Abstract
Objectives
To examine the risk and trends of HPV-related and HPV-unrelated Head and Neck Squamous Cell Carcinoma (HNSCC) in HIV-infected individuals and assess whether immunosuppression (measured through CD4 cell count) and other risk factors impact HNSCC risk.
Materials and methods
Incident HNSCCs at HPV-related and HPV-unrelated anatomic sites were detected in HIV-infected participants from pooled data from 17 prospective studies in the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) between 1996 and 2009. HNSCC cases were validated using chart review or cancer registry matching. Risk factors for incident HPV-related and HPV-unrelated HNSCC were explored using mixed effects Poisson regression in a full prospective analysis, and the effect of CD4 prior to cancer diagnosis was examined in a nested case control analysis.
Results
66 HPV-related and 182 HPV-unrelated incident HNSCCs were detected among 82,375 HIV-infected participants. Standardized incidence ratios (SIRs) for both HPV-related (SIR = 3.2, 95%CI = 2.5–3.4) and HPV-unrelated (SIR = 3.0, 95%CI = 2.5–4.1) HNSCC were significantly elevated in HIV-infected individuals compared with the US general population. Between 1996 and 2009, the age-standardized HPV-related HNSCC incidence increased non-significantly from 6.8 to 11.4 per 100,000 person-years (p-trend = 0.31) while the age-standardized incidence of HPV-unrelated HNSCC decreased nonsignificantly from 41.9 to 29.3 per 100,000 person-years (p-trend = 0.16). Lower CD4 cell count prior to cancer diagnosis was significantly associated with increased HPV-related and HPV-unrelated HNSCC risk.
Conclusion
The standardized incidence of HPV-related and HPV-unrelated HNSCC are both elevated in HIV-infected individuals. Immunosuppression may have a role in the development of both HPV-related and HPV-unrelated HNSCC.
Keywords: Head and Neck Squamous Cell Carcinoma, HPV, CD4 T cell count, NA-ACCORD, HIV, Oropharyngeal cancer
Introduction
Human papillomavirus (HPV) infection, tobacco, and alcohol use are three major causes of Head and Neck Squamous Cell Carcinoma (HNSCC) [1,2]. In the general population of North America, the incidence of many types of HNSCCs, such as oral cavity cancer, have been decreasing over the past several decades due to decreases in tobacco use [3,4]. In contrast, incidence of HNSCCs related primarily to oral HPV infection (i.e. oropharyngeal cancers), have been increasing over the same time period [3–5].
Less is known about HNSCC in the HIV-infected population of North America, as few studies have been large enough to explore this heterogeneous malignancy. Large cohort and HIV/AIDS-Cancer registry match studies have suggested that HIV-infected individuals have a 1.5–4-fold higher risk for HNSCC compared with the general population [6–8]. However, many of these studies have combined HPV-related oropharyngeal cancers with other HPV-unrelated HNSCCs together in one “oral cavity and pharynx cancer” category, so it is unclear whether this increased HNSCC risk may be explained by higher levels of tobacco and alcohol use, higher exposure to HPV, faster progression of carcinogenesis due to immunosuppression (lower CD4 cell count), or a combination of these factors [9,10].
The incidence of several cancers caused by infectious agents, such as Kaposi's sarcoma, has decreased dramatically in HIV-infected individuals after the introduction of effective antiretroviral therapy (ART, also known as HAART or cART) around 1996. In contrast, there is evidence that the incidence of some HPV-related cancers such as anal cancer [11–14], and perhaps oropharyngeal cancer [11], may have increased in HIV-infected individuals over the past two decades.
The role of HIV and immunological factors such as reduced CD4 cell count in the development of HNSCC is unclear as some preliminary studies have suggested an association with increased HNSCC risk [15–17], but another did not [18]. Using data from the NA-ACCORD (North American AIDS Cohort Collaboration on Research and Design) [19], a collaboration of longitudinal studies of HIV-infected individuals, we explored the risk and trends of HPV-related and HPV-unrelated HNSCC, and assessed the degree to which immunosuppression plays a role in increasing HNSCC risk.
Material and methods
Study population and design
The NA-ACCORD is a large collaboration of longitudinal cohort studies involving HIV-infected individuals in North America and is a part of the International Epidemiologic Databases to Evaluate AIDS (IeDEA) initiative [19]. HNSCCs were identified and validated from HIV-infected individuals from three interval cohorts and fourteen clinically based cohort studies between 1996 and 2009. Each cohort submitted demographic, treatment, clinical, and laboratory data to the NA-ACCORD data management center. The data management center harmonized the individual cohort datasets and implemented a series of rigorous quality control procedures [19].
Participants’ follow-up time for this study began at the maximum of: January 1, 1996, the participant's entry date into the cohort or the earliest date of cancer validation in the respective cohort. Participants were followed until the minimum of: the date of their HNSCC diagnosis, loss to follow-up, death, a cohort's specific administrative censoring date or the last date of cancer validation (December 31, 2009 for most cohorts). Data from the Surveillance Epidemiology and End Results (SEER) program (version 9) on anatomical site-specific HNSCC was utilized as a general population comparison [20].
Data in this analysis was restricted to the potent combination ART era (1996 or later), but included both individuals who used and did not use ART. ART use was defined as a regimen involving at least three antiretrovirals, including a protease inhibitor, an entry inhibitor or an integrase inhibitor, or three nucleoside reverse-transcriptase inhibitors, including abacavir or tenofovir as further described in the DHHS Panel [21]. Institutional review boards at each of the participating cohort site locations have reviewed and approved the human subject activities of the NA-ACCORD.
Cancer validation and categorization
HNSCC cases were validated before this analysis through chart review with clinical confirmation from medical records/pathology reports or through cancer registry-linkage. The validation was performed using a standardized abstraction survey which included histologic confirmation of each HNSCC along with the source of cancer confirmation and the date of diagnosis. Cohorts that validated HNSCCs through cancer registry linkage used internal validation systems which adhered either to the Surveillance Epidemiology and End Results (SEER) or the North American Association of Central Cancer Registries quality standards [22].
All HNSCCs [23] were included in the analysis, and were classified as C00.0-C14.8, C30.0-C31.9, and C32.0-32.9 using the International Classification of Disease for Oncology version-3 (ICD-0-3) topography codes. As tumor HPV status was not available in this study, all oropharyngeal cases were considered “HPV-related”, as has been previously defined in several other previous HNC studies [3,4,24]. This assumption is supported by data indicating oncogenic HPV is detectable in approximately 50–80% of HIV-infected [25] and HIV-uninfected [3,5] individuals from the US with oropharyngeal cancer. HPV-related HNSCCs were defined using the following ICD-0-3 topographic codes: base of the tongue (C01.9), lingual tonsil (C02.4), palatine tonsil (C09.0-09.9), oropharynx (C10.2-10.9), pharynx NOS (C14.0), and Waldyer's ring (C14.2) [3]. All other HNSCCs were classified as HPV-unrelated, as recent studies have found that HPV has little to no etiologic role (<20%) in HNSCCs outside the oropharynx in either HIV-uninfected [24,26] or HIV-infected individuals [25]. HNSCCs classified as HPV-unrelated included: lip (C00.0-00.9), oral cavity (C02.0-02.3 and C02.5-06.9), hypopharynx (C12.9-13.9), nasopharynx (C11.0-11.9), salivary gland (C07.9-08.9), anterior epiglottis (C10.1), other overlapping sites (C14.8), nasal cavity and middle ear (C30.0-30.1), accessory sinuses (C31.0-31.9), and larynx (C32.0-32.9) cancers. For these analyses, cancers were restricted to squamous cell carcinomas, (ICD-O-3 morphology codes: 8050-76, 8078, 8083, 8084, 8094) and carcinoma, NOS (8010).
Statistical analysis
The characteristics of participants who developed an incident HPV-related or HPV-unrelated HNSCC were compared to each other and with all other NA-ACCORD participants who did not develop an HNSCC using the x2 test for categorical data and the Wilcoxon–Mann–Whitney test for medians for continuous data. Incidence rates of HPV-related and HPV-unrelated HNSCC were calculated among HIV-infected individuals overall (from 1996 to 2009) and were stratified by calendar period. For descriptive analyses, we performed direct age standardization of these rates using the 2000 US census standard population and restricted to ages 20 or older in order to better match the NA-ACCORD population [20]. We also compared the rate of HNSCC in the NA-ACCORD with the US general population using standardized incidence ratios (SIRs). These SIRs were standardized for age, gender, race, and calendar period and were, calculated by indirect standardization using rates from Surveillance Epidemiology and End Results (SEER) from 1996 to 2009 [20].
Mixed effects Poisson regression was utilized to model the incidence rates and examine risk factors for HNSCC incidence. A random intercept was used to account for differences in HNSCC incidence by cohort. All multivariate Poisson models included age, gender, race, smoking status and baseline CD4 cell count.
We also explored the effect of CD4 cell count prior to cancer diagnosis utilizing a nested case control study similar to previous studies [27,28]. We matched each HNSCC case to up to ten controls on age at study entry (+5 years), cohort, gender, and enrollment year in the study. As incidence density sampling was utilized, the matched controls had the same amount of study follow-up time as the cases for this analysis. Reference dates were defined as the date of cancer diagnosis for HNSCC cases, or for controls as the date after the same length of follow-up time in the NA-ACCORD as the matched HNSCC case. To evaluate the impact of CD4 prior to the reference date, we categorized CD4 measures into time period categories prior to the reference date (less than 2 years, 2–4 years, 5–7 years, or 8–10 years). The results were then evaluated using conditional logistic regression. For this analysis, if more than one CD4 cell count measurement was available for an individual case or control in a specific time period, then the mean of the multiple CD4 measurements was utilized.
Ever smoking was defined as ever reporting smoking cigarettes before or during the study. Data on this variable was non-uniformly collected by sixteen participating cohorts, and in one cohort (hereby called cohort A, which represented 46% of our total NA-ACCORD sample) no smoking information was available on any participants. In the other sixteen contributing cohorts, smoking status was available on 75% of the participants. To account for the missingness in the ever smoking variable in the other 25% of participants, stochastic multiple imputation via logistic regression was performed [29]. The probability of smoking as a time-fixed variable was imputed using PROC MI (SAS version 9.3) from five random draws from a multivariate normal distribution [29,30]. The imputation model included: study site, age, gender, race, HIV transmission risk, death during study, follow-up time, CD4 and HIV RNA at entry, and ever ART use. Proc MIANALYZE was used to obtain correct standard errors for parameter estimates as previously described [29,30]. We did not impute the smoking status for participants in cohort A as the imputation largely relied on having at least some participants with smoking data from the same study site. For the full prospective analysis, a missing category was used for the participants in cohort A, and we also performed sensitivity analyses considering risk factors excluding cohort A.
Results
Participant characteristics
This study included 82,375 HIV-infected participants from 17 cohort studies in the NA-ACCORD. These participants contributed 463,784 person-years between 1996 and 2009, with a median follow-up time of 4.9 (IQR = 2.0–8.9) years. Only a subset of participants were on ART at entry into the study (20%); however most of the study participants had been exposed to ART by the end of follow-up (78%). There were 248 incident HNSCCs ascertained during follow-up. These HNSCC cases were more likely to be older, male, African American, ever smokers, and have a lower baseline CD4 cell count compared with other individuals in the NA-ACCORD (Table 1, all p-values < 0.01). Characteristics of individuals who developed HPV-related HNSCC were similar to those who developed HPV-unrelated HNSCCs (p > 0.05), except the former were modestly younger (Table 1, p = 0.04).
Table 1.
Characteristics of the participants in NA-ACCORD compared with those with HPV-related and HPV-unrelated Head and Neck Squamous Cell Carcinomas (HNSCCs).
| HIV-positive individuals in NA-ACCORD |
|||
|---|---|---|---|
| All cohort (n = 82,375) | HPV-related HNSCC (n = 66) | HPV-unrelated HNSCC (n = 182) | |
| Median age at baseline (IQR) | 42.8 (36.0-49.7) | 48.5 (43.4-52.3)b | 49.0 (44.4-55.3)b |
| Gender/sexual orientation | |||
| Female | 11,308 (14%) | 5 (7.6%) | 14 (7.6%) |
| Male (heterosexual and MSM) | 71,308 (86%) | 61 (92.4%) | 168 (92.3%) |
| MSM onlya | 20,960 (50%) | 8 (47%) | 15 (40%) |
| Race | |||
| Black (non-hispanic) | 31,411 (38%) | 35 (53%) | 86 (47%) |
| White (non-hispanic) | 36,588 (45%) | 24 (36%) | 91 (50%) |
| Other | 13,897 (17%) | 7 (10%) | 5 (2.8%) |
| Median year of entry (IQR) | 2000 (1997-2004) | 1999 (1997-2000) | 1998 (1997-1998) |
| Median years of follow-up (IQR) | 4.9 (2.0-8.9) | 4.7 (3.7-6.0) | 4.5 (3.6-5.1) |
| Median CD4 T cell count at baseline (IQR) | 293 (127-487) | 248 (189-327) | 240 (205-322) |
| Current ART use at baseline | 15,990 (20%) | 12 (19%) | 44 (24%) |
| Ever ART during study | 64,072 (78%) | 54 (82%) | 137 (75%) |
| Median HIV viral load at baseline (IQR) | 15258 (508-91000) | 9479 (400-63608) | 15,100 (971-78032) |
| ≤500 copies/mL | 14,388 (18%) | 15 (23%) | 17 (16%) |
| >500 copies/mL | 64,587 (81%) | 51 (77%) | 92 (84%) |
| Smoking a | |||
| Ever | 31,857 (76%) | 21 (84%) | 44 (92%) |
| Never | 10,016 (24%) | 4 (16%) | 4 (8.3%) |
| Stage of cancer | |||
| T1-T2 | n/a | 38 (58%) | 118 (64%) |
| T3-T4 | n/a | 19 (29%) | 32 (18%) |
| Unknown | n/a | 9 (14%) | 32 (18%) |
Smoking status and sexual orientation is missing from cohort A (n = 37,685, 46 % of the study population).
p Value = 0.04 between HPV related and unrelated HNSCCs.
Incidence of HPV-related and HPV-unrelated HNSCC
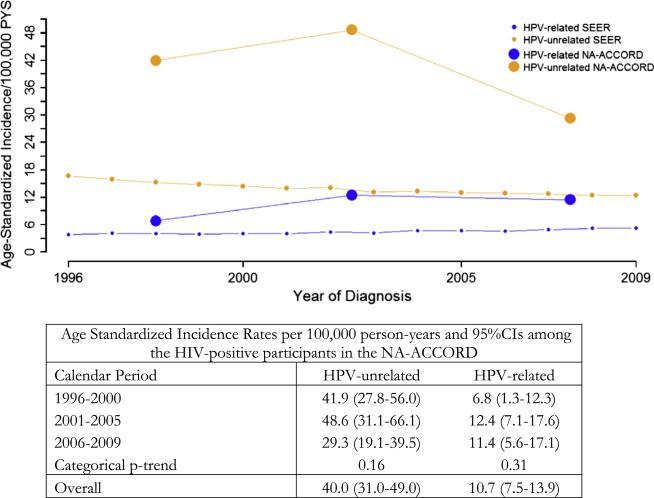
In the HIV-infected participants of NA-ACCORD, the age-standardized incidence rate for overall HNSCC was 50.6 (95%CI = 41.0– 60.1) per 100,000 person-years. Among the 248 incident HNSCCs in the NA-ACCORD, there were 182 HPV-unrelated HNSCCs and 66 HPV-related HNSCCs (Table 2). The majority of HPV-unrelated HNSCCs were larynx and oral cavity cancers, while the most common anatomical sites for HPV-related HNSCCs were the lateral wall of the oropharynx and the base of the tongue (Table 2). The age-standardized incidence of HPV-unrelated HNSCC (40.0, 95%CI = 31.0– 49.0 per 100,000 person-years) was significantly higher than HPV-related HNSCC (10.7, 95%CI = 7.5–13.9 per 100,000 person-years) (table at bottom of Fig. 1).
Table 2.
Total Head and Neck Squamous Cell Carcinomas in NA-ACCORD compared to the US General Population between 1996 and 2009.
| Anatomical site | Observed total | Standardized incidence ratio (SIR)a |
|---|---|---|
| Total Head Neck Squamous Cell Carcinomas (HNSCCs) | 248 | 3.0 (2.7-3.4) |
| HPV-related - Oropharynxb | 66 | 3.2 (2.5-4.1) |
| Oropharynx - lateral wall/tonsil/posterior wall/unspecified | 43 | 3.9 (2.8-5.3) |
| Base of tongue | 20 | 2.4 (1.5-3.7) |
| Pharynx, NOS | 4 | 3.6 (1.0-9.2) |
| HPV-unrelatedb | 182 | 3.0 (2.5-3.4) |
| Larynx/hypopharynx/supraglottis | 95 | 3.5 (2.9-4.3) |
| Oral cavity | 56 | 2.5 (1.9-3.3) |
| Lip | 10 | 1.9 (0.9-3.6) |
| Paranasal sinus/nasal cavity/middle ear | 9 | 4.0 (1.8-7.6) |
| Nasopharynx | 8 | 2.7 (1.1-5.2) |
| Salivary gland | 4 | 2.0 (0.6-5.2) |
Standardized for age, gender, race, and calendar period.
HPV-related and HPV-unrelated HNSCC were categorized by anatomical site, since HPV tumor status was unavailable.
Fig. 1.
Age Standardized Incidence Rates of HPV-related and HPV-unrelated HNSCC between 1996 and 2009 in the NA-ACCORD and SEER.
Compared with the general US population from SEER, HIV-infected individuals in the NA-ACCORD had a threefold higher standardized incidence of HNSCC (SIR = 3.0, 95%CI = 2.7–3.4, Table 2). The threefold increased incidence of cancer was consistent across all head and neck anatomical sites (Table 2), including all of the HPV-related (oropharyngeal) cancer sites (SIR = 3.2, 95%CI = 2.5–4.1) and the HPV-unrelated HNSCC anatomical sites (SIR = 3.0, 95%CI = 2.5–3.4).
Incidence of HNSCC over time
The incidence of HNSCC did not substantially change over the course of this study, but there were non-significant trends which appeared to mirror the trends in the US general population (Fig. 1). The age-standardized incidence of HPV-related HNSCC was close to twice as high in 2001–2005 (IR = 12.4 per 100,000, 95%CI = 7.4–17.6) compared with 1996–2000 (6.8 per 100,000, 95%CI = 1.3–12.3), although this difference was not statistically significant; furthermore the rate did not continue to increase in more recent years (Fig. 1, p-trend = 0.31). The age-standardized incidence of HPV-unrelated HNSCC remained constant between 1996 and 2005, and then non-significantly declined in more recent years (48.6 vs. 29.3 per 100,000, Fig. 1, p-trend = 0.16).
Impact of risk factors on HNSCC incidence
Risk factors were similar for HPV-related and HPV-unrelated HNSCC incidence. Older age appeared to have the strongest impact on HNSCC incidence, as older participants had a significantly higher incidence of HPV-related HNSCC, as well as HPV-unrelated HNSCC, after adjustment for other risk factors (both p < 0.001, Table 3). In addition, ever smokers and those with a lower baseline CD4 cell count both had a non-significantly elevated incidence of HPV-related and HPV-unrelated HNSCC after adjustment (Table 3).
Table 3.
Unadjusted and adjusted incidence rate ratios for risk factors for HPV-related and HPV-unrelated Head and Neck Squamous Cell Carcinoma (HNSCC).
| Variable | HPV-related HNSCC |
HPV-unrelated HNSCC |
||
|---|---|---|---|---|
| IRR | aIRRa | IRR | aIRRa | |
| Age | ||||
| <40 | REF | REF | REF | REF |
| 40-50 | 3.4(1.6-7.1) | 3.3 (1.4-7.5) | 4.9 (2.8-8.2) | 4.5 (2.5-8.2) |
| 50+ | 4.2 (1.9-9.2) | 4.9 (2.0-11.8) | 8.5 (4.9-14.5) | 7.1 (3.8-13.3) |
| Gender/sexual orientation | ||||
| Female | REF | REF | REF | REF |
| Maleb | 1.6 (0.55-4.3) | 1.2 (0.42-3.2) | 1.7 (0.81-3.7) | 1.0 (0.47-2.1) |
| Race | ||||
| Black | REF | REF | REF | REF |
| White | 0.69 (0.41-1.2) | 0.78 (0.41-1.5) | 1.3 (0.78-1.4) | 1.0 (0.67-1.50) |
| Other | 0.63 (0.24-1.7) | 0.76 (0.27-2.2) | 0.23 (0.09-0.60) | 0.29 (0.10-0.82) |
| Smoking status c | ||||
| Never | REF | REF | REF | REF |
| Ever | 2.5 (0.57-10.8) | 2.4 (0.55-11) | 3.0 (1.1-8.4) | 2.6 (0.94-7.4) |
| Baseline CD4 | ||||
| ≥500 cells/μL | REF | REF | REF | REF |
| 200-500 cells/μL | 2.0 (0.86-4.7) | 1.9 (0.82-4.5) | 1.7 (0.97-2.8) | 1.6 (0.93-2.7) |
| <200 cells/μL | 1.8 (0.74-4.5) | 1.6 (0.66-3.9) | 1.9 (1.1-3.3) | 1.7 (0.99-3.0) |
| ART use at baseline | ||||
| Yes | REF | REF | - | |
| No | 1.0 (0.53-1.8) | - | 0.68 (0.48-0.96) | - |
| Baseline HIV viral load | ||||
| <500 copies/mL | REF | - | REF | - |
| 500-100,00 copies/mL | 0.49 (0.22-1.1) | - | 0.74 (0.41-1.3) | - |
| >10,000 copies/mL | 0.49 (0.23-1.0) | - | 0.94 (0.55-1.6) | - |
| Baseline CD8 c | ||||
| Highest (Quartile 1) | REF | - | REF | - |
| Quartile 2 | 2.0 (0.37-11.0) | - | 0.42 (0.13-1.4) | - |
| Quartile 3 | 2.1 (0.38-11.7) | - | 0.74 (0.27-2.0) | - |
| Quartile 4 | 1.1 (0.15-7.5) | - | 0.74 (0.28-2.0) | - |
Adjusted for age, gender, race, smoking status, CD4 at baseline, and cohort.
MSM and other men had a similar incidence of both HPV-related and HPV-unrelated HNSCC men when sexual orientation was available (p > 0.50).
Data not available for these variables in Cohort A.
The incidence of HPV-related and HPV-unrelated HNSCC, respectively, was not associated with gender, sexual orientation, baseline HIV RNA viral load or baseline CD8 T cell count. While the incidence of HPV-related HNSCC was also not associated with race or baseline ART status, the incidence of HPV-unrelated HNSCC was lower among those who were not on ART at baseline compared to those who were on ART at baseline and among those who were an “other” race compared to those who were black or white.
In a sensitivity analysis, we considered the effect of risk factors on HNSCC incidence by cohort and observed modest differences between the cohort A and the other cohorts in the NA-ACCORD, however, these interactions did not approach statistical significance (p-interactions ≥ 0.20, Supplemental Table 1). To determine if this non-significant difference by cohort was due to the lack of smoking data in the cohort A, we ran another sensitivity analysis that excluded cohort A participants. We found that adjusting for ever smoking status did not strongly impact the point estimates between risk factors and HPV-related or HPV-unrelated HNSCC among non-cohort A participants (Supplemental Table 2). In addition, we found that the baseline CD4 cell count in ever and never smokers was similar (p = 0.90).
Impact of CD4 cell count on HNSCC incidence
After finding that reduced baseline CD4 cell count may be associated with increased HNSCC incidence, we further explored the effect of reduced CD4 by conducting a nested case control analysis. When measured up to seven years prior to the reference date, reduced CD4 cell count was significantly associated with HPV-related and HPV-unrelated HNSCC (Table 4, Supplemental Fig. 1). CD4 cell count measured within two years prior to the reference date (i.e. around cancer diagnosis for cases) was associated with both HPV-related (CD4 < 200: aOR = 2.1, 95%CI = 1.0–4.5; continuous p-trend = 0.04) and HPV-unrelated HNSCC (CD4 < 200: aOR = 2.5, 95%CI = 1.5–4.2; continuous p-trend < 0.001). Similar associations were seen when CD4 cell count was measured up to seven years prior to the reference date for both HPV-unrelated and HPV-related HNSCC (Table 4). Additionally, there was a non-significant suggestion that the association may have modestly strengthened further from the reference date for HPV-related HNSCC (5–7 years, CD4 < 200: aOR = 3.9, 95%CI = 1.1–13.0).
Table 4.
Impact of different measures of CD4 T cell count on HPV-related and HPV-unrelated HNSCC in the HIV-infected individuals in the NA-ACCORD.
| CD4 cell count measure | HPV-related HNSCC |
HPV-unrelated HNSCC |
||
|---|---|---|---|---|
| Cases/total | aORa | Cases/total | aORa | |
| CD4 0-1 years before cancer diagnosis/reference date b | ||||
| ≥500 cells/μL | 16/235 | REF | 36/518 | REF |
| 200-500 cells/μL | 36/321 | 1.6 (0.85-3.0) | 72/685 | 1.8 (1.1-2.9) |
| <200 cells/μL | 19/156 | 2.1 (1.0-4.5) | 49/388 | 2.5 (1.5-4.2) |
| CD4 2-4 years before cancer diagnosis/reference date b | ||||
| ≥500 cells/μL | 18/247 | REF | 33/527 | REF |
| 200-500 cells/μL | 37/370 | 1.5 (0.82-2.9) | 71/698 | 1.5 (0.96-2.3) |
| <200 cells/μL | 15/148 | 1.9 (0.86-4.2) | 39/314 | 3.6 (2.2-5.9) |
| CD4, 5-7 years before cancer diagnosis/reference date b | ||||
| ≥500 cells/μL | 7/130 | REF | 17/269 | REF |
| 200-500 cells/μL | 28/234 | 3.3 (1.1-9.9) | 45/442 | 1.8 (0.94-3.3) |
| <200 cells/μL | 11/91 | 3.9 (1.1-13.0) | 30/188 | 3.3 (1.7-6.5) |
| CD4, 8-10 years before cancer diagnosis/reference date b | ||||
| ≥500 cells/μL | 1/23 | REF | 7/76 | REF |
| 200-500 cells/μL | 14/101 | 3.6 (0.41-31.8) | 13/174 | 0.79 (0.28-2.2) |
| <200 cells/μL | 0/30 | - | 14/70 | 3.1 (1.0-9.0) |
10 controls were matched to each HNSCC cases and were matched on age (±5 years), cohort, gender, and enrollment year in the study. Using conditional logistic regression models, the association with each CD4 measure was considered separately and was adjusted for smoking status and current ART status.
Controls were matched to cases utilizing incident density sampling, thus the reference dates for controls had the same amount of study follow-up time as the cancer diagnosis date for cases.
Discussion
In this large prospective study, we determined that the standardized incidence of HPV-related and HPV-unrelated HNSCC were both threefold higher in HIV-infected individuals than the US general population. This study also supports a modest role for immunosuppression (low CD4 cell count) prior to cancer diagnosis. This supports evidence from other studies [7,31], that immunosuppression from HIV may impact the head and neck carcinogenesis process.
The three fold higher incidence of HNSCC in this HIV-infected population compared with the general population is consistent with previous registry-based studies, which have suggested that the incidence of HNSCC is between 1.5 and 4-fold higher in HIV-infected individuals [6,7]. In addition, the age-standardized incidence of HPV-related HNSCC increased while the age-standardized incidence of HPV-unrelated HNSCC decreased among these HIV-infected individuals between 1996 and 2009, which mirrors what has been observed in the general HIV-uninfected population [3,5]. However, the calendar trends in this study were modest and did not reach statistical significance. This may, in part, be explained by the imprecise groupings of cancers in our study by tumor subsite; as some oropharyngeal cases were likely HPV-negative this misclassification may have attenuated the actual HPV-positive oropharyngeal trends similar to what has been seen in the general population [5]. Regardless of the age-standardized trends, it appears likely that the overall number of both HPV-related and HPV-unrelated HNSCCs may increase in the future given the aging of the HIV-infected population in North America [11] and the strong association we observe between older age and both HPV-related and HPV-unrelated HNSCC. Further research is also necessary to examine how providing ART for those with higher CD4 cell counts will impact HNSCC incidence, since it has recently become the more common practice in high income countries. Our study did not observe ART use to be protective against either HPV-related or HPV-unrelated HNSCC; however, since ART has traditionally been given to individuals who are more immunosuppressed there is a possibility of confounding by treatment indication.
It has been speculated that the higher incidence of HNSCC in HIV-infected individuals might be due to multiple different factors including: their increased use of tobacco or alcohol, their higher number sexual partners, and/or from HIV-related immunosuppression [7,32]. However, the effect of immunosuppression on HNSCC incidence has not been extensively explored previously. Most previous studies that include HNSCCs in HIV-infected individuals have been limited to exploring the role of CD4 cell count at one time point, and have not explored the effect immunosuppression trends over time [16,17]. Our study supports the conclusion that immuno-suppression may have a modest role in the development of HNSCC [15–17], and suggests this may be true for both HPV-related and HPV-unrelated HNSCC.
It has been previously speculated that immunosuppression may have its strongest impact earlier in the HPV-related carcinogenesis process (in affecting HPV acquisition and persistence) [33,34] however; initial findings exploring the longitudinal effect of CD4 on other HPV-associated cancers have been mixed. One recent study suggested that immunosuppression may have its largest effect 6– 7 years prior to anal cancer diagnosis [28], while another study suggested a stronger effect close to cervical cancer diagnosis [27]. The results of this NA-ACCORD study found that lower CD4 measured further from HPV-related HNSCC diagnosis (5–7 years vs. 0–2 years) may be more strongly associated with HPV-related HNSCC; however the difference between these CD4 measures was not significant.
The finding that immunosuppression was also associated with HPV-unrelated HNSCC in this study suggests that reduced immune surveillance for malignant cells could have a role in HNSCC development [35,36] There have been several recent studies that have suggested immunosuppression prior to cancer diagnosis may impact infection and non-infection related non-AIDS defining malignances [9,15,37,38]. For example, a study from Kaiser Permante [15] suggested that reduced recent CD4 was associated with a higher risk of both infection related and non-infection related cancers, except prostate cancer. Alternatively, it is also conceivable that undiagnosed HNSCC may be playing a role in reducing a participant's immune status. A recent European study found a higher risk of cancer diagnosis within six months of a detectable decline in CD4 count, suggesting that the decline in CD4 may be a consequence of an undiagnosed cancer rather than a causal agent in the malignancy's development [39].
While we note a modest association between immunosuppression and HPV-related HNSCC risk, the three fold higher standardized incidence rate for HPV-related HNSCC (comparing HIV-infected individuals with the general population) is considerably lower than the SIRs for other HPV-associated cancers including cervical, anal, and vulvar cancer (SIRs > 5) [6,13,16]. It is possible that the SIR of HPV-related HNSCC may be lower due to the demographics (other than age) and sexual orientation of the North American HIV-infected population [6,7]. Specifically, the incidence of HNSCC may be modest in North American HIV-infected individuals as ~75% are either heterosexual women or men who have sex with men (MSM) [40], and there is evidence that performing oral sex on a man may be less likely to transmit oral HPV infection than performing it on a woman [7,41]. However, in this study, heterosexual men had a similar incidence of HPV-related HNSCC compared to MSM and women, although sexual orientation was not available on some of our participants and we were unable to adjust for important risk factors such as number of lifetime oral sex partners.
There are several strengths to this study. The study used prospective data from a large collaboration of HIV-studies that have been suggested to be representative of the HIV-infected population in clinical care in North America [19]. In addition, this study included a rigorous standardized validation procedure to confirm malignancies, included approximately 15 years of data, and is one of the first studies to explore HPV-related and HPV-unrelated HNSCC incidence among HIV-infected individuals. However, we should note that we lacked detailed information on other co-factors such as sexual behavior, alcohol use, and detailed tobacco use (such as current tobacco use and cumulative tobacco use) and thus the risk factor's associations with HNSCC are still prone to residual confounding. In addition, there was outcome misclassification as we lacked the HPV tumor status of these HNSCCs and thus all oropharyngeal cancers were classed HPV-related in this study. While a relatively small recent case series suggests that HPV is detected in the majority of oropharyngeal cancers in HIV-infected individuals [25], there were still likely HPV-negative oropharyngeal cancers that were classified as HPV-related HNSCCs in this study especially given the high rate of tobacco use in HIV-infected individuals [10]. This may impact both the HPV-related SIR and the association of risk factors.
In summary, this study demonstrates that North American HIV-infected individuals have an elevated risk of both HPV-related and HPV-unrelated HNSCC. However these SIRs are relatively modest compared to other carcinomas induced by HPV or other viruses [6,17], suggesting that the North American HIV-infected population as a whole may not be strong candidates for more aggressive HNSCC screening modalities if they were developed. While there has not been a strong decline in the age-standardized incidence of HNSCC in the antiretroviral therapy era, these results and others suggest that immunosuppression likely plays a role during the HNSCC carcinogenesis process.
Supplementary Material
Acknowledgements
NA-ACCORD participating cohorts and representatives (*indicates contributed data to this study): *AIDS Link to the IntraVenous Experience (ALIVE; G. Kirk), Adult AIDS Clinical Trials Group Longitudinal Linked Randomized Trials (ALLRT; C. Benson, R. Bosch, A. Collier, *HIV Research Network (HIVRN; K. Gebo); *Johns Hopkins HIV Clinical Cohort (JHHCC; R. M.), *HAART Observational Medical Evaluation and Research (HOMER; R. Hogg), *HIV Outpatient Study J.T.B.), *Johns Hopkins HIV Clinical Cohort (JHHCC; R.D.M.), *Kaiser Permanente Northern California (M. Horbberg and M.S.), Multicenter Hemophilia Cohort Study-II; J. Goedert), *Multicenter AIDS Cohort Study (MACS; L. Jacobson), *Montreal Chest Institute Immunodeficiency Service Cohort (M. Klein.), Ontario HIV Treatment Network Cohort Study (OHTN; S. Rourke and A. Rachlis), John T. Carey Special Immunology Unit Patient Care and Research Database (PCRD; B. Rodriguez) and Plaris HIV Seroconversion Study (L. Calzavara), *Southern Alberta Clinic Cohort (M.J.G), Studies of the Consequences of the Protease Inhibitor Era (SCOPE; S. Deeks and J. Martin), University of Alabama at Birmingham Clinic Cohort (M. Saag), *University of North Carolina, Chapel Hill (UCHCC; J.J.E. and S.N.), *University of Washington HIV Cohort (M.K.), Veterans Aging Cohort Study (VACS; A. Justice, R.D.), Vanderbilt-Meharry CFAR Cohort (T.R.S), *Women's Interagency HIV Study (K. Anastos, S. Gange). Executive Committee: R. Moore, M. Saag, S. Gange, M. Kitahata, R. McKaig, and A. Freeman; Epidemiology/Biostatistics Core: A. Abraham, K. Althoff, E. Golub, Y. Jing, B. Lau, P. Rebeiro, A. Mendes, S. Modur, and J. Zhnag; Data Management Core; S. Van Rompaey, E. Webster, and B. Simon.
The data were presented in part during the 2012 European Research Organization on Genital Infection and Neoplasia (EUROGIN) conference in Prague, Czech Republic and at the Conference on Retroviruses and Opportunistic Infections (CROI) in Seattle, Washington also in 2012.
Funding support
National Institute of Allergy & Infectious Diseases, with supplemental funding from the National Cancer Institute. This work was supported by grants: U01-AI069918, U01-AA013566, U24-AA020794, U01-AA020790, U01-AI31834, U01-AI34989, U01-AI34993, U01-AI34994, U01-AI35004, U01-AI35039, U01-AI35040, U01-AI35041, U01-AI35042, U01-AI35043, U01-AI37613, U01-AI37984, U01-AI38855, U01-AI38858, U01-AI42590, U01-AI68634, U01-AI68636, U01-AI69432, U01-AI69434, U01-HD32632, U10-EY08057, U10-EY08052, U10- EY08067, UL1-RR024131, UL1-TR000083, U54- MD007587, F31-DA035713, G12- MD007583, K01-AI071754, K01-AI093197, K23 EY013707, K24–00432, MO1-RR-00052, N02-CP55504, P30-AI027763, P30-AI094189, P30-AI27757, P30-AI27767, P30-AI50410, P30-AI54999, P30-AI036219, P30-MH62246, R01-CA165937, R01-AA16893, R01-DA11602, R01-DA04334, R01-DA12568, R24-AI067039, R56-AI102622, Z01-CP010214, and Z01-CP010176 from the National Institutes of Health, USA; contract CDC200–2006-18797 from the Center's for Disease Control and Prevention, USA; contract 90047713 from the Agency for Healthcare Research and Quality, USA; contract 90051652 from the Health Resources and Services Administration, USA; grants TGF-96118, HCP-97105, CBR-86906, CBR-94036 from the Canadian Institutes of Health Research, Canada; Ontario Ministry of Health and Long Term Care; and the Government of Alberta, Canada. The lead author is funded through T32 CA CA009314.
Footnotes
Conflict of interest statement
Michael Silverberg has ongoing grants from Pfizer and Merck, Gypsyamber D'souza received previous received funding from Merck, John Gill is a board member of BMS Abbvie, Janssen, and Gilead Viiv, and Marina Klein has consulted with Glaxo Smith Klein and VIIV and has a current grant with Merck. For the remaining authors no conflicts of interest were declared.
Appendix A. Supplementary material
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.oraloncology.2014.09.011.
References
- 1.D'Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, Koch WM, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356(19):1944–56. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 2.Gillison ML, D'Souza G, Westra W, Sugar E, Xiao W, Begum S, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100(6):407–20. doi: 10.1093/jnci/djn025. [DOI] [PubMed] [Google Scholar]
- 3.Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26(4):612–9. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 4.Auluck A, Hislop G, Bajdik C, Poh C, Zhang L, Rosin M. Trends in oropharyngeal and oral cavity cancer incidence of human papillomavirus (HPV)-related and HPV-unrelated sites in a multicultural population: the British Columbia experience. Cancer. 2010;116(11):2635–44. doi: 10.1002/cncr.25087. [DOI] [PubMed] [Google Scholar]
- 5.Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29(32):4294–301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shiels MS, Cole SR, Kirk GD, Poole C. A meta-analysis of the incidence of non-AIDS cancers in HIV-infected individuals. J Acquir Immune Defic Syndr. 2009;52(5):611–22. doi: 10.1097/QAI.0b013e3181b327ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beachler DC, D'souza G. Oral human papillomavirus infection and head and neck cancers in HIV-infected individuals. Curr Opin Oncol. 2013;25(5):503–10. doi: 10.1097/CCO.0b013e32836242b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silverberg MJ, Chao C, Leyden WA, Xu L, Tang B, Horberg MA, et al. HIV infection and the risk of cancers with and without a known infectious cause. AIDS. 2009;23(17):2337–45. doi: 10.1097/QAD.0b013e3283319184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubrow R, Silverberg MJ, Park LS, Crothers K, Justice AC. HIV infection, aging, and immune function: implications for cancer risk and prevention. Curr Opin Oncol. 2012;24(245):506–16. doi: 10.1097/CCO.0b013e328355e131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirk GD, Merlo CA. Lung H.I.V. Study. HIV infection in the etiology of lung cancer: confounding, causality, and consequences. Proc Am Thorac Soc. 2011;8(3):326–32. doi: 10.1513/pats.201009-061WR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shiels MS, Pfeiffer RM, Gail MH, Hall HI, Li J, Chaturvedi AK, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst. 2011;103(9):753–62. doi: 10.1093/jnci/djr076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shiels MS, Pfeiffer RM, Chaturvedi AK, Kreimer AR, Engels EA. Impact of the HIV epidemic on the incidence rates of anal cancer in the United States. J Natl Cancer Inst. 2012;104(20):1591–8. doi: 10.1093/jnci/djs371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silverberg MJ, Lau B, Justice AC, Engels E, Gill MJ, Goedert JJ, et al. Risk of anal cancer in HIV-infected and HIV-uninfected individuals in North America. Clin Infect Dis. 2012;54(7):1026–34. doi: 10.1093/cid/cir1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piketty C, Selinger-Leneman H, Bouvier A, Belot A, Mary-Krause M, Duvivier C, et al. Incidence of HIV-related anal cancer remains increased despite long-term combined antiretroviral treatment: results from the French hospital database on HIV. J Clin Oncol. 2012;30(35):4360–6. doi: 10.1200/JCO.2012.44.5486. [DOI] [PubMed] [Google Scholar]
- 15.Silverberg MJ, Chao C, Leyden WA, Xu L, Horberg MA, Klein D, et al. HIV infection, immunodeficiency, viral replication, and the risk of cancer. Cancer Epidemiol Biomarkers Prev. 2011;20(12):2551–9. doi: 10.1158/1055-9965.EPI-11-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clifford GM, Polesel J, Rickenbach M, Dal Maso L, Keiser O, Kofler A, et al. Cancer risk in the Swiss HIV Cohort Study: associations with immunodeficiency, smoking, and highly active antiretroviral therapy. J Natl Cancer Inst. 2005;97(6):425–32. doi: 10.1093/jnci/dji072. [DOI] [PubMed] [Google Scholar]
- 17.Engels EA, Biggar RJ, Hall HI, Cross H, Crutchfield A, Finch JL, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123(1):187–94. doi: 10.1002/ijc.23487. [DOI] [PubMed] [Google Scholar]
- 18.Chaturvedi AK, Madeleine MM, Biggar RJ, Engels EA. Risk of human papillomavirus-associated cancers among persons with AIDS. J Natl Cancer Inst. 2009;101(16):1120–3. doi: 10.1093/jnci/djp205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gange SJ, Kitahata MM, Saag MS, Bangsberg DR, Bosch RJ, Brooks JT, et al. Cohort profile: the North American AIDS cohort collaboration on research and design (NA-ACCORD). Int J Epidemiol. 2007;36(2):294–301. doi: 10.1093/ije/dyl286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Howe HL, Edwards BK, Young JL, Shen T, West DW, Hutton M, et al. A vision for cancer incidence surveillance in the United States. Cancer Causes Control. 2003;14(7):663–72. doi: 10.1023/a:1025667524781. [DOI] [PubMed] [Google Scholar]
- 23.Patel SG, Shah JP. TNM staging of cancers of the head and neck: striving for uniformity among diversity. CA Cancer J Clin. 2005;55(4):242, 58. doi: 10.3322/canjclin.55.4.242. quiz 261-2, 264. [DOI] [PubMed] [Google Scholar]
- 24.Joseph AW, D'Souza G. Epidemiology of human papillomavirus-related head and neck cancer. Otolaryngol Clin North Am. 2012;45(4):739–64. doi: 10.1016/j.otc.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 25.D'Souza G, Carey TE, William WN, Jr, Nguyen ML, Ko EC, Riddell J, 4th, et al. Epidemiology of head and neck squamous cell cancer among HIV-infected patients. J Acquir Immune Defic Syndr. 2014;65(5):603–10. doi: 10.1097/QAI.0000000000000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lingen MW, Xiao W, Schmitt A, Jiang B, Pickard R, Kreinbrink P, et al. Low etiologic fraction for high-risk human papillomavirus in oral cavity squamous cell carcinomas. Oral Oncol. 2013;49(1):1–8. doi: 10.1016/j.oraloncology.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 27.Abraham AG, Strickler HD, Jing Y, Gange SJ, Sterling TR, Silverberg M, et al. Invasive cervical cancer risk among HIV-infected women: a North American multi-cohort collaboration prospective study. J Acquir Immune Defic Syndr. 2012;62(4):405–13. doi: 10.1097/QAI.0b013e31828177d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bertisch B, Franceschi S, Lise M, Vernazza P, Keiser O, Schoni-Affolter F, et al. Risk factors for anal cancer in persons infected with HIV: a nested case-control study in the Swiss HIV cohort study. Am J Epidemiol. 2013;178(6):877–84. doi: 10.1093/aje/kwt153. [DOI] [PubMed] [Google Scholar]
- 29.Rubin DB. Multiple Imputation for Nonresponse in Surveys. John Wiley & Sons, Inc.; Hoboken: 1987. [Google Scholar]
- 30.Abraham AG, Lau B, Deeks S, Moore RD, Zhang J, Eron J, et al. Missing data on the estimation of the prevalence of accumulated human immunodeficiency virus drug resistance in patients treated with antiretroviral drugs in North America. Am J Epidemiol. 2011;174(6):727–35. doi: 10.1093/aje/kwr141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tugizov SM, Herrera R, Chin-Hong P, Veluppillai P, Greenspan D, Michael Berry J, et al. HIV-associated disruption of mucosal epithelium facilitates paracellular penetration by human papillomavirus. Virology. 2013;446(1–2):378–88. doi: 10.1016/j.virol.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gillison ML. Oropharyngeal cancer: a potential consequence of concomitant HPV and HIV infection. Curr Opin Oncol. 2009;21(5):439–44. doi: 10.1097/CCO.0b013e32832f3e1b. [DOI] [PubMed] [Google Scholar]
- 33.Engels EA, Madeleine MM. Invited commentary: biological and clinical insights from epidemiologic research into HIV, HPV, and anal cancer. Am J Epidemiol. 2013;178(6):885–7. doi: 10.1093/aje/kwt149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strickler HD, Burk RD, Fazzari M, Anastos K, Minkoff H, Massad LS, et al. Natural history and possible reactivation of human papillomavirus in human immunodeficiency virus-positive women. J Natl Cancer Inst. 2005;97(8):577–86. doi: 10.1093/jnci/dji073. [DOI] [PubMed] [Google Scholar]
- 35.Nakachi K, Hayashi T, Imai K, Kusunoki Y. Perspectives on cancer immunoepidemiology. Cancer Sci. 2004;95(12):921–9. doi: 10.1111/j.1349-7006.2004.tb03178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smyth MJ, Dunn GP, Schreiber RD. Cancer immunosurveillance and immunoediting: the roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv Immunol. 2006;90:1–50. doi: 10.1016/S0065-2776(06)90001-7. [DOI] [PubMed] [Google Scholar]
- 37.Guiguet M, Boue F, Cadranel J, Lang JM, Rosenthal E, Costagliola D, et al. Effect of immunodeficiency, HIV viral load, and antiretroviral therapy on the risk of individual malignancies (FHDH-ANRS CO4): a prospective cohort study. Lancet Oncol. 2009;10(12):1152–9. doi: 10.1016/S1470-2045(09)70282-7. [DOI] [PubMed] [Google Scholar]
- 38.Reekie J, Kosa C, Engsig F, Monforte A, Wiercinska-Drapalo A, Domingo P, et al. Relationship between current level of immunodeficiency and non-acquired immunodeficiency syndrome-defining malignancies. Cancer. 2010;116(22):5306–15. doi: 10.1002/cncr.25311. [DOI] [PubMed] [Google Scholar]
- 39.Helleberg M, Kronborg G, Larsen CS, Pedersen G, Pedersen C, Obel N, et al. CD4 decline is associated with increased risk of cardiovascular disease, cancer, and death in virally suppressed patients with HIV. Clin Infect Dis. 2013;57(2):314–21. doi: 10.1093/cid/cit232. [DOI] [PubMed] [Google Scholar]
- 40.Althoff KN, Buchacz K, Hall HI, Zhang J, Hanna DB, Rebeiro P, et al. U.S. trends in antiretroviral therapy use, HIV RNA plasma viral loads, and CD4 T-lymphocyte cell counts among HIV-infected persons, 2000 to 2008. Ann Intern Med. 2012;157(5):325–3. doi: 10.7326/0003-4819-157-5-201209040-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beachler DC, D'Souza G, Sugar EA, Xiao W, Gillison ML. Natural history of anal versus oral HPV infection in HIV-infected men and women. J Infect Dis. 2013;208(2):330–9. doi: 10.1093/infdis/jit170. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.