Abstract
Metformin (N,N-dimethylbiguanidine) is a widely employed oral hypoglycemic agent for the management of type 2 diabetes mellitus. Its antioxidant properties and safe clinical use raise the possibility of preventing gentamicin-induced hearing loss in patients. Therefore, we screened the usefulness of metformin against gentamicin toxicity in murine cochlear explants and in the guinea pig in vivo. We confirmed in organ culture that metformin blocks the gentamicin-induced translocation of endonuclease G into the nucleus of outer hair cells and attenuates hair cell loss. In vivo, gentamicin treatment with 80, 100, or 130 mg/kg body weight for 14 days induced significant threshold shifts as determined by auditory brain stem responses. Metformin (30, 75, or 100 mg/kg for 14 days) was well tolerated without any indication of auditory side effects. However, co-administration of metformin with gentamicin in various permutations did not prevent loss of auditory function. On the contrary, combined treatment at higher dosages aggravated the gentamicin-induced threshold shifts and caused additional adverse reactions including body weight loss and premature deaths in some animals. These results caution against the use of metformin co-treatment with aminoglycosides and confirm the need for in-vivo studies in order to evaluate potentially protective agents selected by in-vitro screens.
Keywords: metformin, gentamicin, cochlear explants, guinea pigs, outer hair cells, ototoxicity
1. Introduction
The search for effective protection against aminoglycoside ototoxicity is receiving much attention as aminoglycosides maintain their position as powerful antibiotics to treat multi-resistant bacterial infections and tuberculosis [11]. Compelling in-vivo and in-vitro studies have suggested that reactive oxygen species (ROS) are important in aminoglycoside ototoxicity and that antioxidants can significantly protect against hearing loss in animal models [17]. Furthermore, two drugs have attenuated ototoxicity in the clinical setting. Aspirin reduced the incidence of gentamicin-induced hearing loss in a double-blinded randomized clinical trial by 75% [15]. N-acetylcysteine attenuated gentamicin-induced high frequency hearing loss in hemodialysis patients in a small prospective randomized controlled open label study [7].
Proof of the concept of antioxidant protection has encouraged the search for efficacious therapeutics providing safety and easy availability. To this end, screening systems have been devised to detect potentially protective agents without the tedium of in-vivo studies. Cell lines, cochlear explants, and zebra fish larvae have shown promise [21] but also met with problems or criticism [5].
Metformin (N,N-dimethylbiguanidine) is a potential anti-ototoxic agent, based on reports that it reduced gentamicin-induced apoptosis and cisplatin toxicity in a cell line [4]. The drug also prevents experimental gentamicin-induced nephropathy in the rat [12]. Metformin is a widely used oral hypoglycemic agent for the management of type 2 diabetes mellitus [20]. It also prevents oxidative stress-induced cell death through mechanisms related to mitochondrial permeability transition pore opening [22] and inhibition of lipid peroxidation. In addition, metformin scavenges hydroxyl radicals by modulating NADPH oxidase [2] and inhibits apoptotic cascades by increasing the expression of the anti-apoptotic protein Bcl-2 [19].
Metformin should be a good candidate for preventing ototoxicity based on the in-vitro studies on gentamicin and cisplatin and the absence of adverse auditory effects during anti-diabetic therapy [1,18]. Therefore, we probed the usefulness of metformin on gentamicin-induced toxicity in murine cochlear explants and in guinea pigs in vivo.
2. Materials and Methods
2.1 Materials
Gentamicin linked to the fluorophore Texas Red (GTTR) was kindly provided by Dr. Peter S. Steyger [13]. Anti- endonuclease G (endoG) antibody was purchased from Chemicon International (Temicula, CA), rhodamine phalloidin, culture media and Alexa Fluor from Invitrogen (Carlsbad, CA), gentamicin from Sigma Chemical Co. (St. Louis, MO), and metformin from Calbiochem (Merck KGaA, Darmstadt, Germany).
2.2 Animals
For breeding, sexually mature male (6 week-old) and female (8 week-old) CBA/J mice were obtained from Harlan Sprague-Dawley Co. (Indianapolis, IN). Male Hartley guinea pigs (200–250 g) were purchased from Charles River (Wilmington, MA). Animals were maintained on a 12-h light/12-h dark schedule and had free access to water and a regular diet. Experimental protocols were approved by the University of Michigan Committee on the Use and Care of Animals and animal care was under the supervision of the University of Michigan's Unit for Laboratory Animal Medicine.
2.3 Organotypic cultures of post-natal organ of Corti
The culture procedures were previously described in detail [6]. Cochleae from post-natal day 2 or 3 (p2–3) mice were immersed in cold Hank's Balanced Salt Solution, and the lateral wall tissues (stria vascularis and spiral ligament) and the auditory nerve bundle were dissected away leaving the organ of Corti. The explants were placed onto a previously prepared culture dish in 1 mL of culture medium consisting of Basal Medium Eagle, 1% serum-free supplement (Gibco #51500-056; Invitrogen, Carlsbad, CA), 1% bovine serum albumin, 5 mg/mL glucose, and 10 U/mL penicillin G. After 4 h of incubation (37 °C, 5% CO2) 1.5 mL of medium was added to submerge the explants.
2.4 Treatment of explants
Explants were incubated for 2 days to recover from dissection stress before gentamicin treatment. The medium was exchanged for new media containing drugs and incubated for 24–72 h. Final concentrations were 3.5 μM gentamicin, 15 μM metformin, or 3.5 μM GTTR; metformin was added 16 hours prior to gentamicin or GTTR.
2.5 Immunofluorescent assessment
Explants were fixed with 4% paraformaldehyde overnight at 4 °C and permeabilized for 30 min with 3% Triton X-100 in PBS at room temperature (RT). Specimens were washed three times with PBS and blocked with 10% goat serum for 30 min at RT, followed by incubation at 4 °C for 72 h with the primary antibodies (endoG, 1:1,000, or Myo 7a, 1:200, in PBS). After three washes with PBS, secondary Alexa Fluor 488-conjugated goat anti-mouse antibody or Alexa Fluor 546-conjugated goat anti-rabbit antibody (each 1:200 in PBS) were applied overnight in darkness at 4 °C. Hair cells were visualized by incubating with rhodamine phalloidin or Alexa Fluor 488 phalloidin (each 1:100) at RT for 1 h; nuclei with Hoechst 33342 (2 μg/mL in PBS, 40 min at RT). After rinsing in PBS, specimens were mounted on a slide with Prolong Gold anti-fade reagent (Life Technologies, Grand Island, NY) and imaged on an Olympus Fluoview Confocal Laser Scanning Microscope-FV500 (Olympus, Melville, NY).
2.6 Auditory brainstem response (ABR)
Guinea pigs were anesthetized with intra-peritoneal injections of xylazine (2.4 mg/kg body weight), ketamine (58.8 mg/kg), and acepromazine (1.2 mg/kg). Body temperature was maintained near 37 °C with a heating pad. ABRs were measured at 12 and 32 kHz in a sound-isolated and electrically shielded booth (Acoustic Systems, Austin, TX). Sub-dermal electrodes were inserted at the vertex of the skull, under the left ear, and under the right ear (ground). Tucker Davis Technology System III hardware and SigGen/Biosig software were used to present the stimuli (15 ms duration tone bursts with 1 ms rise-fall time) through a Beyer earphone and to average up to 1024 responses for each stimulus level. Thresholds (the lowest stimulus level with a reproducible response) were determined by reducing the intensity in 10-dB steps and in 5-dB close to threshold.
2.7 Drug administration in vivo
Gentamicin and metformin were dissolved in saline, pH was adjusted to 7.5, and the animals received daily subcutaneous injections for 14 days. Body weight was measured before each injection and the drug dose adjusted accordingly. If an animal was losing ∼5% of its weight, 5-6 ml of saline were also subcutaneously injected.
3. Results
3.1 In-vitro studies
3.1.1 Metformin attenuates gentamicin-induced hair cell loss
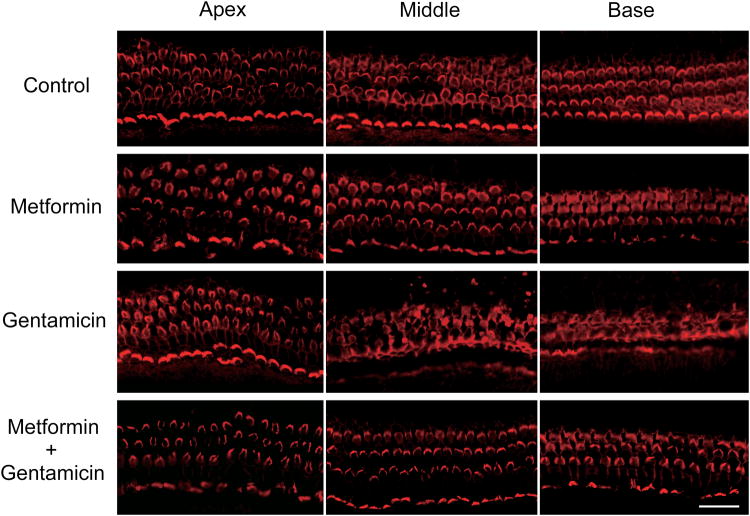
The concentration of gentamicin (3.5 μM for 72 h) was selected from a series of preliminary experiments in murine explants (p2-3). The 72-h culture was chosen over a previous 24-h model [6] because gentamicin concentrations are lower by about two orders of magnitude and the delayed hair cell death better reflects the slow development of ototoxicity in vivo. The treated explants show the typical pattern of chronic aminoglycoside ototoxicity, namely damaged outer hair cells (OHCs) in a graded fashion from the base to the apex of the cochlea with essentially complete loss at the base (fig. 1 A). Inner hair cells showed no noticeable damage. Metformin alone did not cause any OHC loss at concentrations up to 1 mM but preserved OHC structure from gentamicin damage at a concentrations as low as 15 μM.
Figure 1.
Metformin protects explants from gentamicin toxicity.
Gentamicin treatment (3.5 μM for 72 hours) damaged OHCs in a graded fashion with maximal damage to the base of the cochlea (n = 6). Metformin (15 μM for 72 hours) alone did not cause any OHC loss (n = 4) and protected hair cell integrity when co-administered with gentamicin (n = 4). Red: rhodamine phalloidin. Scale bar = 20 μm.
Hair cell loss was quantified in the three sections of the explants and the percentage of missing OHC was calculated against a normative data base. Hair cell loss after metformin only treatment (n=4) remained below 1% in all regions. Gentamicin (n=6) caused a minor loss of 1.8% in the apex but increasing damage from the middle section (37% ± 20; means ± s.d.) to the base (77% ± 14). Importantly, co-administration of 15 uM metformin (n=4) attenuated the damage by gentamicin in the middle (0.3% ± 0.3) and basal sections (2.2 % ±1.1) almost completely and significantly (p<0.01).
3.1.2 Metformin blocks the translocation of endoG but not the entry of gentamicin
We first tested whether the protective effect of metformin might be caused by an inhibition of cellular uptake of gentamicin. Metformin did not block entry of drug into OHCs, as assessed using Texas Red-coupled gentamicin as a fluorescent probe (fig 2).
Figure 2.
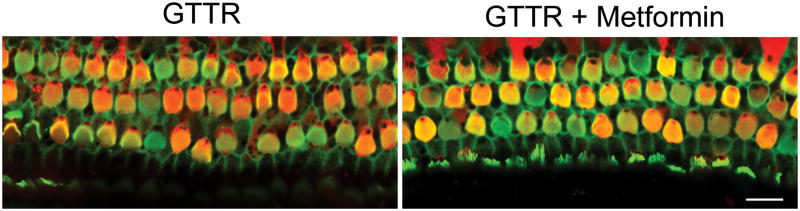
Metformin does not block entry of gentamicin into OHCs in organ culture.
Fluorescently tagged gentamicin (3.5 μM for 24 hours) was used to investigate the entry of gentamicin into explants. Metformin (15 μM) did not block uptake of gentamicin into OHCs (n = 3). Green: Alexa Fluor 488 phalloidin, Red: GTTR. Scale bar = 10 μm.
However, metformin attenuated apoptotic mechanisms associated with aminoglycoside ototoxicity. EndoG translocated into the nucleus of gentamicin-treated cells (fig. 3A), consistent with previous observations in vivo [9]. Middle sections of 4 to 6 different explants per treatment, comprising >100 cells per treatment, were then quantitatively evaluated for endoG-positive nuclei (fig. 3B). Middle sections were chosen as basal sections were unsuitable because of major loss of hair cells in gentamicin treatment. Only a few cells showed nuclear staining in the control and metformin groups. In contrast, gentamicin increased the number of endoG-positive nuclei to 26 ± 4%, and this increase was significantly prevented by the additional presence of metformin in the incubations (2 ± 2%; p<0.01).
Figure 3.
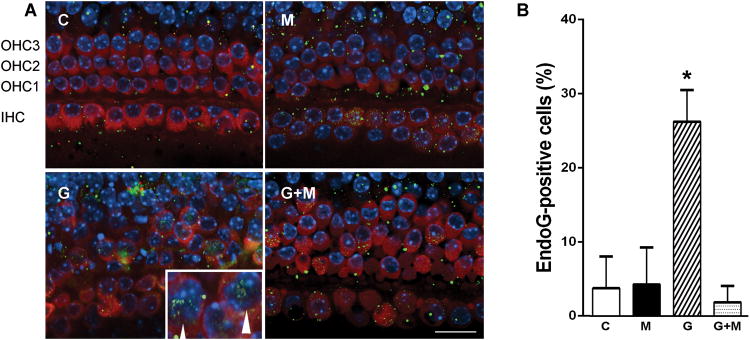
Metformin blocks gentamicin-induced translocation of endoG.
A. EndoG translocated into the nuclei of gentamicin-treated cells (3.5 μM for 72 hours, arrow heads) while little or no translocation of endoG was observed in cells treated with 15 μM metformin alone or metformin in combination with gentamicin. Images are middle sections and representative of 4 to 6 explants per condition. IHC indicates position of inner hair cells, OHC of outer hair cells of rows 1, 2, and 3; respectively. C, control, n =6; M, metformin, n = 4; G, gentamicin, n = 4; G+M, gentamicin + metformin, n = 4. Red: Myo 7a, Green: endoG, Blue: Hoechst 33342. Scale bar = 20 μm. Insert in G shows nuclei at higher magnification with arrow heads pointing to endoG staining.
B. Middle sections of treated explants were quantified for endoG-positive cells and their percentage in each section was calculated and averaged. In each section, between 20and 36 cells could be evaluated, for a total of >100 cells per condition. Data are means+ s.d.; n as in figure 3A. * gentamicin treatment differs from all other conditions, p <0.05; there are no significant differences between metformin or gentamicin plus metformin treatments (One-way ANOVA followed by Student-Newman-Keuls multiple comparisons).
3.2 In-vivo studies
3.2.1 Metformin is devoid of ototoxicity
In a preliminary study, six guinea pigs received daily subcutaneous injections of 100 mg metformin/kg body weight for two weeks. The treatment was well tolerated and body weights of treated animals steadily increased from 334 ± 41 g to 401 ± 53 g (n=6), similar to that of control animals (from 332 ± 18 g to 407 ± 21 g; n=4). Blood samples were collected from three out of the six treated animals on the day after the last injections to check for renal impairment. Blood urea nitrogen (BUN) and creatinine (Cr) were in a normal range: BUN 13.0 ± 4.0 mg/dL (controls, 14.3 ± 1.2 mg/dL) and Cr 0.7 ± 0.1 mg/dL (controls, 0.6 ± 0.1 mg/dL).
Potential effects of metformin on auditory function were tested at 30 mg/kg, 75 mg/kg, or 100 mg drug/kg body weight per day for 14 days, and ABRs were measured three weeks after the last injections (n = 3 each). None of the treatments caused any significant threshold shifts either at 12 or 32 kHz.
3.2.2 Metformin does not attenuate gentamicin-induced loss of auditory function
Next, we investigated whether metformin protected against gentamicin ototoxicity, starting with injections of 130 mg/kg of gentamicin for 14 days, a regimen within the range of our previous studies [10, 16]. Gentamicin produced robust threshold shifts which were not attenuated by concomitant injections with 100 mg/kg metformin. On the contrary, the combined treatment aggravated the observed threshold shifts (47 ± 7 dB at32 kHz with gentamicin alone vs. 60 ± 3 dB for gentamicin plus metformin, n = 3 each; p < 0.05). However, these results were potentially confounded by adverse reactions to the drug combination including body weight loss in 4/7 animals and 2 premature deaths, effects that were not observed with either drug alone.
We consequently reduced the dosage of gentamicin to 100 mg/kg body weight and co-injected two concentrations of metformin, 100 mg/kg or 30 mg/kg body weight. Both combinations were well tolerated as judged by the weight gain of the animals. Consistent with its established pattern of ototoxicity, gentamicin at this dose again caused frequency-dependent threshold shifts averaging ∼50 dB at 32 kHz. Co-treatment with either dose of metformin did not attenuate threshold shifts (fig. 4A).
Figure 4.
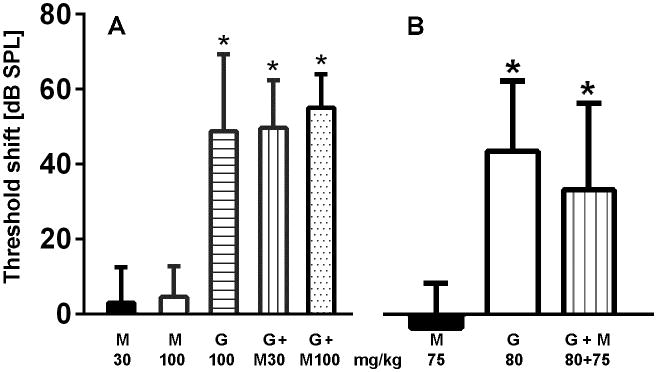
Metformin does not prevent gentamicin-induced loss of auditory function.
A: Gentamicin (100 mg/kg × 14 d) induced a significant threshold shift at 32 kHz which was not attenuated by co-administered metformin. M30, 30 mg/kg metformin (n=3); M100, 100 mg/kg metformin (n=3); G100, 100 mg/kg gentamicin (n=9); G+M30, 100 mg/kg gentamicin + 30 mg/kg metformin (n=3); G+M100, 100 mg/kg gentamicin + 100 mg/kg metformin (n=3).
B: Threshold shift induced by a lower dose of gentamicin (80 mg/kg × 14 d) was also not attenuated by co-administered metformin. M75, 75 mg/kg metformin (n=3); G80, 80 mg/kg gentamicin (n=9); G+M, 80 mg/kg gentamicin + 75 mg/kg metformin (n=3).
A and B. Animals were treated and ABR recorded as described in Methods. Data are means + SD. * threshold shifts of gentamicin-treated animals differ from metformin only treatment, p < 0.05, but there is no significant difference between the various gentamicin treatments in the absence or presence of metformin (One-way ANOVA followed by Student-Newman-Keuls multiple comparisons).
We continued to probe a potential window of protection by further lowering the dose of gentamicin to 80 mg/kg and concurrently reducing metformin to 75 mg/kg. Gentamicin induced a slightly smaller threshold shift but metformin remained unable to protect function (fig. 4B).
The magnitude of threshold shifts induced by gentamicin was generally very consistent at high drug concentrations, such as 130 or 155 mg/kg body weight in our preliminary studies, but inter-animal variability increased as the dosages were lowered. For example, one animal each out of nine in each of the 80 and 100 mg gentamicin groups did not sustain threshold shifts (≤ 0 dB). These outliers were excluded from the analysis.
4. Discussion
The salient finding of this study is the discrepancy between the protection against aminoglycoside-induced hair cell death by metformin in vitro and its inability to provide a functional protection in vivo.
Our in-vitro experiments showing almost complete rescue by metformin of OHCs from gentamicin toxicity are consistent with findings in an auditory cell line [4] and also provide a tentative mechanistic base for a mechanism of this action. Metformin as a biguanidine exists as a cation at physiological pH and might therefore compete with cationic aminoglycosides for uptake into cells. However, metformin does not prevent entry of gentamicin into hair cells, thus suggesting an effect on intracellular pathways.
We selected the translocation of endoG as representative of apoptotic pathways based on our previous observation in mice in vivo following ototoxic treatment with aminoglycosides [9]. Furthermore, endoG is a particularly relevant probe because it is released in response to mitochondrial permeability change, an event that metformin is known to attenuate [8]. Consistent with the in-vivo studies, gentamicin induces the nuclear translocation of endoG in explants, supporting an involvement of mitochondria in aminoglycoside ototoxicity [3]. Metformin indeed blocks the translocation, attesting to its mitochondrial action.
In contrast to its protective action in vitro, metformin failed to protect in vivo although our studies combined a wide range of gentamicin and metformin dosages. The combination of low doses of gentamicin (80 mg/kg) and metformin (75 mg/kg) even had a tendency to increase OHC loss as seen in cytocochleograms (data not shown). There also was a trend towards aggravated threshold shifts at both 12 and 32 kHz when animals received 30 or 100 mg metformin/kg body weight in addition to 100 mg gentamicin/kg. Consistent with this indication of detrimental effects, combining 130 mg gentamicin/kg with 100 mg/kg metformin not only increased the observed threshold shifts but led to further adverse reactions, including death of the animals.
The discrepancy between biochemical and protective actions of metformin is not an isolated observation. Cisplatin-induced functional and histological nephropathy was not prevented by metformin in a rat model in vivo, although metformin significantly attenuated drug-induced lipid peroxidation and reactive oxidant species and preserved enzymatic and non-enzymatic antioxidants [14]. In contrast, metformin prevented experimental gentamicin-induced nephropathy in the rat [12]. The study, however, employed a shorter course of gentamicin (3 or 6 days) than used in our in-vivo experiments combined with a longer metformin exposure (100 mg/kg body weight × 13 d via drinking water). Notwithstanding differences in drug responses between cochlea and kidney (e.g., the latter’s ability to regenerate cells) and different species used in the two studies, these divergent results caution that variations of treatment parameters may introduce negative consequences of this particular drug combination. However, any therapy considered for translation to the clinic must yield robust efficacy with good safety margins. Potential protection by metformin does not conform to such a postulate.
5. Conclusion
Our results confirm protection from gentamicin toxicity by metformin in cochlear explants in vitro. In contrast, metformin did not protect the cochlea from gentamicin-induced ototoxicity in guinea pigs in vivo. These results caution against the use of metformin co-treatment with aminoglycosides and confirm the need for in-vivo studies in order to evaluate potentially protective agents selected by in-vitro screens.
Highlights.
Our results confirm protection from gentamicin toxicity by metformin in vitro
Metformin did not protect the cochlea from gentamicin in guinea pigs in vivo
In-vivo studies are needed to evaluate protective agents selected by in-vitro screens
Acknowledgments
The authors wish to thank Dr. Gao Wei for his help in preparing explant cultures. This work was supported by research grant R01 DC003685 and core grant P30 DC005188 from the National Institutes on Deafness and Other Communication Disorders, National Institutes of Health.
Footnotes
Disclosure: The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bailey CJ, Turner RC. Metformin. The New England journal of medicine. 1996;334:574–579. doi: 10.1056/NEJM199602293340906. [DOI] [PubMed] [Google Scholar]
- 2.Bonnefont-Rousselot D, Raji B, Walrand S, Gardes-Albert M, Jore D, Legrand A, Peynet J, Vasson MP. An intracellular modulation of free radical production could contribute to the beneficial effects of metformin towards oxidative stress. Metabolism: clinical and experimental. 2003;52:586–589. doi: 10.1053/meta.2003.50093. [DOI] [PubMed] [Google Scholar]
- 3.Bottger EC, Schacht J. The mitochondrion: a perpetrator of acquired hearing loss. Hearing research. 2013;303:12–19. doi: 10.1016/j.heares.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang J, Jung HH, Yang JY, Choi J, Im GJ, Chae SW. Protective role of antidiabetic drug metformin against gentamicin induced apoptosis in auditory cell line. Hearing research. 2011;282:92–96. doi: 10.1016/j.heares.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Chen FQ, Hill K, Guan YJ, Schacht J, Sha SH. Activation of apoptotic pathways in the absence of cell death in an inner-ear immortomouse cell line. Hearing research. 2012;284:33–41. doi: 10.1016/j.heares.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen FQ, Schacht J, Sha SH. Aminoglycoside-induced histone deacetylation and hair cell death in the mouse cochlea. Journal of neurochemistry. 2009;108:1226–1236. doi: 10.1111/j.1471-4159.2009.05871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feldman L, Efrati S, Eviatar E, Abramsohn R, Yarovoy I, Gersch E, Averbukh Z, Weissgarten J. Gentamicin-induced ototoxicity in hemodialysis patients is ameliorated by N-acetylcysteine. Kidney international. 2007;72:359–363. doi: 10.1038/sj.ki.5002295. [DOI] [PubMed] [Google Scholar]
- 8.Guigas B, Detaille D, Chauvin C, Batandier C, De Oliveira F, Fontaine E, Leverve X. Metformin inhibits mitochondrial permeability transition and cell death: a pharmacological in vitro study. The Biochemical journal. 2004;382:877–884. doi: 10.1042/BJ20040885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang H, Sha SH, Forge A, Schacht J. Caspase-independent pathways of hair cell death induced by kanamycin in vivo. Cell death and differentiation. 2006;13:20–30. doi: 10.1038/sj.cdd.4401706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matt T, Ng CL, Lang K, Sha SH, Akbergenov R, Shcherbakov D, Meyer M, Duscha S, Xie J, Dubbaka SR, Perez-Fernandez D, Vasella A, Ramakrishnan V, Schacht J, Bottger EC. Dissociation of antibacterial activity and aminoglycoside ototoxicity in the 4-monosubstituted 2-deoxystreptamine apramycin. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:10984–10989. doi: 10.1073/pnas.1204073109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore RD, Smith CR, Lietman PS. Risk factors for the development of auditory toxicity in patients receiving aminoglycosides. The Journal of infectious diseases. 1984;149:23–30. doi: 10.1093/infdis/149.1.23. [DOI] [PubMed] [Google Scholar]
- 12.Morales AI, Detaille D, Prieto M, Puente A, Briones E, Arevalo M, Leverve X, Lopez-Novoa JM, El-Mir MY. Metformin prevents experimental gentamicin-induced nephropathy by a mitochondria-dependent pathway. Kidney international. 2010;77:861–869. doi: 10.1038/ki.2010.11. [DOI] [PubMed] [Google Scholar]
- 13.Myrdal SE, Steyger PS. TRPV1 regulators mediate gentamicin penetration of cultured kidney cells. Hearing research. 2005;204:170–182. doi: 10.1016/j.heares.2005.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sahu BD, Kuncha M, Putcha UK, Sistla R. Effect of metformin against cisplatin induced acute renal injury in rats: a biochemical and histoarchitectural evaluation. Experimental and toxicologic pathology : official journal of the Gesellschaft fur Toxikologische Pathologie. 2013;65:933–940. doi: 10.1016/j.etp.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 15.Sha SH, Qiu JH, Schacht J. Aspirin to prevent gentamicin-induced hearing loss. The New England journal of medicine. 2006;354:1856–1857. doi: 10.1056/NEJMc053428. [DOI] [PubMed] [Google Scholar]
- 16.Sha SH, Schacht J. Antioxidants attenuate gentamicin-induced free radical formation in vitro and ototoxicity in vivo: D-methionine is a potential protectant. Hearing research. 2000;142:34–40. doi: 10.1016/s0378-5955(00)00003-4. [DOI] [PubMed] [Google Scholar]
- 17.Song BB, Schacht J. Variable efficacy of radical scavengers and iron chelators to attenuate gentamicin ototoxicity in guinea pig in vivo. Hearing research. 1996;94:87–93. doi: 10.1016/0378-5955(96)00003-2. [DOI] [PubMed] [Google Scholar]
- 18.Spiller HA, Sawyer TS. Toxicology of oral antidiabetic medications. American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists. 2006;63:929–938. doi: 10.2146/ajhp050500. [DOI] [PubMed] [Google Scholar]
- 19.Ullah I, Ullah N, Naseer MI, Lee HY, Kim MO. Neuroprotection with metformin and thymoquinone against ethanol-induced apoptotic neurodegeneration in prenatal rat cortical neurons. BMC neuroscience. 2012;13:11. doi: 10.1186/1471-2202-13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Viollet B, Guigas B, Sanz Garcia N, Leclerc J, Foretz M, Andreelli F. Cellular and molecular mechanisms of metformin: an overview. Clinical science. 2012;122:253–270. doi: 10.1042/CS20110386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vlasits AL, Simon JA, Raible DW, Rubel EW, Owens KN. Screen of FDA-approved drug library reveals compounds that protect hair cells from aminoglycosides and cisplatin. Hearing research. 2012;294:153–165. doi: 10.1016/j.heares.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zorov DB. Amelioration of aminoglycoside nephrotoxicity requires protection of renal mitochondria. Kidney international. 2010;77:841–843. doi: 10.1038/ki.2010.20. [DOI] [PubMed] [Google Scholar]