Abstract
Objective
To determine whether a 3-month contraceptive vaginal ring (CVR) delivering ulipristal acetate (UPA) can inhibit ovulation in 90% of cycles.
Study Design
This was a randomized dose-finding parallel group clinical trial. Fifty-five healthy women with normal ovulation at baseline were randomized to receive a low-dose (1500μg/day) or a high-dose (2500μg/d) UPA-CVR for two consecutive 12-week treatment periods, followed by a recovery cycle. A subgroup of women received levonorgestrel (LNG) 1.5 mg orally twice (at the end of both 12-week ring periods) or once (at the end of the 24-week treatment). The primary outcome was ovulation suppression assessed by transvaginal ultrasound and hormone levels. Secondary outcomes included endometrial safety and bleeding patterns.
Results
All subjects showed normal ovulation at baseline and recovery. Ovulation suppression was seen in 81.8% (95% CI: 73.3%, 88.5%) and 86.1% (95% CI: 78.1%, 92%) of treatment cycles with low and high-dose, respectively. Benign progesterone receptor modulator associated endometrial changes (PAEC) were seen during treatment; 78.8% at week 24, but resolved at recovery cycle. A few cases of heavy bleeding occurred near the end of the 24-week treatment, but a single dose of LNG every 12weeks reduced the increase in endometrial thickness during the second treatment period and prevented excessive bleeding.
Conclusion
The 3-month UPA-CVR may become an effective long-acting, user-controlled estrogen-free contraceptive. The greatest suppression of ovulation was seen with the 2500 μg/d ring.
Keywords: Ulipristal acetate, contraception, pharmacodynamics, endometrium, vaginal ring
INTRODUCTION
High rates of unintended pregnancy resulting from non-use of contraception or failure of existing methods indicate the need for novel methods with better safety and acceptability [1]. An estrogen-free, long-acting, user-controlled, vaginal ring with a simplified continuous regimen, may improve compliance and contraceptive safety and efficacy.
The progesterone receptor modulators (PRMs) exert contraceptive action through a variety of mechanisms including inhibition of ovulation. Prevention of follicular rupture occurs by blockade of gonadotropin-surge mediated events which are progesterone receptor (PR)- dependent [2]. Ulipristal acetate (UPA), also known as CDB/VA-2914, is a PRM initially developed by the National Institutes of Child Health and Human Development (NICHD) and then by HRA pharma [3,4]. UPA was developed for gynecological applications, and is currently approved as an oral emergency contraceptive in Europe (EU) and in the USA, for use up to 5 days after unprotected intercourse [5], and for treatment of heavy menstrual bleeding due to uterine fibroids (EU) [6]. Oral doses of 5 or 10mg/day, suppress ovulation in about 80% of subjects and induce amenorrhea in 81% and 90% of subjects, respectively [7]. UPA metabolism is predominantly mediated by cytochrome P450 isoenzymes (CYP450) 3A4 with the monodemethylated derivative (CDB-3877) being the main metabolite. This derivative is pharmacologically active but less than its parent compound [4].
A 3-month UPA contraceptive vaginal ring (UPA-CVR) designed for continuous use is under development for contraception by The Population Council (PC). In earlier studies, rings releasing 800μg/day UPA, suppressed ovulation in 68% of cycles, when UPA levels reached >7 ng/mL [8,9]. End of treatment endometrial biopsies (EB) showed benign glandular changes, described as PRM-associated endometrial changes (PAECs) [10], in a background of inactive or weakly proliferative endometrium. Results from these preliminary studies demonstrate tolerability of UPA-CVR and indicate UPA levels at ≥7ng/ml, as threshold for efficacy.
We report here pharmacodynamic results of a dose-finding study testing two new UPA-CVRs releasing 1500 or 2500μg/day. We hypothesized that by increasing the delivery dose of UPA, subjects using the new rings would achieve higher serum levels of UPA than seen with the prior UPA-CVR [9]. Therefore the main objective was to determine the dose capable of suppressing ovulation in 90% of cycles.
MATERIALS and METHODS
Study design
This randomized, parallel-group dose-finding and safety study was conducted at three clinics in Chile (Clinic 2), Dominican Republic [(DR) Clinic 3], and Portland [(OR) Clinic 17], in accordance with ICH Guidelines and the Declaration of Helsinki. Approval was granted by each clinic’s Ethics Committee and PC’s Institutional Review Board.
Eligible subjects were 21–40 years old, with regular menstrual cycles, a body mass index (BMI) ≤ 30kg/m2 and not at risk for pregnancy. Exclusion criteria included breastfeeding, contraindications to hormonal contraceptives, and current use of hormones or intrauterine device. A baseline ovulatory cycle was required.
Eligible subjects were randomized to the first treatment period on day 2–5 of the next cycle to a low-dose (1500μg/d) or a high-dose (2500μg/d) vaginal ring to determine the lowest-effective dose to block ovulation in 90% of cycles. Staff members in clinics 2 and 3, or pharmacists in clinic 17 dispensed either dose of vaginal rings according to a computer generated randomization list (See description in supplemental material section).
The UPA-CVRs made of micronized UPA mixed in a silicone elastomer matrix designed to achieve a first-order release profile of the steroid over 3 months [11]. Each ring was used continuously for 12 consecutive weeks. After 12 weeks (Treatment Period-1), the ring was changed for a new ring of same dose used for 12 additional weeks (Treatment Period-2). A post-treatment recovery cycle followed.
Transvaginal ultrasound (TVUS) and serum hormone levels were used to evaluate ovarian activity. For safety evaluation of the endometrial effect of treatment, an EB was obtained during the luteal phase of the baseline cycle, at the end of each 12-week treatment period, and during the luteal phase of the recovery cycle. Subjects recorded adverse events, bleeding and spotting, in a daily diary. Bleeding categories were: none, spotting (light bleeding, not requiring protection), normal bleeding (as normal menses), and heavy bleeding (excessive).
Measurements
Serum Estradiol (E2) and progesterone (P) were measured twice weekly, while UPA was measured once a week. Clotted whole blood was centrifuged; serum specimens were separated and stored at −80°C until analysis. E2 and P were assayed at each clinic’s laboratory. Serum aliquots were sent to PC’s labs for UPA analysis by radioimmunoassay (RIA) method [12]; samples from Chile were also measured by liquid chromatography–tandem mass spectrometry (LCMS/MS) (see supplemental material). While RIA measures both UPA and metabolites together, LCMS/MS measures separately the parent molecule and one major metabolite [7].
Follicular development and endometrial changes were assessed by TVUS done twice weekly during the baseline cycle, all 12 weeks of treatment period-1, weeks 6–12 only in treatment period-2, and during the recovery cycle (see supplemental material). At each visit, the leading follicle in each ovary was measured in the plane that showed the follicle to be the largest. The two largest perpendicular measurements in one plane were taken and used to calculate the mean diameter. Follicle rupture was defined by at least 50% decrease in maximum diameter or the presence of internal echoes suggesting change to a corpus luteum. Endometrial thickness (ET) was measured in an anterior-posterior plane.
Classification of Ovarian Activity
As rings were used continuously, we defined “treatment cycle” as each 4-week treatment sequence.
Ovarian function was evaluated during six time periods: 1) control cycle; 2) weeks 1–4 treatment period-1; 3) weeks 5–8 treatment period-1; 4) weeks 9–12 treatment period-1; 5) weeks 6–12 treatment period-2; 6) post-treatment cycles. Treatment cycles were classified according to presence or absence of luteal activity, defined as P≥10 nmol/L (~3ng/mL) in at least two consecutive samples. Ultrasound and endocrine profiles in each “treatment cycle” allowed six classifications as previously described [9]: ovulation; ovulatory dysfunction; luteinized un-ruptured follicle (LUF); persistent follicle; no follicular development; or no follicular resolution (see Table-1S in Supplemental material).
Endometrial Evaluation
EB samples were obtained using either a Pipelle (Cooper Surgical, Trumbull, CT) (DR, Chile) or an Explora (Cooper Surgical, Trumbull, CT) (Oregon) device. A portion of each sample was placed for use in 10% neutral buffered formaldehyde for histology and immunohistochemistry studies. The remaining fresh tissue, flash-frozen in liquid nitrogen, was stored at −80°C. Endometrial evaluation included conventional histological examination of hematoxylineosin stained sections. Immunohistochemical assessment included proliferation markers Ki67, PhosphoH3, and bcl2. A semiquantitative method was used to assess immunohistochemical staining [9].
Protocol amendment
During the study, three initial cases of heavy or prolonged bleeding were reported; two in the last month of ring use, while another shortly after discontinuing ring use. These bleeding episodes led to a protocol amendment that included treatment with levonorgestrel (LNG) 1.5 mg single dose at the end of each 12-week period at ring change. We hypothesized that since LNG is a potent androgenic progestin, a single dose would induce decidualization, followed by a withdrawal bleeding, thus reducing the risk of significant endometrial thickening and subsequent heavy bleeding. When the amendment was approved by the IRB’s, 27 participants had already completed both rings’ use and therefore did not receive LNG; eleven participants were in the last three months of the study and received a single 1.5 mg LNG dose after 24 weeks of use on the day of ring removal; 4 participants were in the first month of use and 12 had not started ring use yet, and therefore received both LNG 1.5 mg doses.
Statistics
Each participant was scheduled to contribute four treatment cycles for analysis. Under the assumptions that the ring would prove 90% successful in inhibiting ovulation and 25 women in each dose would complete the study, 100 cycles would be analyzed in each dose. Under this hypothesis, the probability of observing between 83–97 non-ovulatory cycles at each dose would be 96%. To account for drop-outs, planned enrollment was 60 women (30 per dose).
Data were summarized based on intention-to-treat population. Subjects’ baseline characteristics were presented as means(±SD), compared with two samples t-tests between doses, and with analysis of variance (ANOVA) between centers. The primary outcome, ovulation suppression rate was presented as percentage and 95% confidence interval (CI) was calculated for each dose. Fisher’s Exact Tests and Chi-Square tests were used to compare ovulation suppression rate between doses at each center and in total. Continuous longitudinal data were presented as means(±SD). Linear mixed models with Bonferroni adjustment were used to compare the change of mean levels of UPA, estradiol, progesterone, ET over time and between different ovarian activity classifications. The association between means of UPA measured by LCMS/MS and RIA was evaluated by linear regression model. All analyses were performed using SAS 9.1 (Cary, NC, USA). Statistical significance was taken as two-sided p<0.05.
RESULTS
A total of 55 women were randomized to the treatment with 28 and 27 subjects randomized to low- and high-dose rings, respectively. Fifty-three subjects completed the study; (one discontinued after treatment period-2 due to moving and one was lost to follow-up during post-treatment recovery). Therefore the analysis included 110 and 108 cycles, respectively (see diagram in supplemental section, Table-2S). Baseline characteristics were not statistically different between doses (Table 1). The Oregon subjects were significantly younger than subjects from Chile, and taller and heavier than those at the other sites, although the BMI was not significantly different (Range 18.3–30).
Table 1.
Baseline characteristics of subjects by doses and sites (Mean±SD)
| Variable | Age (years) | Height (cm) | Weight (kg) | BMI(kg/m2) | Parity |
|---|---|---|---|---|---|
| Doses | |||||
| Low dose (n=28) | 33.5±3.6 | 157.8±6.1 | 61.0±9.5 | 24.4±2.9 | 2.82±1.79 |
| High dose (n=27) | 33.4±4.8 | 157.4±4.5 | 59.9±8.4 | 24.3±3.1 | 3.00±1.27 |
| P-values* | NS | NS | NS | NS | NS |
| Study sites | |||||
| Chile (n=20) | 35.5±3.5 | 155.6±4.6 | 57.1±8.5 | 23.6±3.2 | 3.3±0.7 |
| DR (n=20) | 32.7±4.3 | 156.7±5.0 | 60.1±7.1 | 24.5±2.9 | 3.5±1.7 |
| Oregon (n=15) | 31.8±4.1 | 161.3±5.0 | 65.4±9.8 | 25.3±2.8 | 1.7±1.4 |
| P-values** | 0.0316 | 0.0049 | 0.0219 | NS | 0.0005 |
t-test.
One-way ANOVA: post hoc p values, Age: Chile vs. Oregon, p=0.0248; Height: Oregon vs. Chile and DR, each p<0.05; Weight: Chile vs. Oregon, p=0.0159; Parity: Chile vs. Oregon, p=0.0036; DR vs. Oregon, p=0.0007 DR, Dominican Republic; ANOVA, analysis of variance; NS, not significant.
Ovarian response
Figure 1 shows percentages of ovulation.
Figure 1. Rate of ovulation (number of ovulatory cycles/total number of cycles) during the study.
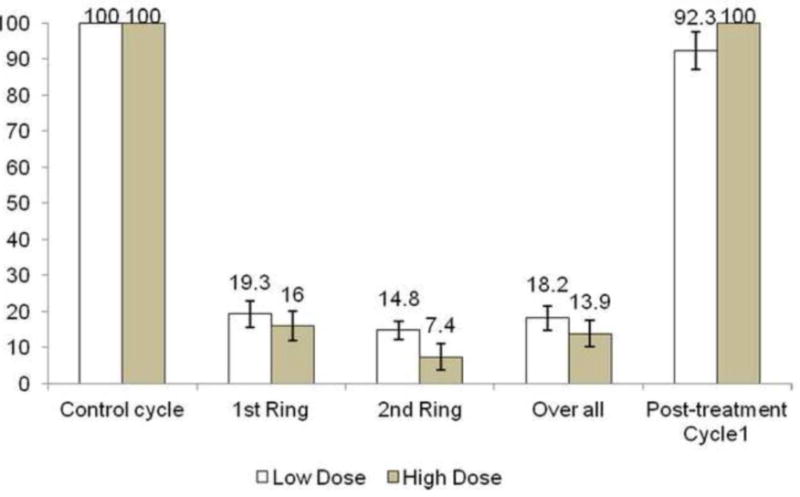
Control cycle was the baseline cycle. 1st and 2nd ring correspond to cycles assessed during Treatment Periods 1 and 2. The overall rate combines all cycles during both treatment periods. The post treatment cycle reflects the first 4 weeks after discontinuation of the ring.
Overall (Table 2), 81.8% (95% CI: 73.3%, 88.5%) of treatment cycles were classified as ovulation suppression in low-dose and 86.1% (95% CI: 78.1%, 92%) in high-dose group with no significant differences between sites.
Table 2.
Ovarian activity classification by treatment groups [n(%)]
| Ovarian activity classification | Low dose (n=110 cycles) |
High dose (n=108 cycles) |
Total (n=218 cycles) |
|---|---|---|---|
| Ovulation | 20 (18.2) | 15 (13.9) | 35 (16.1) |
| Ovulatory dysfunction | 7 (6.4) | 7 (6.5) | 14 (6.4) |
| LUF | 6 (5.5) | 14 (12.3) | 20 (9.2) |
| Persistent follicle | 35 (31.8) | 37 (34.3) | 72 (33.0) |
| No follicular resolution | 19 (17.3) | 27 (25.0) | 46 (21.1) |
| No follicular development | 23 (20.9) | 8 (7.4) | 31 (14.2) |
Chi-Square tests: No statistically significant difference between doses regarding the rate of ovulation (p>0.05) and the distribution of ovarian activity classification (p>0.05).
The highest efficacy was seen in Oregon [0% ovulation in the second 12-week high-dose, treatment period], however all women in this site also received 2 doses of LNG. Since there was a 6-week sample break following the first LNG intake, any effect of LNG over ovarian activity could not be assessed.
Ovarian classifications and serum levels of E2 and P during UPA-CVR use are represented in Table 3. Ovarian activity classification showed no significant difference between doses, and the most common categories observed during UPA ring use were: persistent follicle (33.0%) followed by ovulation (16.1%), no follicular development (14.2%), LUF (9.2%), and ovulatory dysfunction (6.4%). Persistent follicles were associated with prolonged elevation of estradiol, which lasted 2–3 weeks. The mean level of E2 during all cycles in which a persistent follicle was diagnosed was 446.8 ±301 pmol/L, similar to levels seen during ovulatory cycles (449.0 ±187.6 pmol/L) and much greater than in cycles with no follicular development (162.6 ±66.9 pmol/L) (Table 3).
Table 3.
Ovarian activity classification and mean serum level of UPA, Estradiol, Progesterone, and endometrial thickness (Mean±SD)
| Ovarian activity classification | UPA (RIA) (ng/ml) | UPA (LCMS) (ng/ml) | Estradiol (pmol/L) | Progesterone (nmol/L) | Endometrial thickness (mm) |
|---|---|---|---|---|---|
| Ovulation | 4.85±1.85 | 3.21±1.15 | 449.07±187.63† | 14.81±9.32 | 6.98±1.45 |
| Ovulatory dysfunction | 5.03±2.48 | 4.72±1.41 | 376.33±179.40† | 8.77±8.11# | 8.98±4.06 |
| LUF | 7.19±2.86 | 5.31±1.59 | 415.28±213.70† | 8.03±4.27# | 8.07±1.70 |
| Persistent follicle | 7.38±2.38* | 4.78±1.93 | 446.75±300.96†‡ | 1.34±0.56#$& | 11.64±5.80ˆ |
| No follicular resolution | 7.62±2.96 | 5.18±1.92 | 307.59±178.53† | 3.25±4.20#$& | 9.16±4.41 |
| No follicular development | 7.88±2.18 | 5.74±1.92 | 162.64±66.85 | 1.62±0.65#$& | 8.23±3.59 |
The total cycles were 218 while UPA was tested by RIA in 138 cycles and LCMS in 80 cycles.
Linear mixed models with Bonferroni multiple comparisons were employed within each column.
UPA column:
When compared with “Ovulation”, adjusted P <0.05
Estradiol column:
When compared with “No follicular development”, adjusted P <0.05;
when Compared with “No follicular resolution”, adjusted P <0.05
Progesterone column:
When compared with “Ovulation”, adjusted P <0.05;
When compared with “Ovulatory dysfunction”, adjusted P <0.05;
When compared with “LUF (luteinized unruptured follicle)”, adjusted P <0.05.
Endometrial thickness column:
When compared with “Ovulation” and “No follicular development”, adjusted P <0.05.
In 21.1% of the sampling periods, no follicular resolution was observed within the designated 4-week observation cycle; that is, a follicle began to develop, but the resolution (whether rupture, persistent follicle, or LUF) occurred in the following 4-week sequence.
A return to normal ovulation following ring removal was observed during the post-treatment recovery cycle in all subjects except for 2 in the low-dose group. One experienced a LUF in the first post-treatment cycle followed by normal ovulation in the second post-treatment cycle; the other one experienced LUF in four cycles and ovulated during the 5th post-treatment cycle.
Ovarian suppression was directly related to UPA serum levels. The highest levels were observed in cycles with complete ovarian suppression (no follicular development, RIA: 7.88±2.18 ng/mL), while the lowest level was observed in ovulatory cycles (RIA: 4.85±1.85ng/mL) (Table 3). A threshold of 7ng/mL (RIA) seems required for consistent ovulation suppression.
Figure 2 shows the mean levels of UPA during the study. Serum levels of UPA tested by LCMS/MS were significantly lower than those tested by RIA, measuring UPA and metabolites. However, UPA mean levels measured by these two methods were strongly associated (UPA tested by RIA = 0.9445 + 0.9201UPA tested by LCMS/MS, R2 = 0.8209). Among users of the high-dose ring, the UPA serum levels (based on RIA) were not significantly higher in participants from Oregon than from site 3 (Oregon: 7.86±1.83 versus Site3: 6.61±2.70, p values=0.3068). The threshold of UPA level at 7ng/ml showed no association with BMI, ethnicity, race, education level, alcohol use or smoking. (p>0.05 for each variable)
Figure 2.
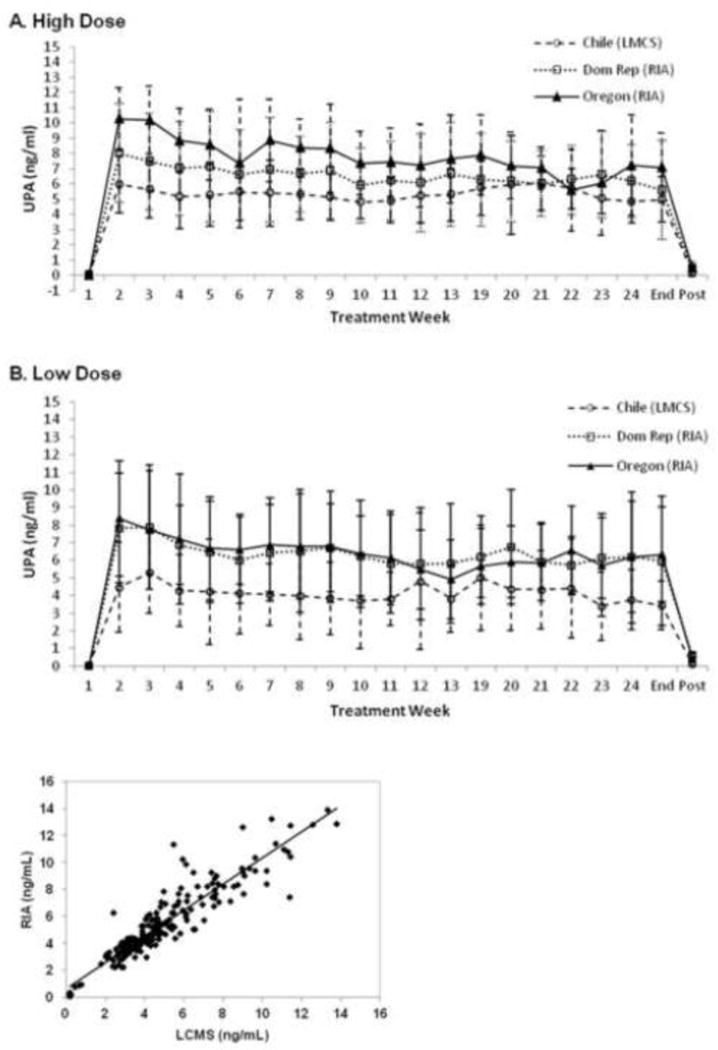
Mean (± SD) serum levels of UPA during treatment with a 2500 μg (high dose) (panel A) and 1500 μg (low dose) (panel B) vaginal ring. Correlation between serum levels of UPA measured with RIA and LCMS (panel C).
Endometrial evaluation
Pre-treatment luteal phase biopsies (n=55) all showed normal secretory endometrium. Since no differences were seen between the two doses, histological results were pooled (Table 4). Conventional histology demonstrated the transition of endometrium from normal physiological baseline secretory state (100%), to specific PAECs on treatment (64.2% at week-12 and 78.8% at week-24), followed by a return to baseline aspects after treatment withdrawal (100%) (Table 4). PAEC were present in 90.9% of biopsies in women without luteal activity in the 30 days preceding the EB, and only near 20% in those with luteal activity in the 30 days preceding the EB. Normal endometrium was seen in all subjects in the recovery cycle. There were no differences in the on-treatment endometrial outcomes whether LNG was used or not, however the biopsy taken 12 weeks after LNG may have missed related changes.
Table 4.
Association of ovulatory status, and presence of PRM-associated endometrial changes (PAEC), in the histological appearance of the endometrium in the control cycle, after 12 and 24 weeks of treatment and post-treatment
| Luteal activity (P > 10 nmol/L) in preceding 30 days | No luteal activity in preceding 30 days | Total | ||
|---|---|---|---|---|
| Control cycle (n=55) | Physiological | 55/55 (100.0%) | 0/0 (0.0%) | 55/55 (100.0%) |
| Week 12 (n=53)* | Physiological | 9/20 (45.0%) | 1/33 (3.3%) | 10/53 (18.9%) |
| PAEC | 4/20 (20.0%) | 30/33 (90.9%) | 34/53 (64.2%) | |
| Other# | 7/20 (35.0%) | 2/33 (6.1%) | 9/53 (17.0%) | |
| Week 24 (n=53)* | Physiological | 4/9 (44.4%) | 3/43 (7.0%) | 7/52 (13.5%) |
| PAEC | 2/9 (22.2%) | 39/43 (90.7%) | 41/52 (78.8%) | |
| Other# | 3/9 (33.3%) | 1/43 (2.3%) | 4/52 (7.7%) | |
| Post-Treatment (n=52)$ | Physiological | 52/52 (100.0%) | 0/0 (0.0%) | 52/52 (100.0%) |
One subject discontinued the study during treatment period-1, and one insufficient biopsy.
One subject was lost to follow up during the post-treatment period, and two insufficient biopsies.
Includes minor histological changes insufficient to be considered as PAEC
As expected Ki67 labeling of glandular and surface epithelia was low in baseline specimens obtained during the luteal phase when epithelial proliferation in endometrium is suppressed by progesterone. Ki67 labeling increased in epithelium during treatment, indicating that an increased proportion of epithelial cells returned to the cell cycle. Phosphorylated Histone H3 (PhosphoH3) antibody is specific to cells in mitosis [14]. As expected, epithelial cells in the baseline secretory endometrium showed very low levels of PhosphoH3 labeling, but increased under UPA. For both Ki67 and PhosphoH3 there was little change in the labeling index of endometrial stromal cells throughout the study. Bcl-2 protein is known to prolong cell survival by preventing apoptosis. During treatment with UPA, Bcl2 glandular staining increased, but surface epithelial and stromal staining decreased. After treatment, a return to normal morphology of luteal phase was observed, and expression of proliferation markers returned to similar patterns seen at baseline.
During the control cycle, mean endometrial thickness (ET) of all subjects was 8.65 (±3.02) mm. Thickness gradually increased during treatment and became significantly elevated from baseline by week-24 (mean 13.65±6.74 mm, p<.0001) (Fig 3).
Figure 3.
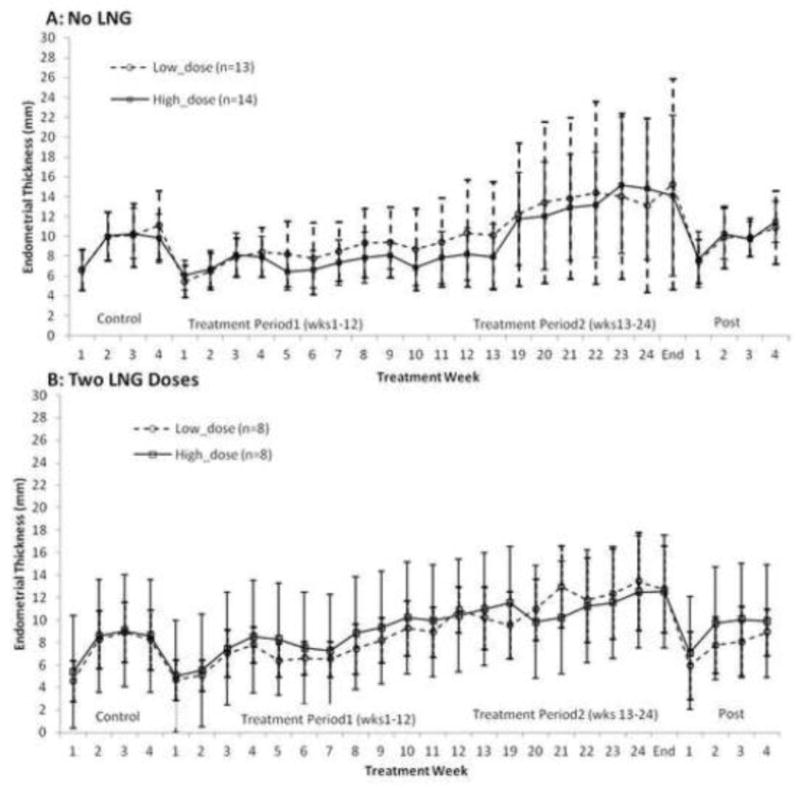
Mean (± SD) values of endometrial thickness change over 6 months use of a 2500 μg (high dose) and 1500 μg (low dose) CVR in subjects who received (panel B) and did not receive (panel A) levonorgestrel (LNG) 1.5 mg at the time of removal of the first and second ring.
The linear mixed model showed that mean ET (log transformation) was significantly different between ovarian activity categories (p=0.0028). The highest ET level was observed in cycles of persistent follicle (11.6±5.8 mm) and mean thickness in these cycles was significantly greater than that in ovulatory and no follicular development cycles (adjusted p = 0.0110 and 0.0385, respectively) (Table 3).
During first ring use, only 3/54 (5.6%), two subjects in the low-dose and one in the high-dose group, had ET >20 mm; while during second ring use, 15/54 (27.8%), 9 in high-dose and 6 in low-dose, had ET >20 mm. ET increased less during the second study period for subjects who used LNG. Fewer subjects using two doses of LNG (18.8%, 3/16) had endometrial thickness of >20 mm at the end of treatment period-2 compared with those that did not receive LNG or received LNG only after the second ring (31.6%, 12/38).
Bleeding patterns
Most subjects reported either a complete absence of bleeding and spotting (47.8% low-dose, 52.2% high-dose group) or infrequent spotting (<7days) (26.1% in both groups) during first treatment period. During treatment period-2, bleeding patterns varied depending on whether the subject had received LNG. For the subjects who did not take LNG at end of first treatment period, 31.6% in low-dose and 52.6% in high-dose group had no bleeding or spotting during the second treatment period. Bleeding episodes were associated with P withdrawal in ovulatory cycles, whereas amenorrhea was common in anovulatory cycles. Of these 16 subjects who used LNG at the end of both ring sequences, 11 experienced LNG withdrawal bleeding at end of treatment period-1, and all 16 subjects bled following the second ring removal. The mean interval from administration of LNG to bleeding was 7.2 (±4.4) days for the first dose of LNG and 7.6 (±4.1) days for the second dose. Mean duration of bleeding was 6.2 (±2.0) days and 7.9 (±4.9) days, respectively.
Heavy bleeding and/or prolonged bleeding
A total of 8 subjects reported heavy or prolonged bleeding as adverse events. The three first cases of heavy bleeding led to protocol amendment one in Chile (SAE with curettage) and two cases from Santo Domingo. Two cases occurred during ring use and one case occurred after the last ring removal. However, before approval of the protocol amendment by the IRB’s and its implementation, five other cases occurred following last ring removal. Table 5 describes the occurrence of heavy and/or prolonged bleeding.
Table 5.
Heavy and/or prolonged bleeding reported as AE during treatment or post treatment
| No LNG received (n=27#) |
One dose LNG* (n=11) |
Two doses LNG** (n=16) |
|
|---|---|---|---|
| Heavy / prolonged bleeding reported as AE during treatment | 2 (7.4) | 0 (0.0) | 0 (0.0) |
| Heavy/prolonged bleeding reported as AE post-treatment | 4 (14.8) | 2 (18.2) | 0 (0.0) |
| No heavy/prolonged bleeding reported as AE during or post treatment | 21 (77.8) | 9 (81.8) | 16 (100.0) |
One subject discontinued prematurely at cycle2
One dose LNG at the end of second ring use.
Two doses LNG at the end of first ring and second ring use.
Two of these occurred in the sixth cycle of ring use, associated with thickened endometrium (30 and 40mm). One of these two bleeding episodes was reported as a serious adverse event and is described below. The other six reports of heavy and/or prolonged bleeding occurred after discontinuing the second ring use. The maximum endometrial thickness in these six cases at end of second ring use ranged from 17 to 37mm. Table 5 shows the cases of heavy and/or prolonged bleeding according to LNG use or not. No apparent difference in such reports was seen between subjects who received none or only one dose of LNG at end of treatment. None of the 16 subjects who used LNG at the end of both ring sequences reported adverse events related to heavy and/or prolonged bleeding.
Safety reports
As mentioned above, one of the subjects with heavy bleeding required hospitalization and this was assessed as a drug-related SAE. The bleeding occurred after using the ring for 144 days with a thick endometrium (40mm, then 33mm at hospitalization) showing PAEC. Treatment was discontinued and the subject underwent uterine curettage and blood transfusion.
Two other reported SAEs: thyroiditis and incisional hernia were judged to be unrelated to treatment.
Other adverse events
A variety of vaginal adverse events (AEs) were reported including vulvovaginal mycotic infection (18.2%), bacterial vaginosis (14.5%) and vaginal discharge (10.9%). These common side-effects were evaluated and identified more frequently in this longer 6-month study with more visits than in our previous 3-month ring study [9].
No significant overall change was seen in either systolic or diastolic blood pressure, or in hemoglobin (mean difference from baseline −0.46 (±0.03) low and −0.26 (±0.04) g/dL high dose. Clinical chemistry and hematological parameters also remained within normal ranges at end of treatment. Three participants developed anemia.
DISCUSSION
This study assessed the pharmacodynamic effects of two doses of UPA delivered from a novel vaginal ring system. Although there was a trend towards greater ovulation suppression with the high versus low-dose (86.1 % vs. 81.8%) UPA ring, this was not statistically significant. However, with a 95% Confidence Interval (78%, 92%) for the high-dose, including the targeted 90% ovulation suppression, we can conclude the 2500 μg/d was the most effective dose. This high-dose achieved 92.6% suppression in the second period.
Ovarian suppression correlated with serum UPA >7ng/mL (RIA) as previously reported [9]. Subjects at the Oregon study site had higher mean serum levels of UPA consistently >7ng/mL, but these levels were not significantly different from the site in Santo Domingo where the measures were made with the same RIA technique. Also there were no ovulations in the high dose ring group in Oregon. Since the mean BMI of Oregon subjects was greater than that of subjects at the other sites, it is not likely that this influenced serum levels. Data from subject diaries did not suggest a difference in compliance as an explanation. Moreover, the follow-up of subjects was intense with serial visits that showed stable UPA levels indicating continuous ring use in all three centers.
Although all subjects at this site also received treatment with a single dose LNG 1.5 mg at the end of each treatment period, it is not likely that this improved the results with ovulation suppression, because the first LNG dose was administered after evaluation of the first three 28-day “cycles” of ring use, suggesting that LNG could not have influenced these observations. The observations for the final cycle occurred during weeks 6–12 of ring 2, more than 6 weeks after LNG levels were expected to have cleared [13]. Although a statistical difference in parity and height was found between women from Oregon and the other sites, there was no effect of age, BMI or parity on UPA-CVR effect on ovulation.
PRMs act both at the hypothalamic-pituitary level and at the ovarian level to prevent follicle rupture [2]. We did not measure gonadotrophins in this study as we could not conduct daily measures. However, higher serum levels of UPA were associated with greater follicular suppression. Moreover, delivery of UPA through a vaginal ring was far more effective at suppressing ovulation than the same dose (2.5mg/d) given orally in a previous study (9.1% suppression) [7], suggesting higher bioavailability with the ring or a more direct ovarian action [2].
Bleeding patterns were generally favorable during treatment. A complete absence of bleeding and spotting occurred in almost half of subjects. In general, predictable withdrawal bleeding occurred following ovulatory cycles. Heavy bleeding occurred in eight women with increased endometrial thickness, at the end of treatment or shortly after discontinuing ring use. The administration of a single dose of LNG every 3 months reduced thickening and induced a withdrawal bleeding 7-days following intake. The 1.5mg-dose was selected based on pharmacokinetic data showing LNG levels >1,000 pmol/L for 5 days after oral intake [13]. We hypothesized that this dose would induce decidualization and withdrawal bleeding, and reduce the risk of endometrial thickening and heavy bleeding. After LNG use was implemented, fewer subjects had ET >20 mm, and none experienced heavy bleeding. Although this strategy represents a simple approach to manage endometrial changes during PRM use, further safety and acceptability studies are needed. Also, LNG treatment may not be needed in women who achieve serum levels of UPA >7 ng/mL, as follicle development is uncommon, E2 levels remain low, and endometrial thickening is limited. In our study, persistent follicles occurred with levels of UPA that suppressed ovulation but were insufficient to prevent follicle growth. Persistent follicles were associated with high E2 levels which may explain the endometrial thickening after prolonged treatment, followed by bleeding upon ring removal.
Biopsies showed characteristic PAEC [10], mostly in participants with no luteal activity. These changes are considered benign, as there is no hyperplasia or cytological atypia. Expression of proliferation markers Ki-67 and PhosphoH3 was significantly increased in the endometrial glandular epithelium from samples obtained on treatment compared to baseline. However, baseline biopsies were taken during secretory phase when proliferative activity is low. While the EB taken on treatment showed lower levels of proliferation than would be seen in physiological endometrium in the follicular phase, it was not possible to compare these directly.
In our study, all of the endometrial changes reverted to normal after ovulation in the post-treatment recovery samples, and there were no instances of PAEC in baseline samples. In the PEARL studies where UPA was used for treatment of leiomyomas [6, 15, 17], non-physiological changes were seen at low frequency at screening (8–10%) including in the placebo group.
Whether high rates of ovulation suppression are required for contraceptive efficacy with UPA is unclear, and other mechanisms may be involved. A possible direct effect on sperm was not confirmed [18, 19]. An effect on endometrial receptivity seems more likely [20–22].
With the hypothesis that PRM in general and UPA in particular would prevent breast cell proliferation [16, 23], the in vivo effects of UPA on the mammary gland need to be studied further.
In conclusion, the 3-month UPA-CVR may become an effective long-acting, user-controlled estrogen-free non-daily contraceptive and the 95% CI of ovulation suppression rate at the high dose of 2500μg/d included the 90% targeted efficacy. Confirmation of safety and prevention of excessive bleeding, either by a progestin or by using higher UPA levels to increase follicle suppression may permit prolonged treatment. Endometrial findings differ from classic endometrial hyperplasia; however long-term studies are needed to confirm the safety of chronic use of PRMs for contraception.
Supplementary Material
Implications.
The 3-month CVR delivering UPA 2500μg per day can become an effective user-controlled estrogen-free contraceptive method. Benign PAEC during treatment returns to normal after discontinuation. The prevention of occasional excessive withdrawal bleeding, either by a progestin or by using higher UPA levels to increase follicle suppression may permit prolonged treatment.
Acknowledgments
The study was supported by NICHD U54 Grant # 5U54HD9990 and 3U54HD029990-17S1, and a complementary clinical grant from HRA Pharma, France as well as an educational grant from HRA Pharma contributing to the fellowship of Dr YongMei Huang at the Population Council.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure summary:
Dr. Jensen has received payments for consulting from HRA Pharma and the Population Council, a not-for-profit organization. He has also received research funding from the Population Council. These companies and organizations may have a commercial or financial interest in the results of this research and technology. These potential conflicts of interest have been reviewed and managed by OHSU.
Vivian Brache has received payments for consulting from the Population Council, FHI360, CONRAD and HRA Pharma. She has also received research funding from the Population Council, FHI360, and HRA Pharma. These companies and organizations may have a commercial or financial interest in the results of this research and technology.
Alistair Williams currently consults for and has received consultancy payments from HRA Pharma, PregLem SA, Gedeon Richter and Bayer.
Regine Sitruk-Ware received a grant from the National Institute of Child and Health Development (NICHD) to conduct the research project described in this manuscript. She is co-inventor of the UPA vaginal ring in a patent owned by the Population Council and HRA Pharma. She is employed by The Population Council a not-for-profit organization developing the UPA vaginal ring in a joint development agreement with HRA Pharma, a company producing UPA that may have a commercial interest in the results of this study.
Dr. Diana Blithe is a principal investigator on the Collaborative Research and Development Agreement (CRADA) of the NICHD with HRA Pharma for the development of ulipristal acetate for therapeutic indications. Inventions under research in the CRADA are joint property of NICHD and HRA Pharma. Funds from the CRADA were not used in the conduct of this research; however, the company supplied the drug for the studies.
Other authors have nothing to declare and their disclosures are also reported separately on appropriate forms.
Clinical Trial Registration Number: NCT00791297
References
- 1.Mosher WD, Jones J. Use of contraception in the United States: 1982–2008. Vital Health Stat 23. 2010;29:1–44. [PubMed] [Google Scholar]
- 2.Nallasamy S, Kim J, Sitruk-Ware R, Bagchi M, Bagchi I. Ulipristal blocks ovulation by inhibiting progesterone receptor-dependent pathways intrinsic to the ovary. Reprod Sci. 2013;4:371–381. doi: 10.1177/1933719112459239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gainer EE, Ulmann A. Pharmacologic properties of CDB(VA)-2914. Steroids. 2003:10–13. 1005–11. doi: 10.1016/s0039-128x(03)00130-2. [DOI] [PubMed] [Google Scholar]
- 4.Attardi BJ, Burgenson J, Hild SA, Reel JR. In vitro antiprogestational/antiglucocorticoid activity and progestin and glucocorticoid receptor binding of the putative metabolites and synthetic derivatives of CDB-2914, CDB-4124, and mifepristone. J Steroid Biochem Mol Biol. 2004;3:277–288. doi: 10.1016/j.jsbmb.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Glasier AF, Cameron ST, Fine PM, Logan SJ, Casale W, Van Horn J, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomised non-inferiority trial and meta-analysis. Lancet. 2010;9714:555–562. doi: 10.1016/S0140-6736(10)60101-8. [DOI] [PubMed] [Google Scholar]
- 6.Donnez J, Tatarchuk TF, Bouchard P, Puscasiu L, Zakharenko NF, Ivanova T, et al. Ulipristal acetate versus placebo for fibroid treatment before surgery. N Engl J Med. 2012;5:409–420. doi: 10.1056/NEJMoa1103182. [DOI] [PubMed] [Google Scholar]
- 7.Chabbert-Buffet N, Pintiaux-Kairis A, Bouchard P, VA2914 Study Group Effects of the progesterone receptor modulator VA2914 in a continuous low dose on the hypothalamic- pituitary-ovarian axis and endometrium in normal women: a prospective, randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2007;9:3582–3589. doi: 10.1210/jc.2006-2816. [DOI] [PubMed] [Google Scholar]
- 8.Croxatto HB, Brache V, Sitruk-Ware R, Kumar N, Sivin I. Protocol. Vol. 312. New York, New York: Population Council; A study to evaluate the effect of a contraceptive vaginal ring delivering a daily dose of 400–500μg of CDB-2914 on pharmacokinetics and pharmacodynamics in normal cycling women. 2003. [data on file] [Google Scholar]
- 9.Brache V, Sitruk-Ware R, Williams A, Blithe D, Croxatto H, Kumar N, et al. Effects of a novel estrogen-free, progesterone receptor modulator contraceptive vaginal ring on inhibition of ovulation, bleeding patterns and endometrium in normal women. Contraception. 2012;5:480–488. doi: 10.1016/j.contraception.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mutter GL, Bergeron C, Deligdisch L, Ferenczy A, Glant M, Merino M, et al. The spectrum of endometrial pathology induced by progesterone receptor modulators. Mod Pathol. 2008;5:591–598. doi: 10.1038/modpathol.2008.19. [DOI] [PubMed] [Google Scholar]
- 11.Jensen JT. Vaginal ring delivery of selective progesterone receptor modulators for contraception. Contraception. 2013;3:314–318. doi: 10.1016/j.contraception.2012.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larner JM, Reel JR, Blye RP. Circulating concentrations of the antiprogestin CDB-2914 and mifepristone in the female rhesus monkey following various routes of administration. Hum Reprod. 2000;5:1100–1106. doi: 10.1093/humrep/15.5.1100. [DOI] [PubMed] [Google Scholar]
- 13.Johansson E, Brache V, Alvarez F, Faundes A, Cochon L, Ranta S, et al. Pharmacokinetic study of different dosing regimens of levonorgestrel for emergency contraception in healthy women. Hum Reprod. 2002;6:1472–1476. doi: 10.1093/humrep/17.6.1472. [DOI] [PubMed] [Google Scholar]
- 14.Brenner RM, Slayden OD, Rodgers WH, Critchley HO, Carroll R, Nie XJ, et al. Immunocytochemical assessment of mitotic activity with an antibody to phosphorylated histone H3 in the macaque and human endometrium. Hum Reprod. 2003;6:1185–1193. doi: 10.1093/humrep/deg255. [DOI] [PubMed] [Google Scholar]
- 15.Williams AR, Bergeron C, Barlow DH, Ferenczy A. Endometrial morphology after treatment of uterine fibroids with the selective progesterone receptor modulator, ulipristal acetate. Int J Gynecol Pathol. 2012;6:556–69. doi: 10.1097/PGP.0b013e318251035b. [DOI] [PubMed] [Google Scholar]
- 16.Baird DT, Brown A, Critchley HOD, Williams AR, Lin S, Cheng L. Effect of long-term treatment with low-dose mifepristone on the endometrium. Hum Reprod. 2003;18:61–68. doi: 10.1093/humrep/deg022. [DOI] [PubMed] [Google Scholar]
- 17.Chabbert-Buffet N, Pintiaux A, Bouchard P. The immninent dawn of SPRMs in obstetrics and gynecology. Mol Cell Endocrinol. 2012;2:232–243. doi: 10.1016/j.mce.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 18.Munuce MJ, Cicare J, Zumoffen C, Caille A, Ghersevich S, Bahamondes L. Effects of ulipristal acetate on sperm DNA fragmentation during in vitro incubation. Eur J Contracept Reprod Health Care. 2013;5:355–363. doi: 10.3109/13625187.2013.813930. [DOI] [PubMed] [Google Scholar]
- 19.Munuce MJ, Zumoffen C, Cicare J, Caille A, Ghersevich S, Bahamondes L. Effect of exposure to ulipristal acetate on sperm function. Eur J Contracept Reprod Health Care. 2012;6:428–437. doi: 10.3109/13625187.2012.725877. [DOI] [PubMed] [Google Scholar]
- 20.Gemzell-Danielsson K, Berger C, P GLL. Emergency contraception – mechanisms of action. Contraception. 2013;3:300–308. doi: 10.1016/j.contraception.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 21.Stratton P, Levens ED, Hartog B, Piquion J, Wei Q, Merino M, et al. Endometrial effects of a single early luteal dose of the selective progesterone receptor modulator CDB-2914. Fertil Steril. 2010;6:2035–2041. doi: 10.1016/j.fertnstert.2008.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams ARW, Bergeron C, Chabbert-Buffet N, Ferenczy A. Progesterone receptor modulator associated endometrial changes: a pilot dose escalation study of ulipristal acetate. Facts, Views and Vision in ObGyn. Sci J Flemish Soc Obstet Gynecol. 2010:99. abstract 163, EBCOG, Antwerp. [Google Scholar]
- 23.Communal L, Vilasco M, Hugon-Rodin J, Courtin A, Mourra N, Lahlou N, et al. Ulipristal acetate does not impact human normal breast tissue. Hum Reprod. 2012;9:2785–2798. doi: 10.1093/humrep/des221. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


