Abstract
Substantial evidence has suggested that the brain structures of the medial prefrontal cortex (mPFC) and amygdala (AMYG) are implicated in the pathophysiology of stress-related disorders. However, little is known with respect to the system-level adaptation of their neural circuitries to the perturbations of traumatic stressors. By utilizing behavioral tests and an awake animal imaging approach, in the present study we non-invasively investigated the impact of single-episode predator odor exposure in an inescapable environment on behaviors and neural circuits in rodents. We found that predator odor exposure significantly increased the freezing behavior. In addition, animals exhibited heightened anxiety levels seven days after the exposure. Intriguingly, we also found that the intrinsic functional connectivity within the AMYG-mPFC circuit was considerably compromised seven days after the traumatic event. Our data provide neuroimaging evidence suggesting that prolonged neuroadaptation induced by a single episode of traumatic stress can be non-invasively detected in rodents. These results also support the face validity and construction validity of using the paradigm of single trauma exposure in an inescapable environment as an animal model for post-traumatic stress disorder. Taken together, the present study has opened a new avenue to investigating animal models of stress-related mental disorders by going beyond static neuroanatomy, and ultimately bridging the gap between basic biomedical and human imaging research.
Keywords: trauma, intrinsic functional connectivity, rat, awake, amygdala, medial prefrontal cortex, PTSD
Introduction
Stress is initiated by an event that challenges an organism and threatens to exceed available coping resources. Psychophysiological responses to stress are directed towards adaptation (McEwen, 2000). However, in the event of maladaptation, chronic stress-related disorders can develop, including anxiety disorders such as post-traumatic stress disorder (PTSD), phobia, excessive fear and panic, and mood disorders such as depression.
Stress can induce significant functional and structural alterations in brain regions such as the amygdala (AMYG) and medial prefrontal cortex (mPFC) in both humans and animal models (Shin et al., 2006). For instance, animal research has shown that stressful experiences produce functionally relevant effects on the dendritic arbor, spine, and synapse numbers in both the mPFC and AMYG (McEwen, 1999, 2000; McEwen and Morrison, 2013; Roozendaal et al., 2009). In humans, AMYG hyper-responsivity has been reported in PTSD patients during the presentation of trauma-related visual and auditory cues, or even trauma-unrelated affect cues (Liberzon et al., 1999; Shin et al., 2004), and AMYG activation is positively correlated with the severity of PTSD symptom (Shin et al., 2004). In addition, abnormal neuronal morphology and deficient activity of mPFC were observed in patients suffering from major depression (Frodl et al., 2010). Further, structural neuroimaging studies have reported decreased volumes of the anterior cingulate cortex (ACC) in PTSD patients when compared to trauma-exposed control groups (Woodward et al., 2006; Yamasue et al., 2003). Also, the severity of PTSD symptoms is inversely correlated with the ACC volume (Woodward et al., 2006; Yamasue et al., 2003) and mPFC activation (Britton et al., 2005).
Although it is well accepted that stress can induce considerable morphological and functional changes in neurons of AMYG and mPFC, little is known with respect to the macroscopic circuit-level mechanisms during perturbations of different stressors. Lack of such knowledge has highlighted a critical gap to successful identification of circuit-level dysfunction in stress-related disorders. To address this issue, it is necessary to trace the stress-induced dynamic alterations of connectivity between AMYG and mPFC. This goal can be achieved using the technique of resting-state functional magnetic resonance imaging (rsfMRI). rsfMRI measures intrinsic functional connectivity (FC) in the absence of external stimulation by detecting synchronized spontaneous fluctuations of the rsfMRI signal between different brain regions (Biswal et al., 1995; Fox and Raichle, 2007). This technique is unique in its noninvasiveness, whole-brain coverage and sensitivity to neuroadaptation in various conditions. Therefore, intrinsic FC measurement may provide an ideal tool for noninvasively assessing the stress-related neural circuitry alterations. Indeed, Milad and coworkers recently showed that the intrinsic FC between AMYG and dACC in the human predicted subsequent functional activation of the ventral mPFC and skin conductance responses in a fear extinction task (Linnman et al., 2012), suggesting that intrinsic FC can predict the function of anxiety circuits.
By utilizing an awake animal rsfMRI approach (Liang et al., 2011, 2012a, b; Zhang et al., 2010), we previously showed the connectivity pattern of the neural circuit between AMYG and mPFC in rats (Liang et al., 2012a). In the present study, we have employed the same rsfMRI technique to directly assess the long-lasting impact of traumatic stress on the AMYG-mPFC circuit in a rodent model of PTSD (Cohen and Zohar, 2004; Cohen et al., 2003; Cohen et al., 2005). Animals were exposed to a single episode of predator odor in an inescapable environment. The anxiety level and AMYG-mPFC connectivity of the animal were measured seven days after the predator odor exposure experiment. We found that the traumatic stressor had significant prolonged effects on both the anxiety level and the AMYG-mPFC circuit in animals.
Methods
Animals
Thirty two male Long-Evans (LE) adult rats (250 – 350 g) were obtained from Charles River Laboratories. Animals were housed in Plexiglas cages (two per cage) and maintained in ambient temperature (22–24 °C) on a 12-h light:12-h dark schedule. Food and water were provided ad libitum. All 32 rats were used in the behavioral experiments, 16 of which were also imaged.
Single-episode predator odor exposure experiment
Rats were randomly divided into control and predator odor groups. Before the exposure experiment, each rat was habituated to the exposure environment for 10 min each day for 2 consecutive days. On Day 3, rats were exposed to a piece of cat collar in an inescapable chamber for 10 min. In the predator odor group, cat collars were worn by the same cat for at least 3 months. In the control group, cat collars were never in contact with cats. During the exposure experiment, animals were videotaped to record the freezing behavior, and the freezing time was used to evaluate the behavioral response to the traumatic stressor.
EPM experiment
Seven days after the exposure experiment, the anxiety level of rats was assessed by using the EPM test. The maze employed was a four-armed black platform, elevated 50 cm above the ground. The two closed arms were enclosed by 40-cm-high walls on both sides and on the outer edges of the platform; the two remaining arms were open. The apparatus was illuminated by dim light to ensure that the rat could see its surroundings. The maze was cleaned with 5% ethanol and water solution and dried thoroughly between test sessions.
Each rat was initially placed at the center of the maze and allowed to explore freely for 5 min. Movement was video-tracked from directly above. Time in the open arms and closed arms were separately evaluated. Arm entry was defined as all four paws crossing the center square line. The “ratio time”, defined as the time spent in open arms versus the time spent in (open+closed) arms, was calculated. A higher ratio indicates a lower anxiety level.
rsfMRI experiment
Intrinsic FC was measured shortly after the EPM experiments in 16 of the 32 rats. Before the imaging experiment, these rats were acclimated to the environment and imaging acoustic noise produced by the MR scanner using the procedure previously described (Liang et al., 2011, 2012a, b; Liang et al., 2013; Zhang et al., 2010). Briefly, rats were anesthetized with isoflurane (2%) and secured in a head holder using plastic bite bar and ear bars. EMLA cream was topically applied to relieve any pain associated with the head holder. Animals were then placed into a black opaque tube ‘mock scanner’ with tape-recorded scanner noise played. Animals were acclimated for seven days, one session per day. The time of acclimation was 15 minutes on day 1 with an increment of 15 minutes per day up until day 4. A maximum of 60 minutes was used on days 4, 5, 6 and 7.
All MRI experiments were conducted on a Bruker 4.7 T magnet. A dual 1H radiofrequency (RF) coil configuration (Insight NeuroImaging Systems, Worcester, MA) consisting of a volume coil and a surface coil was used. During the experimental setup, the rat was briefly anesthetized by isoflurane (2%), and the head was secured in a head restrainer with a built-in coil. The body was then fit into a body tube. After completing the setup, isoflurane was removed, and the whole system was positioned inside the magnet. Rats were all fully awake during imaging sessions. For each session, RARE sequence was used to acquire anatomical images with the following parameters: TR = 2125ms, TE = 50ms, matrix size = 256×256, FOV = 3.2cm×3.2cm, slice number = 18, slice thickness = 1mm, RARE factor = 8. To collect rsfMRI data, gradient-echo images were then acquired using the echo-planar imaging (EPI) sequence with the following parameters: TR = 1s, TE = 30ms, flip angle = 60°, matrix size = 64×64, FOV = 3.2cm×3.2cm, slice number=18, slice thickness = 1mm. Two hundred rsfMRI volumes were acquired for each run, and 18 runs were obtained for each rsfMRI experiment.
This experimental design is summarized in Figure 1.
Figure 1.
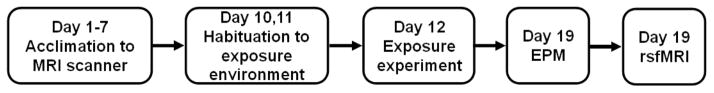
Schematic of the experimental design.
rsfMRI data analysis
rsfMRI images of all rats were first co-registered to a fully segmented rat atlas based on anatomical images by using Medical Image Visualization and Analysis (Liang et al., 2011, 2012a, b; Liang et al., 2013; Zhang et al., 2010). Preprocessing steps included motion correction with SPM8 (http://www.fil.ion.ucl.ac.uk/spm/), spatial smoothing (FWHM = 1mm), regression of motion parameters and the signals of white matter and ventricles, and 0.002–0.1Hz band-pass filtering. Scans with excessive motion (>0.25 mm, equivalent to half voxel size) were discarded. Notably, there was no significant difference in animal’s motion between the predator odor group and control group. Before discarding any scans, the mean volume-to-volume displacement was 0.065±0.0061mm (mean±sem) and 0.057±0.0051mm (mean±sem) in control and predator odor groups, respectively (p=0.41, two sample t-test). After discarding scans with excessive movement, the mean volume-to-volume displacement was 0.051±0.0033mm (mean±sem) and 0.049±0.0038mm (mean±sem) in control and predator odor groups, respectively (p=0.43, two sample t-test).
Functional connectivity was estimated by either seed-based correlational analysis in a voxel-by-voxel manner or by ROI analysis (Liang et al., 2012a; Zhang et al., 2010). For the seed-based analysis, the infralimbic (IL) subdivision of mPFC and AMYG were selected as separate seed regions of interest (ROIs). The anatomical definition of the seed ROIs was based on the Swanson atlas (Swanson, 2004). For each scan the regionally averaged reference time course was obtained by averaging time courses from all voxels inside each seed region. Pearson cross-correlation coefficients between the reference time course and the time courses of individual voxels were then calculated for each seed. Correlation coefficients were transformed to z scores using Fisher’s z transformation and then averaged across scans and animals belonging to the same group. Subsequently, the averaged z values were transformed back to r values, yielding a mean correlation map for each seed. Intrinsic FC maps were displayed by thresholding the correlation coefficients at 0.21 and a cluster size of 10 voxels (p<0.001, uncorrected). For the ROI analysis, the Pearson cross-correlation coefficient (r value) between regionally averaged time courses from two ROIs was calculated for each individual scan. This r value was transformed to the z score using Fisher’s z transformation, and then entered further statistical analysis.
Statistics
To examine the impact of traumatic stress on neural circuits, we contrasted the FC strength within individual circuits obtained by the ROI analysis between the predator odor group and the control group using two-sample t-tests. In order to take into account both intra-subject and inter-subject variances, a linear mixed-effect model was calculated using the lme4 package in the R environment (http://www.r-project.org, version 2.15.1) with the random effect of rats and the fixed effect of z scores for individual scans. The p value of the fixed effect was then calculated by using the Markov chain Monte Carlo (MCMC) method with 10000 samples (implemented in the language R package in R). Behavioral scores were also compared between the two groups using two-sample t-tests. Statistical significance was thresholded at p value < 0.05.
Results
Behavioral responses to the traumatic stressor
In this study, we exposed the rat to a piece of worn cat collar for 10 minutes in an inescapable environment (Adamec and Shallow, 1993; Cohen et al., 2008; Cohen and Zohar, 2004; Cohen et al., 2003). Control rats were exposed to a piece of new cat collar of a similar size. Figure 2 (left panel) shows that rats (n=16) exposed to predator odor in an inescapable environment demonstrated significantly more fearful behaviors as determined by their increased freezing time during the exposure compared to control rats (n=16, two-sample t-test, p<0.05). Seven days after the predator odor exposure, the predator odor rats still displayed significantly heightened anxiety level reflected by their significantly smaller open/(open+closed) arm ratio in the EPM test (two-sample t- test, p<0.05, Fig. 2, right panel). This result clearly indicates a prolonged effect on animal’s behaviors due to the trauma exposure.
Figure 2. Behavior measures in control and predator odor rats.
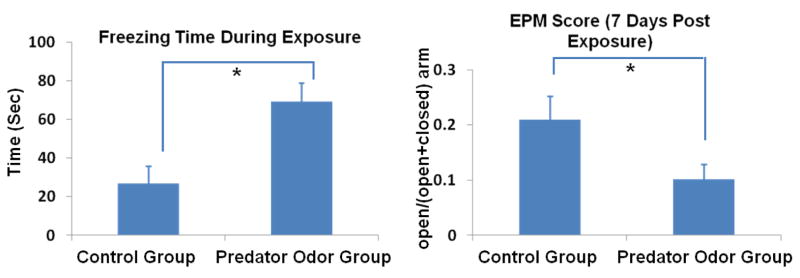
Rats (n=16) exposed to a single episode (10 min) of predator odor in an inescapable environment demonstrated significantly more freezing behaviors than rats exposed to air (n=16, two-sample t-test, p<0.05). Seven days after the traumatic event, these rats still exhibited significantly higher anxiety levels in the EPM test (i.e. smaller open/(open+closed) arm ratio, p<0.05). Bars are SEM. *: p<0.05.
Impact of trauma exposure on neural circuits
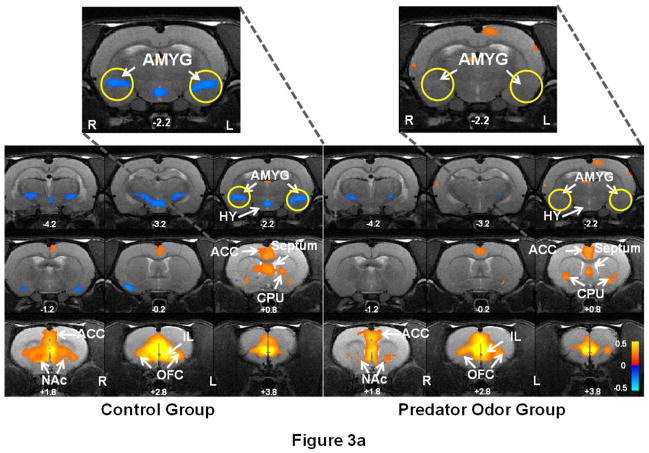
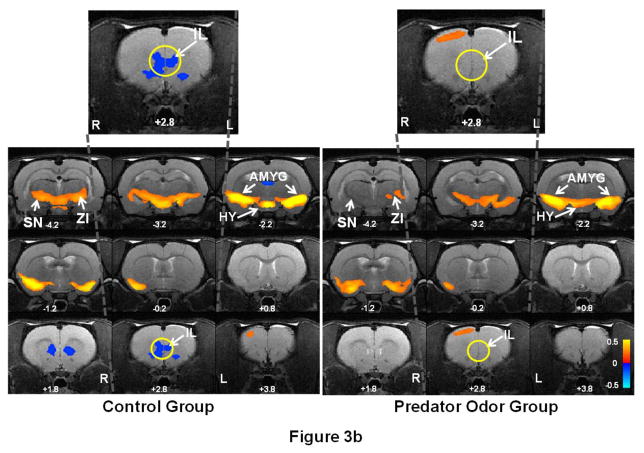
Following the EPM experiment, we measured the intrinsic FC within the mPFC-AMYG circuit in both predator odor and control rat groups to assess the long-lasting effect of the traumatic stressor on the neural circuit. By separately selecting IL and AMYG as seed ROIs, Figure 3 shows the spatial patterns of the IL (Fig. 3a) and AMYG (Fig. 3b) FC circuits in both predator odor (right panels) and control (left panels) rats. Consistent with the results in our previous publication (Liang et al., 2012a), pronounced anticorrelated connectivity between IL and AMYG (as shown in blue colors) was observed in control rats. By contrast, this connectivity was considerably weakened in predator odor rats 7 days after the trauma exposure. In addition to the IL-AMYG circuit, changes in connectivity between AMYG and zona incerta (ZI) as well as substantia nigra (SN) were also observed (Fig 3b). We also quantitatively compared the FC strength in the IL-AMYG circuit between the two groups using ROI-based analysis. Figure 4 shows the averaged FC strength (in absolute values) in the two groups. Two-sample t-test reveals that the FC strength in the IL-AMYG circuit was significantly lower in predator odor rats compared to non-traumatized controls (p < 0.05, n=8). By contrast, trauma exposure did not induce any changes of FC in the visual system estimated by the cross hemispheric connectivity between the left and right primary visual cortices (Fig. 4, p = 0.82, two sample t-test). Taken together, these results strongly indicate that exposure to predator odor in an inescapable environment can induce neuroadaptation in the IL-AMYG circuit that can last at least 7 days in rats.
Figure 3. Prolonged impact of trauma stressor on the IL-AMYG circuit in rats.
Right panel shows the neural circuits of (a) IL and (b) AMYG in rats that were exposed cat odor (n=8). Left panel shows the same neural circuits in rats that were exposed to air (n=8). Warm colors (red-yellow) indicate positively correlated FC, and cold colors (blue) indicate anticorrelated FC. Intrinsic FC was measured seven days after the exposure experiment in both groups. Strong anticorrelated FC was observed between IL and AMYG in control rats, whereas this connectivity was considerably weaker in predator odor rats. Distance to bregma (in mm) is labeled at the bottom of each slice. L, left, R, right. ACC: anterior cingulate cortex; AMYG: amygdala; CPU: caudate-putamen; NAc: nucleus accumbens; OFC: orbital cortex; HY: hypothalamus; ZI: zona incerta; SN: substantia nigra.
Figure 4. Quantitative evaluations of the neural impact of trauma exposure.
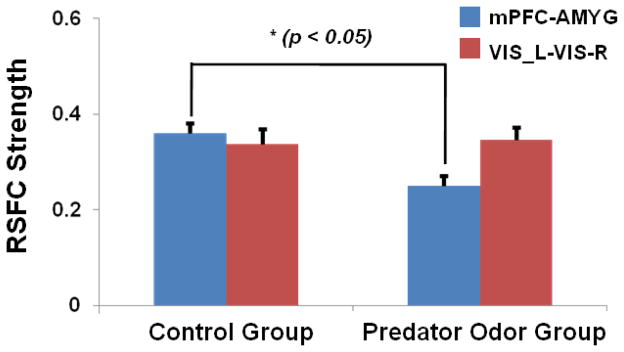
The FC strength in the mPFC-AMYG circuit was statistically significantly weaker in predator odor rats compared to control rats (p < 0.05, n = 8 for each group). By contrast, trauma exposure did not induce any changes in the FC of the visual system measured by cross-hemispheric FC between the left and right primary visual cortices (p = 0.82, two sample t-test). Bars are SEM.
All quantitative measures mentioned above are summarized in Table 1.
Table 1.
Summary of all quantitative measures.
| Measures (mean + SEM) | Predator Odor Group | Control Group | p value |
|---|---|---|---|
| Freezing time (s) | 69.1+14.9 | 26.7+9.0 | <0.05 |
| EPM (open/open+close arms) | 0.1+0.03 | 0.21+0.04 | <0.05 |
| IL-AMYG RSFC (a.u.) | 0.25+0.02 | 0.36+0.04 | <0.05 |
| VIS_L-VIS_R RSFC (a.u.) | 0.35+0.03 | 0.34+0.03 | =0.82 |
Potential influences of acclimation-related stress
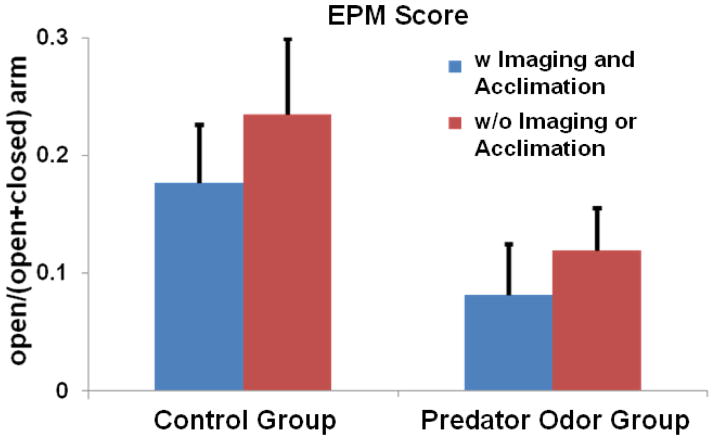
Although our prevsious study showed that the method of acclimation can minimize the stress during imaging (King et al., 2005), it is not known how this procedure influences the effect of traumatic stress. To examine the potential influence of stress attributed to the acclimation procedure, we separately examined the EPM scores in four groups of rats: (i) exposed to predator odor and acclimated (n=8), (ii) exposed to air and acclimated (n=8), (iii) exposed to predator odor but not acclimated (or imaged) (n=8), and (iv) exposed to air but not acclimated (or imaged) (n=8, Figure 5). The results suggest that the stress induced by acclimation was much smaller than the stress induced by a single trauma exposure. Two-way ANOVA with the factors of acclimation and predate odor further confirmed that exposure to predator odor alone significantly increased the anxiety level while acclimation alone did not (p_acclimation = 0.34, p_trauma < 0.05). Importantly, these data showed that there was no interaction between trauma-induced stress and acclimation-related stress (p_interaction = 0.85), indicating that any potential acclimation- and imaging-related stress can be subtracted out with non-traumatized controls that underwent the identical imaging and acclimation procedures except that they were only exposed to air.
Figure 5. Relative stress induced by a single trauma exposure and acclimation.
In all rats of the present study, 16 rats were imaged and thus underwent the acclimation and imaging procedures (among them 8 rats were exposed to predator odor and the other 8 rats were exposed to air). The other 16 rats were not acclimated or imaged (8 of them were exposed to predator odor and the other 8 were controls). The EPM score (open/(open+closed) arm) was separately calculated for all four subgroups. Two-way ANOVA was applied to the four groups with the factors of acclimation and trauma exposure. Statistical results (p_acclimation = 0.34, p_trauma exposure = 0.05 and p_interaction = 0.85) confirmed a much smaller effect of acclimation than trauma exposure. They also indicated a minimal interaction between the trauma-induced stress and acclimation- and imaging-related stress.
Discussion
Animal model of PTSD
Exposure of rodents to predator odor is stressful and produces long-lasting behavioral and physiologic responses (Cohen and Zohar, 2004; Cohen et al., 2003; Cohen et al., 2005). In fact, the single-episode predator odor exposure paradigm has been used as an animal model of PTSD (Cohen and Zohar, 2004; Cohen et al., 2003; Cohen et al., 2005). This model was chosen because “predator exposure trauma in an inescapable environment” is a “potentially life-threatening situation” (Cohen and Zohar, 2004; Cohen et al., 2003; Cohen et al., 2005). In the present study we have investigated the impact of a single episode of predator odor exposure on behaviors and neural circuitries in rodents. Consistent with the previous findings (Cohen and Zohar, 2004; Cohen et al., 2003; Cohen et al., 2005), the traumatic stressor of predator odor exposure induced not only significantly more fearful behaviors, but also heightened anxiety levels that could be detected seven days later in the rat. Intriguingly, similar prolonged effects were also observed on the mPFC-AMYG neural circuit. The functional connectivity within this circuit was significantly impaired seven days post trauma exposure, as shown through pronounced neuroplasticity following a single episode of traumatic stress. Given the critical roles that mPFC and AMYG play in PTSD, these results have provided evidence supporting the face validity and construction validity of this animal model for PTSD.
Circuit-level neuroadaptation identified by the awake animal imaging approach
The alterations of intrinsic FC in humans in pathophysiologic conditions or induced by interventions have been repeatedly demonstrated by the rsfMRI technique (Greicius, 2008). However, this imaging technique has not been as commonly used in assessing different animal models for mental disorders (Heffernan et al., 2013; Huang et al., 2011). A major factor that precludes the applicability of rsfMRI methods in animal models involves the compounding effects of anesthetic agents used in most animal experiments on intrinsic FC. In particular, since behavioral assessments are usually performed on awake animals, imaging animals in anesthetized states makes it difficult to link imaging results with animal behavioral measurements, especially those involving emotional and/or cognitive functions. Therefore, for the purpose of understanding the animal’s brain function at the basal condition and its specific alterations in different animal models, it is essential to image awake animals. Our lab has recently established an approach that allows animals to be imaged in the awake state (Liang et al., 2011, 2012a, b; Zhang et al., 2010). By utilizing this approach, we previously demonstrated robust anticorrelated FC within the IL-AMYG circuit that was anatomically specific, reproducible and independent of preprocessing methods in awake rats. Interestingly, the anticorrelated FC within this frontolimbic circuit was disrupted in anesthetized rats, further supporting the importance of imaging animals at the awake state (Liang et al., 2012a). In the present study, the spatial pattern of the IL-AMYG circuit in the control group (Fig. 3, left panel) was remarkably consistent with that shown in our previous publication (Liang et al., 2012a), again confirming the robustness of intrinsic FC measurement in awake rodents. Importantly, consistent intrinsic FC patterns revealed in independent groups of animals imaged in the same awake state (also see Figure 6 from (Liang et al., 2012a)) warrant the reliability of this rsfMRI approach for detecting neural circuitry changes associated with perturbed states. Indeed, in the predator odor rat, the FC within the IL-AMYG circuit was considerably weaker, indicating that this neural circuit was functionally impaired by the trauma exposure.
In addition to AMYG, several other brain regions such as nucleus accumbens (NAc) and hypothalamus (HY) showed considerable changes in the IL connectivity maps (Fig. 3a) when comparing predator odor rats to control rats. Interestingly, both NAc and HY are known to be sensitive to different stressors. Particularly, HY is one of the key components of the hypothalamic-pituitary-adrenal axis, which is a major part of the neuroendocrine system controlling reactions to stress. In addition, severe stress can switch the action of corticotropin-releasing factor in the NAc from appetitive to aversive (Lemos et al., 2012), which could be central to stress-induced depressive disorders. These results collectively highlight the advantage of using the whole-brain coverage of rsfMRI when studying the impact of stress on multiple neural circuits.
Potential confounding effects of acclimation in awake rats
Many types of measurements by themselves can be stressful. Consequently, when studying the neurobiological effects of a particular stressor, it is critical to take into account potential confounding effects of measurement-related stress. In the present study, our goal was to examine the impact of the traumatic stressor on neural circuitries by utilizing the awake animal rsfMRI paradigm (Liang et al., 2011, 2012a, b; Liang et al., 2013; Zhang et al., 2010). In this imaging paradigm, animal motion and stress during MRI scanning were minimized by using an entirely noninvasive restraining system and a routine acclimation procedure (King et al., 2005) (refer to the review paper (Febo, 2011) for more discussions on this paradigm). However, in order to control possible residual stress in animals, we divided control and predator odor rats into subgroups that were acclimated and imaged and subgroups that were not acclimated or imaged, and separately examined the EPM scores among these subgroups. The data showed that the stress attributed to acclimation was much less and insignificant relative to the traumatic stress introduced to animals (Figure 5). Importantly, we found that there was no interaction between the acclimation stress and traumatic stress, suggesting that any possible effects from acclimation- and imaging- related stress would be parceled out in predator odor animals with control rats.
Summary and Conclusions
The neural mechanism underlying stress-related anxiety and mood disorders has been intensively and extensively studied at different levels. Tremendous progress has been made in discovering the cellular and molecular mechanisms in animal models. Human imaging studies have also provided complementary information in the neural mechanism at the systems level. However, functional neuroimaging studies in animals are rather sparse due to confounding effects of widely used anesthesia on evaluating brain function in animals. By utilizing the awake animal rsfMRI approach, the present study demonstrated that trauma-induced neuroplasticity in neural circuitries can be non-invasively detected. Along with behavioral measurements, the present study has opened a new avenue to investigating stress-related animal models of mental disorders by going beyond static neuroanatomy, and ultimately bridging the gap between basic biomedical and human imaging research.
Acknowledgments
Authors would like to thank Drs. Gregory Quirk and Bruce McEwen for their helpful scientific discussions. Authors would like to thank Mr. Samuel Cramer for editing the manuscript. The work was also supported by the National Institutes of Health Grant Numbers R01MH098003 (PI: Nanyin Zhang, PhD) from the National Institute of Mental Health and R01NS085200 (PI: Nanyin Zhang, PhD) from the National Institute of Neurological Disorders and Stroke.
Footnotes
Financial Disclosures:
Authors have no financial conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamec RE, Shallow T. Lasting effects on rodent anxiety of a single exposure to a cat. Physiol Behav. 1993;54:101–109. doi: 10.1016/0031-9384(93)90050-p. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Britton JC, Phan KL, Taylor SF, Fig LM, Liberzon I. Corticolimbic blood flow in posttraumatic stress disorder during script-driven imagery. Biol Psychiatry. 2005;57:832–840. doi: 10.1016/j.biopsych.2004.12.025. [DOI] [PubMed] [Google Scholar]
- Cohen H, Matar MA, Buskila D, Kaplan Z, Zohar J. Early post-stressor intervention with high-dose corticosterone attenuates posttraumatic stress response in an animal model of posttraumatic stress disorder. Biol Psychiatry. 2008;64:708–717. doi: 10.1016/j.biopsych.2008.05.025. [DOI] [PubMed] [Google Scholar]
- Cohen H, Zohar J. An animal model of posttraumatic stress disorder: the use of cut-off behavioral criteria. Ann N Y Acad Sci. 2004;1032:167–178. doi: 10.1196/annals.1314.014. [DOI] [PubMed] [Google Scholar]
- Cohen H, Zohar J, Matar M. The relevance of differential response to trauma in an animal model of posttraumatic stress disorder. Biol Psychiatry. 2003;53:463–473. doi: 10.1016/s0006-3223(02)01909-1. [DOI] [PubMed] [Google Scholar]
- Cohen H, Zohar J, Matar MA, Kaplan Z, Geva AB. Unsupervised fuzzy clustering analysis supports behavioral cutoff criteria in an animal model of posttraumatic stress disorder. Biol Psychiatry. 2005;58:640–650. doi: 10.1016/j.biopsych.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Febo M. Technical and conceptual considerations for performing and interpreting functional MRI studies in awake rats. Front Psychiatry. 2011;2:43. doi: 10.3389/fpsyt.2011.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Frodl T, Bokde AL, Scheuerecker J, Lisiecka D, Schoepf V, Hampel H, Moller HJ, Bruckmann H, Wiesmann M, Meisenzahl E. Functional connectivity bias of the orbitofrontal cortex in drug-free patients with major depression. Biol Psychiatry. 2010;67:161–167. doi: 10.1016/j.biopsych.2009.08.022. [DOI] [PubMed] [Google Scholar]
- Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol. 2008;21:424–430. doi: 10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- Heffernan ME, Huang W, Sicard KM, Bratane BT, Sikoglu EM, Zhang N, Fisher M, King JA. Multi-modal approach for investigating brain and behavior changes in an animal model of traumatic brain injury. J Neurotrauma. 2013;30:1007–1012. doi: 10.1089/neu.2012.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Heffernan ME, Li Z, Zhang N, Overstreet DH, King JA. Fear induced neuronal alterations in a genetic model of depression: an fMRI study on awake animals. Neurosci Lett. 2011;489:74–78. doi: 10.1016/j.neulet.2010.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JA, Garelick TS, Brevard ME, Chen W, Messenger TL, Duong TQ, Ferris CF. Procedure for minimizing stress for fMRI studies in conscious rats. J Neurosci Methods. 2005;148:154–160. doi: 10.1016/j.jneumeth.2005.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos JC, Wanat MJ, Smith JS, Reyes BA, Hollon NG, Van Bockstaele EJ, Chavkin C, Phillips PE. Severe stress switches CRF action in the nucleus accumbens from appetitive to aversive. Nature. 2012;490:402–406. doi: 10.1038/nature11436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z, King J, Zhang N. Uncovering intrinsic connectional architecture of functional networks in awake rat brain. J Neurosci. 2011;31:3776–3783. doi: 10.1523/JNEUROSCI.4557-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z, King J, Zhang N. Anticorrelated resting-state functional connectivity in awake rat brain. Neuroimage. 2012a;59:1190–1199. doi: 10.1016/j.neuroimage.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z, King J, Zhang N. Intrinsic organization of the anesthetized brain. J Neurosci. 2012b;32:10183–10191. doi: 10.1523/JNEUROSCI.1020-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z, Li T, King J, Zhang N. Mapping thalamocortical networks in rat brain using resting-state functional connectivity. Neuroimage. 2013;83:237–244. doi: 10.1016/j.neuroimage.2013.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon I, Taylor SF, Amdur R, Jung TD, Chamberlain KR, Minoshima S, Koeppe RA, Fig LM. Brain activation in PTSD in response to trauma-related stimuli. Biol Psychiatry. 1999;45:817–826. doi: 10.1016/s0006-3223(98)00246-7. [DOI] [PubMed] [Google Scholar]
- Linnman C, Zeidan MA, Furtak SC, Pitman RK, Quirk GJ, Milad MR. Resting amygdala and medial prefrontal metabolism predicts functional activation of the fear extinction circuit. Am J Psychiatry. 2012;169:415–423. doi: 10.1176/appi.ajp.2011.10121780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- McEwen BS. The neurobiology of stress: from serendipity to clinical relevance. Brain Res. 2000;886:172–189. doi: 10.1016/s0006-8993(00)02950-4. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Morrison JH. The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron. 2013;79:16–29. doi: 10.1016/j.neuron.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci. 2009;10:423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- Shin LM, Orr SP, Carson MA, Rauch SL, Macklin ML, Lasko NB, Peters PM, Metzger LJ, Dougherty DD, Cannistraro PA, Alpert NM, Fischman AJ, Pitman RK. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch Gen Psychiatry. 2004;61:168–176. doi: 10.1001/archpsyc.61.2.168. [DOI] [PubMed] [Google Scholar]
- Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann N Y Acad Sci. 2006;1071:67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Brain Maps: Structure of the Rat Brain. Elsevier; 2004. [Google Scholar]
- Woodward SH, Kaloupek DG, Streeter CC, Martinez C, Schaer M, Eliez S. Decreased anterior cingulate volume in combat-related PTSD. Biol Psychiatry. 2006;59:582–587. doi: 10.1016/j.biopsych.2005.07.033. [DOI] [PubMed] [Google Scholar]
- Yamasue H, Kasai K, Iwanami A, Ohtani T, Yamada H, Abe O, Kuroki N, Fukuda R, Tochigi M, Furukawa S, Sadamatsu M, Sasaki T, Aoki S, Ohtomo K, Asukai N, Kato N. Voxel-based analysis of MRI reveals anterior cingulate gray-matter volume reduction in posttraumatic stress disorder due to terrorism. Proc Natl Acad Sci U S A. 2003;100:9039–9043. doi: 10.1073/pnas.1530467100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Rane P, Huang W, Liang Z, Kennedy D, Frazier JA, King J. Mapping resting-state brain networks in conscious animals. J Neurosci Methods. 2010;189:186–196. doi: 10.1016/j.jneumeth.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]