Abstract
The FLT3-ITD mutation is associated with a high relapse rate for patients with AML even after allogeneic hematopoietic stem cell transplantation (HSCT). Sorafenib is a tyrosine kinase inhibitor which inhibits the FLT3 tyrosine kinase and has shown encouraging activity in FLT3-ITD AML. We conducted a phase I trial of maintenance sorafenib after HSCT in patients with FLT3-ITD AML (ClinicalTrials.gov NCT01398501). Patients received a variety of conditioning regimens and graft sources. A dose escalation 3+3 cohort design was used to define the maximum tolerated dose (MTD) with an additional 10 patients treated at the MTD. Sorafenib was initiated between days 45 and 120 after HSCT continued for twelve 28-day cycles. Twenty-two patients were enrolled (status at HSCT: CR1=16, CR2=3, refractory=3). The MTD was established at 400 mg BID with one DLT observed (pericardial effusion). Two patients died of transplant-related causes, both unrelated to sorafenib. Two patients stopped sorafenib after relapse and 5 stopped due to attributable toxicities after the DLT period. Median follow-up for surviving patients is 16.7 months after HSCT (range, 8.1–35.0). There was one case of grade II acute GVHD after starting sorafenib and the 12-month cumulative incidence of chronic GVHD was 38% (90% CI, 21%–56%). For all patients, one-year progression-free survival (PFS) is 85% (90% CI, 66%–94%) and one-year overall survival (OS) is 95% (90% CI, 79%–99%) after HSCT. For patients in CR1 / CR2 prior to HSCT (n=19), one-year PFS is 95% (90% CI, 76%–99%) and one-year OS is 100% with only one patient who has relapsed. Sorafenib is safe after HSCT for FLT3-ITD AML and merits further investigation for the prevention of relapse.
INTRODUCTION
Fms-like tyrosine kinase 3 internal tandem duplication (FLT3-ITD) mutations are found in approximately one quarter of patients with AML.1, 2, 3 While initial remission rates are comparable for patients with FLT3-ITD relative to FLT3-wildtype (WT), patients with a FLT3-ITD mutation are significantly more likely to relapse and do so more rapidly. Upon relapse, disease is often refractory to further treatment.
There is no accepted optimal treatment for patients with FLT3-ITD AML.4, 5 No randomized prospective clinical trials have ever been conducted to confirm a benefit for allogeneic HSCT in first remission. While a few retrospective studies have suggested that there is no benefit for allogeneic HSCT in first remission (CR1),6, 7 the majority of such analyses have indicated an advantage for early HSCT.8, 9, 10, 11, 12, 13 These analyses have reported a durable disease-free survival after HSCT ranging from 19%–58%.7, 9, 12, 13 Therefore, allogeneic HSCT has become the standard of care at many institutions for patients with FLT3-ITD AML in CR1; however, relapse remains the main obstacle to long-term survival.
Given the success of tyrosine kinase inhibitors (TKIs) incorporated into the standard treatment of chronic myeloid leukemia (CML) and Philadelphia chromosome positive acute lymphoblastic leukemia (Ph+ ALL), several inhibitors of the FLT3 tyrosine kinase have been studied in recent years. These include sorafenib, lestaurtinib (CEP-701), midostaurin (PKC412), and quizartinib (AC220).14, 15, 16, 17, 18, 19, 20 The ideal time to incorporate FLT3 inhibitors into the treatment of FLT3-ITD AML remains unclear, although evidence based on the kinetics of endogenous levels of FLT3 ligand suggests that maintenance may be optimal.21 As very little data has been published on using sorafenib after HSCT and before frank relapse, we conducted a phase I study investigating sorafenib as maintenance therapy after allogeneic HSCT for patients with FLT3-ITD AML.
METHODS
Patients
This study was approved by the Institutional Review Board at the Dana-Farber Harvard Cancer Center (DFHCC) and conducted at Dana-Farber Brigham & Women’s Cancer Center and Massachusetts General Hospital Cancer Center. Informed consent was obtained from all patients. This trial was registered at ClinicalTrials.gov (NCT01398501). Patients were between the ages of 18 and 75, had a diagnosis of AML with a FLT3-ITD mutation, underwent first allogeneic HSCT, and had an Eastern Cooperative Oncology Group performance status score of 0–2. Any conditioning regimen and GVHD prophylaxis regimen was allowed and left at the discretion of the treating physician. Donors were 7/8 or 8/8 HLA-matched related or unrelated, double umbilical cord blood, or haploidentical. Stem cell sources were peripheral blood or bone marrow grafts. No pre-HSCT disease status was specified, but patients were only eligible if they were in morphological complete remission by bone marrow biopsy assessment after day +30, and if they had all-cell peripheral blood chimerism ≥ 70% of donor origin. Participants had recovered hematopoietic function defined by absolute neutrophil count (ANC) ≥ 1000 / µl without growth factor (G-CSF) support in the previous 7 days and platelet count ≥ 50,000 / µl without transfusion support in the previous 7 days. Patients with recent significant bleeding events, uncontrolled hypertension, serious non-healing wounds and active GVHD requiring ≥ 0.5 mg/kg/day of prednisone (or equivalent) or therapy beyond systemic corticosteroids were excluded. Use of sorafenib or other FLT3 inhibitors prior to HSCT was allowed.
Treatment
Sorafenib was started between 45 and 120 days after HSCT to allow for variable recovery from the conditioning regimen and other toxicities. Sorafenib was given daily in 28-day cycles by continuous dosing. Patients were assigned a fixed dose based on cohort with no dose escalations allowed with dose interruptions and reductions defined below. Patients were seen weekly during the first cycle to assess for toxicity and to collect correlative studies. Patients were then evaluated monthly within the first week of each subsequent cycle. After completion of 12 cycles of therapy, continuation of sorafenib was allowed at the discretion of the treating physician.
Correlative Studies
Correlative studies were performed to analyze pharmacokinetic (PK) and pharmacodynamic (PD) effects of sorafenib in the HSCT patient population. Whole blood from patients at designated time points was collected into heparinized vacuum tubes. The samples were centrifuged and the plasma was stored frozen while awaiting analysis, and shipped in batches to the reference laboratory at Johns Hopkins University. Three assays were performed on each sample: the plasma inhibitory activity (PIA) assay for FLT3, FLT3 ligand concentration, and sorafenib concentration as described.22 PIA data takes into account protein binding, active metabolite levels and cytokine levels, which may influence target sensitivity to inhibition.
Statistical Methods
A standard 3+3 study design was used with the primary endpoint to determine the maximum tolerated dose (MTD) of sorafenib in the post-HSCT setting. Three escalating doses of sorafenib were used: 200 mg PO BID (dose level 1), 400 mg PO QAM / 200 mg PO QPM (dose level 2), and 400 mg PO BID (dose level 3). The period of dose-limiting toxicity (DLT) evaluation was the first 28-day cycle. Patients were considered not evaluable for the determination of MTD if they were removed from the study for reasons unrelated to therapy or died within 28 days of starting treatment without developing a DLT, and the cause of death was unrelated to sorafenib. Any occurrence of NCI Common Terminology Criteria for Adverse Events (CTCAE v4.0) grade 3 or 4 adverse event (AE) mandated temporary hold of sorafenib with investigation conducted as to etiology of the AE. DLT was defined as drug-related AE which did not resolve to grade or less in 14 days or recurs after resuming sorafenib. Exceptions included grade 3 nausea, vomiting, or diarrhea that lasted < 48 hours and responded to medical intervention or grade 3 hypertension which responded to medical therapy. Lastly, hematological DLTs only included grade 4 neutropenia or thrombocytopenia. Dose reductions were undertaken for recurrent AEs or if AE required > 14 days to resolve without any other explanation. Only two dose reductions were allowed per participant and the lowest dose allowed was 200 mg daily. After the MTD was established, an additional 10 patients were treated at the MTD. Secondary endpoints included progression-free survival (PFS), overall survival (OS) and cumulative incidence rates of acute and chronic GVHD.
PFS and OS were estimated using the Kaplan-Meier method. PFS was calculated from the date of transplant to disease progression or death from any cause. Patients who were alive without relapse or progression were censored at the time of last clinical evaluation. OS was calculated from the date of transplant to death or censored at last clinical evaluation. Incidence of acute GVHD prior to and after the start of sorafenib was reported. The cumulative incidence of chronic GVHD (all events occurred after the start of sorafenib) was estimated with early relapse or death as competing risks.
RESULTS
Patients
Twenty-two patients were enrolled with clinical characteristics shown in Table 1. The median age was 54 (range, 20–67) with 6 patients older than the age of 60. Three patients were in second complete remission (CR2) and 16 patients were in CR1 at the time of HSCT. Three patients were classified as having primary refractory disease although two of them had achieved < 5% bone marrow blasts prior to HSCT through the use of salvage single-agent sorafenib. The third patient was aplastic after conventional reinduction chemotherapy without evidence of hematopoietic recovery. One additional patient received sorafenib prior to HSCT as part of a clinical trial combining sorafenib with conventional induction chemotherapy. In total, 3 of the 22 patients received sorafenib prior to HSCT, and no other patients were treated with any other FLT3 inhibitors. Fifteen patients had a normal karyotype, and 12 patients had a concurrent nucleophosmin1 (NPM1) mutation. Twelve patients underwent myeloablative conditioning while 10 patients were treated with reduced intensity conditioning. Nineteen patients received grafts from HLA-matched related or unrelated donors (2 bone marrow, 17 peripheral blood stem cells). One patient each received grafts from a single antigen mismatched unrelated donor, double umbilical cord blood, and haploidentical bone marrow, respectively.
Table 1.
Clinical Characteristics (n=22)
| Age | Karyotype | FLT3 | NPM1 | Disease Status |
Conditioning Regimen |
Donor Type |
GVHD ppx |
Outcome |
|---|---|---|---|---|---|---|---|---|
| 54 | Normal | ITD | Mutant | Refractory1 | CyTBI | MUD | Tac/Mtx | Died (relapse) |
| 20 | Normal | ITD | WT | Refractory1 | BuCy | MUD | CsA Mtx | Died (relapse) |
| 54 | +8 | ITD | Mutant | Refractory2 | CyTBI | MRD | Tac/Mtx | Died (NRM) |
| 62 | t(1; 14) | ITD | Mutant | CR2 | BuFlu (RIC) | MUD | Tac/Mtx | Alive (CR) |
| 35 | Normal | ITD | Mutant | CR2 | Flu/Mel/TBI | dUCB | Tac/Siro | Alive (CR) |
| 49 | +6p, −21 | ITD | WT | CR2 | BuCy | MUD | Tac/Mtx | Alive (CR) |
| 21 | Normal | ITD | Unknown | CR1 | BuCy | MUD | Tac/Mtx/ATG | Alive (CR) |
| 54 | Normal | ITD | Mutant | CR1 | CyTBI | MRD | Tac/Mtx | Alive (CR) |
| 49 | Normal | ITD | Mutant | CR1 | BuCy | MUD | Tac/Mtx/ATG | Alive (CR) |
| 42 | Normal | ITD | Mutant | CR1 | CyTBI | MUD | Tac/Mtx | Alive (CR) |
| 62 | Normal | ITD | Mutant | CR1 | BuFlu (RIC) | MUD | Tac/Siro/Mtx | Alive (CR) |
| 58 | Normal | ITD | WT | CR1 | BuClo (RIC) | MRD | Tac/Mtx | Alive (CR) |
| 53 | +8 | ITD | Unknown | CR1 | FluMel (RIC) | MRD | Tac/Mtx | Alive (CR) |
| 67 | Normal | ITD | WT | CR1 | FluCyTBI (RIC) | Haplo | Cy/Tac/MMF | Alive (CR) |
| 59 | Normal | ITD | Mutant | CR1 | BuClo (RIC) | MRD | Tac/Mtx | Alive (CR) |
| 34 | Normal | ITD | WT | CR1 | BuCy | MRD | Tac/Mtx | Alive (Relapsed) |
| 40 | Normal | ITD | Mutant | CR1 | BuCy | MUD | Tac/Mtx | Alive (CR) |
| 65 | Unknown | ITD | Mutant | CR1 | BuClo (RIC) | MUD | Tac/Mtx | Alive (CR) |
| 54 | Normal | ITD | WT | CR1 | BuCy | MRD | Tac/Mtx | Alive (CR) |
| 50 | t(2;3) | ITD | WT | CR1 | BuCy | MUD | Tac/Mtx | Alive (CR) |
| 62 | +11 | ITD | WT | CR1 | BuFlu (RIC) | MMUD | Tac/Mtx/ATG | Alive (CR) |
| 63 | Normal | ITD | Mutant | CR1 | BuFlu (RIC) | MRD | Tac/Mtx | Alive (CR) |
Achieved < 5% bone marrow blasts with sorafenib prior to HSCT
In BM aplasia after reinduction chemotherapy
Abbreviations:
GVHD = graft-vs.-host disease; ppx = prophylaxis; Cy = cyclophosphamide; TBI = total body irradiation; MUD = matched unrelated donor; Tac = tacrolimus; Mtx = methotrexate; NPM1 = nucleophosmin 1; WT = wild-type; Bu = busulfan; CsA = cyclosporine; MRD = matched related donor; NRM = non-relapsed mortality; t = translocation; CR = complete remission; Flu = fludarabine; RIC = reduced intensity conditioning; Mel = melphalan; dUCB = double umbilical cord blood; Siro = sirolimus; ATG = anti-thymocyte globulin; Clo = clofarabine; Haplo = haploidentical; MMF = mycophenolate mofetil; MMUD = mismatched unrelated donor
Toxicity / GVHD
The median day of starting sorafenib was 69.5 days after HSCT (range, 46–112). Three patients were treated on the first cohort (200 mg BID), three patients on the second cohort (400 mg QAM / 200 mg QPM), and six patients on the third cohort (400 mg BID). One DLT was observed at the 400 mg BID dose. This was a pericardial effusion which on subsequent evaluation was deemed unlikely to be related to sorafenib. The MTD, based on the initial 28-day DLT period, was found to be 400 mg BID. Ten additional patients were then treated at the 400 mg BID dose, making a total of 16 patients treated at the 400 mg BID dose. Nine patients finished the planned 12 cycles of therapy and 3 patients remain on active therapy, all of whom have been on sorafenib therapy for at least 6 cycles. Five patients stopped sorafenib due to causes unrelated to sorafenib including two cases of relapsed AML, one case of nephrotic syndrome, one case of graft failure, and one case of idiopathic pneumonia syndrome. Five patients stopped sorafenib after the initial 28-day DLT period due to related toxicities which included weight loss, tongue / facial swelling, and persistent gastrointestinal symptoms manifesting mainly as nausea and diarrhea.
Of the 16 patients treated at the MTD of 400 mg BID, 4 have not required a dose reduction - 3 finished all 12 cycles and 1 remains on active therapy; 6 patients required two dose reductions to 200 mg BID which was then much better tolerated; the remaining 5 patients came off therapy – 1 DLT, 1 relapse, 3 due to GI toxicity even at the 200 mg BID dose. As shown in Table 2, the most common toxicities observed were skin rashes and GI symptoms which improved with drug interruptions or dose reductions. The majority of the skin rashes appeared clinically consistent with acute GVHD; however, they all resolved with temporary holding of sorafenib. There were no obvious effects of sorafenib on tacrolimus drug levels; however, the single patient who was taking cyclosporine did have a significant increase in her level after starting sorafenib.
Table 2.
Clinically significant toxicities (CTCAE v4.3 grades 2–4) encountered (n=22 patients)
| Toxicity | Grade 2 | Grade 3 | Grade 4 | Total (2–4) |
|---|---|---|---|---|
| Rash | 4 | 4 | 0 | 8 |
| Diarrhea | 6 | 1 | 0 | 7 |
| Anemia | 1 | 5 | 0 | 6 |
| Nausea | 4 | 1 | 0 | 5 |
| Thrombocytopenia | 3 | 2 | 0 | 5 |
| Weight loss | 3 | 2 | 0 | 5 |
| Abdominal pain | 1 | 3 | 0 | 4 |
| Hypertension | 1 | 3 | 0 | 4 |
| Anorexia | 3 | 0 | 0 | 3 |
| Arthralgia/Myalgias | 4 | 1 | 0 | 3 |
| Fatigue | 3 | 0 | 0 | 3 |
| Leukopenia | 1 | 1 | 1 | 3 |
| Transaminitis | 2 | 1 | 0 | 3 |
| Dyspnea | 2 | 0 | 0 | 2 |
| Hand-Foot | 2 | 0 | 0 | 2 |
| Headache | 1 | 1 | 0 | 2 |
| Sensory neuropathy | 2 | 0 | 0 | 2 |
| Pericardial effusion | 0 | 0 | 1 | 1 |
| Vomiting | 1 | 0 | 0 | 1 |
Four patients had acute GVHD requiring systemic therapy prior to starting sorafenib, and none of these patients experienced a flare of acute GVHD symptoms while on sorafenib. Of the remaining 18 patients, only one patient developed acute GVHD after starting sorafenib and this was grade II disease of the skin treated with topical therapy. The cumulative incidence of any chronic GVHD in the 12 months after starting sorafenib was 38% (90% CI, 21%, 56%).
Outcomes
There have been 3 relapses observed thus far. Two of these were the two patients with primary refractory AML who had achieved < 5% bone marrow blasts with single agent sorafenib prior to myeloablative HSCT. The first relapse occurred 13 months after HSCT. This patient had taken sorafenib for 5 months but then discontinued therapy for the 6 months prior to relapse due to the development of nephrotic syndrome. The FLT3-ITD was found to be present at relapse and the patient died shortly thereafter. The second relapse occurred 12 months after HSCT while the patient was still taking 200 mg BID of sorafenib. The FLT3-ITD was also present in this case at relapse and she died 10 months later. The third relapse occurred in a patient who was in CR1 prior to HSCT while taking sorafenib 400 mg BID at 6 months after HSCT. FLT3-ITD was present at relapse and he remains alive on other treatment.
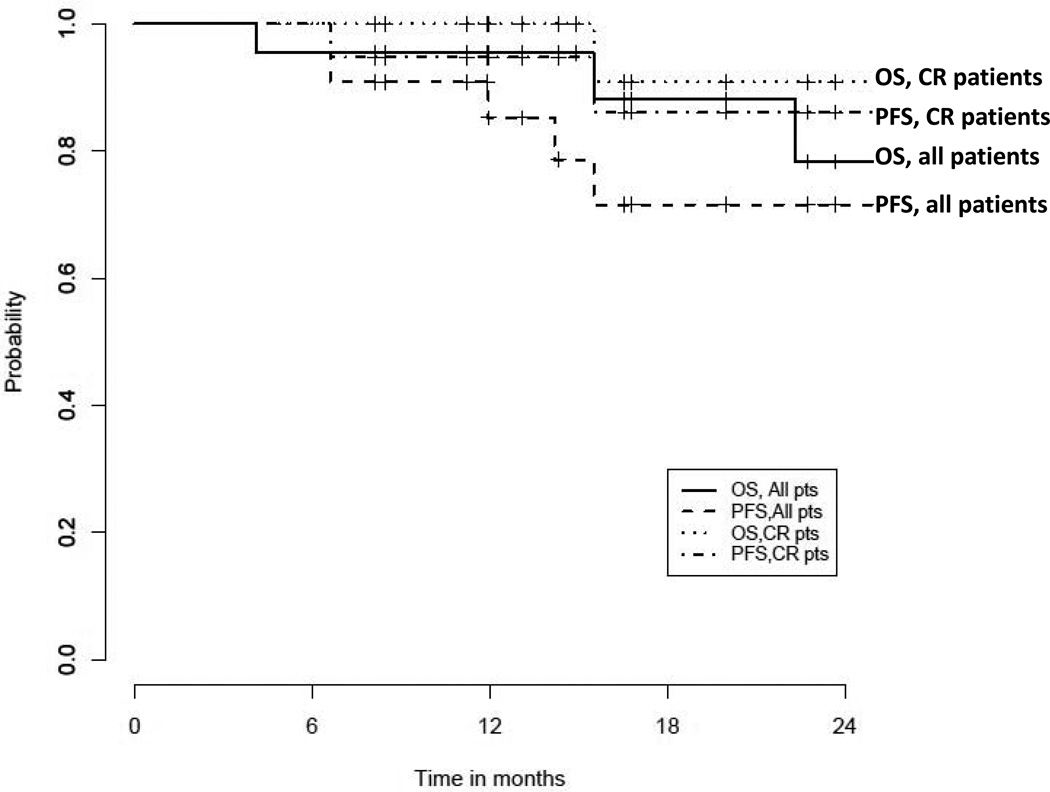
There have been a total of four deaths. All three patients with primary refractory disease prior to HSCT have died – two from relapse described above and the other from idiopathic pneumonia syndrome (IPS) occurring about 3 months after HSCT. The last death was in a patient who survived 15 months after HSCT with no evidence of recurrent AML, but who did develop secondary graft failure and subsequently died of cirrhosis from secondary iron overload. Sorafenib had been stopped for 12 months prior to his death due to unrelated cytopenias. For the entire cohort, 1-year PFS is 85% (90% CI, 66%–94%) and 1-year OS is 95% (90% CI, 79%–99%) while 2-year PFS is 72% (49%–86%) and 2-year OS is 78% (51%–91%) (Figure 1). For the 19 patients who were in a conventional complete remission (CR1/CR2) prior to HSCT, 1-year PFS is 95% (90% CI, 76%–99% and 1-year OS is 100%, while 2-year PFS is 86% (90% CI, 61%–96%) and 2-year OS is 78% (51%–91%) (Figure 1).
Figure 1.
PFS and OS for all patients (n=22) and for patients in CR1/CR2 (n=19)
Correlative Studies
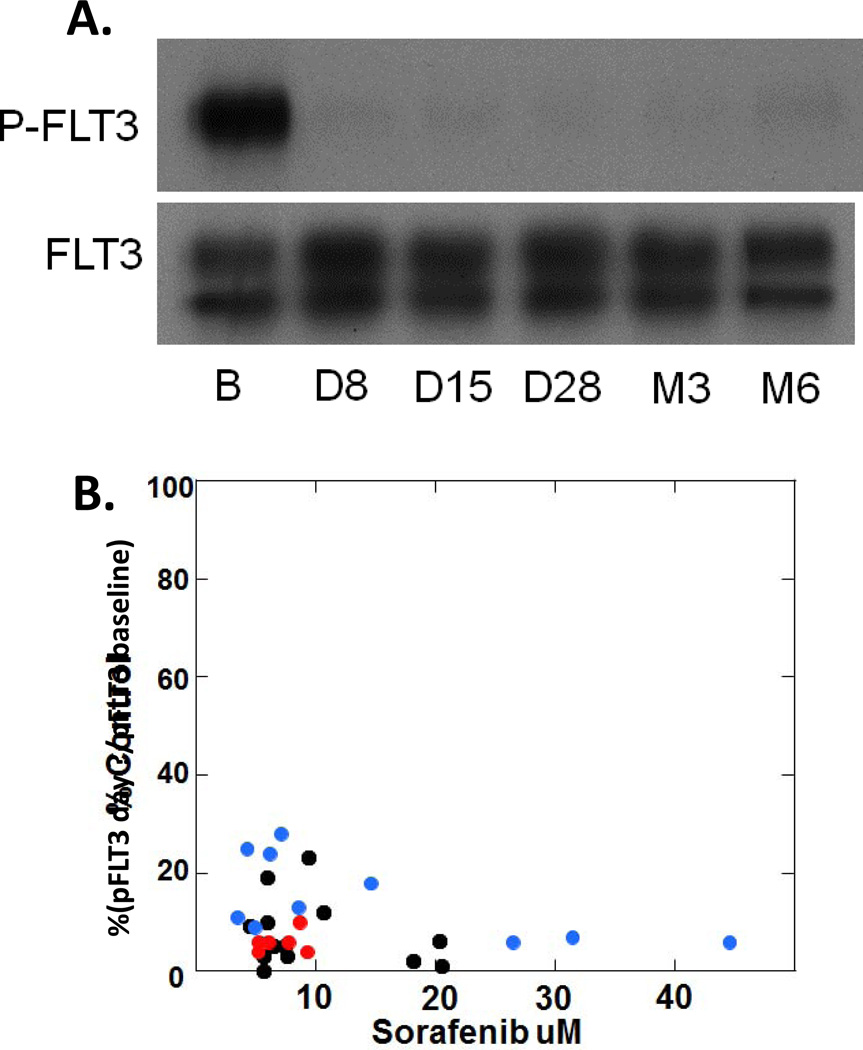
Serial peripheral blood samples were collected from available patients at established timepoints. FLT3 inhibitory activity was examined in a plasma inhibitory activity assay (PIA) using an FLT3 mutant cell line to assess target inhibition potential ex vivo. FLT3 inhibition was observed in patients at all dose levels tested as shown in Figure 2A. Inhibition of P-FLT3 (phosphorylated FLT3) to less than 15% of baseline was observed in 25 out of 30 samples evaluated by PIA. There was no clear correlation between effective inhibition and dose level (Figure 2B).
Figure 2.
A. Plasma inhibitory activity (PIA) assay of a patient treated with 200 mg twice daily sorafenib. B = baseline; D = treatment day (e.g., C1D8 = Day 8 of cycle 1); M3 = Cycle 3; M6 = Cycle 6. B. PIA results (expressed as % P-FLT3 at baseline) plotted against plasma sorafenib levels measured from the same time points. The color of the dot denotes the sorafenib dose the patient was receiving at the time of the measurement (not the original cohort assigned). Black dots = 200 mg twice daily; red dots = 400 mg in the AM, 200 mg in the PM; blue dots = 400 mg twice daily. Note that several patients had multiple samples represented at different time points.
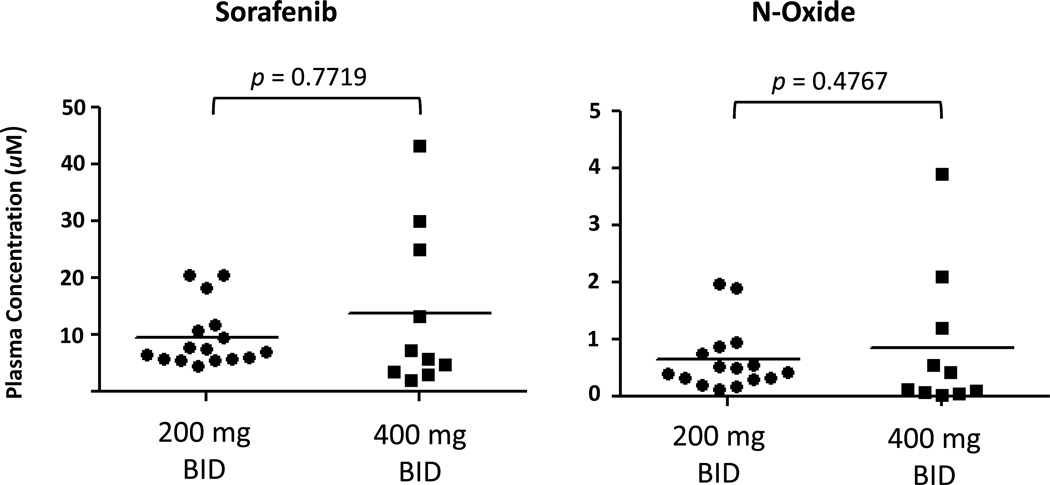
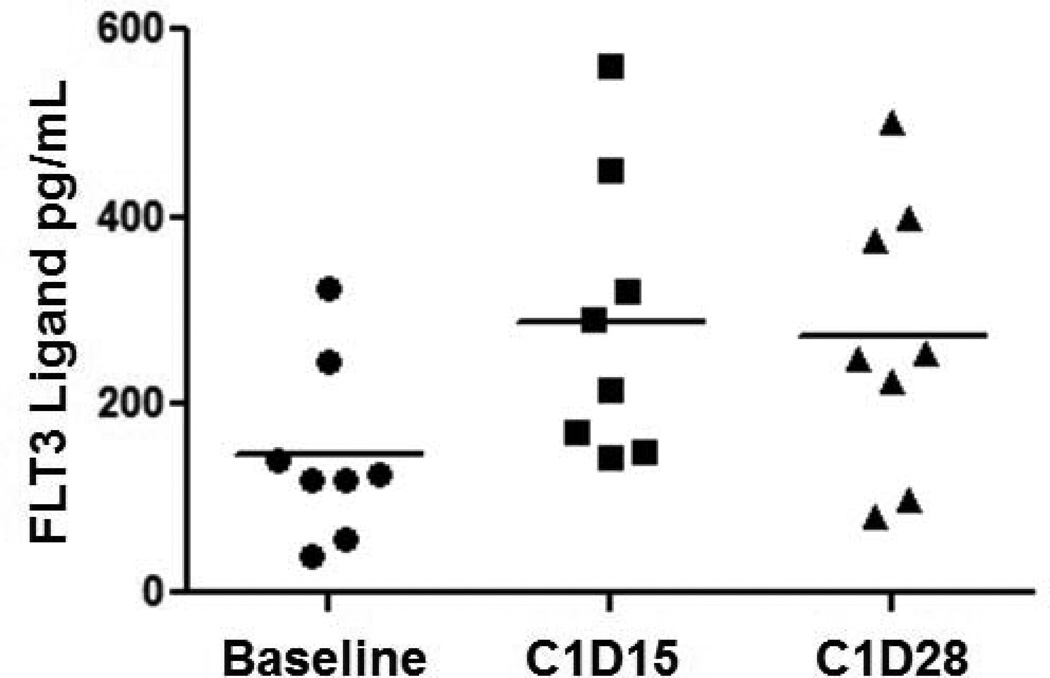
Pharmacokinetic analysis was performed on concentrations of both sorafenib and its metabolite sorafenib N-oxide. There was no significant correlation between concentration of either sorafenib or sorafenib N-oxide with dose of sorafenib administered, and both exhibited moderate inter-individual variability (Figure 3). Plasma concentrations of FLT3 ligand were also measured serially in 7 patients. Median level at baseline and prior to any sorafenib administration was 125 pg/ml (range 40–323) and this significantly increased to a median level of 254 pg/ml (range 80–500) (p = 0.016) measured on day 29 of cycle 1 (Figure 4).
Figure 3.
Pharmacokinetics of sorafenib and N-oxide. Plasma concentrations of sorafenib and N-oxide were measured at several time points. Note that several patients had multiple samples represented at different time points.
Figure 4.
Plasma levels of FLT3 ligand in patients treated with sorafenib. (p =0.016 comparing baseline to day 28 levels)
DISCUSSION
Sorafenib is an oral tyrosine kinase inhibitor which is FDA-approved for the treatment of metastatic hepatocellular, thyroid, and renal cell carcinoma with inhibitory activity demonstrated against the FLT3 tyrosine kinase. We conducted a phase I trial to define the maximum tolerated dose of sorafenib given as maintenance therapy after HSCT as there has been very little experience using sorafenib soon after HSCT and before relapse of AML. In addition, one previous study had suggested that use of sorafenib after HSCT might potentially trigger significant rates of acute GVHD.23 While the number of patients we studied is small, our results are compelling, showing good tolerability of sorafenib at doses between 200–400 mg BID. In addition, 1-year PFS of 85% for all patients, including several patients transplanted beyond CR1 and many undergoing reduced intensity HSCT. Most notably, out of the 19 patients who had achieved a conventional CR1 or CR2 prior to transplant, there has been only one relapse, yielding a 1-year PFS of 95% and 2-year PFS of 86% at a median follow-up of 16.7 months after HSCT.
Increasingly, HSCT in CR1 has become the standard treatment for patients with FLT3-ITD AML. This is based on the results of several retrospective analyses which have suggested superior outcomes with HSCT compared to standard chemotherapy approaches.8, 9, 10, 11, 12, 13 To date the largest series describing the outcome of FLT3-ITD AML after HSCT is an observational study from the European Group for Blood and Marrow Transplantation (EBMT) registry which analyzed 120 younger patients in CR1 who underwent myeloablative matched related or unrelated donor HSCT. This study demonstrated a 2-year DFS of 58% with the majority of relapses occurring in the first year after HSCT.13 Thus, relapse remains the biggest obstacle to achieving long-term survival.
Use of sorafenib around HSCT was first reported by Metzelder et al. in 6 patients with FLT3-ITD AML showing that it induced meaningful and rapid responses in overt leukemia either before or after HSCT.24 Others have also described case reports illustrating the ability of sorafenib to bring about sustained remissions at relapse of FLT3-ITD AML after HSCT.25, 26 A larger report of 16 patients by Sharma et al., however, suggested that most responses achieved at relapse after HSCT are transient.27 In contrast, Metzelder et al. published a registry analysis of 65 patients with FLT3-ITD AML treated with sorafenib at 23 centers, of which 29 had undergone prior HSCT. Findings showed that patients with prior HSCT developed sorafenib resistance less frequently, and at a later time relative to patients without prior HSCT. Additionally, cases of sustained remission were seen exclusively in those who had previously undergone HSCT.28
The optimal time to deliver sorafenib or other FLT3 inhibitors for patients with FLT3-ITD AML is unclear. Recently, several clinical trials have incorporated sorafenib in combination with conventional chemotherapy,29, 30, 31, 32 yet none have shown any clear benefit with the addition of sorafenib. Administering sorafenib in the maintenance setting rather than in combination with chemotherapy is supported by the observation that chemotherapy-induced marrow aplasia leads to elevated FLT3-ligand levels that may overwhelm on-target activity of any FLT3 inhibitor.21, 33 In addition, the observation that the only sustained remissions achieved with sorafenib in relapsed FLT3-ITD AML have been in patients following HSCT suggest that sorafenib can potentially synergize with allo-immune effects provided by the donor graft,28 and raise the possibility that inhibition of FLT3 is not the only mechanism of action. For instance, direct donor T-cell activation by sorafenib was suggested in a mouse model23 and FLT3-ligand induced activation of dendritic cells34 may stimulate graft-vs.-leukemia (GVL). As shown here, the administration of sorafenib does induce a significant increase in FLT3-ligand, although such levels are an order of magnitude less than levels measured during induction chemotherapy. Other studies have shown that the exogenous administration of FLT3 ligand causes an increase in proliferation of regulatory T-cells leading to inhibition of GVHD,35, 36, 37 however, the levels of FLT3 ligand described in these studies are far greater than what was measured here. Lastly, a variety of kinases in addition to FLT3 are inhibited by sorafenib, and these actions could obviously be playing a role in maintaining remission.
The use of maintenance agents following HSCT is routine with available inhibitors of the BCR-ABL tyrosine kinase in patients with CML or Ph+ALL even without any prospective trials showing a clear benefit to such a strategy.38, 39 There is, however, clear evidence in patients with CML, that introducing BCR-ABL inhibitors at the time of detectable minimal residual disease after HSCT can induce long-term remissions.40 Thus, many practitioners routinely incorporate TKIs into standard post-HSCT care. As an increasing number of targeted and well-tolerated therapies are developed which do not cause significant cytopenias or immunosuppression, the paradigm of maintenance therapy after HSCT will likely become increasingly popular, leading to the need to conduct careful clinical trials to assess the efficacy, tolerability, and cost-effectiveness of such an approach.
The goal of this phase I study was to study the safety and to define the maximum tolerated dose of sorafenib when given as maintenance following HSCT. It is limited by the small sample size and heterogeneity of patient characteristics, yet the inclusion of patients who are older, in CR2, and received reduced intensity conditioning regimens should only lead to increased rates of relapse compared to other series such as the previously mentioned EBMT registry study. From our experience, it does appear that sorafenib is safe when started in the first 120 days after HSCT. One previous report had cautioned against potentially inducing GVHD based on a mouse model23, but we observed no episodes of significant acute GVHD after starting sorafenib and a comparable incidence of chronic GVHD relative to our historical experience (20–40%). Upon starting sorafenib therapy, several patients experienced an erythematous skin rash which resembled acute GVHD clinically; however, the majority of such rashes resolved when sorafenib was temporarily held. Drug interruptions and dose reductions were common and mostly due to skin and GI toxicities, similar to side effects attributed to sorafenib in other clinical studies. Unfortunately, initial AML samples were not available for analysis. We therefore lack information on the FLT3-ITD allelic ratio at diagnosis, which some have demonstrated to be prognostically important.41, 42 However, it should be noted that there is currently no standardized manner in which to measure the allelic ratio,5 and, thus, incorporating this into future trials may not be feasible.
In conclusion, maintenance therapy with sorafenib following allogeneic HSCT for patients with FLT3-ITD AML is safe and feasible. The maximum tolerated dose was found to be 400 mg twice daily, although drug interruptions and dose reductions were common and FLT3 inhibition appeared to be as effective at the 200 mg twice daily dose as well, which was better tolerated. Our rates of progression-free and overall survival compare favorably with the historical experience. This is especially the case for patients in a conventional complete remission prior to HSCT, where only one of 19 cases has relapsed. Maintenance therapy with sorafenib after HSCT for patients with FLT3-ITD AML merits further study, and a large multicenter study is planned.
Highlights.
Sorafenib is safe to administer as maintenance therapy after HSCT for FLT3-ITD AML.
The MTD of sorafenib was 400 mg BID, but 200 mg BID was better tolerated.
Maintenance sorafenib for FLT3-ITD AML after HSCT is promising.
Acknowledgements
Dr. Chen reports receiving support for this clinical trial in terms of funding and drug supply from Bayer Pharmaceuticals, Inc.
SL’s work is partially supported by NIH grant CA 006516. This work was supported in part by the NCI Leukmia SPORE P50 CA100632-11, the Jock and Bunny Adams Research and Education Endowment and the Analytical Pharmacology Core of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins (M.A.R.; NIH grants P30 CA006973 and UL1 RR025005; Shared Instrument Grant (1S10RR026824-01). This publication was made possible in part by Grant Number UL1RR025005 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. Preliminary results of this study were presented in abstract form at the 2014 American Society of Bone Marrow Transplant Tandem Meetings, Grapevine, TX. Feburary 25th-March 2nd, 2014. The authors would like to thank Dr. Timothy Graubert for his thoughtful review of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures/COI: The remaining authors report no relevant conflicts of interest.
Authorship: Y.C. and R.S. designed the research, enrolled patients, analyzed data, and wrote the paper. S.L. designed the research, analyzed data and wrote the paper. A.A.L. designed the research, collected data, and wrote the paper. C.C., C.D.R., B.V., and M.C. collected data and wrote the paper. K.B., C.C., B.R.D., A.E.J., A.T.F., V.T.H., A.J., and S.M. enrolled patients and wrote the paper. M.R., T.R., and S.V. collected and analyzed data and wrote the paper. J.A. and T.R.S. designed the research and wrote the paper. M.L. designed the research, analyzed data, and wrote the paper.
REFERENCES
- 1.Gilliland DG, Griffin JD. The roles of FLT3 in hematopoiesis and leukemia. Blood. 2002;100(5):1532–1542. doi: 10.1182/blood-2002-02-0492. [DOI] [PubMed] [Google Scholar]
- 2.Kiyoi H, Towatari M, Yokota S, Hamaguchi M, Ohno R, Saito H, et al. Internal tandem duplication of the FLT3 gene is a novel modality of elongation mutation which causes constitutive activation of the product. Leukemia. 1998;12(9):1333–1337. doi: 10.1038/sj.leu.2401130. [DOI] [PubMed] [Google Scholar]
- 3.Levis M, Small D. FLT3: ITDoes matter in leukemia. Leukemia. 2003;17(9):1738–1752. doi: 10.1038/sj.leu.2403099. [DOI] [PubMed] [Google Scholar]
- 4.Fathi AT, Chen YB. Treatment of FLT3-ITD acute myeloid leukemia. American journal of blood research. 2011;1(2):175–189. [PMC free article] [PubMed] [Google Scholar]
- 5.Levis M. FLT3 mutations in acute myeloid leukemia: what is the best approach in 2013? Hematology / the Education Program of the American Society of Hematology American Society of Hematology Education Program. 2013;2013:220–226. doi: 10.1182/asheducation-2013.1.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gale RE, Hills R, Kottaridis PD, Srirangan S, Wheatley K, Burnett AK, et al. No evidence that FLT3 status should be considered as an indicator for transplantation in acute myeloid leukemia (AML): an analysis of 1135 patients, excluding acute promyelocytic leukemia, from the UK MRC AML10 and 12 trials. Blood. 2005;106(10):3658–3665. doi: 10.1182/blood-2005-03-1323. [DOI] [PubMed] [Google Scholar]
- 7.Sengsayadeth SM, Jagasia M, Engelhardt BG, Kassim A, Strickland SA, Goodman S, et al. Allo-SCT for high-risk AML-CR1 in the molecular era: impact of FLT3/ITD outweighs the conventional markers. Bone Marrow Transplant. 2012;47(12):1535–1537. doi: 10.1038/bmt.2012.88. [DOI] [PubMed] [Google Scholar]
- 8.Bornhauser M, Illmer T, Schaich M, Soucek S, Ehninger G, Thiede C. Improved outcome after stem-cell transplantation in FLT3/ITD-positive AML. Blood. 2007;109(5):2264–2265. doi: 10.1182/blood-2006-09-047225. author reply 2265. [DOI] [PubMed] [Google Scholar]
- 9.DeZern AE, Sung A, Kim S, Smith BD, Karp JE, Gore SD, et al. Role of allogeneic transplantation for FLT3/ITD acute myeloid leukemia: outcomes from 133 consecutive newly diagnosed patients from a single institution. Biol Blood Marrow Transplant. 2011;17(9):1404–1409. doi: 10.1016/j.bbmt.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laboure G, Dulucq S, Labopin M, Tabrizi R, Guerin E, Pigneux A, et al. Potent graft-versus-leukemia effect after reduced-intensity allogeneic SCT for intermediate-risk AML with FLT3-ITD or wild-type NPM1 and CEBPA without FLT3-ITD. Biol Blood Marrow Transplant. 2012;18(12):1845–1850. doi: 10.1016/j.bbmt.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 11.Lin PH, Lin CC, Yang HI, Li LY, Bai LY, Chiu CF, et al. Prognostic impact of allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia patients with internal tandem duplication of FLT3. Leuk Res. 2013;37(3):287–292. doi: 10.1016/j.leukres.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Schlenk RF, Dohner K, Krauter J, Frohling S, Corbacioglu A, Bullinger L, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358(18):1909–1918. doi: 10.1056/NEJMoa074306. [DOI] [PubMed] [Google Scholar]
- 13.Brunet S, Labopin M, Esteve J, Cornelissen J, Socie G, Iori AP, et al. Impact of FLT3 internal tandem duplication on the outcome of related and unrelated hematopoietic transplantation for adult acute myeloid leukemia in first remission: a retrospective analysis. J Clin Oncol. 2012;30(7):735–741. doi: 10.1200/JCO.2011.36.9868. [DOI] [PubMed] [Google Scholar]
- 14.Knapper S, Mills KI, Gilkes AF, Austin SJ, Walsh V, Burnett AK. The effects of lestaurtinib (CEP701) and PKC412 on primary AML blasts: the induction of cytotoxicity varies with dependence on FLT3 signaling in both FLT3-mutated and wild-type cases. Blood. 2006;108(10):3494–3503. doi: 10.1182/blood-2006-04-015487. [DOI] [PubMed] [Google Scholar]
- 15.Levis M, Allebach J, Tse KF, Zheng R, Baldwin BR, Smith BD, et al. A FLT3-targeted tyrosine kinase inhibitor is cytotoxic to leukemia cells in vitro and in vivo. Blood. 2002;99(11):3885–3891. doi: 10.1182/blood.v99.11.3885. [DOI] [PubMed] [Google Scholar]
- 16.Levis M, Smith BD, Beran M, Baer MR, Erba HP, Cripe L, et al. A randomized, open-label study of lestaurtinib (CEP-701), an oral FLT3 inhibitor, administered in sequence with chemotherapy in patients with relapsed AML harboring FLT3 activating mutations: Clinical response correlates with successful FLT3 inhibition. Blood. 2005;106:121a. [Google Scholar]
- 17.Ravandi F, Cortes JE, Jones D, Faderl S, Garcia-Manero G, Konopleva MY, et al. Phase I/II study of combination therapy with sorafenib, idarubicin, and cytarabine in younger patients with acute myeloid leukemia. Journal of Clinical Oncology. 2010;28(11):1856–1862. doi: 10.1200/JCO.2009.25.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stone RM, DeAngelo DJ, Klimek V, Galinsky I, Estey E, Nimer SD, et al. Patients with acute myeloid leukemia and an activating mutation in FLT3 respond to a small-molecule FLT3 tyrosine kinase inhibitor, PKC412. Blood. 2005;105(1):54–60. doi: 10.1182/blood-2004-03-0891. [DOI] [PubMed] [Google Scholar]
- 19.Yee KW, O’Farrell AM, Smolich BD, Cherrington JM, McMahon G, Wait CL, et al. SU5416 and SU5614 inhibit kinase activity of wild-type and mutant FLT3 receptor tyrosine kinase. Blood. 2002;100(8):2941–2949. doi: 10.1182/blood-2002-02-0531. [DOI] [PubMed] [Google Scholar]
- 20.Cortes J, Foran J, Ghirdaladze D, DeVetten MP, Zodelava M, Holman P, et al. AC220, a potent, selective, second generation FLT3 receptor tyrosine kinase (RTK) inhibitor, in a first-in-human (FIH) phase 1 AML study. Blood (ASH Annual Meeting Abstracts) 2009;114 Abstract 636. [Google Scholar]
- 21.Sato T, Yang X, Knapper S, White P, Smith BD, Galkin S, et al. FLT3 ligand impedes the efficacy of FLT3 inhibitors in vitro and in vivo. Blood. 2011;117(12):3286–3293. doi: 10.1182/blood-2010-01-266742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ravandi F, Alattar ML, Grunwald MR, Rudek MA, Rajkhowa T, Richie MA, et al. Phase 2 study of azacytidine plus sorafenib in patients with acute myeloid leukemia and FLT-3 internal tandem duplication mutation. Blood. 2013;121(23):4655–4662. doi: 10.1182/blood-2013-01-480228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yokoyama H, Lundqvist A, Su S, Childs R. Toxic effects of sorafenib when given early after allogeneic hematopoietic stem cell transplantation. Blood. 2010;116(15):2858–2859. doi: 10.1182/blood-2010-06-291104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Metzelder S, Wang Y, Wollmer E, Wanzel M, Teichler S, Chaturvedi A, et al. Compassionate use of sorafenib in FLT3-ITD-positive acute myeloid leukemia: sustained regression before and after allogeneic stem cell transplantation. Blood. 2009;113(26):6567–6571. doi: 10.1182/blood-2009-03-208298. [DOI] [PubMed] [Google Scholar]
- 25.Kruger WH, Hirt C, Kiefer T, Neumann T, Busemann C, Dolken G. Molecular remission of FLT3-ITD(+) positive AML relapse after allo-SCT by acute GVHD in addition to sorafenib. Bone Marrow Transplant. 2012;47(1):137–138. doi: 10.1038/bmt.2011.7. [DOI] [PubMed] [Google Scholar]
- 26.Winkler J, Rech D, Kallert S, Rech J, Meidenbauer N, Roesler W, et al. Sorafenib induces sustained molecular remission in FLT3-ITD positive AML with relapse after second allogeneic stem cell transplantation without exacerbation of acute GVHD: a case report. Leuk Res. 2010;34(10):e270–e272. doi: 10.1016/j.leukres.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 27.Sharma M, Ravandi F, Bayraktar UD, Chiattone A, Bashir Q, Giralt S, et al. Treatment of FLT3-ITD-positive acute myeloid leukemia relapsing after allogeneic stem cell transplantation with sorafenib. Biol Blood Marrow Transplant. 2011;17(12):1874–1877. doi: 10.1016/j.bbmt.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Metzelder SK, Schroeder T, Finck A, Scholl S, Fey M, Gotze K, et al. High activity of sorafenib in FLT3-ITD-positive acute myeloid leukemia synergizes with allo-immune effects to induce sustained responses. Leukemia. 2012;26(11):2353–2359. doi: 10.1038/leu.2012.105. [DOI] [PubMed] [Google Scholar]
- 29.Ravandi F, Cortes JE, Jones D, Faderl S, Garcia-Manero G, Konopleva MY, et al. Phase I/II study of combination therapy with sorafenib, idarubicin, and cytarabine in younger patients with acute myeloid leukemia. J Clin Oncol. 2010;28(11):1856–1862. doi: 10.1200/JCO.2009.25.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Macdonald DA, Assouline SE, Brandwein J, Kamel-Reid S, Eisenhauer EA, Couban S, et al. A phase I/II study of sorafenib in combination with low dose cytarabine in elderly patients with acute myeloid leukemia or high-risk myelodysplastic syndrome from the National Cancer Institute of Canada Clinical Trials Group: trial IND.186. Leuk Lymphoma. 2013;54(4):760–766. doi: 10.3109/10428194.2012.737917. [DOI] [PubMed] [Google Scholar]
- 31.Serve H, Krug U, Wagner R, Sauerland MC, Heinecke A, Brunnberg U, et al. Sorafenib in combination with intensive chemotherapy in elderly patients with acute myeloid leukemia: results from a randomized, placebo-controlled trial. J Clin Oncol. 2013;31(25):3110–3118. doi: 10.1200/JCO.2012.46.4990. [DOI] [PubMed] [Google Scholar]
- 32.Uy GL, Sanford B, Marcucci G, Zhao W, Geyer S, Klepin HD, et al. Initial Resutls of a Phase II Trial of Sorafenib Plus Standard Induction in Older Adults with Mutatnt FLT3 Acute Myeloid Leukemia (AML) (Alliance Trial C11001) American Society of Hematology Annual Meeting. 2013 abstract 2653. [Google Scholar]
- 33.Levis M, Ravandi F, Wang ES, Baer MR, Perl A, Coutre S, et al. Results from a randomized trial of salvage chemotherapy followed by lestaurtinib for patients with FLT3 mutant AML in first relapse. Blood. 2011;117(12):3294–3301. doi: 10.1182/blood-2010-08-301796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whartenby KA, Calabresi PA, McCadden E, Nguyen B, Kardian D, Wang T, et al. Inhibition of FLT3 signaling targets DCs to ameliorate autoimmune disease. Proc Natl Acad Sci U S A. 2005;102(46):16741–16746. doi: 10.1073/pnas.0506088102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klein O, Ebert LM, Zanker D, Woods K, Tan BS, Fucikova J, et al. Flt3 ligand expands CD4+ FoxP3+ regulatory T cells in human subjects. European journal of immunology. 2013;43(2):533–539. doi: 10.1002/eji.201242603. [DOI] [PubMed] [Google Scholar]
- 36.Swee LK, Bosco N, Malissen B, Ceredig R, Rolink A. Expansion of peripheral naturally occurring T regulatory cells by Fms-like tyrosine kinase 3 ligand treatment. Blood. 2009;113(25):6277–6287. doi: 10.1182/blood-2008-06-161026. [DOI] [PubMed] [Google Scholar]
- 37.Teshima T, Reddy P, Lowler KP, KuKuruga MA, Liu C, Cooke KR, et al. Flt3 ligand therapy for recipients of allogeneic bone marrow transplants expands host CD8 alpha(+) dendritic cells and reduces experimental acute graft-versus-host disease. Blood. 2002;99(5):1825–1832. doi: 10.1182/blood.v99.5.1825. [DOI] [PubMed] [Google Scholar]
- 38.Klyuchnikov E, Kroger N, Brummendorf TH, Wiedemann B, Zander AR, Bacher U. Current status and perspectives of tyrosine kinase inhibitor treatment in the posttransplant period in patients with chronic myelogenous leukemia (CML) Biol Blood Marrow Transplant. 2010;16(3):301–310. doi: 10.1016/j.bbmt.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 39.Kebriaei P, Saliba R, Rondon G, Chiattone A, Luthra R, Anderlini P, et al. Long-term follow-up of allogeneic hematopoietic stem cell transplantation for patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: impact of tyrosine kinase inhibitors on treatment outcomes. Biol Blood Marrow Transplant. 2012;18(4):584–592. doi: 10.1016/j.bbmt.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hess G, Bunjes D, Siegert W, Schwerdtfeger R, Ledderose G, Wassmann B, et al. Sustained complete molecular remissions after treatment with imatinib-mesylate in patients with failure after allogeneic stem cell transplantation for chronic myelogenous leukemia: results of a prospective phase II open-label multicenter study. J Clin Oncol. 2005;23(30):7583–7593. doi: 10.1200/JCO.2005.01.3110. [DOI] [PubMed] [Google Scholar]
- 41.Pratcorona M, Brunet S, Nomdedeu J, Ribera JM, Tormo M, Duarte R, et al. Favorable outcome of patients with acute myeloid leukemia harboring a low-allelic burden FLT3-ITD mutation and concomitant NPM1 mutation: relevance to post-remission therapy. Blood. 2013;121(14):2734–2738. doi: 10.1182/blood-2012-06-431122. [DOI] [PubMed] [Google Scholar]
- 42.Pratz KW, Sato T, Murphy KM, Stine A, Rajkhowa T, Levis M. FLT3-mutant allelic burden and clinical status are predictive of response to FLT3 inhibitors in AML. Blood. 2010;115(7):1425–1432. doi: 10.1182/blood-2009-09-242859. [DOI] [PMC free article] [PubMed] [Google Scholar]