Abstract
Major Depressive Disorder (MDD) is among the most prevalent of all psychiatric disorders and is the single most burdensome disease worldwide. In attempting to understand the profound deficits that characterize MDD across multiple domains of functioning, researchers have identified aberrations in brain structure and function in individuals diagnosed with this disorder. In this review we synthesize recent data from human neuroimaging studies in presenting an integrated neural network framework for understanding the impairments experienced by individuals with MDD. We discuss the implications of these findings for assessment of and intervention for MDD. We conclude by offering directions for future research that we believe will advance our understanding of neural factors that contribute to the etiology and course of depression, and to recovery from this debilitating disorder.
Keywords: Depression, Neuroscience, Neuroimaging, Assessment, Treatment, Intervention, risk
INTRODUCTION
Major Depressive Disorder (MDD) is a prevalent psychiatric disorder characterized by significant role impairment, suicide risk, economic burden, and the largest number of years lived with disability in the US (US Burden of Disease Collaborators, 2013). Almost 20% of Americans will experience a major depressive episode in their lifetime (Hirschfeld, 2012); moreover, up to 80% of these individuals will have multiple depressive episodes (Bulloch, Williams, Lavorato, & Patten, 2014). Given its high prevalence, recurrence, and enormous personal and societal costs, it is not surprising that the World Health Organization projects that MDD will be the single most burdensome disease in the world in this century (Moussavi et al., 2007).
In this review, we synthesize multimodal neuroimaging data that inform the diagnosis and intervention of MDD, taking into consideration recent advances in the nosology and treatment of depression. With respect to diagnosis, we specifically consider the advantages of addressing the heterogeneity of MDD by integrating a dimensional Research Domain Criteria (RDoC) approach in evaluating neuroimaging characteristics of depression. In terms of intervention, we review recent research demonstrating the neural effects of the most evidence-based interventions, of rapid-acting antidepressants (e.g. ketamine), and the regional and global effects of targeting neural networks.
According to the Diagnostic and Statistical Manual-Fifth Edition (DSM-5), a diagnosis of MDD requires a persistent disturbance of mood (sadness, and/or in children, irritability) or a loss of interest or pleasure in virtually all activities, in addition to at least four of the following symptoms: sleep disturbance, guilt, loss of energy, impaired concentration, change in appetite, psychomotor agitation or retardation, and suicidal ideation (American Psychiatric Association, 2013). Given these varied symptoms, it is not surprising that depression is a heterogeneous disorder; indeed, each of these symptoms has specific risk factors, severities, and trajectories (Fried, Nesse, Zivin, Guille, & Sen, 2013). Further, both MDD and its individual symptoms often co-occur with other psychiatric disorders (Curry et al., 2014); they also manifest differently both across developmental stages (Dekker et al., 2007) and between males and females (Goodwin & Gotlib, 2004). This heterogeneity and comorbidity has posed significant challenges for the diagnosis and treatment of depression and has hindered our ability to predict long-term outcome of MDD. Although investigators and clinicians have made significant advancements in identifying depressive symptoms and in managing MDD with multimodal pharmacological and psychological treatments, there are wide variations in the efficacy and tolerability of interventions (Perlis, 2014), and problems related to symptom relapse and medication non-adherence (Sato & Yeh, 2013). Further, despite the well-documented burden of MDD, we do not yet understand the pathophysiology of this disorder.
In attempting to address this issue, researchers have begun to examine psychobiological aspects of MDD in the context of RDoC. For example, in order to advance our understanding of the pathophysiology and outcome of MDD, investigators have attempted to deconstruct depression along unitary psychopathological dimensions, such as anhedonia (Downar et al., 2013) or negative affect (Vrieze et al., 2014). In a recent review, Dillon et al. (2014) related anhedonic behavior to deficits in psychological functions that rely heavily on dopamine signaling, especially cost/benefit decision-making and reward learning, influenced negatively both by acute threats and chronic stress. Other studies have documented shared characteristics between MDD and other disorders associated with blunted or negative affect, such as schizophrenia, by focusing on similar reduced expressive behaviors measured by computerized acoustic analysis of speech (Cohen, Najolia, Kim, & Dinzeo, 2012). Although these are recent efforts, these dimensions of depression may aid in predicting treatment outcome. Researchers have also characterized cognitive impairments associated with depression. For example, Gotlib and Joormann (2010) noted that depressed individuals have been found consistently to be characterized by difficulties with inhibition of negative information and deficits in working memory, ruminative responses to negative mood states and life events, and the inability to use positive stimuli to regulate negative mood.
Importantly, recent advances in brain imaging have allowed researchers to augment studies of cognitive and behavioral impairments in MDD with an examination of neural circuit-level mechanisms that may underlie these difficulties (Foland-Ross & Gotlib, 2012). Specifically, neuroimaging studies have documented structural and functional neural characteristics critical to the pathogenesis of MDD (see Hamilton, Chen, & Gotlib, 2013 for a recent review). These studies have also demonstrated that anomalies in distributed, integrated neural networks that involve multiple brain regions, linked structurally and functionally, underlie the disturbances in cognitive functioning that have been documented in MDD (Sacher et al., 2012). Only recently, however, have researchers begun to examine explicitly the nature of the relation between clinical and neural network markers of MDD at various points during the onset and course of the disorder, and to use neural characteristics to predict treatment outcome. We believe that neuroimaging is a promising tool for elucidating the pathogenesis of MDD; it is a safe, noninvasive procedure that is ideally suited for simultaneously identifying aberrant behavior, brain structure, and brain function in MDD. Indeed, with neuroimaging, we can bridge a clinical assessment of depressive symptoms with an examination of brain abnormalities to advance our understanding of the pathophysiology of MDD.
We have three broad goals in this review. We describe anomalies in neural structure and function in adults and youth with MDD and discuss the implications of these abnormalities, first, for the assessment of depression, and second, for approaches to intervention with this disorder. Finally, we offer directions for future research that we believe will advance our understanding of biological factors that are implicated in the etiology and course of MDD, and in recovery from depression. We begin by presenting a brief review of neural aspects of unipolar depression and their implications for assessment of MDD.
HOW THE NEUROSCIENCE OF DEPRESSION CAN INFORM ASSESSMENT
Researchers have consistently documented impairments in emotional functioning and emotion regulation in MDD (Gotlib & Joormann, 2010). Moreover, these difficulties have been found to predict the early onset (Klein et al., 2013) and the recurrence of depressive episodes (Lewinsohn, Allen, Seeley, & Gotlib, 1999), suggesting that impairments in specific domains of emotional functioning reflect stable vulnerabilities that place individuals at increased risk for experiencing recurrent episodes of MDD. Neuroimaging studies have complemented these clinical findings, documenting aberrant structure, function, and connectivity in brain regions that subserve these aspects of emotion and emotional regulation. Specifically, investigators have reported structural anomalies in MDD in the amygdala and hippocampus, and functional abnormalities in the subgenual anterior cingulate cortex (sgACC), dorsolateral prefrontal cortex (DLPFC), amygdala, and ventral striatum in MDD (Sacher et al., 2012). Further, there is growing recognition that depressed individuals are characterized by abnormalities in the anatomical and functional connections among these brain regions (Hamilton et al., 2013). In the following sections we describe these neural aberrations in depressed individuals, their relation to the clinical syndrome of MDD, and their implications for assessment of depression. Key findings from this section are summarized in Table 1.
Table 1.
Common neuroimaging findings that characterize individuals diagnosed with depression relative to controls.
Structural Neuroimaging in MDD
Researchers using structural magnetic resonance imaging (MRI) have reported abnormalities in specific brain regions in depressed individuals. Using such tools as manual volumetry, voxel-based morphometry (VBM), and cortical thickness, investigators have documented differences in neural structure between individuals with depression and healthy controls, primarily involving depression-related reductions in brain regions important for the generation and regulation of emotion. Meta-analyses that have pooled structural neuroimaging data in depressed individuals have consistently identified focal gray matter volumetric reductions in the rostral anterior cingulate cortex (rACC; Bora, Fornito, Pantelis, & Yücel, 2012), hippocampus (Cole, Costafreda, McGuffin, & Fu, 2011), striatum and, more broadly, basal ganglia (Bora, Harrison, Davey, Yücel, & Pantelis, 2012), insula (Liu et al., 2014), subregions of the prefrontal cortex (PFC; Kempton et al., 2011), and amygdala (Sacher et al., 2012), although reports of decreased amygdala volume in depressed individuals are less consistent in medicated samples (Hamilton, Siemer, & Gotlib, 2008). Trait-specific structural differences between depressed and nondepressed individuals have been reported as relative thickening in MDD the temporal pole, caudate, and posterior cingulate, thinning of the medial PFC (van Eijndhoven et al., 2013), and reduced volumes in the anterior insula (Takahashi et al., 2010) and the left anterior cingulate (Caetano et al., 2006). Larger gray matter volume in the bilateral amygdala, hippocampus, and dorsolateral PFC have been found in first-degree relatives of patients with MDD, suggesting a mechanism of risk for MDD. The most consistent state-dependent structural finding is reduction in hippocampal volume (Arnone et al., 2013).
Through inhibitory connections with other subcortical structures, the hippocampus is involved in the appraisal and regulation of stress and in the generation of emotion. Indeed, investigators have demonstrated that gray matter reductions in hippocampal volume are associated with an increased number of depressive episodes (Videbech & Ravnkilde, 2004), and with greater symptom severity and longer illness duration (Cheng et al., 2010), including longer durations during which depressive episodes went untreated (Sheline, Gado, & Kraemer, 2003). Depression-associated reductions in gray matter volume in emotion regulatory regions of the PFC, which include DLPFC (Li et al., 2010) and ventrolateral PFC (VLPFC; Salvadore et al., 2011), ACC and sgACC, and orbitofrontal cortex (OFC), reflect the involvement of these structures in impairments in reasoning and other executive functions, regulation of emotion and attention, and processing of rewards and motivation in MDD. In particular, reductions in ACC volume have been associated with poorer executive functioning (Vasic, Walter, Höse, & Wolf, 2008). Some studies suggest that volumetric reductions in frontal regions, especially in the ACC and OFC, are more pronounced than are decreases in hippocampal and striatal volumes (e.g., Koolschijn, van Haren, Lensvelt-Mulders, Hulshoff Pol, & Kahn, 2009). In fact, in examining large-scale structural brain networks, researchers have consistently found widespread abnormalities in depressed individuals in gray matter in the ACC, DLPFC and dorsomedial PFC (DMPFC), and lateral and medial OFC (Singh et al., 2013).
These gray matter anomalies in prefrontal and subcortical brain regions may be associated with abnormalities within white matter tracts that connect these regions. White matter hyperintensities, areas of increased signal intensity on T2 MRI images that may be indicative of neuropathological changes in white matter tracts, have been found to be more common in depressed than in healthy individuals (Arnone, McIntosh, Ebmeier, Munafò, & Anderson, 2012). White matter hyperintensities may represent early signs of demyelination (Hajek et al., 2005), and have been reported to be associated with greater clinical severity (Tham, Woon, Sum, Lee, & Sim, 2011). These anatomical abnormalities are poorly understood, however, and may be confused with vascular abnormalities, particularly in late-life depression. For example, post-mortem studies of depression have documented pathology in prefrontal white matter, including decreased oligodentrocyte density, reduced expression of genes related to oligodendrocyte function, molecular changes in intercellular adhesion molecule expression levels, and ischemic changes (Tham et al., 2011). It is difficult to distinguish, however, whether signal intensity differences reflect myelin changes, reduced axon density, or an entirely different process related to regional differences in vasculature. Thus, to characterize quantitatively white matter tracts between prefrontal and subcortical regions, researchers have used alternative approaches such as diffusion tensor imaging (DTI).
DTI uses measurement of water diffusion to study white matter tracts in vivo. Water diffusion in white matter is highly anisotropic (i.e., not equal in all directions); diffusion tends to be greater parallel than perpendicular to the fiber axis. Using DTI, investigators have documented abnormalities in MDD in white matter tracts that connect portions of the PFC to subcortical structures (Liao et al., 2013), suggesting that MDD is a syndrome of white matter disconnection. Researchers have also reported lower levels of white matter integrity in the limbic system, DLPFC, thalamic projection fibers, corpus callosum, and other association fibers in depressed than in nondepressed individuals (but see also Choi et al., 2013).
Functional Neuroimaging in MDD
Functional MRI (fMRI) generates data regarding magnitude of brain activation in specific regions, as indexed by levels of oxygenated versus de-oxygenated hemoglobin, referred to as the Blood Oxygen Level Dependent (BOLD) signal. Investigators using fMRI have documented aberrations in regional brain activation in MDD in response to a variety of emotional and cognitive stimuli, suggesting that depression is associated with dysfunction in emotion regulation (Rive et al., 2013). Other researchers have reported findings that suggest more complex patterns of neural dysfunction in depression. For example, using both task-activated and resting-state data, investigators have documented hypoactive clusters in the anterior insula and rACC in MDD that appear to be linked to affectively biased information processing and poor cognitive control (Diener et al., 2012). In addition, frontal brain areas have been found to show both under- and over-activation during cognitive-emotional challenges in MDD and, subcortically, thalamic and striatal activations are associated with biased processing of emotional stimuli (Hamilton et al., 2012). Similar to the structural neuroimaging studies described above, some investigators argue that aberrant activations in medial prefrontal cortex (mPFC) show larger effect sizes than do limbic activations (Steele, Currie, Lawrie, & Reid, 2007). The mPFC and rACC have been implicated in self-referential processing and are emphasized in theoretical models of MDD that posit biases toward negative emotional processing and increased self-focus (Nejad, Fossati, & Lemogne, 2013). We now describe specific tasks used in fMRI studies of depression, discussing cognitive and emotional functioning and their interplay in MDD.
Researchers have examined neural responses as depressed individuals complete an emotional Stroop task in the scanner to examine neural aspects of the interference in cognitive processing associated with this task. Wagner et al. (2013) found that patients with MDD are characterized by an inability to deactivate the rACC during cognitive processing, leading to a compensatory prefrontal hyperactivation and interfering with cognitive control. This interaction between affective and cognitive circuitry may contribute to the reduced capacity for affect regulation and cognitive control in MDD. Interestingly, the rACC was activated during negative self-referential processing, and this activation was related to the severity of patients’ depressive symptoms. Other investigators have found hypoactivity in MDD during an emotional Stroop task in regions important for conflict resolution, including the DLPFC, parietal, and extrastriate cortices combined with amygdala hyperactivity (Chechko et al., 2013).
Given memory difficulties that often characterize depressed individuals, researchers have also examined neural aspects of working memory (WM) in MDD. Anomalous activations in bilateral hippocampus during verbal WM tasks have been documented in unmedicated and remitted depressed patients compared to controls, suggesting that both depression and a vulnerability to recurrence of depression are characterized by a reduced capacity to ignore task-irrelevant stimuli (Foland-Ross et al., 2013; Norbury, Godlewska, & Cowen, 2013). Other investigators have reported hypoactivation in the DLPFC and hyperactivation in the DMPFC during WM tasks (Korgaonkar, Grieve, Etkin, Koslow, & Williams, 2013). When a WM task is combined with emotional distracter stimuli, remitted depressed individuals show hyperactivation of the DLPFC in response to negative emotional distracters but hypoactivation of the DLPFC and VLPFC in response to positive emotional distracters, suggesting an attentional bias toward negatively valenced information (Kerestes et al., 2012).
Several researchers have examined reward-related brain functions and deficits in depressed individuals. A recent meta-analysis found that a reward network is characterized in MDD by decreased striatal and limbic activity and increased cortical activity (Zhang, Chang, Guo, Zhang, & Wang, 2013). More specifically, depressed individuals consistently show reduced caudate response to anticipation and outcome of monetary rewards, and increased activation in the middle frontal gyrus and dorsal ACC (dACC) during anticipation of reward. Variability in tasks and mood states were found to contribute to heterogeneity across studies, highlighting the importance in future investigations of carefully specifying the types of reward used and the different components of reward processing that are being examined.
It appears, therefore, that emotional processing in MDD is characterized by hyperactivity in the amygdala and ACC, and hypoactivity in the striatum and DLPFC. Findings in this area have been inconsistent, however, due in part to valence-related effects. A recent meta-analysis found that depressed individuals show hyperactivity in the amygdala, striatum, parahippocampal gyrus, cerebellum, fusiform gyrus, and ACC in response to positive stimuli, but hypoactivity in these regions in response to negative stimuli (Groenewold, Opmeer, de Jonge, Aleman, & Costafreda, 2013). In addition, activation in the ACC appears to be modulated both by valence and by facial versus non-facial stimuli. These findings suggest that emotional valence moderates neural abnormalities in depression and is a critical factor in understanding emotional dysfunction in depression. Indeed, a recent meta-analysis of fMRI studies using emotional faces found that whereas depressed patients have significantly increased activations in the striatum and parahippocampal gyrus, nondepressed controls have increased activations in the medial and middle frontal gyrus, thalamus, ACC, and superior frontal gyrus (Lai, 2014).
Other cognitive functions are also affected in MDD. Given the documented deficits in inhibitory control in MDD, researchers have also examined patterns of response inhibition in depression using tasks such as the Go/NoGo task. Depressed individuals have been found to show greater neural activation than do controls in frontal, limbic, and temporal regions during successful response inhibition (Langenecker et al., 2007). Other cognitive functions including response flexibility, reversal learning, and emotional reactivity have similarly been examined to understand the pathophysiology of depression. Theorists have suggested that depressed individuals shift from episodic to semantic autobiographical memory retrieval in which they overgeneralize and, consequently, reduce executive resources that lead to a pervasive negative self-representation (Watson, Berntsen, Kuyken, & Watkins, 2013). Interestingly, healthy persons with a rigid negative self-image mimic patterns of altered neurobehavioral functioning found in depression, including hypoactivity of the VLPFC and ACC (Sperduti et al., 2013). These latter findings suggest that a negative self-image represents a risk factor for depression. Importantly, aberrations in any of these cognitive functions may represent important targets for intervention.
The functional imaging studies that we described above all used tasks that target specific brain regions and functions. Task-independent spontaneous resting-state functional connectivity (RSFC) has recently been used to gain a better understanding of intrinsic brain activity and relations among prefrontal and subcortical structures at rest. Researchers have postulated that abnormal resting-state activity may affect stimulus-induced neural activity in core medial prefrontal systems important for self-representation as well as for interactions with external stimuli such as stress (Northoff, Wiebking, Feinberg, & Panksepp, 2011). Indeed, unlike task-activation studies, RSFC can directly assess neural systems without the confound of differences in performance that might increase variability within study groups. RSFC is assessed by correlating this activity temporally; such patterns are believed to reflect synchronous interactions among brain regions. Using RSFC, researchers have found depressed individuals to exhibit broad abnormalities in interhemispheric connectivity (Wang et al., 2013a), as well as focal deficits in connectivity at rest. One specific RSFC network is the default mode network (DMN), which includes the ACC and a large portion of mPFC extending inferiorly into the OFC. This network is posited to be involved with internally generated thought and is inhibited when individuals attend to external stimuli that require attention and cognition. The first RSFC finding in depression reported increased connectivity between the DMN and the sgACC and the thalamus (Greicius et al., 2007). Given these and similar subsequent findings, the DMN has been hypothesized to play a critical role in the pathophysiology and recurrence of depression (Marchetti, Koster, Sonuga-Barke, & De Raedt, 2012). In fact, the salience network (SN) and central executive network (CEN) have also been found to be disrupted in MDD (Tahmasian et al., 2013). Depressed individuals exhibit increased intrinsic connectivity between bilateral dorsal medial PFC and each of cognitive control, default mode, and affective networks, suggesting that a coordinated engagement of diverse networks might explain the development and expression of specific symptoms of depression (Sheline, Price, Yan, & Mintun, 2010). A recent analysis of subsystems of the DMN suggest that patients with MDD exhibit more focal increased within-network connectivity in posterior, ventral, and core DMN subsystems (Sambataro, Wolf, Pennuto, Vasic, & Wolf, 2013). Other studies have documented decreased intrinsic corticolimbic connectivity, including between the pregenual ACC and the thalamus, amygdala, and pallidostriatum, in depressive and bipolar mood disorders (Anand, Li, Wang, Lowe, & Dzemidzic, 2009). These findings highlight the possibility that mood-state and treatment-related factors such as treatment status (Anand, Li, Wang, Gardner, & Lowe, 2007) and treatment response (Guo et al., 2013), and exposure to early life stress (Cisler et al., 2013), influence the nature of the relation between MDD and neural activation. Despite the specific directional discrepancies, these findings collectively suggest that depression is a disorder of disrupted global and focal connectivity.
Recent advances in analytic techniques have increased our ability to use RSFC to inform the assessment of MDD. For example, RSFC has been used in conjunction with support vector machine (SVM) classification methods, which have discriminated MDD patients from healthy controls with 84-93% accuracy, based on connectivity in key areas of the DMN and SN (Zeng, Shen, Liu, & Hu, 2013). Cortical thinning in the DMPFC has been found to directly and selectively affect RSFC to the precuneus in depressed individuals (van Tol et al., 2013), and reductions in RSFC have been found to overlap with volume reductions in the ACC and right inferior frontal gyrus that are correlated with rumination (Kühn, Vanderhasselt, De Raedt, & Gallinat, 2012). Finally, investigators have recently tried to bridge data from RSFC and white matter based structural connectivity data to construct the resting-state structural connectome. In a small sample of seven depressed individuals and seven matched controls, function-by-structure hierarchical mapping demonstrated that depressed individuals had stronger associations in bilateral posterior cingulate and right precuneus than did controls, both regions important for self-referential functions (Ajilore et al., 2013). These studies illustrate the increased sensitivity gained by using integrative multimodal imaging data and analyses to examine aberrations in brain connectomes that characterize MDD.
Although researchers may disagree about the specific dimensions or brain regions that are most relevant to the onset and maintenance of depressive symptoms, there is general consensus that altered interactions between key prefrontal and subcortical regions contribute to dysfunctional cognition and regulation of emotion in MDD. Importantly, altered interactions among these regions may be a developmental phenomenon. For example, in typical neurodevelopment, subcortical gray matter development precedes prefrontal development and pruning, and there appears to be a shift in functional dependence from subcortical to prefrontal structures around puberty (Thompson et al., 2000). Consequently, puberty may represent a critical sensitive period when such functional shifts are vulnerable to alterations and the development of psychopathology (Casey, Jones, & Hare, 2008). Perhaps not coincidentally, the onset of depression occurs most commonly in adolescence (Shanahan, Copeland, Costello, & Angold, 2011). Neuroimaging findings indicate that reductions in hippocampal volume (Chen, Hamilton, & Gotlib, 2010) and blunted striatal activation (Sharp et al., 2014) may precede the onset of MDD, suggesting that certain neural features are endophenotypic for MDD. Further, the results of several studies suggest that chronic depression can lead to neurodegeneration, highlighting the potential for early intervention to prevent neuronal loss and regenerate neural tissue to restore function (Bewernick & Schlaepfer, 2013). Specifically, experiencing a greater number of depressive episodes influences the organization of striatal networks (Meng et al., 2014) and may be associated with impaired hippocampal activation during a recollection memory task (Milne, MacQueen, & Hall, 2012) and with selective deficits in down-regulating amygdala responses to negative emotional stimuli using reappraisal (Kanske, Heissler, Schönfelder, & Wessa, 2012), factors that also contribute to risk for relapse. Vulnerability for recurrence of depressive episodes has been found to be associated with poorer outcomes in patients with earlier onset of depression, with increased number and length of depressive episodes (Moylan, Maes, Wray, & Berk, 2013), and with history of childhood trauma (Barnhofer, Brennan, Crane, Duggan, & Williams, 2014). Thus, in such patients, longer and more frequent depressive episodes appear to increase vulnerability for further episodes, precipitating an accelerating and progressive course of illness, leading to functional decline.
By studying youth with MDD, we can examine the neurobiological features of depression without the confounds associated with chronic illness course, including medication exposure and comorbidities, and can investigate etiological factors associated with the onset, early stages, and progression of MDD. Although meta-analytic data in adults suggest that hippocampal volume reductions occur only after the onset of depression (McKinnon, Yucel, Nazarov, & MacQueen, 2009), studies of youth with MDD indicate that reductions in hippocampal volume are a consequence of depressive symptoms detected as early as the preschool years (Suzuki et al., 2013), and in young offspring of mothers with depression even before the onset of symptoms (Chen, Hamilton, & Gotlib, 2010). Across tasks assessing emotion processing, cognitive control, affective cognition, reward processing, and resting state, researchers have found elevated neural activity in the ACC, VMPFC and OFC, and amygdala in children and adolescents with MDD (Kerestes, Davey, Stephanou, Whittle, & Harrison, 2013). Longitudinal studies that contextualize functional activation patterns according to neurodevelopmental changes and pubertal maturation will be critical in providing a more comprehensive picture of the trajectories of anomalies in neural development in depression.
HOW THE NEUROSCIENCE OF DEPRESSION CAN INFORM INTERVENTION
Investigators have begun to use imaging techniques to examine neural aspects of different interventions in individuals diagnosed with MDD. Across most imaging studies, treatment such as antidepressants, and especially those affecting the serotonergic system, have been shown to modulate the volumes, functions and biochemistry of brain regions implicated in MDD, including the DLFPC, ACC and amygdala (Bellani, Dusi, Yeh, Soares, & Brambilla, 2011). In the following sections, we describe changes in neural structure and function in MDD in response to a variety of interventions, and discuss how these changes can inform the development of more effective treatments for this disorder. Key findings from this section are summarized in Table 2.
Table 2.
Neuroimaging findings that characterize response to treatment for depression
| Finding | Region/Network | Reference/Review/Meta-analysis |
|---|---|---|
| Antidepressants increase gray matter volume | Hippocampus | MacQueen et al., 2008, Fu et al., 2007, Delaveau et al., 2011 |
| Orbitofrontal cortex | Kong et al. 2014, Lisiecka et al., 2011, Delaveau et al., 2011 | |
| Anterior cingulate cortex | Chen et al. 2007, Lisiecka et al., 2011, Fu et al., 2013, Fu et al., 2008 | |
| Middle frontal gyrus | Kong et al. 2014 | |
| Antidepressants normalize fMRI activation | Anterior cingulate cortex (including subgenual and rostral) | Chen et al. 2007, Lisiecka et al., 2011, Fu et al., 2013, Fu et al., 2008, Delaveau et al., 2011, Mayberg et al., 2005, Langenecker et al., 2007, Brody et al., 1999; Miller et al., 2008 |
| Hippocampus | MacQueen et al., 2008, Fu et al., 2007, Delaveau et al., 2011 | |
| Parahippocampus | Delaveau et al., 2011 | |
| Amygdala | Rosenblau et al., 2012, Victor et al., 2013, Victor et al., 2013, Delaveau et al., 2011, Miller et al., 2006 | |
| Striatum (including caudate, nucleus accumbens) | Samson et al., 2011, Lisiecka et al., 2011, Langenecker et al., 2007, Stoy et al., 2012 | |
| Insula | Samson et al., 2011, Rizvi et al., 2013, Langenecker et al., 2007, Delaveau et al., 2011 | |
| Prefrontal cortex (including ventrolateral and dorsolateral) | Fitzgerald et al., 2008, Samson et al., 2011, Delaveau et al., 2011, Rosenblau et al., 2012, Victor et al., 2013, Langenecker et al., 2007, Brody et al., 1999, Carlson et al., 2013, Drevets, 1998, van Wingen et al, 2010 | |
| Superior frontal gyrus | Samson et al., 2011 | |
| Posterior cingulate cortex | Samson et al., 2011, Rizvi et al., 2013 | |
| Antidepressants improve functional connectivity | Prefrontal-subcortical networks | Wang et al., 2013b |
| Anterior cingulate cortex | Salvadore et al., 2010, Delaveau et al., 2011 | |
| Posterior cingulate cortex | Delaveau et al., 2011 | |
| Precuneus | Delaveau et al., 2011 | |
| Inferior parietal lobe | Delaveau et al., 2011 | |
| Thalamus | Lisiecka et al., 2011 | |
| Cerebellum | Lisiecka et al., 2011 | |
| Structured psychotherapy normalizes fMRI activation | Anterior cingulate cortex (including dorsal and subgenual) | Siegle et al., 2006, Chen et al., 2007, Fu et al., 2008, Buchheim et al., 2012 |
| Medial prefrontal cortex | Buchheim et al., 2012 | |
| Amygdala | Siegle et al., 2006, Buchheim et al., 2012 | |
| Medial temporal lobe | Buchheim et al., 2012, Amsterdam et al., 2013 | |
| Electroconvulsive therapy increases brain connectivity | Increased fiber integrity of dorsal fronto-limbic pathways | Lyden et al., 2014 |
| Transcranial magnetic stimulation improves function at target and transynaptically connected networks | Subgenual anterior cingulate | Pascual-Leone et al., 1998, Liston et al., 2014 |
| Connectivity between central executive and salience networks and default mode network | Chen et al., 2013 | |
| Deep brain stimulation reduces symptoms in significantly treatment refractory patients | Subgenual anterior cingulate | Anderson et al., 2012 |
Structural MRI and intervention for MDD
As we described above, MDD is characterized by structural abnormalities that are associated with clinical features of the disorder. Recently, researchers have examined whether these abnormalities can be reversed with intervention. Using multivariate pattern analyses of structural MRI data to predict treatment response in depressed individuals, researchers have distinguished treatment-resistant from treatment-sensitive individuals with 82-91% accuracy (Liu et al., 2012). Researchers have also found greater volumes of the hippocampal body in medicated than in unmedicated depressed individuals (Malykhin, Carter, Seres, & Coupland, 2010). Further, researchers have found that greater baseline hippocampal volumes predict remission of MDD following eight weeks of antidepressant treatment (MacQueen, Yucel, Taylor, Macdonald, & Joffe, 2008). Other studies have shown no effects of treatment on hippocampal volume (Vythilingam et al., 2004), but have reported that hippocampal memory function improves with treatment. Kong et al. (2014) found increased gray matter volume in the left middle frontal gyrus and right OFC in depressed individuals relative to controls following eight weeks of fluoxetine administration, and Chen et al. (2007) reported that faster rates of improvement in symptoms and lower residual symptom scores in depressed individuals were associated with greater gray matter volume in the ACC. Interestingly, patients with treatment-responsive MDD continue to have abnormalities in white matter tracts in the projection fibers and corpus callosum (Guo et al., 2012); similarly, white matter hyperintensities have been found to predict failure to recover following antidepressant therapy (Sneed et al., 2011). These latter findings suggest that white matter abnormalities associated with MDD are less responsive to pharmacological intervention than are gray matter abnormalities. Other investigators, however, have found that four weeks of guided imagery psychotherapy not only significantly reduces depressive symptoms, but also increases prefrontal fractional anisotropy, suggesting that psychotherapy contributes to white matter remodeling through a top-down regulatory mechanism (Wang et al., 2013). Discrepant findings across studies may be due to differences in treatment targets across interventions, to heterogeneous levels of depression severity across studies, or to other factors that influence treatment response.
fMRI and intervention for MDD
Many of the cross-sectional functional neuroimaging studies in MDD reviewed above were confounded by varying levels of exposure of participants to psychotropic medications at the time of the scan. A growing number of researchers are now examining the effects of intervention on neural function in depression. Across a range of tasks, depressed individuals have been found to exhibit patterns of limbic hyperactivity and prefrontal hypoactivity that reverse with intervention (Fitzgerald, Laird, Maller, & Daskalakis, 2008). For example, as early as four weeks after treatment with mirtazapine or venlafaxine, depressed individuals show increased activations in the DMPFC, posterior cingulate cortex, superior frontal gyrus, caudate, and insula during an emotion perception task (Samson et al., 2011). Other researchers have found that relative to controls, depressed individuals had less activity in the amygdala and PFC during the explicit processing of negative pictures after eight weeks of escitalopram (Rosenblau et al., 2012), and during the implicit processing of emotional faces after eight weeks of sertraline (Victor, Furey, Fromm, Öhman, & Drevets, 2013). Selective serotonin reuptake inhibitors (SSRIs) may be most beneficial for patients who are less able to engage cognitive control networks while they process negative stimuli (Miller et al., 2013) or while they perform verbal WM tasks (Walsh et al., 2007). Indeed, researchers have reported that after eight weeks of fluoxetine, depressed individuals have reversed attenuated activations in the hippocampus and extrastriate visual regions as they process positively valenced facial stimuli (Fu et al., 2007). Interestingly, treatment with SSRIs in depressed individuals has also been shown to attenuate a bias toward attending to masked sad faces and to lead to a positive bias toward masked happy faces, along with corresponding mood-congruent amygdala activation (Victor, Furey, Fromm, Ohman, & Drevets, 2010). Further, using an inhibitory control task, Langenecker et al. (2007) found that activation during successful inhibition in bilateral inferior frontal, left amygdala, insula, and nucleus accumbens, and during unsuccessful inhibition in the rACC, predicted improvement in depressive symptoms following treatment with escitalopram; Stoy et al. (2012) found escitalopram to also normalize ventral striatal hypoactivity during reward processing. In another study researchers showed that after eight weeks of SSRI treatment, depressed individuals no longer differed from controls in either DLPFC or amygdala activity during an emotional interference task (Fales et al., 2009). Baseline activations in posterior cingulate cortex, anterior insula, and premotor cortex while processing affective images have been found to predict response to combination pharmacotherapies such as fluoxetine and olanzapine (Rizvi et al., 2013). Researchers have also demonstrated that fMRI can be used to distinguish neural responses to interventions that have different mechanisms of action in the brain than do SSRIs (Wagner et al., 2010).
Studies using positron emission tomography (PET) and single photon emission computed tomography (SPECT) have yielded information about the effect of specific neurochemical challenges on regional brain function at rest or under activation. Different PET methods demonstrate consistent abnormalities in depressed individuals in the prefrontal, cingulate, and amygdala regions (Kennedy, Javanmard, & Vaccarino, 1997). For example, symptom improvement from paroxetine is correlated with normalized inferior frontal gyrus and VLPFC metabolism, and lower metabolism in the left ventral anterior cingulate gyrus is associated with better treatment response (Brody et al., 1999). These studies also provide information about the activity of neurotransmitters and the regional expression of specific molecular targets in depression, including their modulation by drug treatment, and the kinetics of drug disposition and activity directly in the target organ (Moresco, Matarrese, & Fazio, 2006). For example, lower pretreatment serotonin (5-HTT) binding by PET may predict non-remission from MDD following one year of naturalistic antidepressant treatment, demonstrating the power of functional PET to distinguish treatment remitters from nonremitters (Miller, Oquendo, Ogden, Mann, & Parsey, 2008).
In this context, functional neuroimaging has been used to document changes in neural activation in response to newer pharmacological agents for depression such as scopolamine (Furey et al., 2013) and ketamine (Carlson et al., 2013). Imaging markers have the potential to provide a powerful assay to investigate how novel therapies work, and have validated the function of ketamine as an N-methyl-D-aspartate receptor antagonist (Doyle et al., 2013) that leads to decreased metabolism in the right habenula and the extended medial and orbital prefrontal networks in association with rapid antidepressant response (Carlson et al., 2013). Ketamine-related symptom improvement is predicted by pretreatment functional connectivity between the pgACC and the left amygdala during a working-memory task (Salvadore et al., 2010). The rapid and long-lasting (up to 10 days) antidepressant response to ketamine has been demonstrated consistently in treatment-resistant patients (Bortolozzi, Celada, & Artigas, 2013; Hayley & Litteljohn, 2013), in electroconvulsive therapy (ECT) and non-ECT studies (Fond et al., 2014), and in unipolar and bipolar depression (McGirr et al., 2014).
Increased functional connectivity between prefrontal (e.g., OFC or ACC) and other emotion processing regions including the caudate, thalamus, and cerebellum, has also been found to predict response to antidepressant therapy (Lisiecka et al., 2011). Further, in the only fMRI study to examine changes in brain activity with treatment in pediatric depression, (Tao et al., 2012) documented that overactivation in prefrontal and temporal regions in depressed youth normalized after eight weeks of fluoxetine treatment.
In sum, therefore, most studies have found normalizing effects on neural activation in response to emotional stimuli following antidepressant treatment. In a recent meta-analysis, (Delaveau et al., 2011) found treatment-related increases in activation in DLFPC, DMPFC, and VLPFC and decreases in activation in limbic structures such as the amygdala, hippocampus, parahippocampus, sgACC, OFC, and insula. This meta-analysis also found decreased activation in the anterior and posterior cingulate cortices, precuneus, and inferior parietal lobule, suggesting restored deactivation of the DMN in MDD following antidepressant treatment. In a different meta-analysis of studies of participants who were medication-free before their initial scan, Fu et al. (2013) found distinct regional activations that predicted antidepressant response. Specifically, Fu et al. found that increased baseline activity in the ACC predicted a higher likelihood of improvement, whereas increased baseline activation in the insula and striatum was associated with poorer clinical response. Indeed, anomalous activation in the sgACC has been a treatment target for patients with refractory depression (Mayberg et al., 2005) and has been found to predict treatment response (Korb, Hunter, Cook, & Leuchter, 2011). Although it is difficult to disentangle whether improved fMRI response following treatment is due to symptom reduction or to the intervention itself, these findings highlight the promise of fMRI data to provide prognostic markers of clinical response to treatment in MDD. Further, there are limited neurobiological data to guide treatment choices for individuals with depression, and even fewer data that are not biased by treatment histories (Dunlop et al., 2012). Studies in which depressed patients are selected or stratified on the basis of these neural characteristics and that accurately predict which patients respond to treatment will advance the field significantly. Similarly, large controlled studies of individuals who were previously naïve to any intervention will increase our knowledge of the specific effects of antidepressant treatment on neural activation.
Functional neuroimaging is limited by its relative sensitivity to state-dependent features of depression, which are often difficult to distinguish from trait-related phenomena. Importantly, however, the results of a recent meta-analysis investigating three neurobiological models of depression, including limbic-cortical, cortico-striatal, and the DMN, suggest that state and trait neural markers of depression can be distinguished based on the responsiveness of activations in these neural regions to intervention (Graham et al., 2013). This meta-analysis found broad support for limbic-cortical and cortico-striatal models of depression, and noted that lateral frontal areas were most sensitive to treatment. In contrast, subcortical regions appeared relatively insensitive to treatment, suggesting that they are indicators of trait vulnerability rather than state or treatment-sensitive markers of depression. Other studies suggest that aberrant amygdala activation in response to sad faces (Arnone et al., 2012) and memory formation and retrieval (van Wingen et al., 2010) are specific to the depressed state and are responsive to treatment. Portions of the prefrontal cortex are also responsive to treatment (Drevets, 1998; van Wingen et al., 2010). As Stuhrmann, Suslow, & Dannlowski (2011) note, it is likely that inconsistencies among studies are due to the heterogeneity of patient samples and paradigms. Changes in resting-state function in response to eight weeks of escitalopram also support a prefrontal-subcortical model of depression (Wang et al., 2013b), suggesting that biomarkers of treatment response are not limited to task-based fMRI studies.
Although psychotherapy is a mainstay intervention for depression, relatively few studies have examined the effects of structured psychotherapy on neural function. One early study found that depressed individuals with low sustained reactivity to emotional stimuli in the sgACC and high reactivity in the amygdala exhibited the strongest improvement following 16 weeks of cognitive behavioral therapy (CBT) (Siegle, Carter, & Thase, 2006). Another study found that depressed individuals with higher levels of baseline dACC activity during an affect recognition task had better clinical response to 16 weeks of CBT (Fu et al., 2008), suggesting that ACC activity is an important predictor of treatment response for both psychotherapeutic and pharmacological interventions (Chen et al., 2007). Using SPECT, Amsterdam et al. (2013) found that low pretreatment serotonin transporter binding in the medial temporal lobe bilaterally increased over time in depressed individuals who experienced symptom improvement with 12 weeks of CBT. Neural benefits of psychotherapy seem to be sustained even beyond the acute phase of treatment. Depressed patients who had increased activation in the left anterior hippocampus/amygdala, sgACC, and mPFC before treatment with psychodynamic psychotherapy showed reduction in activation in these areas after 15 months of treatment, which was associated with improvement in depressive symptoms (Buchheim et al., 2012). Importantly, researchers who directly compared psychotherapy with pharmacotherapy found dissociable patterns of treatment response. For example, whereas response to CBT was associated with a reciprocal modulation of cortical-limbic connectivity, pharmacological treatment with venlafaxine engaged additional cortical and striatal regions (Kennedy et al., 2007). It appears that, much like pharmacological interventions, CBT affects clinical recovery by modulating the functioning of specific sites in limbic and cortical regions; however, unique directional changes in frontal cortex, cingulate, and hippocampus with CBT relative to paroxetine may reflect modality-specific effects (Goldapple et al., 2004). These results suggest that neuroimaging can identify treatment-specific biomarkers (McGrath et al., 2013), a formulation that is particularly important for a disorder that is typically characterized by equivalent responses to different kinds of intervention.
Neuromodulation
Aberrant functional activations in key brain regions have led to the development of neuromodulatory treatment options for severe and treatment refractory depression (Kennedy & Giacobbe, 2007). In contrast to medications that putatively work by altering neurochemistry throughout the brain, neuromodulation offers a more targeted approach to treatment that modulates specific networks in the brain (Schlaepfer & Bewernick, 2014). Neuromodulatory interventions include ECT, magnetic seizure therapy, repetitive transcranial magnetic stimulation (rTMS), transcranial direct current stimulation (tDCS), vagal nerve stimulation (VNS), and deep brain stimulation (DBS). ECT is considered the first-line treatment for more severe forms of depression (Kellner et al., 2014). Recent MRI studies indicate that ECT may increase brain connectivity in depression, demonstrated by neuroplasticity of white matter microstructure suggesting increased fiber integrity of dorsal fronto-limbic pathways involved in mood regulation (Lyden et al., 2014). Further, depressed individuals who do not respond to ECT are characterized by a poorer overall clinical response, more sustained reported depression with anxiety features, and higher left temporal regional cerebral blood flow (Berggren, Gustafson, Höglund, & Johanson, 2014). Unfortunately, extant data reveal considerable variations among studies, which limits our understanding of a unified mechanism by which ECT might achieve its positive clinical effects (Bolwig, 2014).
rTMS and tDCS have comparable effects to antidepressant drugs, but have a relatively benign profile of side effects (Brunoni et al., 2010). Importantly, the effects of rTMS are not limited to the cortical area that is targeted directly, but affect a wider neural network transynaptically (Pascual-Leone et al., 1998). For example, although targeted modulation of subgenual cingulate connectivity has been shown to play an important mechanistic role in alleviating depression (Liston et al., 2014), rTMS has also been shown to selectively modulate functional connectivity both within and between the broader CEN and DMN. Chen and colleagues (2013) recently combined TMS with functional MRI to causally excite or inhibit TMS-accessible prefrontal nodes within the CEN or SN to determine the effects on the DMN. Single-pulse excitatory stimulations delivered to only the CEN node induced negative DMN connectivity with the CEN and SN, consistent with the hypothesized negative regulation of the DMN by the CEN and the SN. Conversely, low-frequency inhibitory repetitive TMS to the CEN node resulted in a shift of DMN signal from its normally low-frequency range to a higher frequency, suggesting disinhibition of DMN activity. Moreover, the CEN node exhibited this causal regulatory relation primarily with the medial prefrontal portion of the DMN. These findings advance our understanding of the causal mechanisms by which major brain networks coordinate information processing, and how these systems might be aberrant in MDD.
DBS and VNS are invasive strategies with a possible role in treatment-resistant depression (Brunoni et al., 2010). In fact, DBS has yielded significant reductions in depressive symptomatology and high rates of remission in severely treatment-resistant patients (Anderson et al., 2012). Some researchers have suggested that neuromodulation would be more efficacious if used early to alter the developmental course of dysfunctional neurocircuitry; however, safety and tolerability are important concerns, especially for pediatric populations (Croarkin et al., 2010). Some researchers have found benefit for anterior cingulotomy in treatment refractory patients, demonstrating that a stronger clinical response was associated with more anterior lesions and with smaller lesion volumes (Steele, Christmas, Eljamel, & Matthews, 2008). Other investigators have proposed trigeminal nerve stimulation as a promising adjunct to pharmacotherapy in depression (Cook et al., 2013). Additional comparative studies are needed to determine the relative advantages of each of these approaches and how best to match patients to specific neuromodulation strategies.
LIMITATIONS AND DIRECTIONS FOR FUTURE RESEARCH
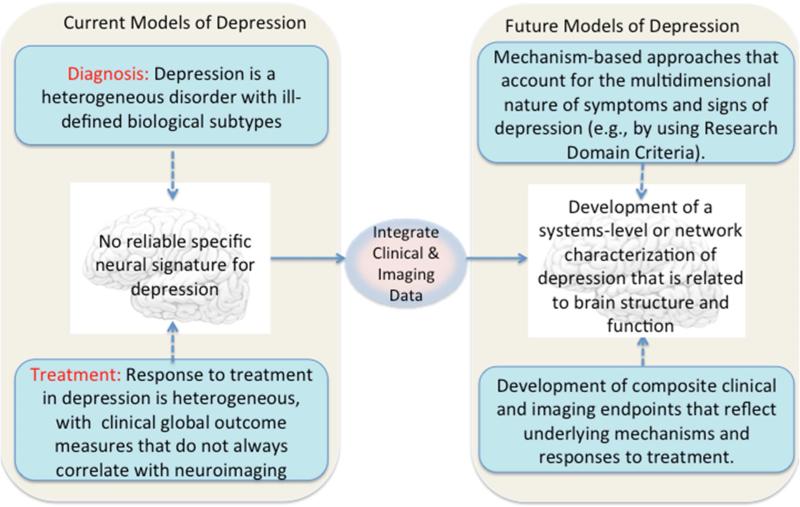
In this article we have described implications of neuroimaging for assessment and treatment of MDD. There are several challenges for research in this field. For example, patients are diagnosed and treated on the basis of symptom clusters that are heterogeneous and are likely to be caused by numerous and divergent biological factors. Two patients can exhibit non-overlapping clusters of symptoms, but share the same diagnosis and treatment recommendation. Another challenge involves distinguishing depression from other commonly co-occurring disorders such as anxiety. Patients may be better served by a diagnostic system that is based on underlying genetic and neurobiological factors that define subtypes of broad syndromes instead of on symptom clusters. One way to apply this concept would be to identify biomarker signatures that accompany specific domains of behavioral abnormalities and that predict distinct treatment responses. The identification of new biomarker targets based on disease mechanisms has the potential to significantly improve the treatment of depression. In Figure 1 we present a conceptual framework for developing new models of depression grounded in neuroscience.
Figure 1.
Conceptual framework for developing neuroscience models of depression
Given the complexity of the depressed brain, it is also important to integrate neuroimaging findings with results of gene and protein expression analyses that suggest that depression involves disruptions in various components of a complex signaling network (Villanueva, 2013). Components of this network include the hypothalamic-pituitary-adrenal axis; the production of neurotrophins and growth factors; the expression of miRNAs; the production of proinflammatory cytokines; and the abnormal delivery of gastrointestinal signaling peptides, all of which may contribute to significant alterations in mood. Indeed, cytokines have been implicated in early-onset depression in adolescents, suggesting important developmental vulnerabilities in the immune and inflammatory systems (Mills, Scott, Wray, Cohen-Woods, & Baune, 2013). Similarly, animal models of stress exposure have been used to advance our understanding of the pathophysiology of depression (Abelaira, Réus, & Quevedo, 2013). These factors also modulate neurogenesis in brain regions involved in MDD, and are functionally interconnected in a fashion that creates cascading abnormalities. Neuroimaging findings are also being associated with specific genotypes (Flint & Kendler, 2014). Advances in genetic and imaging technology may enable us to gain a better understanding of the role of genetics in contributing to brain abnormalities that increase risk for the development of depression.
Some investigators have argued that progress in assessment and intervention for depressive disorders has been limited by a lack of valid diagnostic and treatment biomarkers (Niciu et al., 2013). In addition to integrating macro- and micro-level information about neural systems, there are several additional solutions that might strengthen the impact of studies of depression on our understanding of assessment and intervention. For example, many neuroimaging studies have used univariate analytic techniques to examine single structures and specific task-based neural functions, which has limited our ability to integrate neurobiological findings across modalities. A multivariate analytic approach, using techniques such as graph theory and multivariate pattern analyses, is likely to be more sensitive to subtle neurobiologic changes than are traditional univariate analyses, and can be used to develop a more sophisticated model of depression based on neural network dysfunction. Similarly, as we noted above, investigators have assessed clinically heterogeneous samples cross-sectionally (e.g., variable pubertal status, ages, gender, medication exposures, number of episodes, and comorbidities). While informative, findings of these studies are limited in furthering our understanding of the neural consequences of MDD, which are likely to be better modeled with longitudinal study designs. Finally, researchers should integrate approaches across biological systems including genetics, peripheral measures, proteomics/metabolomics, multimodal neuroimaging, neuropsychopharmacological challenge paradigms, and clinical predictors. These systems can be integrated to explore potential predictor and mediator/moderator biomarkers for novel intervention approaches, which could then be selected for a priori use in larger multisite controlled studies.
Other challenges associated with fMRI research in MDD include the appropriate selection of cognitive tasks and the use of standardized tasks that take into account differences in performance of individuals at different life stages. Future studies will benefit from careful selection of tasks and from generating a priori hypotheses about specific mechanisms of dysfunction to advance our understanding of functional impairments in depression. Additional functional and resting state connectivity studies are important to evaluate networks involved in clinical and neurocognitive aspects of depression, such as executive function, self-monitoring, and emotion regulation. In addition, paradigms that highlight core symptoms of depression, such as anhedonia, could expand how we conceptualize subtypes of depression. In addition, more studies are needed assessing symptomatic and never-ill youth at familial risk for depression to determine whether there is functional impairment prior to the onset of depression (e.g., Gotlib, Joormann, & Foland-Ross, 2014). Neuroimaging studies of healthy and subsyndromal youth at risk for depression can aid in determining endophenotypes of depression.
As we noted above, studies of youth at familial risk for depression indicate that structural and functional neural changes precede onset of depressive symptoms, stressing the importance of early identification of risk factors to prevent disease onset and progression. Compared to low-risk counterparts, healthy daughters of depressed mothers selectively attend to negative facial expressions after a negative mood induction (Joormann, Talbot, & Gotlib, 2007), interpret ambiguous words and stories more negatively (Dearing & Gotlib, 2009), and have poorer accuracy in identifying sad facial expressions (Joormann, Gilbert, & Gotlib, 2010). These negative cognitive biases in high-risk youth have been coupled with increased stress reactivity (Gotlib, Joormann, Minor, & Hallmayer, 2008) and with abnormalities in brain structure and function, including reduced hippocampal volumes (Chen et al., 2010), amygdala and VLPFC hyperactivity during negative mood induction combined with DLPFC and dACC hypoactivity during automatic mood regulation (Joormann, Cooney, Henry, & Gotlib, 2012), putamen and right insula hypoactivity while anticipating rewards (Gotlib et al., 2010), and aberrant frontolimbic responses to emotional faces (Monk et al., 2008). Other studies have found familial risk for depression to be associated with cortical thinning (Peterson et al., 2009), altered white matter integrity (Huang, Fan, Williamson, & Rao, 2011), and lower levels of intrinsic connectivity at rest (Clasen, Beevers, Mumford, & Schnyer, 2014). Importantly, these abnormalities in neural function in high-risk youth mimic those found in depressed adults and, consequently, may represent risk factors for the development of MDD. Studies of high-risk populations may also suggest clues for resilience factors that may be important in preventing the onset of illness, but longitudinal observations are required for this purpose.
Less is known about how abnormalities in structure and function in depression are directly related to each other on a network level. Nor do we understand how depression-associated impairment in specific brain regions affect other areas to which they are functionally and structurally connected to form neural networks. Indeed, we do not yet understand precisely how these anomalies are related to MDD. Inconsistent results across studies may be due to variations in how brain volume was measured and non-uniform scaling across brain regions. To correct for this variation, most structural studies provide volumetric measurements as a ratio to overall brain volume. Variation in whole brain measurements between groups is therefore an important consideration when evaluating specific brain regions (Barnes et al., 2010). Nevertheless, these studies collectively demonstrate structural and functional deficits in multiple cortical and subcortical brain regions that may lead to emotional dysfunction during critical periods of neurodevelopment. Further, volumetric changes in portions of PFC may have a significant impact on cognitive development and the regulation of emotion, and suggest vulnerability factors that affect chronicity of the course of depressive illness.
We are also limited in our understanding of how the neural characteristics of depression described above can be useful in predicting treatment response and long-term prognosis. Some researchers attribute the lack of progress in this area to the gap between current clinical nosology and classification suggested by neuroimaging (Farah & Gillihan, 2012). Other investigators have begun to link neurobiological assessments to an understanding of treatment response (Fu & Costafreda, 2013), which may be an early proxy for prognosis. Clearly, we need to conduct more innovative examinations of MDD that integrate structural and functional properties of the brain in order to provide a strong empirical basis for the development of effective early and lasting interventions that prevent progression and recurrence of MDD.
Finally, despite the progress that we have made in understanding the neurobiological basis of depression, longitudinal controlled studies are needed to examine more explicitly the nature of the relation between depression and neuroimaging findings, to identify neurobiomarkers in order to establish rational treatment strategies in individuals with and at risk for developing depression, and to elucidate the mechanisms that contribute to illness onset. Through such investigations, we should be better able to clarify the complex etiopathophysiology of depression in order to diagnose more accurately, and treat more effectively, depressed individuals. Given the enormous costs of MDD and the modest effects of current interventions for depression, it is imperative that we develop a more comprehensive and integrative neural conceptualization of this debilitating disorder.
Highlights.
Major Depressive Disorder is the single most burdensome disease worldwide.
Depressed individuals are characterized by aberrant brain structure and function.
We present an integrative neural network framework for understanding depression.
Understanding neural networks should improve depression assessment and intervention.
ACKNOWLEDGEMENTS
Preparation of this article was facilitated by NIMH Grants MH085919 to MKS and MH074849, MH101495, and MH101545 to IHG.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abelaira HM, Réus GZ, Quevedo J. Animal models as tools to study the pathophysiology of depression. Revista brasileira de psiquiatria (São Paulo, Brazil: 1999) 2013;35(Suppl 2):S112–120. doi: 10.1590/1516-4446-2013-1098. [DOI] [PubMed] [Google Scholar]
- Ajilore O, Zhan L, Gadelkarim J, Zhang A, Feusner JD, Yang S, Leow A. Constructing the resting state structural connectome. Frontiers in neuroinformatics. 2013;7:30. doi: 10.3389/fninf.2013.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association, & American Psychiatric Association . Diagnostic and statistical manual of mental disorders: DSM-5. 5th ed. American Psychiatric Association; Washington, D.C: 2013. [Google Scholar]
- Amsterdam JD, Newberg AB, Newman CF, Shults J, Wintering N, Soeller I. Change over time in brain serotonin transporter binding in major depression: effects of therapy measured with [(123) I]-ADAM SPECT. Journal of Neuroimaging: Official Journal of the American Society of Neuroimaging. 2013;23:469–476. doi: 10.1111/jon.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand A, Li Y, Wang Y, Gardner K, Lowe MJ. Reciprocal effects of antidepressant treatment on activity and connectivity of the mood regulating circuit: an FMRI study. The Journal of Neuropsychiatry and Clinical Neurosciences. 2007;19:274–282. doi: 10.1176/appi.neuropsych.19.3.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand A, Li Y, Wang Y, Lowe MJ, Dzemidzic M. Resting state corticolimbic connectivity abnormalities in unmedicated bipolar disorder and unipolar depression. Psychiatry Research. 2009;171:189–198. doi: 10.1016/j.pscychresns.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RJ, Frye MA, Abulseoud OA, Lee KH, McGillivray JA, Berk M, Tye SJ. Deep brain stimulation for treatment-resistant depression: efficacy, safety and mechanisms of action. Neuroscience and biobehavioral reviews. 2012;36:1920–1933. doi: 10.1016/j.neubiorev.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Arnone D, McIntosh AM, Ebmeier KP, Munafò MR, Anderson IM. Magnetic resonance imaging studies in unipolar depression: systematic review and meta-regression analyses. European neuropsychopharmacology: the journal of the European College of Neuropsychopharmacology. 2012;22:1–16. doi: 10.1016/j.euroneuro.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Arnone D, McKie S, Elliott R, Juhasz G, Thomas EJ, Downey D, Anderson IM. State-dependent changes in hippocampal grey matter in depression. Molecular Psychiatry. 2013;18:1265–1272. doi: 10.1038/mp.2012.150. [DOI] [PubMed] [Google Scholar]
- Arnone D, McKie S, Elliott R, Thomas EJ, Downey D, Juhasz G, Anderson IM. Increased amygdala responses to sad but not fearful faces in major depression: relation to mood state and pharmacological treatment. The American Journal of Psychiatry. 2012;169:841–850. doi: 10.1176/appi.ajp.2012.11121774. [DOI] [PubMed] [Google Scholar]
- Barnes J, Ridgway GR, Bartlett J, Henley SMD, Lehmann M, Hobbs N, Fox NC. Head size, age and gender adjustment in MRI studies: a necessary nuisance? NeuroImage. 2010;53:1244–1255. doi: 10.1016/j.neuroimage.2010.06.025. [DOI] [PubMed] [Google Scholar]
- Barnhofer T, Brennan K, Crane C, Duggan D, Williams JMG. A comparison of vulnerability factors in patients with persistent and remitting lifetime symptom course of depression. Journal of Affective Disorders. 2014;152-154:155–161. doi: 10.1016/j.jad.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellani M, Dusi N, Yeh PH, Soares JC, Brambilla P. The effects of antidepressants on human brain as detected by imaging studies. Focus on major depression. Progress in neuro-psychopharmacology & biological psychiatry. 2011;35:1544–1552. doi: 10.1016/j.pnpbp.2010.11.040. [DOI] [PubMed] [Google Scholar]
- Berggren A, Gustafson L, Höglund P, Johanson A. A long-term follow-up of clinical response and regional cerebral blood flow changes in depressed patients treated with ECT. Journal of Affective Disorders. 2014;167:235–243. doi: 10.1016/j.jad.2014.06.005. [DOI] [PubMed] [Google Scholar]
- Bewernick BH, Schlaepfer TE. Chronic depression as a model disease for cerebral aging. Dialogues in clinical neuroscience. 2013;15:77–85. doi: 10.31887/DCNS.2013.15.1/bbewernick. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolwig TG. Neuroimaging and electroconvulsive therapy: a review. The Journal of ECT. 2014;30:138–142. doi: 10.1097/YCT.0000000000000140. [DOI] [PubMed] [Google Scholar]
- Bora E, Fornito A, Pantelis C, Yücel M. Gray matter abnormalities in Major Depressive Disorder: a meta-analysis of voxel based morphometry studies. Journal of affective disorders. 2012;138:9–18. doi: 10.1016/j.jad.2011.03.049. [DOI] [PubMed] [Google Scholar]
- Bora E, Harrison BJ, Davey CG, Yücel M, Pantelis C. Meta-analysis of volumetric abnormalities in cortico-striatal-pallidal-thalamic circuits in major depressive disorder. Psychological medicine. 2012;42:671–681. doi: 10.1017/S0033291711001668. [DOI] [PubMed] [Google Scholar]
- Bortolozzi A, Celada P, Artigas F. Novel Therapeutic Strategies in Major Depression: Focus on RNAi and Ketamine. Current Pharmaceutical Design. 2013;20:3848–3860. doi: 10.2174/13816128113196660137. [DOI] [PubMed] [Google Scholar]
- Brody AL, Saxena S, Silverman DH, Alborzian S, Fairbanks LA, Phelps ME, Baxter LR., Jr. Brain metabolic changes in major depressive disorder from preto post-treatment with paroxetine. Psychiatry Research. 1999;91:127–139. doi: 10.1016/s0925-4927(99)00034-7. [DOI] [PubMed] [Google Scholar]
- Brunoni AR, Teng CT, Correa C, Imamura M, Brasil-Neto JP, Boechat R, Fregni F. Neuromodulation approaches for the treatment of major depression: challenges and recommendations from a working group meeting. Arquivos de neuro-psiquiatria. 2010;68:433–451. doi: 10.1590/s0004-282x2010000300021. [DOI] [PubMed] [Google Scholar]
- Buchheim A, Viviani R, Kessler H, Kächele H, Cierpka M, Roth G, Taubner S. Changes in prefrontal-limbic function in major depression after 15 months of long-term psychotherapy. PloS one. 2012;7:e33745. doi: 10.1371/journal.pone.0033745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulloch A, Williams J, Lavorato D, Patten S. Recurrence of major depressive episodes is strongly dependent on the number of previous episodes. Depression and anxiety. 2014;31:72–76. doi: 10.1002/da.22173. [DOI] [PubMed] [Google Scholar]
- Caetano SC, Kaur S, Brambilla P, Nicoletti M, Hatch JP, Sassi RB, Soares JC. Smaller cingulate volumes in unipolar depressed patients. Biological Psychiatry. 2006;59:702–706. doi: 10.1016/j.biopsych.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Carlson PJ, Diazgranados N, Nugent AC, Ibrahim L, Luckenbaugh DA, Brutsche N, Drevets WC. Neural correlates of rapid antidepressant response to ketamine in treatment-resistant unipolar depression: a preliminary positron emission tomography study. Biological psychiatry. 2013;73:1213–1221. doi: 10.1016/j.biopsych.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B. j., Jones RM, Hare TA. The Adolescent Brain. Annals of the New York Academy of Sciences. 2008;1124:111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chechko N, Augustin M, Zvyagintsev M, Schneider F, Habel U, Kellermann T. Brain circuitries involved in emotional interference task in major depression disorder. Journal of affective disorders. 2013;149:136–145. doi: 10.1016/j.jad.2013.01.013. [DOI] [PubMed] [Google Scholar]
- Chen AC, Oathes DJ, Chang C, Bradley T, Zhou Z-W, Williams LM, Etkin A. Causal interactions between fronto-parietal central executive and default-mode networks in humans. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:19944–19949. doi: 10.1073/pnas.1311772110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Ridler K, Suckling J, Williams S, Fu CHY, Merlo-Pich E, Bullmore E. Brain imaging correlates of depressive symptom severity and predictors of symptom improvement after antidepressant treatment. Biological psychiatry. 2007;62:407–414. doi: 10.1016/j.biopsych.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Chen MC, Hamilton JP, Gotlib IH. Decreased hippocampal volume in healthy girls at risk of depression. Archives of general psychiatry. 2010;67:270–276. doi: 10.1001/archgenpsychiatry.2009.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng YQ, Xu J, Chai P, Li H-J, Luo C-R, Yang T, Xu L. Brain volume alteration and the correlations with the clinical characteristics in drug-naïve first-episode MDD patients: a voxel-based morphometry study. Neuroscience letters. 2010;480:30–34. doi: 10.1016/j.neulet.2010.05.075. [DOI] [PubMed] [Google Scholar]
- Choi KS, Holtzheimer PE, Franco AR, Kelley ME, Dunlop BW, Hu XP, Mayberg HS. Reconciling Variable Findings of White Matter Integrity in Major Depressive Disorder. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2013;39:1332–1339. doi: 10.1038/npp.2013.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler JM, James GA, Tripathi S, Mletzko T, Heim C, Hu XP, Kilts CD. Differential functional connectivity within an emotion regulation neural network among individuals resilient and susceptible to the depressogenic effects of early life stress. Psychological Medicine. 2013;43:507–518. doi: 10.1017/S0033291712001390. [DOI] [PubMed] [Google Scholar]
- Clasen PC, Beevers CG, Mumford JA, Schnyer DM. Cognitive control network connectivity in adolescent women with and without a parental history of depression. Developmental cognitive neuroscience. 2014;7:13–22. doi: 10.1016/j.dcn.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AS, Najolia GM, Kim Y, Dinzeo TJ. On the boundaries of blunt affect/alogia across severe mental illness: implications for Research Domain Criteria. Schizophrenia Research. 2012;140:41–45. doi: 10.1016/j.schres.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Cole J, Costafreda SG, McGuffin P, Fu CHY. Hippocampal atrophy in first episode depression: a meta-analysis of magnetic resonance imaging studies. Journal of affective disorders. 2011;134:483–487. doi: 10.1016/j.jad.2011.05.057. [DOI] [PubMed] [Google Scholar]
- Cook IA, Schrader LM, Degiorgio CM, Miller PR, Maremont ER, Leuchter AF. Trigeminal nerve stimulation in major depressive disorder: acute outcomes in an open pilot study. Epilepsy & behavior: E&B. 2013;28:221–226. doi: 10.1016/j.yebeh.2013.05.008. [DOI] [PubMed] [Google Scholar]
- Croarkin PE, Wall CA, McClintock SM, Kozel FA, Husain MM, Sampson SM. The emerging role for repetitive transcranial magnetic stimulation in optimizing the treatment of adolescent depression. The journal of ECT. 2010;26:323–329. doi: 10.1097/YCT.0b013e3181dd17eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry JF, Aubuchon-Endsley N, Brancu M, Runnals JJ, VA Mid-Atlantic Mirecc Women Veterans Research Workgroup, VA Mid-Atlantic Mirecc Registry Workgroup. Fairbank JA. Lifetime major depression and comorbid disorders among current-era women veterans. Journal of affective disorders. 2014;152-154:434–440. doi: 10.1016/j.jad.2013.10.012. [DOI] [PubMed] [Google Scholar]
- Dearing KF, Gotlib IH. Interpretation of ambiguous information in girls at risk for depression. Journal of abnormal child psychology. 2009;37:79–91. doi: 10.1007/s10802-008-9259-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker MC, Ferdinand RF, van Lang NDJ, Bongers IL, van der Ende J, Verhulst FC. Developmental trajectories of depressive symptoms from early childhood to late adolescence: gender differences and adult outcome. Journal of child psychology and psychiatry, and allied disciplines. 2007;48:657–666. doi: 10.1111/j.1469-7610.2007.01742.x. [DOI] [PubMed] [Google Scholar]
- Delaveau P, Jabourian M, Lemogne C, Guionnet S, Bergouignan L, Fossati P. Brain effects of antidepressants in major depression: a meta-analysis of emotional processing studies. Journal of affective disorders. 2011;130:66–74. doi: 10.1016/j.jad.2010.09.032. [DOI] [PubMed] [Google Scholar]
- Diener C, Kuehner C, Brusniak W, Ubl B, Wessa M, Flor H. A meta-analysis of neurofunctional imaging studies of emotion and cognition in major depression. NeuroImage. 2012;61:677–685. doi: 10.1016/j.neuroimage.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Dillon DG, Rosso IM, Pechtel P, Killgore WDS, Rauch SL, Pizzagalli DA. Peril and pleasure: an rdoc-inspired examination of threat responses and reward processing in anxiety and depression. Depression and Anxiety. 2014;31:233–249. doi: 10.1002/da.22202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle OM, De Simoni S, Schwarz AJ, Brittain C, O'Daly OG, Williams SCR, Mehta MA. Quantifying the attenuation of the ketamine pharmacological magnetic resonance imaging response in humans: a validation using antipsychotic and glutamatergic agents. The Journal of Pharmacology and Experimental Therapeutics. 2013;345:151–160. doi: 10.1124/jpet.112.201665. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Functional neuroimaging studies of depression: the anatomy of melancholia. Annual Review of Medicine. 1998;49:341–361. doi: 10.1146/annurev.med.49.1.341. [DOI] [PubMed] [Google Scholar]
- Dunlop BW, Binder EB, Cubells JF, Goodman MM, Kelley ME, Kinkead B, Mayberg HS. Predictors of remission in depression to individual and combined treatments (PReDICT): study protocol for a randomized controlled trial. Trials. 2012;13:106. doi: 10.1186/1745-6215-13-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fales CL, Barch DM, Rundle MM, Mintun MA, Mathews J, Snyder AZ, Sheline YI. Antidepressant treatment normalizes hypoactivity in dorsolateral prefrontal cortex during emotional interference processing in major depression. Journal of affective disorders. 2009;112:206–211. doi: 10.1016/j.jad.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah MJ, Gillihan SJ. The Puzzle of Neuroimaging and Psychiatric Diagnosis: Technology and Nosology in an Evolving Discipline. AJOB Neuroscience. 2012;3:31–41. doi: 10.1080/21507740.2012.713072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald PB, Laird AR, Maller J, Daskalakis ZJ. A meta-analytic study of changes in brain activation in depression. Human Brain Mapping. 2008;29:683–695. doi: 10.1002/hbm.20426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint J, Kendler KS. The Genetics of Major Depression. Neuron. 2014;81:484–503. doi: 10.1016/j.neuron.2014.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foland-Ross LC, Gotlib IH. Cognitive and neural aspects of information processing in major depressive disorder: an integrative perspective. Frontiers in psychology. 2012;3:489. doi: 10.3389/fpsyg.2012.00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foland-Ross LC, Hamilton JP, Joormann J, Berman MG, Jonides J, Gotlib IH. The neural basis of difficulties disengaging from negative irrelevant material in major depression. Psychological science. 2013;24:334–344. doi: 10.1177/0956797612457380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fond G, Loundou A, Rabu C, Macgregor A, Lançon C, Brittner M, Boyer L. Ketamine administration in depressive disorders: a systematic review and meta-analysis. Psychopharmacology. 2014 doi: 10.1007/s00213-014-3664-5. doi:10.1007/s00213-014-3664-5. [DOI] [PubMed] [Google Scholar]
- Fried EI, Nesse RM, Zivin K, Guille C, Sen S. Depression is more than the sum score of its parts: individual DSM symptoms have different risk factors. Psychological medicine. 2013:1–10. doi: 10.1017/S0033291713002900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu CHY, Costafreda SG. Neuroimaging-based biomarkers in psychiatry: clinical opportunities of a paradigm shift. Canadian journal of psychiatry. Revue canadienne de psychiatrie. 2013;58:499–508. doi: 10.1177/070674371305800904. [DOI] [PubMed] [Google Scholar]
- Fu CHY, Steiner H, Costafreda SG. Predictive neural biomarkers of clinical response in depression: a meta-analysis of functional and structural neuroimaging studies of pharmacological and psychological therapies. Neurobiology of disease. 2013;52:75–83. doi: 10.1016/j.nbd.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Fu CHY, Williams SCR, Brammer MJ, Suckling J, Kim J, Cleare AJ, Bullmore ET. Neural responses to happy facial expressions in major depression following antidepressant treatment. The American journal of psychiatry. 2007;164:599–607. doi: 10.1176/ajp.2007.164.4.599. [DOI] [PubMed] [Google Scholar]
- Fu CHY, Williams SCR, Cleare AJ, Scott J, Mitterschiffthaler MT, Walsh ND, Murray RM. Neural responses to sad facial expressions in major depression following cognitive behavioral therapy. Biological psychiatry. 2008;64:505–512. doi: 10.1016/j.biopsych.2008.04.033. [DOI] [PubMed] [Google Scholar]
- Furey ML, Drevets WC, Hoffman EM, Frankel E, Speer AM, Zarate CA., Jr. Potential of pretreatment neural activity in the visual cortex during emotional processing to predict treatment response to scopolamine in major depressive disorder. JAMA psychiatry. 2013;70:280–290. doi: 10.1001/2013.jamapsychiatry.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldapple K, Segal Z, Garson C, Lau M, Bieling P, Kennedy S, Mayberg H. Modulation of cortical-limbic pathways in major depression: treatment-specific effects of cognitive behavior therapy. Archives of General Psychiatry. 2004;61:34–41. doi: 10.1001/archpsyc.61.1.34. [DOI] [PubMed] [Google Scholar]
- Goodwin RD, Gotlib IH. Gender differences in depression: the role of personality factors. Psychiatry research. 2004;126:135–142. doi: 10.1016/j.psychres.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Hamilton JP, Cooney RE, Singh MK, Henry ML, Joormann J. Neural processing of reward and loss in girls at risk for major depression. Archives of general psychiatry. 2010;67:380–387. doi: 10.1001/archgenpsychiatry.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Joormann J. Cognition and depression: current status and future directions. Annual review of clinical psychology. 2010;6:285–312. doi: 10.1146/annurev.clinpsy.121208.131305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Joormann J, Foland-Ross LC. Understanding familial risk for depression: A 25-year perspective. Perspectives on Psychological Science. 2014;9:94–108. doi: 10.1177/1745691613513469. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Joormann J, Minor KL, Hallmayer J. HPA axis reactivity: a mechanism underlying the associations among 5-HTTLPR, stress, and depression. Biological psychiatry. 2008;63:847–851. doi: 10.1016/j.biopsych.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham J, Salimi-Khorshidi G, Hagan C, Walsh N, Goodyer I, Lennox B, Suckling J. Meta-analytic evidence for neuroimaging models of depression: state or trait? Journal of affective disorders. 2013;151:423–431. doi: 10.1016/j.jad.2013.07.002. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Schatzberg AF. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biological Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewold NA, Opmeer EM, de Jonge P, Aleman A, Costafreda SG. Emotional valence modulates brain functional abnormalities in depression: evidence from a meta-analysis of fMRI studies. Neuroscience and biobehavioral reviews. 2013;37:152–163. doi: 10.1016/j.neubiorev.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Guo W, Liu F, Xue Z, Gao K, Liu Z, Xiao C, Zhao J. Abnormal resting-state cerebellar-cerebral functional connectivity in treatment-resistant depression and treatment sensitive depression. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2013;44:51–57. doi: 10.1016/j.pnpbp.2013.01.010. [DOI] [PubMed] [Google Scholar]
- Guo W, Liu F, Xue Z, Gao K, Wu R, Ma C, Zhao JP. Altered white matter integrity in young adults with first-episode, treatment-naive, and treatment-responsive depression. Neuroscience letters. 2012;522:139–144. doi: 10.1016/j.neulet.2012.06.027. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Chen MC, Gotlib IH. Neural systems approaches to understanding major depressive disorder: an intrinsic functional organization perspective. Neurobiology of disease. 2013;52:4–11. doi: 10.1016/j.nbd.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, Gotlib IH. Functional neuroimaging of Major Depressive Disorder: A meta-analysis and new integration of baseline activation and neural response data. American Journal of Psychiatry. 2012;169:693–703. doi: 10.1176/appi.ajp.2012.11071105. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Siemer M, Gotlib IH. Amygdala volume in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Molecular psychiatry. 2008;13:993–1000. doi: 10.1038/mp.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayley S, Litteljohn D. Neuroplasticity and the next wave of antidepressant strategies. Frontiers in Cellular Neuroscience. 2013;7:218. doi: 10.3389/fncel.2013.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschfeld RMA. The epidemiology of depression and the evolution of treatment. The Journal of clinical psychiatry. 2012;73(Suppl 1):5–9. doi: 10.4088/JCP.11096su1c.01. [DOI] [PubMed] [Google Scholar]
- Huang H, Fan X, Williamson DE, Rao U. White matter changes in healthy adolescents at familial risk for unipolar depression: a diffusion tensor imaging study. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2011;36:684–691. doi: 10.1038/npp.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J, Cooney RE, Henry ML, Gotlib IH. Neural correlates of automatic mood regulation in girls at high risk for depression. Journal of abnormal psychology. 2012;121:61–72. doi: 10.1037/a0025294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J, Gilbert K, Gotlib IH. Emotion identification in girls at high risk for depression. Journal of child psychology and psychiatry, and allied disciplines. 2010;51:575–582. doi: 10.1111/j.1469-7610.2009.02175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J, Talbot L, Gotlib IH. Biased processing of emotional information in girls at risk for depression. Journal of abnormal psychology. 2007;116:135–143. doi: 10.1037/0021-843X.116.1.135. [DOI] [PubMed] [Google Scholar]
- Kanske P, Heissler J, Schönfelder S, Wessa M. Neural correlates of emotion regulation deficits in remitted depression: the influence of regulation strategy, habitual regulation use, and emotional valence. NeuroImage. 2012;61:686–693. doi: 10.1016/j.neuroimage.2012.03.089. [DOI] [PubMed] [Google Scholar]
- Kellner CH, Kaicher DC, Banerjee H, Knapp RG, Shapiro RJ, Briggs MC, Liebman LS. Depression Severity in Electroconvulsive Therapy (ECT) Versus Pharmacotherapy Trials. The Journal of ECT. 2014 doi: 10.1097/YCT.0000000000000135. doi:10.1097/YCT.0000000000000135. [DOI] [PubMed] [Google Scholar]
- Kempton MJ, Salvador Z, Munafò MR, Geddes JR, Simmons A, Frangou S, Williams SCR. Structural neuroimaging studies in major depressive disorder. Meta-analysis and comparison with bipolar disorder. Archives of general psychiatry. 2011;68:675–690. doi: 10.1001/archgenpsychiatry.2011.60. [DOI] [PubMed] [Google Scholar]
- Kennedy SH, Giacobbe P. Treatment resistant depression--advances in somatic therapies. Annals of clinical psychiatry: official journal of the American Academy of Clinical Psychiatrists. 2007;19:279–287. doi: 10.1080/10401230701675222. [DOI] [PubMed] [Google Scholar]
- Kennedy SH, Javanmard M, Vaccarino FJ. A review of functional neuroimaging in mood disorders: positron emission tomography and depression. Canadian Journal of Psychiatry. Revue Canadienne De Psychiatrie. 1997;42:467–475. doi: 10.1177/070674379704200502. [DOI] [PubMed] [Google Scholar]
- Kennedy SH, Konarski JZ, Segal ZV, Lau MA, Bieling PJ, McIntyre RS, Mayberg HS. Differences in brain glucose metabolism between responders to CBT and venlafaxine in a 16-week randomized controlled trial. The American Journal of Psychiatry. 2007;164:778–788. doi: 10.1176/ajp.2007.164.5.778. [DOI] [PubMed] [Google Scholar]
- Kerestes R, Davey CG, Stephanou K, Whittle S, Harrison BJ. Functional brain imaging studies of youth depression: A systematic review. NeuroImage. Clinical. 2013;4:209–231. doi: 10.1016/j.nicl.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerestes R, Ladouceur CD, Meda S, Nathan PJ, Blumberg HP, Maloney K, Phillips ML. Abnormal prefrontal activity subserving attentional control of emotion in remitted depressed patients during a working memory task with emotional distracters. Psychological medicine. 2012;42:29–40. doi: 10.1017/S0033291711001097. [DOI] [PubMed] [Google Scholar]
- Klein DN, Glenn CR, Kosty DB, Seeley JR, Rohde P, Lewinsohn PM. Predictors of first lifetime onset of major depressive disorder in young adulthood. Journal of abnormal psychology. 2013;122:1–6. doi: 10.1037/a0029567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong L, Wu F, Tang Y, Ren L, Kong D, Liu Y, Wang F. Frontal-Subcortical Volumetric Deficits in Single Episode, Medication-Naïve Depressed Patients and the Effects of 8 Weeks Fluoxetine Treatment: A VBM-DARTEL Study. PloS one. 2014;9:e79055. doi: 10.1371/journal.pone.0079055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolschijn PCMP, van Haren NEM, Lensvelt-Mulders GJLM, Hulshoff Pol HE, Kahn RS. Brain volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Human Brain Mapping. 2009;30:3719–3735. doi: 10.1002/hbm.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korb AS, Hunter AM, Cook IA, Leuchter AF. Rostral anterior cingulate cortex activity and early symptom improvement during treatment for major depressive disorder. Psychiatry research. 2011;192:188–194. doi: 10.1016/j.pscychresns.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korgaonkar MS, Grieve SM, Etkin A, Koslow SH, Williams LM. Using standardized fMRI protocols to identify patterns of prefrontal circuit dysregulation that are common and specific to cognitive and emotional tasks in major depressive disorder: first wave results from the iSPOT-D study. Neuropsychopharmacology. 2013;38:863–871. doi: 10.1038/npp.2012.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn S, Vanderhasselt M-A, De Raedt R, Gallinat J. Why ruminators won't stop: the structural and resting state correlates of rumination and its relation to depression. Journal of Affective Disorders. 2012;141:352–360. doi: 10.1016/j.jad.2012.03.024. [DOI] [PubMed] [Google Scholar]
- Lai CH. Patterns of cortico-limbic activations during visual processing of sad faces in depression patients: a coordinate-based meta-analysis. The Journal of Neuropsychiatry and Clinical Neurosciences. 2014;26:34–43. doi: 10.1176/appi.neuropsych.12060143. [DOI] [PubMed] [Google Scholar]
- Langenecker SA, Kennedy SE, Guidotti LM, Briceno EM, Own LS, Hooven T, Zubieta JK. Frontal and limbic activation during inhibitory control predicts treatment response in major depressive disorder. Biological Psychiatry. 2007;62:1272–1280. doi: 10.1016/j.biopsych.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn PM, Allen NB, Seeley JR, Gotlib IH. First onset versus recurrence of depression: differential processes of psychosocial risk. Journal of Abnormal Psychology. 1999;108:483–489. doi: 10.1037//0021-843x.108.3.483. [DOI] [PubMed] [Google Scholar]
- Li CT, Lin CP, Chou KH, Chen IY, Hsieh JC, Wu CL, Su TP. Structural and cognitive deficits in remitting and non-remitting recurrent depression: a voxel-based morphometric study. NeuroImage. 2010;50:347–356. doi: 10.1016/j.neuroimage.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Liao Y, Huang X, Wu Q, Yang C, Kuang W, Du M, Gong Q. Is depression a disconnection syndrome? Meta-analysis of diffusion tensor imaging studies in patients with MDD. Journal of Psychiatry & Neuroscience. 2013;38:49–56. doi: 10.1503/jpn.110180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisiecka D, Meisenzahl E, Scheuerecker J, Schoepf V, Whitty P, Chaney A, Frodl T. Neural correlates of treatment outcome in major depression. The International Journal of Neuropsychopharmacology. 2011;14:521–534. doi: 10.1017/S1461145710001513. [DOI] [PubMed] [Google Scholar]
- Liston C, Chen AC, Zebley BD, Drysdale AT, Gordon R, Leuchter B, Dubin MJ. Default Mode Network Mechanisms of Transcranial Magnetic Stimulation in Depression. Biological Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.01.023. doi:10.1016/j.biopsych.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CH, Jing B, Ma X, Xu PF, Zhang Y, Li F, Wang CY. Voxel-based morphometry study of the insular cortex in female patients with current and remitted depression. Neuroscience. 2014;262:190–199. doi: 10.1016/j.neuroscience.2013.12.058. [DOI] [PubMed] [Google Scholar]
- Liu F, Guo W, Yu D, Gao Q, Gao K, Xue Z, Chen H. Classification of different therapeutic responses of major depressive disorder with multivariate pattern analysis method based on structural MR scans. PloS One. 2012;7:e40968. doi: 10.1371/journal.pone.0040968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyden H, Espinoza RT, Pirnia T, Clark K, Joshi SH, Leaver AM, Narr KL. Electroconvulsive therapy mediates neuroplasticity of white matter microstructure in major depression. Translational Psychiatry. 2014;4:e380. doi: 10.1038/tp.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen GM, Yucel K, Taylor VH, Macdonald K, Joffe R. Posterior hippocampal volumes are associated with remission rates in patients with major depressive disorder. Biological Psychiatry. 2008;64:880–883. doi: 10.1016/j.biopsych.2008.06.027. [DOI] [PubMed] [Google Scholar]
- Malykhin NV, Carter R, Seres P, Coupland NJ. Structural changes in the hippocampus in major depressive disorder: contributions of disease and treatment. Journal of Psychiatry & Neuroscience. 2010;35:337–343. doi: 10.1503/jpn.100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti I, Koster EHW, Sonuga-Barke EJ, De Raedt R. The default mode network and recurrent depression: a neurobiological model of cognitive risk factors. Neuropsychology Review. 2012;22:229–251. doi: 10.1007/s11065-012-9199-9. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- McGirr A, Berlim MT, Bond DJ, Fleck MP, Yatham LN, Lam RW. A systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials of ketamine in the rapid treatment of major depressive episodes. Psychological Medicine. 2014:1–12. doi: 10.1017/S0033291714001603. [DOI] [PubMed] [Google Scholar]
- McGrath CL, Kelley ME, Holtzheimer PE, Dunlop BW, Craighead WE, Franco AR, Mayberg HS. Toward a neuroimaging treatment selection biomarker for major depressive disorder. JAMA Psychiatry. 2013;70:821–829. doi: 10.1001/jamapsychiatry.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnon MC, Yucel K, Nazarov A, MacQueen GM. A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. Journal of Psychiatry & Neuroscience. 2009;34:41–54. [PMC free article] [PubMed] [Google Scholar]
- Meng C, Brandl F, Tahmasian M, Shao J, Manoliu A, Scherr M, Sorg C. Aberrant topology of striatum's connectivity is associated with the number of episodes in depression. Brain. 2014;137:598–609. doi: 10.1093/brain/awt290. [DOI] [PubMed] [Google Scholar]
- Miller JM, Oquendo MA, Ogden RT, Mann JJ, Parsey RV. Serotonin transporter binding as a possible predictor of one-year remission in major depressive disorder. Journal of Psychiatric Research. 2008;42:1137–1144. doi: 10.1016/j.jpsychires.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JM, Schneck N, Siegle GJ, Chen Y, Ogden RT, Kikuchi T, Parsey RV. fMRI response to negative words and SSRI treatment outcome in major depressive disorder: a preliminary study. Psychiatry Research. 2013;214:296–305. doi: 10.1016/j.pscychresns.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills NT, Scott JG, Wray NR, Cohen-Woods S, Baune BT. Research review: The role of cytokines in depression in adolescents: a systematic review. Journal of Child Psychology and Psychiatry. 2013;54:816–835. doi: 10.1111/jcpp.12080. [DOI] [PubMed] [Google Scholar]
- Milne AMB, MacQueen GM, Hall GBC. Abnormal hippocampal activation in patients with extensive history of major depression: an fMRI study. Journal of Psychiatry & Neuroscience: JPN. 2012;37:28–36. doi: 10.1503/jpn.110004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk CS, Klein RG, Telzer EH, Schroth EA, Mannuzza S, Moulton JL, 3rd, Ernst M. Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. The American Journal of Psychiatry. 2008;165:90–98. doi: 10.1176/appi.ajp.2007.06111917. [DOI] [PubMed] [Google Scholar]
- Moresco RM, Matarrese M, Fazio F. PET and SPET molecular imaging: focus on serotonin system. Current Topics in Medicinal Chemistry. 2006;6:2027–2034. doi: 10.2174/156802606778522140. [DOI] [PubMed] [Google Scholar]
- Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet. 2007;370:851–858. doi: 10.1016/S0140-6736(07)61415-9. [DOI] [PubMed] [Google Scholar]
- Moylan S, Maes M, Wray NR, Berk M. The neuroprogressive nature of major depressive disorder: pathways to disease evolution and resistance, and therapeutic implications. Molecular Psychiatry. 2013;18:595–606. doi: 10.1038/mp.2012.33. [DOI] [PubMed] [Google Scholar]
- Nejad AB, Fossati P, Lemogne C. Self-Referential Processing, Rumination, and Cortical Midline Structures in Major Depression. Frontiers in Human Neuroscience. 2013;7:666. doi: 10.3389/fnhum.2013.00666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niciu MJ, Mathews DC, Nugent AC, Ionescu DF, Furey ML, Richards EM, Zarate CA., Jr. Developing biomarkers in mood disorders research through the use of rapid-acting antidepressants. Depression and Anxiety. 2013;31:297–307. doi: 10.1002/da.22224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norbury R, Godlewska B, Cowen PJ. When less is more: a functional magnetic resonance imaging study of verbal working memory in remitted depressed patients. Psychological Medicine. 2013:1–7. doi: 10.1017/S0033291713001682. [DOI] [PubMed] [Google Scholar]
- Northoff G, Wiebking C, Feinberg T, Panksepp J. The “resting-state hypothesis” of major depressive disorder-a translational subcortical-cortical framework for a system disorder. Neuroscience and biobehavioral reviews. 2011;35:1929–1945. doi: 10.1016/j.neubiorev.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Tormos JM, Keenan J, Tarazona F, Cañete C, Catalá MD. Study and modulation of human cortical excitability with transcranial magnetic stimulation. Journal of Clinical Neurophysiology: Official Publication of the American Electroencephalographic Society. 1998;15:333–343. doi: 10.1097/00004691-199807000-00005. [DOI] [PubMed] [Google Scholar]
- Perlis RH. Pharmacogenomic testing and personalized treatment of depression. Clinical chemistry. 2014;60:53–59. doi: 10.1373/clinchem.2013.204446. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Warner V, Bansal R, Zhu H, Hao X, Liu J, Weissman MM. Cortical thinning in persons at increased familial risk for major depression. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:6273–6278. doi: 10.1073/pnas.0805311106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rive MM, van Rooijen G, Veltman DJ, Phillips ML, Schene AH, Ruhé HG. Neural correlates of dysfunctional emotion regulation in major depressive disorder. A systematic review of neuroimaging studies. Neuroscience and Biobehavioral Reviews. 2013;37:2529–2553. doi: 10.1016/j.neubiorev.2013.07.018. [DOI] [PubMed] [Google Scholar]
- Rizvi SJ, Salomons TV, Konarski JZ, Downar J, Giacobbe P, McIntyre RS, Kennedy SH. Neural response to emotional stimuli associated with successful antidepressant treatment and behavioral activation. Journal of Affective Disorders. 2013;151:573–581. doi: 10.1016/j.jad.2013.06.050. [DOI] [PubMed] [Google Scholar]
- Rosenblau G, Sterzer P, Stoy M, Park S, Friedel E, Heinz A, Strohle A. Functional neuroanatomy of emotion processing in major depressive disorder is altered after successful antidepressant therapy. Journal of Psychopharmacology. 2012;26:1424–1433. doi: 10.1177/0269881112450779. [DOI] [PubMed] [Google Scholar]
- Sacher J, Neumann J, Fünfstück T, Soliman A, Villringer A, Schroeter ML. Mapping the depressed brain: a meta-analysis of structural and functional alterations in major depressive disorder. Journal of affective disorders. 2012;140:142–148. doi: 10.1016/j.jad.2011.08.001. [DOI] [PubMed] [Google Scholar]
- Salvadore G, Cornwell BR, Sambataro F, Latov D, Colon-Rosario V, Carver F, Zarate CA. Anterior cingulate desynchronization and functional connectivity with the amygdala during a working memory task predict rapid antidepressant response to ketamine. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2010;35:1415–1422. doi: 10.1038/npp.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvadore G, Nugent AC, Lemaitre H, Luckenbaugh DA, Tinsley R, Cannon DM, Drevets WC. Prefrontal cortical abnormalities in currently depressed versus currently remitted patients with major depressive disorder. NeuroImage. 2011;54:2643–2651. doi: 10.1016/j.neuroimage.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambataro F, Wolf ND, Pennuto M, Vasic N, Wolf RC. Revisiting default mode network function in major depression: evidence for disrupted subsystem connectivity. Psychological Medicine. 2013:1–11. doi: 10.1017/S0033291713002596. [DOI] [PubMed] [Google Scholar]
- Samson AC, Meisenzahl E, Scheuerecker J, Rose E, Schoepf V, Wiesmann M, Frodl T. Brain activation predicts treatment improvement in patients with major depressive disorder. Journal of Psychiatric Research. 2011;45:1214–1222. doi: 10.1016/j.jpsychires.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Sato S, Yeh TL. Challenges in treating patients with major depressive disorder: the impact of biological and social factors. CNS Drugs. 2013;27(Suppl 1):S5–10. doi: 10.1007/s40263-012-0028-8. [DOI] [PubMed] [Google Scholar]
- Schlaepfer TE, Bewernick BH. Neuromodulation for treatment resistant depression: state of the art and recommendations for clinical and scientific conduct. Brain Topography. 2014;27:12–19. doi: 10.1007/s10548-013-0315-9. [DOI] [PubMed] [Google Scholar]
- Shanahan L, Copeland WE, Costello EJ, Angold A. Child-, adolescent- and young adult-onset depressions: differential risk factors in development? Psychological Medicine. 2011;41:2265–2274. doi: 10.1017/S0033291711000675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp C, Kim S, Herman L, Pane H, Reuter T, Strathearn L. Major depression in mothers predicts reduced ventral striatum activation in adolescent female offspring with and without depression. Journal of Abnormal Psychology. 2014;123:298–309. doi: 10.1037/a0036191. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. The American Journal of Psychiatry. 2003;160:1516–1518. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Price JL, Yan Z, Mintun MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:11020–11025. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle GJ, Carter CS, Thase ME. Use of FMRI to predict recovery from unipolar depression with cognitive behavior therapy. The American Journal of Psychiatry. 2006;163:735–738. doi: 10.1176/ajp.2006.163.4.735. [DOI] [PubMed] [Google Scholar]
- Singh MK, Kesler SR, Hadi Hosseini SM, Kelley RG, Amatya D, Hamilton JP, Gotlib IH. Anomalous gray matter structural networks in major depressive disorder. Biological Psychiatry. 2013;74:777–785. doi: 10.1016/j.biopsych.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperduti M, Martinelli P, Kalenzaga S, Devauchelle A-D, Lion S, Malherbe C, Piolino P. Don't be Too Strict with Yourself! Rigid Negative Self-Representation in Healthy Subjects Mimics the Neurocognitive Profile of Depression for Autobiographical Memory. Frontiers in Behavioral Neuroscience. 2013;7:41. doi: 10.3389/fnbeh.2013.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele JD, Christmas D, Eljamel MS, Matthews K. Anterior cingulotomy for major depression: clinical outcome and relationship to lesion characteristics. Biological Psychiatry. 2008;63:670–677. doi: 10.1016/j.biopsych.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Steele JD, Currie J, Lawrie SM, Reid I. Prefrontal cortical functional abnormality in major depressive disorder: a stereotactic meta-analysis. Journal of affective disorders. 2007;101:1–11. doi: 10.1016/j.jad.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Stoy M, Schlagenhauf F, Sterzer P, Bermpohl F, Hägele C, Suchotzki K, Strohle A. Hyporeactivity of ventral striatum towards incentive stimuli in unmedicated depressed patients normalizes after treatment with escitalopram. Journal of Psychopharmacology. 2012;26:677–688. doi: 10.1177/0269881111416686. [DOI] [PubMed] [Google Scholar]
- Stuhrmann A, Suslow T, Dannlowski U. Facial emotion processing in major depression: a systematic review of neuroimaging findings. Biology of Mood & Anxiety Disorders. 2011;1:10. doi: 10.1186/2045-5380-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Botteron KN, Luby JL, Belden AC, Gaffrey MS, Babb CM, Barch DD. Structural-functional correlations between hippocampal volume and cortico-limbic emotional responses in depressed children. Cognitive, Affective & Behavioral Neuroscience. 2013;13:135–151. doi: 10.3758/s13415-012-0121-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahmasian M, Knight DC, Manoliu A, Schwerthöffer D, Scherr M, Meng C, Sorg C. Aberrant intrinsic connectivity of hippocampus and amygdala overlap in the fronto-insular and dorsomedial-prefrontal cortex in major depressive disorder. Frontiers in Human Neuroscience. 2013;7:639. doi: 10.3389/fnhum.2013.00639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Yücel M, Lorenzetti V, Tanino R, Whittle S, Suzuki M, Allen NB. Volumetric MRI study of the insular cortex in individuals with current and past major depression. Journal of Affective Disorders. 2010;121:231–238. doi: 10.1016/j.jad.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Tao R, Calley CS, Hart J, Mayes TL, Nakonezny PA, Lu H, Emslie GJ. Brain activity in adolescent major depressive disorder before and after fluoxetine treatment. The American Journal of Psychiatry. 2012;169:381–388. doi: 10.1176/appi.ajp.2011.11040615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tham MW, Woon PS, Sum MY, Lee T-S, Sim K. White matter abnormalities in major depression: evidence from post-mortem, neuroimaging and genetic studies. Journal of affective disorders. 2011;132:26–36. doi: 10.1016/j.jad.2010.09.013. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Giedd JN, Woods RP, MacDonald D, Evans AC, Toga AW. Growth patterns in the developing brain detected by using continuum mechanical tensor maps. Nature. 2000;404:190–193. doi: 10.1038/35004593. [DOI] [PubMed] [Google Scholar]
- US Burden of Disease Collaborators The state of US health, 1990-2010: burden of diseases, injuries, and risk factors. Journal of the American Medical Association. 2013;310:591–608. doi: 10.1001/jama.2013.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Tol M-J, Li M, Metzger CD, Hailla N, Horn DI, Li W, Walter M. Local cortical thinning links to resting-state disconnectivity in major depressive disorder. Psychological Medicine. 2013:1–13. doi: 10.1017/S0033291713002742. [DOI] [PubMed] [Google Scholar]
- Van Eijndhoven P, van Wingen G, Katzenbauer M, Groen W, Tepest R, Fernández G, Tendolkar I. Paralimbic cortical thickness in first-episode depression: evidence for trait-related differences in mood regulation. The American Journal of Psychiatry. 2013;170:1477–1486. doi: 10.1176/appi.ajp.2013.12121504. [DOI] [PubMed] [Google Scholar]
- Van Wingen GA, van Eijndhoven P, Cremers HR, Tendolkar I, Verkes RJ, Buitelaar JK, Fernández G. Neural state and trait bases of mood-incongruent memory formation and retrieval in first-episode major depression. Journal of Psychiatric Research. 2010;44:527–534. doi: 10.1016/j.jpsychires.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Vasic N, Walter H, Höse A, Wolf RC. Gray matter reduction associated with psychopathology and cognitive dysfunction in unipolar depression: a voxel-based morphometry study. Journal of affective disorders. 2008;109:107–116. doi: 10.1016/j.jad.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Victor TA, Furey ML, Fromm SJ, Ohman A, Drevets WC. Relationship between amygdala responses to masked faces and mood state and treatment in major depressive disorder. Archives of General Psychiatry. 2010;67:1128–1138. doi: 10.1001/archgenpsychiatry.2010.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor TA, Furey ML, Fromm SJ, Öhman A, Drevets WC. Changes in the neural correlates of implicit emotional face processing during antidepressant treatment in major depressive disorder. The International Journal of Neuropsychopharmacology. 2013;16:2195–2208. doi: 10.1017/S146114571300062X. [DOI] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. The American Journal of Psychiatry. 2004;161:1957–1966. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- Villanueva R. Neurobiology of major depressive disorder. Neural Plasticity. 2013;2013:873278. doi: 10.1155/2013/873278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrieze E, Demyttenaere K, Bruffaerts R, Hermans D, Pizzagalli DA, Sienaert P, Claes S. Dimensions in major depressive disorder and their relevance for treatment outcome. Journal of Affective Disorders. 2014;155:35–41. doi: 10.1016/j.jad.2013.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vythilingam M, Vermetten E, Anderson GM, Luckenbaugh D, Anderson ER, Snow J, Bremner JD. Hippocampal volume, memory, and cortisol status in major depressive disorder: effects of treatment. Biological Psychiatry. 2004;56:101–112. doi: 10.1016/j.biopsych.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Wagner G, Koch K, Schachtzabel C, Peikert G, Schultz CC, Reichenbach JR, Schlosser RG. Self-referential processing influences functional activation during cognitive control: an fMRI study. Social Cognitive and Affective Neuroscience. 2013;8:828–837. doi: 10.1093/scan/nss074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner G, Koch K, Schachtzabel C, Sobanski T, Reichenbach JR, Sauer H, Schlösser RGM. Differential effects of serotonergic and noradrenergic antidepressants on brain activity during a cognitive control task and neurofunctional prediction of treatment outcome in patients with depression. Journal of Psychiatry & Neuroscience. 2010;35:247–257. doi: 10.1503/jpn.090081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh ND, Williams SCR, Brammer MJ, Bullmore ET, Kim J, Suckling J, Fu CH. A longitudinal functional magnetic resonance imaging study of verbal working memory in depression after antidepressant therapy. Biological Psychiatry. 2007;62:1236–1243. doi: 10.1016/j.biopsych.2006.12.022. [DOI] [PubMed] [Google Scholar]
- Wang L, Li K, Zhang QE, Zeng YW, Jin Z, Dai WJ, Si TM. Interhemispheric functional connectivity and its relationships with clinical characteristics in major depressive disorder: a resting state fMRI study. PloS One. 2013a;8:e60191. doi: 10.1371/journal.pone.0060191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Li K, Zhang Q, Zeng Y, Dai W, Su Y, Si T. Short-term effects of escitalopram on regional brain function in first-episode drug-naive patients with major depressive disorder assessed by resting-state functional magnetic resonance imaging. Psychological Medicine. 2013b;44:1417–1426. doi: 10.1017/S0033291713002031. [DOI] [PubMed] [Google Scholar]
- Wang T, Huang X, Huang P, Li D, Lv F, Zhang Y, Xie P. Early-stage psychotherapy produces elevated frontal white matter integrity in adult major depressive disorder. PloS One. 2013;8:e63081. doi: 10.1371/journal.pone.0063081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson LA, Berntsen D, Kuyken W, Watkins ER. Involuntary and voluntary autobiographical memory specificity as a function of depression. Journal of Behavior Therapy and Experimental Psychiatry. 2013;44:7–13. doi: 10.1016/j.jbtep.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Zeng LL, Shen H, Liu L, Hu D. Unsupervised classification of major depression using functional connectivity MRI. Human Brain Mapping. 2013;35:1630–1641. doi: 10.1002/hbm.22278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WN, Chang SH, Guo LY, Zhang KL, Wang J. The neural correlates of reward-related processing in major depressive disorder: a meta-analysis of functional magnetic resonance imaging studies. Journal of Affective Disorders. 2013;151:531–539. doi: 10.1016/j.jad.2013.06.039. [DOI] [PubMed] [Google Scholar]