Abstract
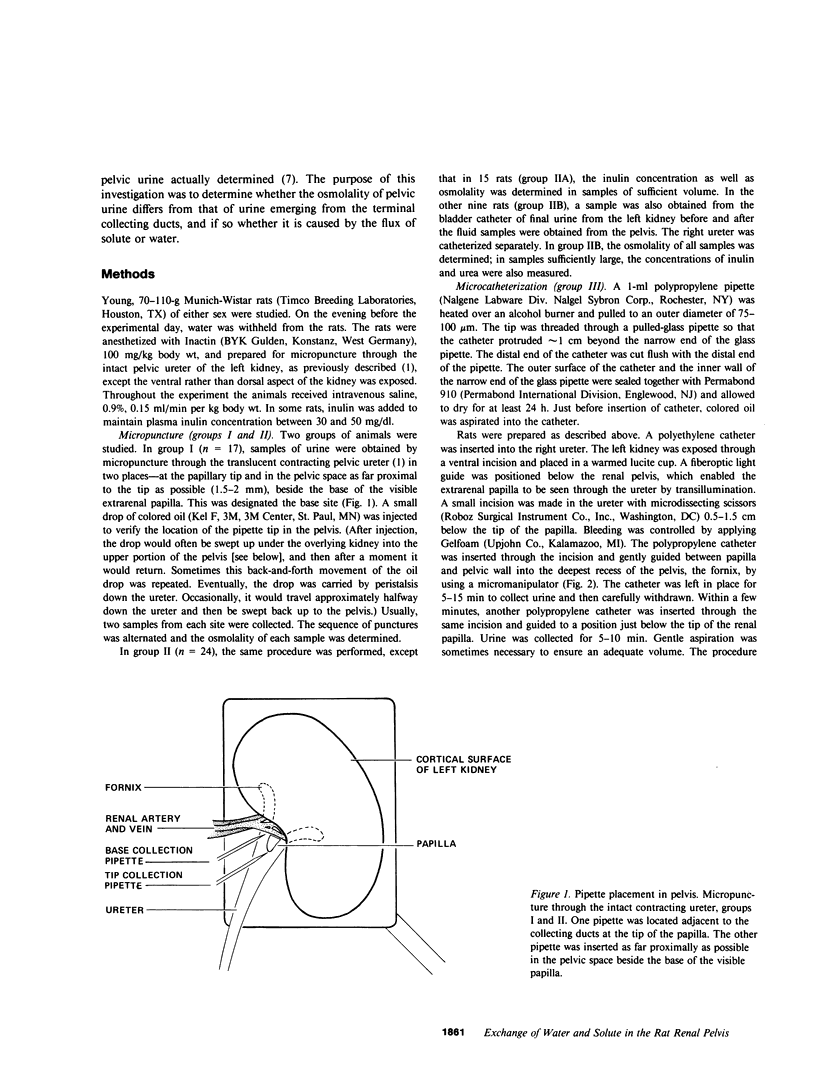
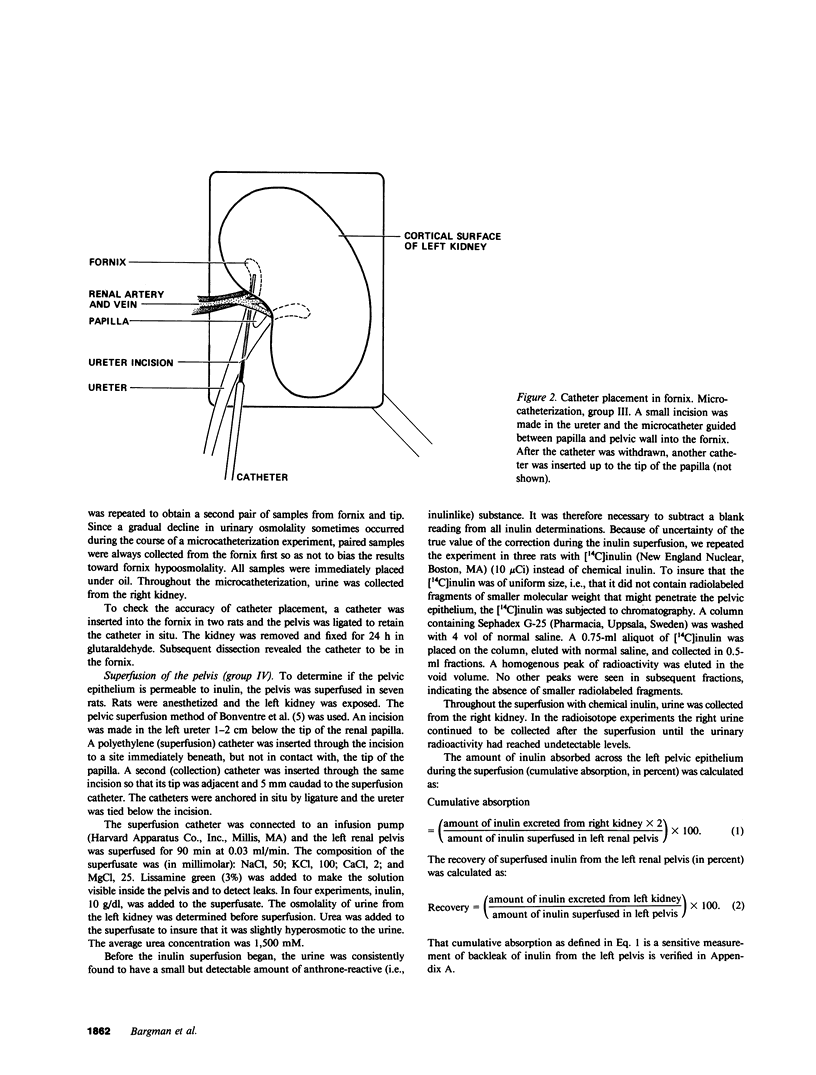
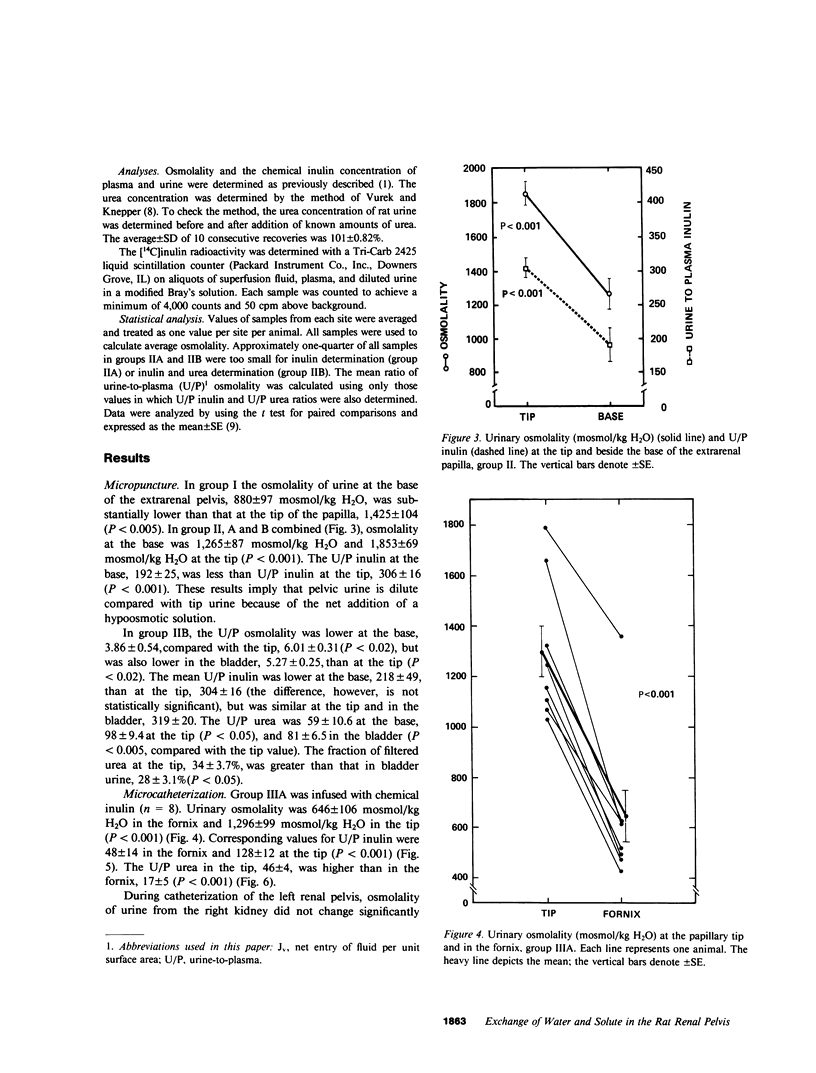
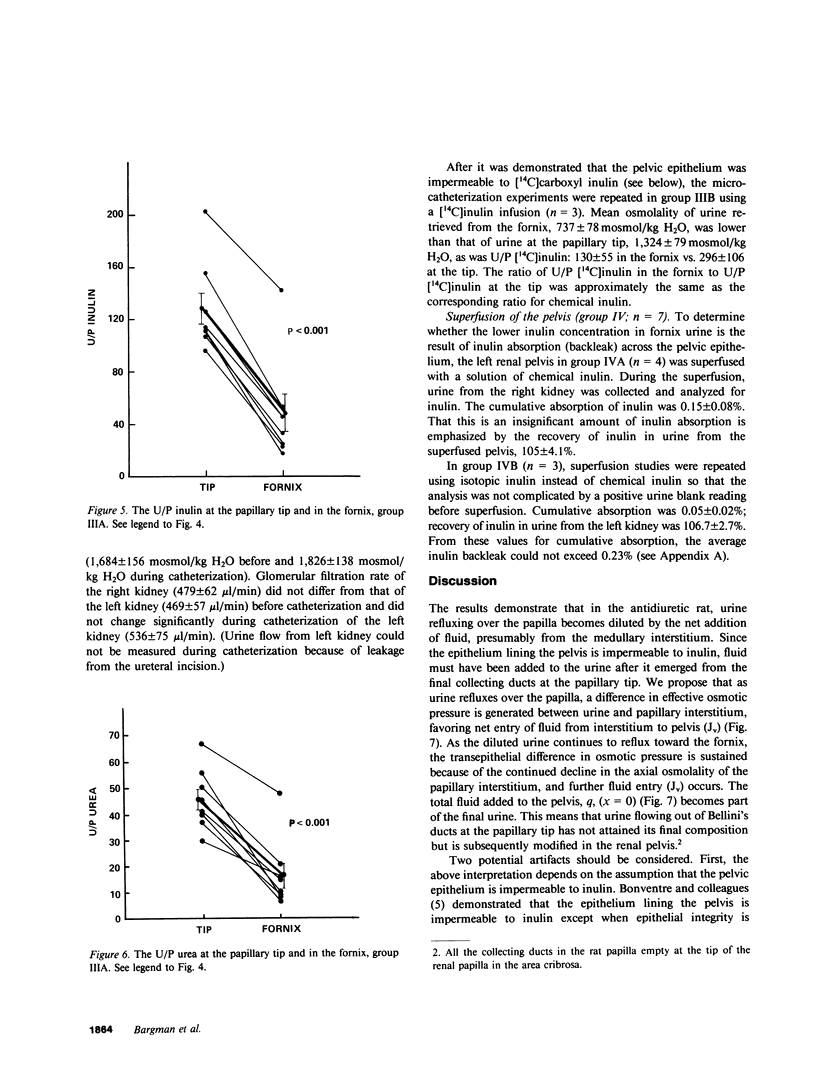
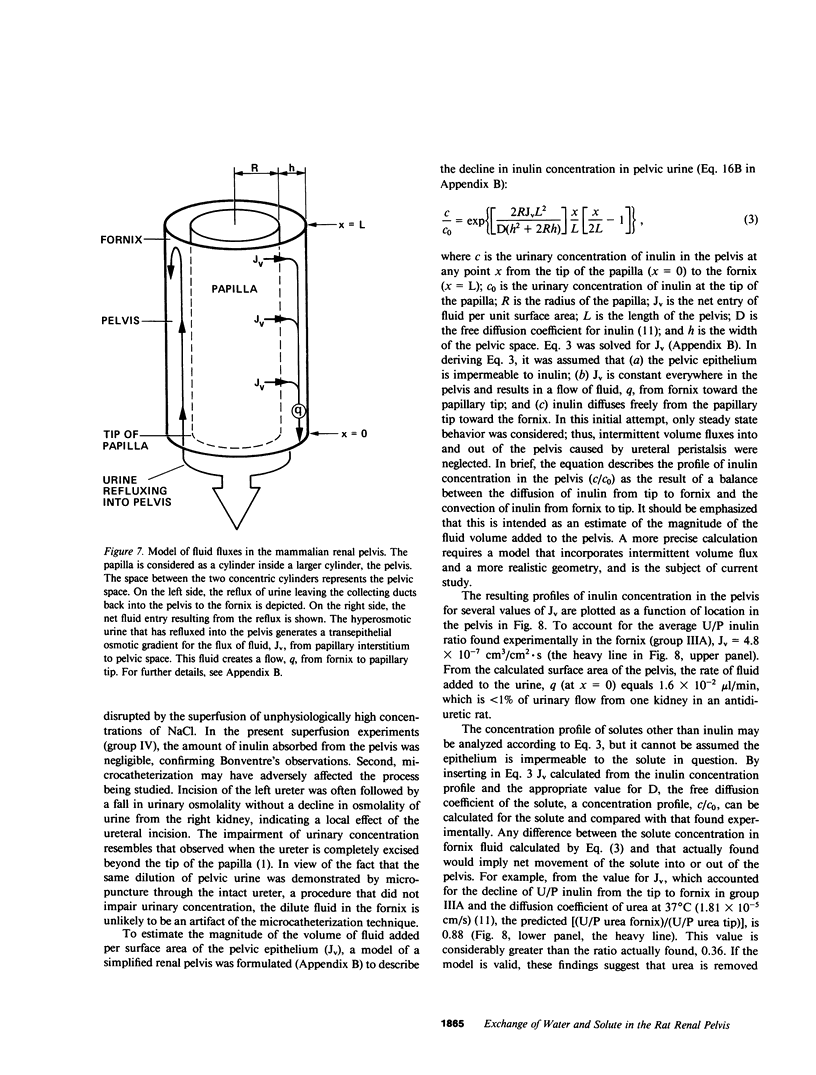
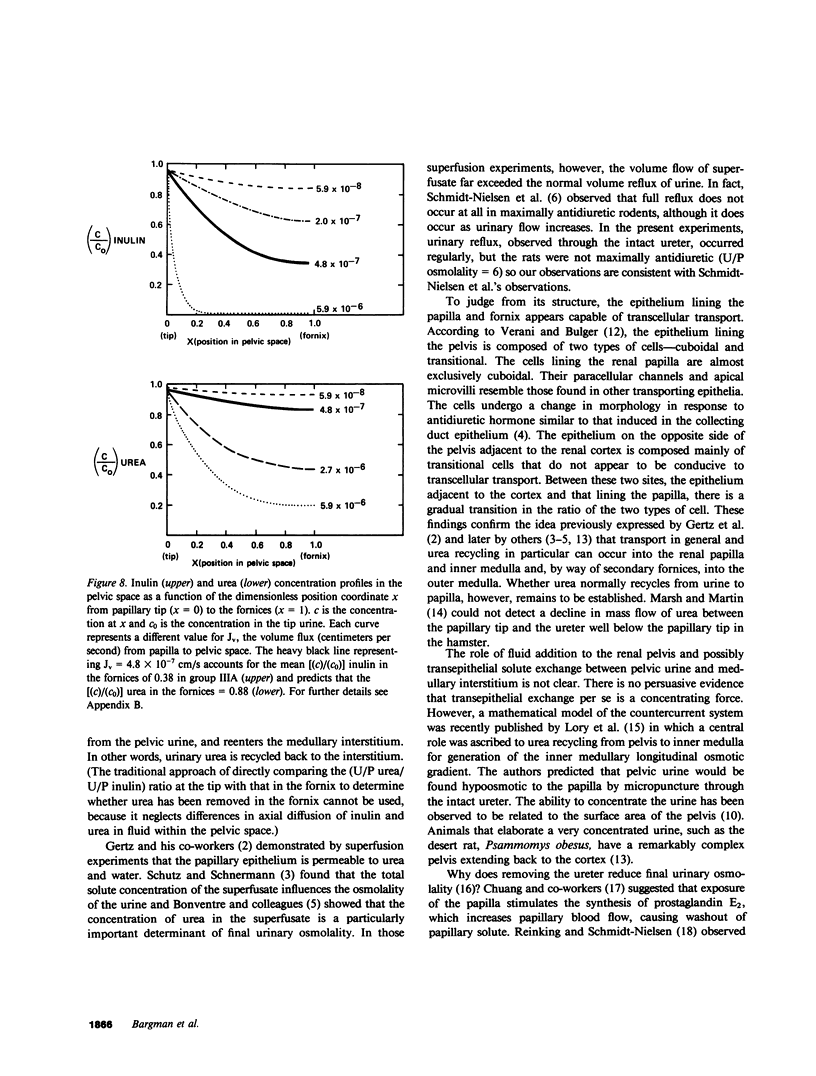
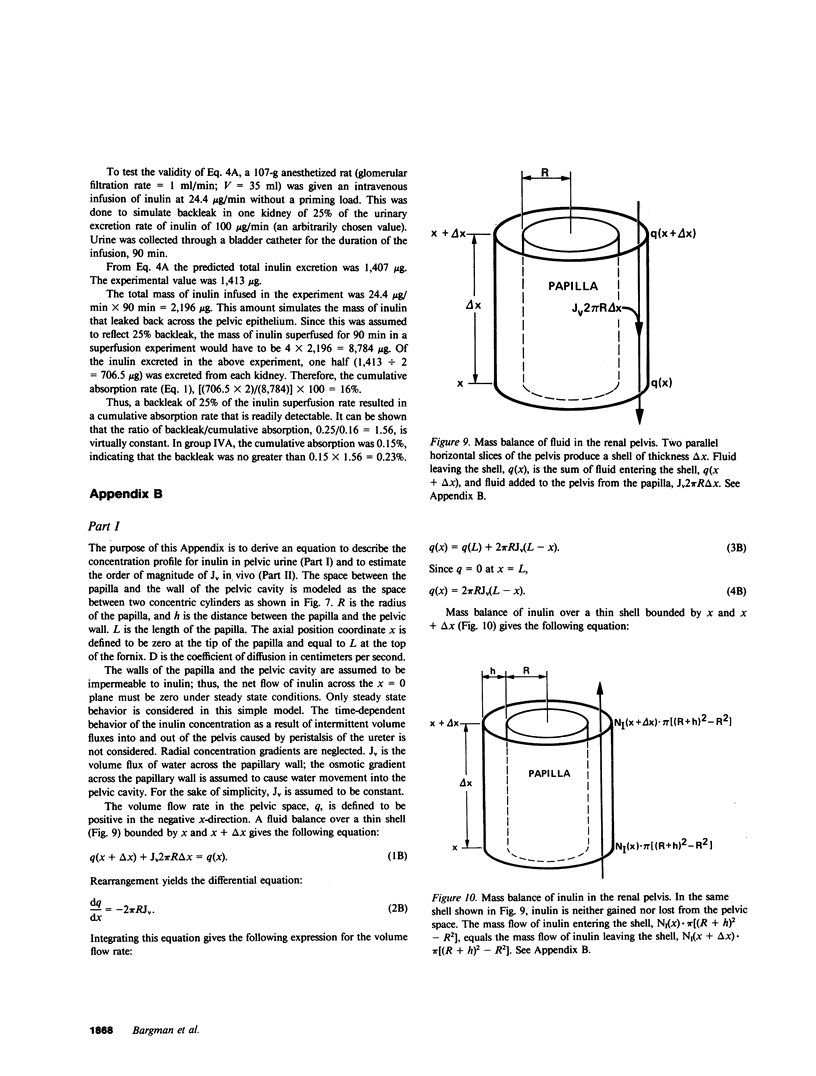
Severance of the ureter beyond the renal papilla causes a fall in urinary osmolality, which suggests that exchange of water or solute between urine and renal parenchyma normally occurs in the intact renal pelvis. We examined water and solute flux in the renal pelvis with micropuncture and microcatheterization techniques. Four groups of antidiuretic rats were studied. Group I (n = 17) underwent micropuncture through the intact contracting ureter. Urine samples were obtained at the papillary tip, and in the pelvis beside the base of the extrarenal papilla. Urinary osmolality at the base, 880 +/- 97 mosmol/kg H2O (mean +/- SE), was less than that at the tip, 1,425 +/- 104 mosmol/kg H2O (P less than 0.005). In group II (n = 24), samples were analyzed for inulin and osmolality. In 15 rats (group IIA), comparison was made between base and tip samples. In the other nine animals (group IIB), comparisons were made among base, tip, and bladder samples and urea was also measured. In group II (A and B combined) urine-to-plasma (U/P) osmolality was lower at the base, 4.31 +/- 0.27, than at the tip, 6.08 +/- 0.23 (P less than 0.001), and U/P inulin was lower at the base, 192 +/- 25, than at the tip, 306 +/- 16 (P less than 0.001). In group IIB, the bladder urine had a lower U/P osmolality, 5.27 +/- 0.25, than the tip, 6.01 +/- 0.31 (P less than 0.02). The U/P urea was 59 +/- 10.6 (base), 98 +/- 9.4 (tip) (base vs. tip, P less than 0.05), and 81 +/- 6.5 (bladder, P less than 0.005, compared with tip). In group III (n = 8), samples were obtained by microcatheter from the fornices, the deepest intrarenal extensions of the pelvis, and compared with samples at the tip. Urinary osmolality was lower in the fornix, 646 +/- 106 mosmol/kg H2O, than at the tip, 1,296 +/- 99 mosmol/kg H2O (P less than 0.001). Similarly, U/P inulin was lower in the fornix, 48 +/- 14, than at the tip, 128 +/- 12 (P less than 0.001). The lower U/P inulin in the pelvic urine is the result of either the addition of fluid to the pelvis, or the backleak of inulin across the epithelium lining the pelvis. To verify that the pelvic epithelium was impermeable to inulin, in group IVA (n = 4) the left renal pelvis was superfused with a solution of chemical inulin. Cumulative absorption of inulin from the left kidney was 0.15 +/- 0.08% of that superfused. Using [14C]inulin in group IVB (n= 3), similar results were obtained (0.05 +/- 0.02%). These findings indicate that in the renal pelvis, fluid is added to urine after it emerges from the collecting ducts. We suggest that reflux of hyperosmotic urine over the renal papilla creates a transepithelial gradient for the flux of water into the pelvis. A model that incorporates diffusive and convective forces for water and solute transport is proposed to account for these findings.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonventre J. V., Karnovsky M. J., Lechene C. P. Renal papillary epithelial morphology in antidiuresis and water diuresis. Am J Physiol. 1978 Jul;235(1):F69–F76. doi: 10.1152/ajprenal.1978.235.1.F69. [DOI] [PubMed] [Google Scholar]
- Bonventre J. V., Roman R. J., Lechene C. Effect of urea concentration of pelvic fluid on renal concentrating ability. Am J Physiol. 1980 Dec;239(6):F609–F618. doi: 10.1152/ajprenal.1980.239.6.F609. [DOI] [PubMed] [Google Scholar]
- Chuang E. L., Reineck H. J., Osgood R. W., Kunau R. T., Jr, Stein J. H. Studies on the mechanism of reduced urinary osmolality after exposure of renal papilla. J Clin Invest. 1978 Mar;61(3):633–639. doi: 10.1172/JCI108974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamison R. L. Micropuncture study of superficial and juxtamedullary nephrons in the rat. Am J Physiol. 1970 Jan;218(1):46–55. doi: 10.1152/ajplegacy.1970.218.1.46. [DOI] [PubMed] [Google Scholar]
- Kaissling B., de Rouffignac C., Barrett J. M., Kriz W. The structural organization of the kidney of the desert rodent Psammomys obesus. Anat Embryol (Berl) 1975 Dec 23;148(2):121–143. doi: 10.1007/BF00315265. [DOI] [PubMed] [Google Scholar]
- Lacy E. R. The mammalian renal pelvis: physiological implications from morphometric analyses. Anat Embryol (Berl) 1980;160(2):131–144. doi: 10.1007/BF00301856. [DOI] [PubMed] [Google Scholar]
- Lory P., Gilg A., Horster M. Renal countercurrent system: role of collecting duct convergence and pelvic urea predicted from a mathematical model. J Math Biol. 1983;16(3):281–304. doi: 10.1007/BF00276508. [DOI] [PubMed] [Google Scholar]
- Oliver R. E., Roy D. R., Jamison R. L. Urinary concentration in the papillary collecting duct of the rat. Role of the ureter. J Clin Invest. 1982 Jan;69(1):157–164. doi: 10.1172/JCI110426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinking L. N., Schmidt-Nielsen B. Peristaltic flow of urine in the renal capillary collecting ducts of hamsters. Kidney Int. 1981 Jul;20(1):55–60. doi: 10.1038/ki.1981.104. [DOI] [PubMed] [Google Scholar]
- Schmidt-Nielsen B., Churchill M., Reinking L. N. Occurrence of renal pelvic refluxes during rising urine flow rate in rats and hamsters. Kidney Int. 1980 Oct;18(4):419–431. doi: 10.1038/ki.1980.155. [DOI] [PubMed] [Google Scholar]
- Schmidt-Nielsen B., Reinking L. N. Morphometry and fluid reabsorption during peristaltic flow in hamster renal papillary collecting ducts. Kidney Int. 1981 Dec;20(6):789–798. doi: 10.1038/ki.1981.212. [DOI] [PubMed] [Google Scholar]
- Schütz W., Schnermann J. Pelvic urine composition as a determinant of inner medullary solute concentration and urine osmolarity. Pflugers Arch. 1972;334(2):154–166. doi: 10.1007/BF00586788. [DOI] [PubMed] [Google Scholar]
- Verani R., Bulger R. E. The pelvic epithelium of the rat kidney: a scanning and transmission electron microscopic study. Am J Anat. 1982 Mar;163(3):223–233. doi: 10.1002/aja.1001630303. [DOI] [PubMed] [Google Scholar]
- Vurek G. G., Knepper M. A. A colorimeter for measurement of picomole quantities of urea. Kidney Int. 1982 Apr;21(4):656–658. doi: 10.1038/ki.1982.74. [DOI] [PubMed] [Google Scholar]



