab. 2010;12:250–259. doi: 10.1016/j.cmet.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hatakeyama H, Kanzaki M. Molecular basis of insulin-responsive GLUT4 trafficking systems revealed by single molecule imaging. Traffic. 2011;12:1805–1820. doi: 10.1111/j.1600-0854.2011.01279.x. [DOI] [PubMed] [Google Scholar]
- 8.Bryant NJ, Gould GW. SNARE proteins underpin insulin-regulated GLUT4 traffic. Traffic. 2011;12:657–664. doi: 10.1111/j.1600-0854.2011.01163.x. [DOI] [PubMed] [Google Scholar]
- 9.Sadler JB, et al. Posttranslational Modifications of GLUT4 Affect Its Subcellular Localization and Translocation. Int J Mol Sci. 2013;14:9963–9978. doi: 10.3390/ijms14059963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ostenson CG, et al. Impaired gene and protein expression of exocytotic soluble N-ethylmaleimide attachment protein receptor complex proteins in pancreatic islets of type 2 diabetic patients. Diabetes. 2006;55:435–440. doi: 10.2337/diabetes.55.02.06.db04-1575. [DOI] [PubMed] [Google Scholar]
- 11.Bostrom P, et al. The SNARE protein SNAP23 and the SNARE-interacting protein Munc18c in human skeletal muscle are implicated in insulin resistance/type 2 diabetes. Diabetes. 2010;59:1870–1878. doi: 10.2337/db09-1503. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Andersson SA, et al. Reduced insulin secretion correlates with decreased expression of exocytotic genes in pancreatic islets from patients with type 2 diabetes. Mol Cell Endocrinol. 2012;364:36–45. doi: 10.1016/j.mce.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 13.Jewell JL, et al. Exocytosis mechanisms underlying insulin release and glucose uptake: conserved roles for Munc18c and syntaxin 4. AJP Regul Integr Comp Physiol. 2010;298:R517–R531. doi: 10.1152/ajpregu.00597.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang C, et al. Syntaxin 4 heterozygous knockout mice develop muscle insulin resistance. J Clin Invest. 2001;107:1311–1318. doi: 10.1172/JCI12274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawaguchi T, et al. The t-SNAREs syntaxin4 and SNAP23 but not v-SNARE VAMP2 are indispensable to tether GLUT4 vesicles at the plasma membrane in adipocyte. Biochem Biophys Res Commun. 2010;391:1336–1341. doi: 10.1016/j.bbrc.2009.12.045. [DOI] [PubMed] [Google Scholar]
- 16.Williams D, Pessin JE. Mapping of R-SNARE function at distinct intracellular GLUT4 trafficking steps in adipocytes. J Cell Biol. 2008;180:375–387. doi: 10.1083/jcb.200709108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sudhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science. 2009;323:474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jahn R, Fasshauer D. Molecular machines governing exocytosis of synaptic vesicles. Nature. 2012;490:201–207. doi: 10.1038/nature11320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hernandez JM, et al. Membrane fusion intermediates via directional and full assembly of the SNARE complex. Science. 2012;336:1581–1584. doi: 10.1126/science.1221976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weber T, et al. SNAREpins: minimal machinery for membrane fusion. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- 21.Rizo J, Sudhof TC. The membrane fusion enigma: SNAREs, Sec1/Munc18 proteins, and their accomplices--guilty as charged? Annu Rev Cell Dev Biol. 2012;28:279–308. doi: 10.1146/annurev-cellbio-101011-155818. [DOI] [PubMed] [Google Scholar]
- 22.Yu H. Synip arrests soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE)-dependent membrane fusion as a selective target membrane SNARE-binding inhibitor. J Biol Chem. 2013;288:18885–18893. doi: 10.1074/jbc.M113.465450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kioumourtzoglou D, et al. Insulin stimulates syntaxin4 SNARE complex assembly via a novel regulatory mechanism. Mol Cell Biol. 2014;34:1271–1279. doi: 10.1128/MCB.01203-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tellam JT, et al. Molecular identification of two novel Munc-18 isoforms expressed in non-neuronal tissues. J Biol Chem. 1995;270:5857–5863. doi: 10.1074/jbc.270.11.5857. [DOI] [PubMed] [Google Scholar]
- 25.Thurmond DC, et al. Regulation of insulin-stimulated GLUT4 translocation by munc18c in 3T3L1 adipocytes. J Biol Chem. 1998;273:33876–33883. doi: 10.1074/jbc.273.50.33876. [DOI] [PubMed] [Google Scholar]
- 26.Tamori Y, et al. Inhibition of insulin-induced GLUT4 translocation by Munc18c through interaction with syntaxin4 in 3T3-L1 adipocytes. J Biol Chem. 1998;273:19740–19746. doi: 10.1074/jbc.273.31.19740. [DOI] [PubMed] [Google Scholar]
- 27.Shen J, et al. Selective activation of cognate SNAREpins by Sec1/Munc18 proteins. Cell. 2007;128:183–195. doi: 10.1016/j.cell.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 28.Yu H. Comparative studies of Munc18c and Munc18-1 reveal conserved and divergent mechanisms of Sec1/Munc18 proteins. Proc Natl Acad Sci U S A. 2013;110:E3271–E3280. doi: 10.1073/pnas.1311232110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hutagalung AH, Novick PJ. Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev. 2011;91:119–149. doi: 10.1152/physrev.00059.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klip A, et al. Signal transduction meets vesicle traffic: the software and hardware of GLUT4 translocation. Am J Physiol Cell Physiol. 2014;306:C879–C886. doi: 10.1152/ajpcell.00069.2014. [DOI] [PubMed] [Google Scholar]
- 31.Boura-Halfon S, Zick Y. Phosphorylation of IRS proteins, insulin action, and insulin resistance. Am J Physiol Endocrinol Metab. 2009;296:E581–E591. doi: 10.1152/ajpendo.90437.2008. [DOI] [PubMed] [Google Scholar]
- 32.Rowland AF, et al. Mapping insulin/GLUT4 circuitry. Traffic. 2011;12:672–681. doi: 10.1111/j.1600-0854.2011.01178.x. [DOI] [PubMed] [Google Scholar]
- 33.Boucher J, et al. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb Perspect Biol. 2014;6 doi: 10.1101/cshperspect.a009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramalingam L, et al. Novel roles for insulin receptor (IR) in adipocytes and skeletal muscle cells via new and unexpected substrates. Cell Mol Life Sci. 2013;70:2815–2834. doi: 10.1007/s00018-012-1176-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oh E, Thurmond DC. The stimulus-induced tyrosine phosphorylation of Munc18c facilitates vesicle exocytosis. J Biol Chem. 2006;281:17624–17634. doi: 10.1074/jbc.M601581200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmelzle K, et al. Temporal dynamics of tyrosine phosphorylation in insulin signaling. Diabetes. 2006;55:2171–2179. doi: 10.2337/db06-0148. [DOI] [PubMed] [Google Scholar]
- 37.Aran V, et al. Tyrosine phosphorylation of Munc18c on residue 521 abrogates binding to Syntaxin 4. BMC Biochem. 2011;12:19. doi: 10.1186/1471-2091-12-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jewell JL, et al. Munc18c phosphorylation by the insulin receptor links cell signaling directly to SNARE exocytosis. J Cell Biol. 2011;193:185–199. doi: 10.1083/jcb.201007176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu SH, et al. Structure of the Munc18c/Syntaxin4 N-peptide complex defines universal features of the N-peptide binding mode of Sec1/Munc18 proteins. Proc Natl Acad Sci U S A. 2007;104:8773–8778. doi: 10.1073/pnas.0701124104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu SH, et al. Possible roles for Munc18-1 domain 3a and Syntaxin1 N-peptide and C-terminal anchor in SNARE complex formation. Proc Natl Acad Sci U S A. 2011;108:1040–1045. doi: 10.1073/pnas.0914906108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collins MO. Cell biology. Evolving cell signals. Science. 2009;325:1635–1636. doi: 10.1126/science.1180331. [DOI] [PubMed] [Google Scholar]
- 42.Collins MO, et al. Phosphoproteomic analysis of the mouse brain cytosol reveals a predominance of protein phosphorylation in regions of intrinsic sequence disorder. Mol Cell Troteomics. 2008;7:1331–1348. doi: 10.1074/mcp.M700564-MCP200. [DOI] [PubMed] [Google Scholar]
- 43.Bakke J, et al. Regulation of the SNARE-interacting protein Munc18c tyrosine phosphorylation in adipocytes by protein-tyrosine phosphatase 1B. Cell Commun Signal. 2013;11:57. doi: 10.1186/1478-811X-11-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yip SC, et al. PTP1B: a double agent in metabolism and oncogenesis. Trends Biochem Sci. 2010;35:442–449. doi: 10.1016/j.tibs.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cho H. Protein tyrosine phosphatase 1B (PTP1B) and obesity. Vitamins Hormones. 2013;91:405–424. doi: 10.1016/B978-0-12-407766-9.00017-1. [DOI] [PubMed] [Google Scholar]
- 46.Monteleone MC, et al. ER-bound protein tyrosine phosphatase PTP1B interacts with Src at the plasma membrane/substrate interface. PLoS One. 2012;7:e38948. doi: 10.1371/journal.pone.0038948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Toonen RF, Verhage M. Vesicle trafficking: pleasure and pain from SM genes. Trends Cell Biol. 2003;13:177–186. doi: 10.1016/s0962-8924(03)00031-x. [DOI] [PubMed] [Google Scholar]
- 48.Spurlin BA, et al. Insulin resistance in tetracycline-repressible Munc18c transgenic mice. Diabetes. 2003;52:1910–1917. doi: 10.2337/diabetes.52.8.1910. [DOI] [PubMed] [Google Scholar]
- 49.Khan AH, et al. Munc18c regulates insulin-stimulated glut4 translocation to the transverse tubules in skeletal muscle. J Biol Chem. 2001;276:4063–4069. doi: 10.1074/jbc.M007419200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brandie FM, et al. Negative regulation of syntaxin4/SNAP-23/VAMP2-mediated membrane fusion by Munc18c in vitro. PLoS One. 2008;3:e4074. doi: 10.1371/journal.pone.0004074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thurmond DC, et al. Munc18c function is required for insulin-stimulated plasma membrane fusion of GLUT4 and insulin-responsive amino peptidase storage vesicles. Mol Cell Biol. 2000;20:379–388. doi: 10.1128/mcb.20.1.379-388.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jain SS, et al. Munc18c provides stimulus- selective regulation of GLUT4 but not fatty acid transporter trafficking in skeletal muscle. FEBS Lett. 2012;586:2428–2435. doi: 10.1016/j.febslet.2012.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sadacca LA, et al. Specialized sorting of GLUT4 and its recruitment to the cell surface are independently regulated by distinct Rabs. Mol Biol Cell. 2013;24:2544–2557. doi: 10.1091/mbc.E13-02-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oh E. Munc18c heterozygous knockout mice display increased susceptibility for severe glucose intolerance. Diabetes. 2005;54:638–647. doi: 10.2337/diabetes.54.3.638. [DOI] [PubMed] [Google Scholar]
- 55.Kanda H, et al. Adipocytes from Munc18c-null mice show increased sensitivity to insulin-stimulated GLUT4 externalization. J Clin Invest. 2005;115:291–301. doi: 10.1172/JCI22681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fukuda N, et al. DOC2B: a novel syntaxin-4 binding protein mediating insulin-regulated GLUT4 vesicle fusion in adipocytes. Diabetes. 2009;58:377–384. doi: 10.2337/db08-0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ramalingam L, et al. Doc2b enrichment enhances glucose homeostasis in mice via potentiation of insulin secretion and peripheral insulin sensitivity. Diabetologia. 2014;57:1476–1484. doi: 10.1007/s00125-014-3227-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ramalingam L, et al. Doc2b is a key effector of insulin secretion and skeletal muscle insulin sensitivity. Diabetes. 2012;61:2424–2432. doi: 10.2337/db11-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu H. Doc2b promotes GLUT4 exocytosis by activating the SNARE-mediated fusion reaction in a calcium- and membrane bending-dependent manner. Mol Biol Cell. 2013;24:1176–1184. doi: 10.1091/mbc.E12-11-0810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jewell JL, et al. The tyrosine phosphorylation of Munc18c induces a switch in binding specificity from syntaxin 4 to Doc2beta. J Biol Chem. 2008;283:21734–21746. doi: 10.1074/jbc.M710445200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aran V, et al. Characterization of two distinct binding modes between syntaxin 4 and Munc18c. Biochem J. 2009;419:655–660. doi: 10.1042/BJ20082293. [DOI] [PubMed] [Google Scholar]
- 62.D'Andrea-Merrins M, et al. Munc18c interaction with syntaxin 4 monomers and SNARE complex intermediates in GLUT4 vesicle trafficking. J Biol Chem. 2007;282:16553–16566. doi: 10.1074/jbc.M610818200. [DOI] [PubMed] [Google Scholar]
- 63.Christie MP, et al. Low-resolution solution structures of Munc18:Syntaxin protein complexes indicate an open binding mode driven by the Syntaxin N-peptide. Proc Natl Acad Sci U S A. 2012;109:9816–9821. doi: 10.1073/pnas.1116975109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smithers NP, et al. Insulin-triggered repositioning of munc18c on syntaxin-4 in GLUT4 signalling. Biochem J. 2008;410:255–260. doi: 10.1042/BJ20070802. [DOI] [PubMed] [Google Scholar]
- 65.Umahara M, et al. Tyrosine phosphorylation of Munc18c regulates platelet-derived growth factor-stimulated glucose transporter 4 translocation in 3T3L1 adipocytes. Endocrinology. 2008;149:40–49. doi: 10.1210/en.2006-1549. [DOI] [PubMed] [Google Scholar]
- 66.Ren W. Proteomic analysis of protein palmitoylation in adipocytes. Adipocyte. 2013;2:17–28. doi: 10.4161/adip.22117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schlaepfer IR, et al. Increased expression of the SNARE accessory protein Munc18c in lipid-mediated insulin resistance. J Lipid Res. 2003;44:1174–1181. doi: 10.1194/jlr.M300003-JLR200. [DOI] [PubMed] [Google Scholar]
- 68.Garrido-Sanchez L, et al. Munc18c in adipose tissue is downregulated in obesity and is associated with insulin. PLoS One. 2013;8:e63937. doi: 10.1371/journal.pone.0063937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Spurlin BA, et al. Syntaxin 4 transgenic mice exhibit enhanced insulin-mediated glucose uptake in skeletal muscle. Diabetes. 2004;53:2223–2231. doi: 10.2337/diabetes.53.9.2223. [DOI] [PubMed] [Google Scholar]
- 70.Torres J, et al. The syntaxin 4 N terminus regulates its basolateral targeting by munc18c-dependent and -independent mechanisms. J Biol Chem. 2011;286:10834–10846. doi: 10.1074/jbc.M110.186668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wei C. miR-153 regulates SNAP-25, synaptic transmission, and neuronal development. PLoS One. 2013;8:e57080. doi: 10.1371/journal.pone.0057080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Paschou M, et al. miRNA regulons associated with synaptic function. PLoS One. 2012;7:e46189. doi: 10.1371/journal.pone.0046189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Oh E, Thurmond DC. Munc18c Depletion Selectively Impairs the Sustained Phase of Insulin Release. Diabetes. 2009;58:1165–1174. doi: 10.2337/db08-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Oh E. Syntaxin 4 up-regulation increases efficiency of insulin release in pancreatic islets from humans with and without type 2 diabetes mellitus. J Clin Endocrinol Metab. 2014;99:E866–E870. doi: 10.1210/jc.2013-2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Degtyar V, et al. Dance of the SNAREs: assembly and rearrangements detected with FRET at neuronal synapses. J Neurosci. 2013;33:5507–5523. doi: 10.1523/JNEUROSCI.2337-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Graczyk A, Rickman C. Exocytosis through the Lens. Front Endocrinology. 2013;4:147. doi: 10.3389/fendo.2013.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kalwat MA, et al. A p21-activated kinase (PAK1) signaling cascade coordinately regulates F-actin remodeling and insulin granule exocytosis in pancreatic beta cells. Biochem Pharmacol. 2013;85:808–816. doi: 10.1016/j.bcp.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schachtner H, et al. Tissue inducible Lifeact expression allows visualization of actin dynamics in vivo and ex vivo. Eur J Cell Biol. 2012;91:923–929. doi: 10.1016/j.ejcb.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cosen-Binker LI, et al. Alcohol/cholecystokinin-evoked pancreatic acinar basolateral exocytosis is mediated by protein kinase C alpha phosphorylation of Munc18c. J Biol Chem. 2007;282:13047–13058. doi: 10.1074/jbc.M611132200. [DOI] [PubMed] [Google Scholar]
- 80.Macaulay SL, et al. Cellular munc18c levels can modulate glucose transport rate and GLUT4 translocation in 3T3L1 cells. FEBS Lett. 2002;528:154–160. doi: 10.1016/s0014-5793(02)03279-9. [DOI] [PubMed] [Google Scholar]
- 81.Chen G, et al. Glucosamine-induced insulin resistance is coupled to O-linked glycosylation of Munc18c. FEBS Lett. 2003;534:54–60. doi: 10.1016/s0014-5793(02)03774-2. [DOI] [PubMed] [Google Scholar]