Abstract
Acute myeloid leukemia (AML) patients harboring internal tandem duplication (ITD) mutations of the FMS-like tyrosine kinase 3 (FLT3) gene carry a poor prognosis. While allogeneic transplantation may improve outcomes, relapse occurs frequently. The FLT3/ITD mutation has been deemed an unsuitable minimal residual disease (MRD) marker because it is unstable and because the standard assay for the mutation is relatively insensitive. The FLT3 mutation is undetectable by polymerase chain reaction (PCR) at pre- or post-transplant time points in many FLT3/ITD AML patients who subsequently relapse following transplant. We report the application of a new technique, tandem duplication PCR (TD-PCR), for detecting MRD in FLT3/ITD AML patients. Between October 2004 and January 2012, 54 FLT3/ITD AML patients in remission underwent transplantation at our institution. Of 37 patients with available Day 60 marrow samples, 28 (76%) were evaluable for MRD detection. In seven (25%) of the 28 patients, the FLT3/ITD mutation was detectable by TD-PCR, but not by standard PCR, on day 60. Six out of the seven patients (86%) with MRD by TD-PCR have relapsed to date compared with only 2 of 21 (10%) patients who were negative for MRD (p = 0.0003). The ability to detect MRD by this sensitive technique may provide an opportunity for early clinical intervention.
Keywords: acute myeloid leukemia (AML), FLT3, internal tandem duplication (ITD), minimal residual disease (MRD), tandem duplication PCR (TD-PCR), bone marrow transplant
Introduction
FLT3/ITD mutations are present in approximately one quarter of adult AML cases. Patients with these mutations carry a particularly poor prognosis, with an estimated four-year overall survival of approximately 20 percent.1–4 For this reason, a number of small molecule FLT3 inhibitors are currently being evaluated in clinical trials. It has become an increasingly common practice to pursue allogeneic bone marrow transplantation during first remission in patients with FLT3/ITD mutations.2, 5–7
Among the FLT3/ITD AML patient population, relapse following transplant occurs frequently.8–10 As a result, there are numerous ongoing efforts to investigate the potential role of maintenance therapy with FLT3 inhibitors in the post-transplant setting. Historically, post-transplant remission in AML patients has been assessed using bone marrow morphology as well as hematologic recovery.11 Morphologic techniques, however, are insensitive, and better methods are needed to assess minimal residual disease (MRD) in AML patients.
Because FLT3 status can change over time, it has been suggested that FLT3 mutational status may not be a good marker for MRD in FLT3/ITD AML.12 The standard method of detecting the FLT3/ITD mutation with polymerase chain reaction (PCR) has a limit of detection of approximately 1 in 100 cells.13 The relatively low sensitivity of this assay is, in part, due to competition between the mutant and wild type alleles,13, 14 which we here refer to as PCR bias. The standard FLT3 PCR amplification product is significantly longer in patients with the ITD mutation than in patients with a wild type FLT3 gene.15 The shorter, wild type amplification product will, therefore, be multiplied preferentially by PCR as compared to the longer, mutant product. The FLT3 mutation is still not detectable by standard PCR assay at time points before and after transplant in many FLT3/ITD AML patients who ultimately relapse with FLT3-mutated disease after transplant, because their burden of disease is lower than the detection threshold of the standard assay. Thus, the change in FLT3 status may be due, at least in part, to the low sensitivity of this assay.
The presence of pre-transplant MRD by flow cytometry is known to carry an adverse prognosis.16 In addition, in the core binding factor leukemias and nucleophosmin (NPM1) mutated AML, MRD as assessed by PCR has been shown to predict relapse.17, 18 In spite of the widely held belief that FLT3 status is not a good marker for MRD, multiple groups have reported that identification of MRD by FLT3 status using patient-specific PCR serves as a marker of relapse.19–22 However, patient-specific PCR is cumbersome to perform, difficult to standardize, and is not routinely available outside of a research setting.
A better method of MRD detection in FLT3/ITD AML would be useful both for determining prognosis and for guiding therapy, such as determining whether transplantation should be performed and whether FLT3 inhibitors should be introduced. We report here the retrospective application of a novel technique, tandem duplication PCR (TD-PCR), to a cohort of consecutive FLT3/ITD AML patients who underwent allogeneic transplant at our institution, allowing us to correlate the presence of the FLT3/ITD mutation with clinical outcomes. Our analysis demonstrates that the FLT3/ITD mutation is a useful marker for MRD and can be used to guide clinical decision making for these patients, including, potentially, the timely use of FLT3 inhibitors.
Materials and Methods
Selection of patients
We performed a chart review to evaluate all patients diagnosed with FLT3/ITD AML between October 2004 and January 2012 who underwent allogeneic bone marrow transplant at our institution while in morphologic and immunophenotypic remission. Patients who had more than 5% blasts by morphology or flow cytometry immediately prior to transplant were excluded from the analysis. Patients followed at our institution but transplanted elsewhere were also excluded.
Polymerase Chain Reaction (PCR)
The conventional PCR for detecting an ITD mutation was performed as previously described.13
Tandem Duplication PCR (TD-PCR)
TD-PCR was modified from an original proof-of-principle version.23 TD-PCR employs a pair of primers for amplification, but oriented in the opposite direction from standard PCR. This technique allows exponential amplification only when the targeted region is duplicated, in a manner analogous to inverse PCR, thus eliminating the background wild-type amplicons obtained with standard approaches and improving the limit of detection to a single molecule. In our previous design, only ITD mutants with large duplications extending into intron 14 could be amplified. We revised the design to improve the clinical applicability of TD-PCR for MRD detection. We found that overlapping primers pointing in opposite directions sufficed for TD-PCR, particularly when a few bases were altered in each primer so that primer-primer annealing is disfavored. This design improved the capability of TD-PCR to detect shorter ITD mutants, although duplications under 30–40 bases remained too small to anneal both primers. With this approach, we constructed a series of 7 primer pairs that cover exon 14 and proximal intron 14. The redesigned TD-PCR has a limit of detection of a single ITD molecule. In a cohort of 58 newly diagnosed or relapsed FLT3/ITD AML patients, we found that up to 70% of ITD mutants can be amplified by 1 or more of the 7 primer pairs (manuscript in preparation).
For the current study, the initial diagnostic specimen of each patient was first tested with all 7 primer pairs to determine informativeness. One or two informative primer pairs were subsequently used to test pre-transplant specimens and day 60 post-transplant bone marrow specimens in quadruplicate (total 1 µg DNA examined, equivalent to approximately 160,000 cells). PCR products were subjected to capillary electrophoresis for analysis as described previously.23
Of note, day 60 TD-PCR results were not available to clinicians and, therefore, did not impact clinical decision-making in the patient population studied.
Statistics
The logrank test was used to assess differences in relapse free survival and overall survival between patients with positive TD-PCR and negative TD-PCR results.
Results
In order to assess whether it is possible to detect MRD at pre- and post-transplant time points in FLT3/ITD AML patients, we identified 54 patients who were diagnosed between October 2004 and January 2012 with FLT3/ITD AML, subsequently underwent allogeneic bone marrow transplant while in morphologic remission, survived the transplant, and were confirmed to be in complete remission (CR) with a bone marrow biopsy obtained 60 days post-transplant. This group represents a consecutive series of such patients from our institution. The demographics of these patients are reported in Table 1. Median duration of follow-up was 35.1 months.
Table 1.
Patient Demographics
| Number of Patients | 54 |
| Mean Age in Years (Standard Deviation) | 46 (13) |
| Sex (Number of Male Patients) | 20 (37%) |
| Relapsed/refractory AML prior to transplant | 11 (20%) |
| Cytogenetics* | |
| Favorable Cytogenetics | 1 (2%) |
| Intermediate Cytogenetics | 49 (91%) |
| Unfavorable Cytogenetics | 4 (7%) |
| Normal Cytogenetics | 36 (67%) |
| NPM1 Mutational Status | |
| Positive | 17 (31%) |
| Negative | 7 (13%) |
| Unknown | 30 (56%) |
| Preparatory Regimen | |
| Myeloablative | 44 (81%) |
| Nonmyeloablative | 10 (19%) |
| Type of Transplant | |
| Matched Related | 19 (35%) |
| Matched Unrelated | 15 (28%) |
| Mismatched Unrelated | 1 (2%) |
| Haploidentical | 19 (35%) |
Cytogenetic risk was determined according to the classification scheme described in Blood 1998; 92:2322–2333.
All patients receiving myeloablative preparative regimens received busulfan and cyclophosphamide, except for one patient who received a myeloablative conditioning regimen consisting of busulfan and fludarabine. All patients receiving nonmyeloablative regimens were treated with fludarabine, cyclophosphamide, and total body irradiation (TBI). For graft versus host disease (GVHD) prophylaxis, all patients received post-transplant cyclophosphamide. For myeloablative matched related and unrelated donor transplants, no further prophylaxis was administered. For myeloablative haploidentical transplants, patients received tacrolimus through day +180. For nonmyeloablative transplants, patients received tacrolimus through day +180 and mycophenolate mofetil through day +35.
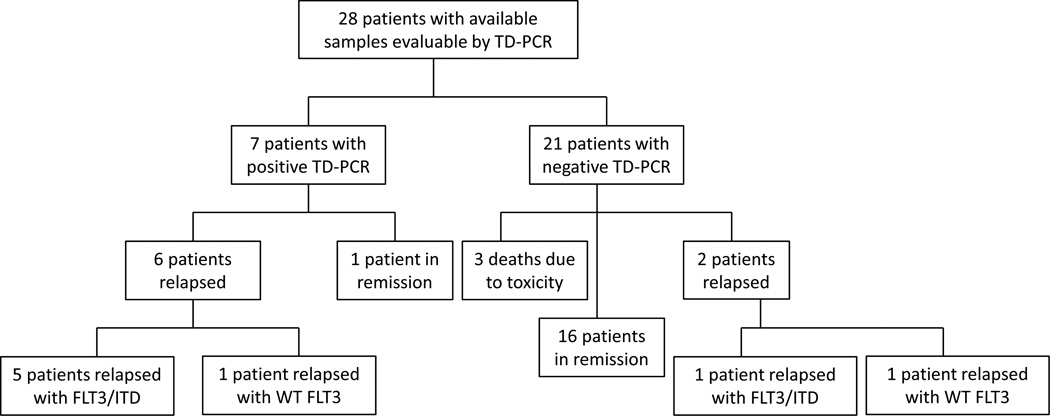
Of the 54 patients studied, 23 patients (43%) were alive and in remission at the time of data analysis, and 13 (24%) had died of transplant-related toxicity. One patient (2%) died of an unknown cause, thought likely related to relapse of an underlying myeloproliferative neoplasm. 17 patients (31%) had relapsed following transplant (six of whom were still alive at the time of data analysis). Of these 17 relapsed patients, 16 (94%) harbored the identical FLT3/ITD mutation present at diagnosis, as detected by the standard clinical PCR assay for FLT3 mutations. DNA samples prepared from bone marrow collected at Day 60 were available for 37 of the 54 patients (69%). Because of the lengths of patients’ ITDs, the FLT3 TD-PCR assay was informative for 28 of the 37 (76%) patients with available samples. FLT3 TD-PCR results for these 28 patients are shown in Table 2. Outcomes and MRD results for the 28 evaluable patients are shown in Figure 1. At the time of transplantation, 24 of the 28 evaluable patients were in CR. Three patients were in CR with incomplete platelet recovery (CRp). One patient was in CR with incomplete hematologic recovery (CRi).
Table 2.
FLT3 TD-PCR Results for 28 Evaluable Patients
| Patient Number |
Pre-Transplant TD-PCR Result |
Post-Transplant TD-PCR Result (Day 60) |
Duration of Follow-Up (Days) |
Outcome |
|---|---|---|---|---|
| 1 | − | − | 1185+ | Alive, in remission |
| 2 | + | + | 589+ | Alive, but relapsed |
| 3 | + | − | 225 | Death due to toxicity |
| 4 | + | − | 2376+ | Alive, in remission |
| 5 | + | − | 1732+ | Alive, in remission |
| 6 | + | − | 821+ | Alive, in remission |
| 7 | − | − | 542+ | Alive, in remission |
| 8 | + | − | 751+ | Alive, in remission |
| 9 | + | + | 844 | Death due to AML |
| 10 | + | − | 2024+ | Alive, in remission |
| 11 | + | + | 656 | Death due to AML |
| 12 | − | − | 3048+ | Alive, in remission |
| 13 | + | − | 1645+ | Alive, in remission |
| 14 | + | − | 541+ | Alive, in remission |
| 15 | + | − | 826+ | Alive, in remission |
| 16 | + | − | 979+ | Alive, in remission |
| 17 | − | − | 343 | Death due to toxicity |
| 18 | + | − | 1341+ | Alive, in remission |
| 19 | − | − | 505 | Death due to toxicity |
| 20 | − | − | 2456+ | Alive, in remission |
| 21 | + | + | 735 | Death due to AML |
| 22 | − | − | 1162+ | Alive, in remission |
| 23 | + | + | 602+ | Alive, but relapsed |
| 24 | + | − | 597 | Death due to AML |
| 25 | + | − | 1039+ | Alive, in remission |
| 26 | + | + | 463 | Death due to AML |
| 27 | no sample | − | 1585 | Death due to AML |
| 28 | + | + | 1855+ | Alive, in remission |
Figure 1.
MRD results and outcomes for 28 evaluable patients.
Among the 28 patients, seven (25%) were positive for MRD by FLT3 TD-PCR on their day 60 bone marrow specimens. The standard clinical PCR for FLT3 mutation was performed and was negative in all seven cases. Of these seven patients, six (86%) have relapsed to date, while one (14%) remains in remission 1855 days following transplant. The remaining 21 evaluable patients (75%) were negative for MRD by TD-PCR on their day 60 bone marrow specimens. Of these 21 patients, only two (10%) have relapsed.
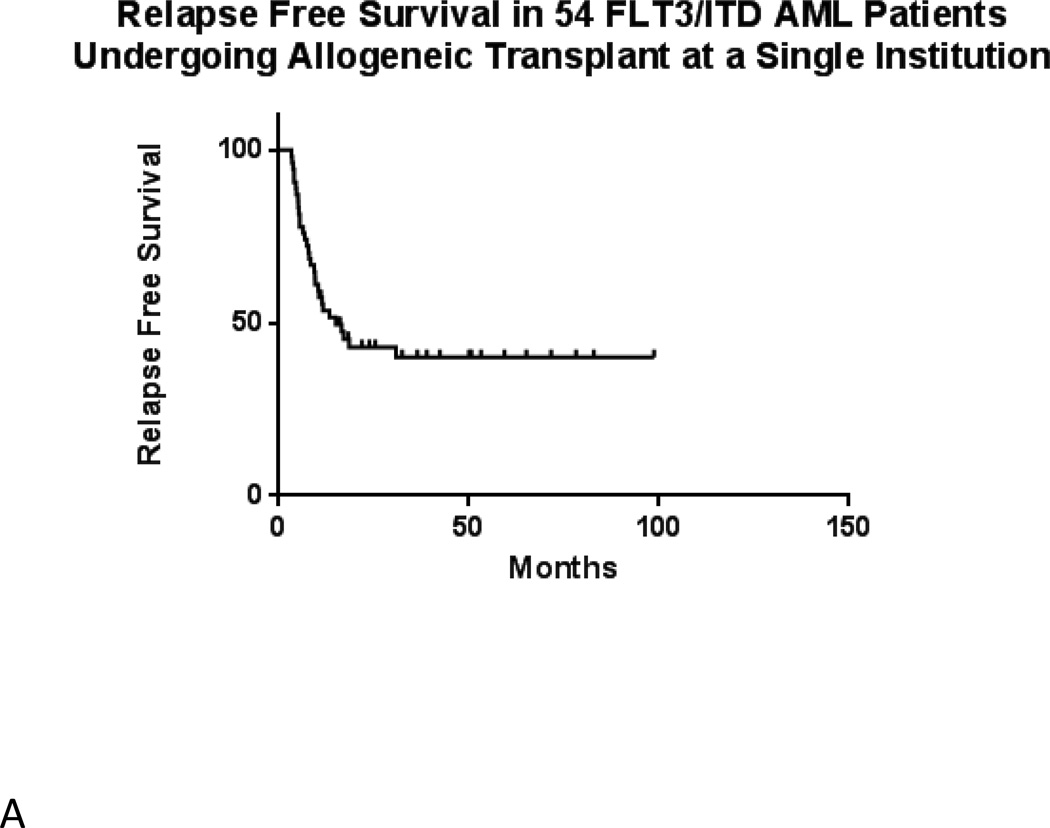
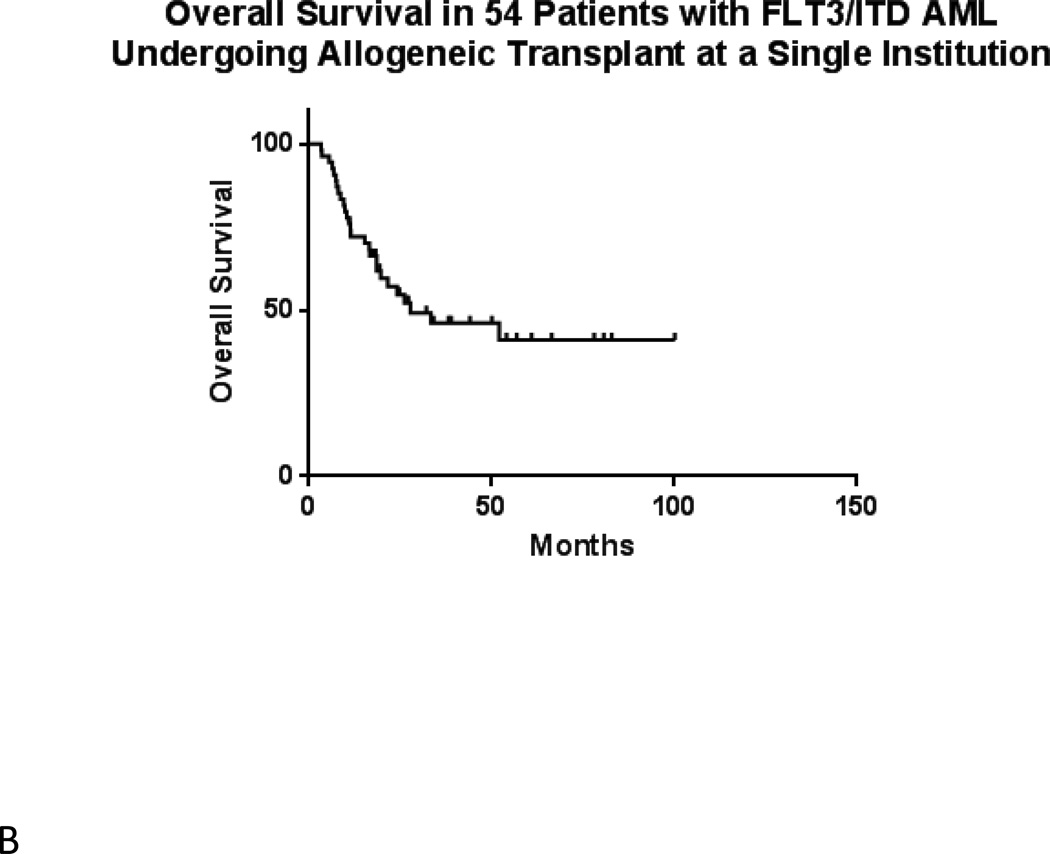
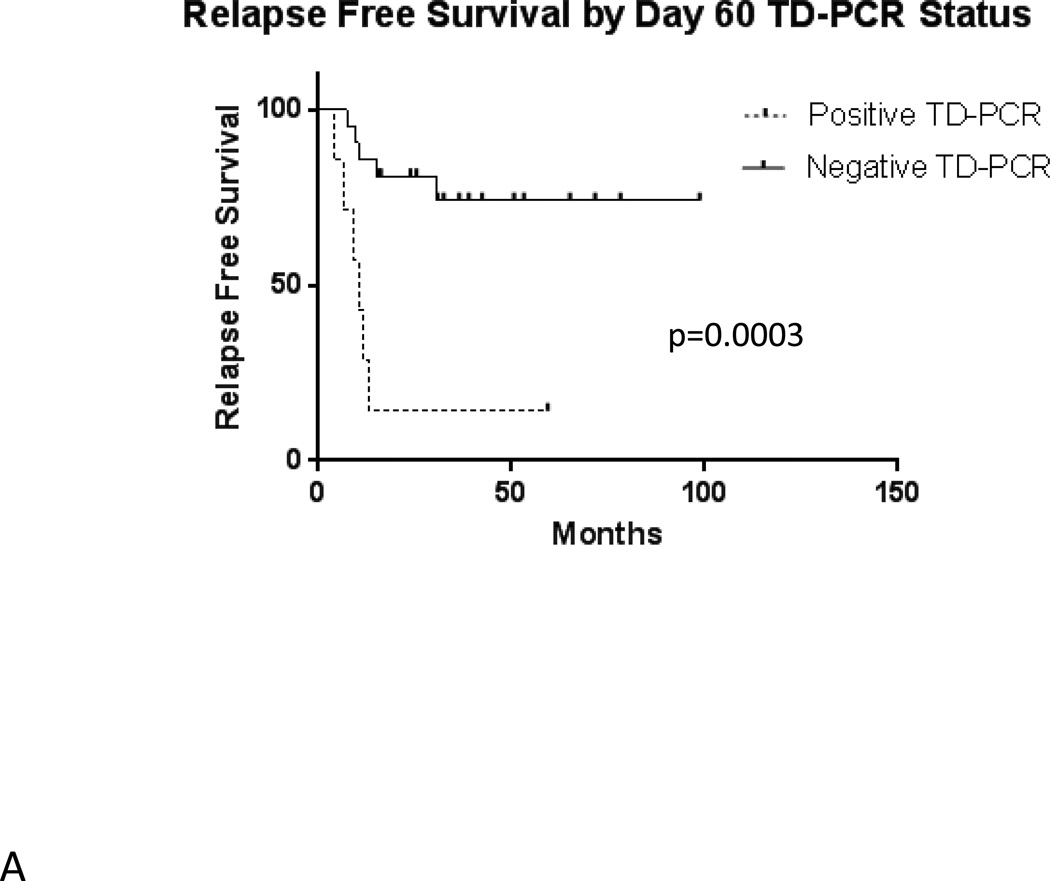
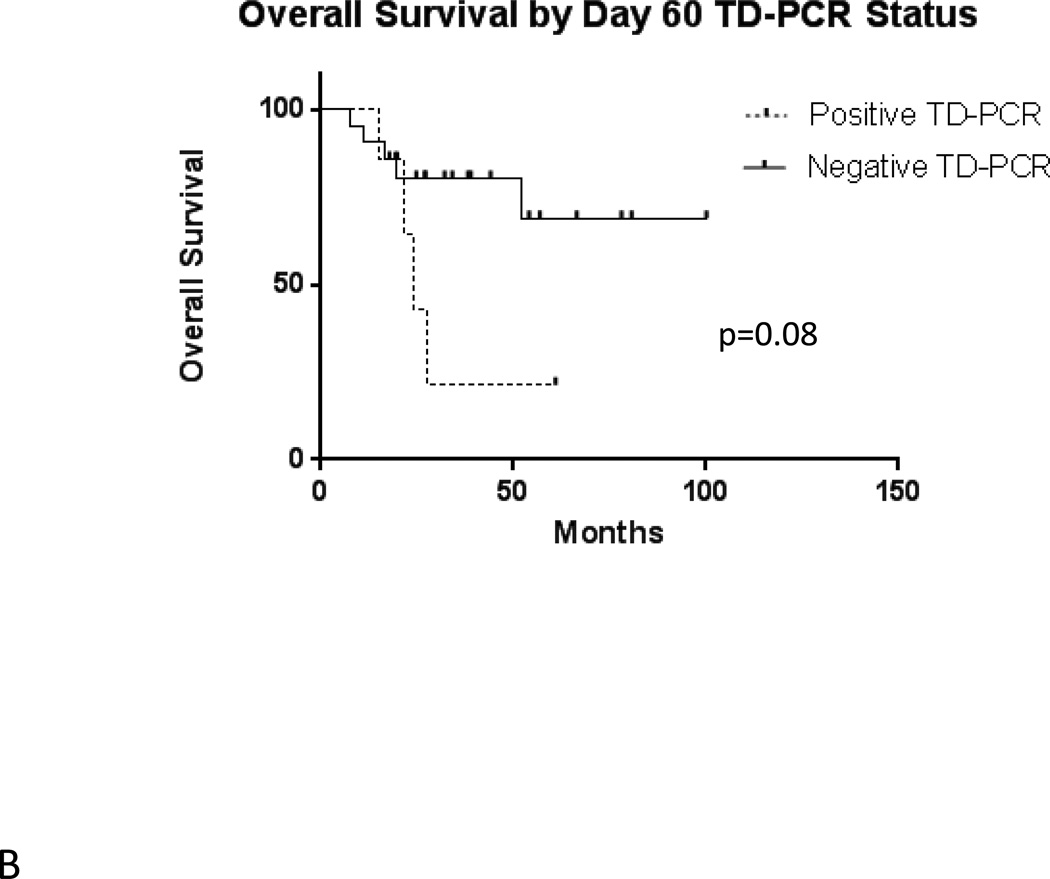
In the total population of 54 transplanted patients, median estimated relapse-free survival is 15.0 months (Figure 2A). Median overall survival is 27.7 months (Figure 2B). Among the 28 patients with evaluable results, evidence of MRD by FLT3 TD-PCR was highly prognostic (p = 0.0003) of relapse following allogeneic transplant (Figure 3A). Overall survival by TD-PCR status is shown in Figure 3B.
Figure 2.
A) Relapse Free Survival in 54 FLT3/ITD AML Patients Undergoing Transplant at a Single Institution. B) Overall Survival in 54 FLT3/ITD AML Patients Undergoing Transplant at a Single Institution.
Figure 3.
A) Relapse Free Survival by Day 60 TD-PCR Status. B) Overall Survival by Day 60 TD-PCR Status.
The results shown in Figure 3 indicate that FLT3/ITD mutations can be used as important markers of MRD. In interpreting our findings, we noted that patients could be divided by MRD status (as determined by FLT3 TD-PCR) into three groups, each with unique characteristics.
Group 1: Patients Negative for Pre-Transplant MRD and Negative for Post-Transplant MRD
Of the seven patients who were negative for MRD both prior to transplant and at day 60, none (0%) have relapsed. One patient (Patient 19) died due to complications of suspected cytomegalovirus pneumonitis approximately 10 months following transplant. No patients with a negative pre-transplant MRD assay became positive for MRD following transplant.
Group 2: Patients Positive for Pre-Transplant MRD and Negative for Post-Transplant MRD
Unlike the patients who were negative for MRD before transplant, some of the patients with positive pre-transplant MRD assays underwent conversion of their MRD status post-transplant. Of the 20 patients with positive pre-transplant MRD assays, 13 (65%) were negative for MRD by FLT3 TD-PCR at day 60. Of these 13 patients, only one (Patient 24 in Table 2) has relapsed. This patient had an ITD allele burden of less than 5% in the initial diagnostic specimen and relapsed approximately 10 months following transplant. Interestingly, this patient relapsed without the ITD mutation by either the standard assay or TD-PCR. Of the other 12 patients who converted from positive MRD status pre-transplant to negative MRD status post-transplant, 11 are alive and in remission, and one died of sepsis four months following transplant.
One patient (Patient 27) did not have a pre-transplant bone marrow sample available. This patient had a negative post-transplant MRD assay at day 60, but a marrow sample collected 12 months after transplant was positive by TD-PCR for the FLT3/ITD mutation. This patient relapsed (with the FLT3/ITD mutation) 28 months following transplant, suggesting that the presence of this MRD marker heralds relapse.
Group 3: Patients Positive for Pre-Transplant MRD and Positive for Post-Transplant MRD
Seven of the 20 patients (35%) who were positive for pre-transplant MRD continued to be positive for MRD by FLT3 mutational status at day 60 following transplant. Six (86%) of these patients have relapsed. One patient (Patient 28) who was positive for the FLT3/ITD mutation at pre- and post-transplant time points has not relapsed. This patient developed cutaneous GVHD and has now been in remission for longer than four years. The ITD mutation was not detected by TD-PCR in bone marrow specimens from 1-year and 3-year post-transplant time points, potentially indicative of a graft-versus-leukemia effect.
Five of the six patients with post-transplant MRD by TD-PCR who ultimately relapsed underwent bone marrow aspirate and biopsy at the time of relapse. Among these five patients, four had metaphase cytogenetics identical to the metaphase cytogenetics seen at diagnosis. One patient with normal cytogenetics at diagnosis had an additional copy of chromosome 13 at relapse.
Having established that TD-PCR represents a sensitive, convenient assay for MRD in FLT3/ITD patients, the question arises as to what type of clinical intervention could be performed when MRD is detected. FLT3 inhibitors represented an obvious possibility. We therefore examined our dataset for patients who received FLT3 inhibitors in the peri-transplant setting.
Patients who Received FLT3 Inhibitors in the Peri-Transplant Setting
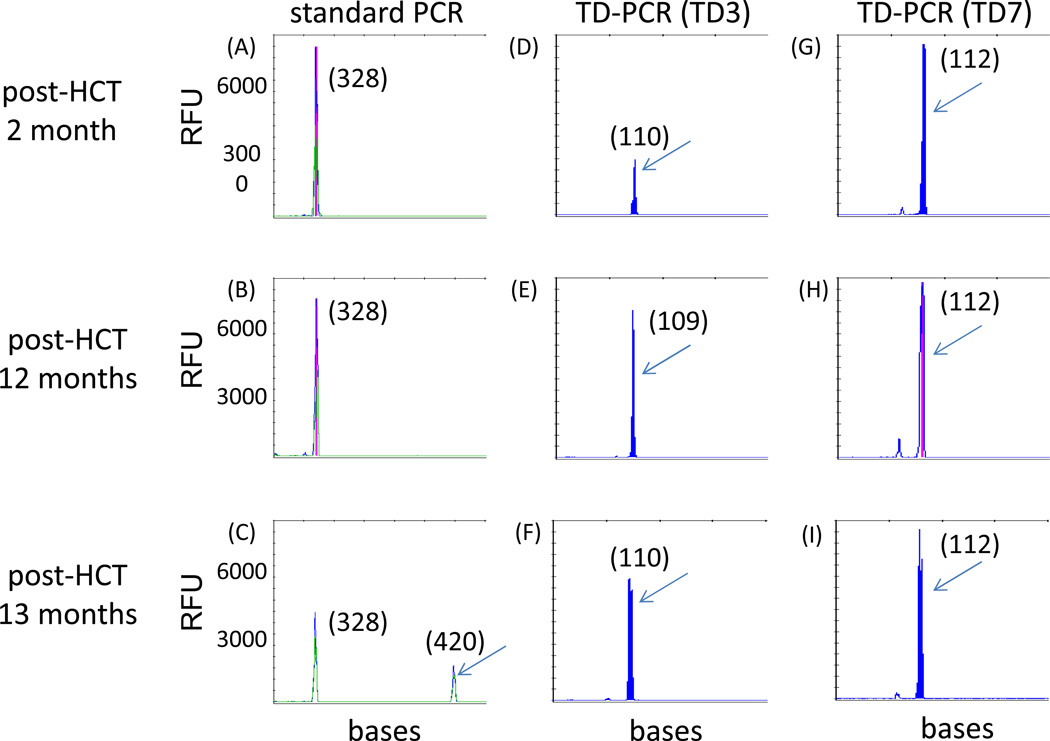
Three patients received small molecule FLT3 inhibitors in the peri-transplant setting. Patient 2, who was positive for MRD both pre- and post-transplant, received sorafenib as post-transplant maintenance therapy on a clinical trial from four months until 12 months following transplantation. Sorafenib was discontinued due to intolerability. During the period in which the patient was on sorafenib, retrospective testing revealed that the patient had been positive for the FLT3/ITD mutation by TD-PCR, despite being in clinical/morphologic remission and despite the standard clinical assay for FLT3 remaining negative. The patient relapsed with FLT3/ITD AML one month after discontinuing sorafenib (Figure 4).
Figure 4. TD-PCR detected MRD at 2 months post-transplant and predicted relapse.
In the case of patient 2, standard PCR showed relapse of the original ITD mutant migrating at 420 bases at 13 months post-transplant (C), but not at 2 months (A) or 12 months (B) post-transplant. TD-PCR using primer pairs TD3 and TD7, however, detected the ITD mutant at 2 (D and G), 12 (E and H) and 13 months (F and I) post-transplant. TD-PCR products from the relapse specimen at 13 months were diluted 30 fold before electrophoresis. The red vertical lines indicate off-scale peaks. Arrows indicate amplicons from the same ITD mutant (with sizes in nucleotides provided in parentheses). RFU, relative fluorescence units.
Patient 21, who was positive for MRD by FLT3 TD-PCR both before and after transplant, received FLT3 inhibitors on two occasions. The patient received the FLT3 inhibitor quizartinib as a single agent on a clinical trial, ultimately achieving a morphologic remission on this drug prior to transplant. The patient was initiated on off-label sorafenib approximately three months following transplant as maintenance therapy. The patient self-discontinued sorafenib after approximately six weeks on this medication. A bone marrow biopsy three weeks after stopping sorafenib revealed AML in early relapse, with morphologic evidence of disease (approximately 4% of cells were leukemic blasts) and with the presence of the FLT3/ITD mutation detectable by standard PCR.
Patient 25 was positive for MRD both by standard PCR and by FLT3 TD-PCR prior to transplant. As a precaution to prevent relapse prior to transplant, this patient received sorafenib for approximately one month prior to the start of the transplant preparative regimen. The patient was negative for MRD by TD-PCR at day 60 following transplant. The patient did not receive a FLT3 inhibitor following transplant but experienced grade 2 GVHD and remains in remission approximately 30 months following transplant.
Discussion
Given the poor prognosis of patients with FLT3/ITD AML and recent reports of some success with allogeneic bone marrow transplant, transplant has become the standard of care for this disease.2, 5–7 However, there remains a significant relapse rate following transplant for FLT3/ITD AML. Thus, it would be useful to have a marker for the presence of MRD in patients after transplant. Such a prognostic biomarker might help guide early intervention with a strategy such as FLT3 inhibition and/or alteration in GVHD prophylaxis in patients who are at high risk for relapse.
Investigators have questioned whether FLT3 mutational status is an adequate marker for MRD in FLT3/ITD AML patients receiving chemotherapy and/or undergoing allogeneic transplant. For instance, it has been shown in multiple studies that the FLT3/ITD mutational status of AML patients can be different at relapse than at diagnosis.24–26 Not only can FLT3/ITD patients relapse without the ITD mutation, but AML patients without the mutation can relapse with FLT3/ITD AML. This phenomenon is not unique to FLT3/ITD AML and also applies to other mutations occurring in leukemia, including N-ras and p53 mutations.24 It has been argued that, because of this “instability” of FLT3 mutations, FLT3/ITD mutational status is not a valuable indicator of MRD or predictor of relapse.12
In spite of the potential for an AML patient’s FLT3/ITD mutational status to change over time, such alterations are not common. Among patients who are FLT3/ITD negative at diagnosis, approximately 10% acquire the mutation at relapse.12 When relapse occurs in patients harboring the FLT3/ITD mutation at diagnosis, the mutation is present in the large majority of cases. In one study, 13 of 16 (81%) relapsing FLT3/ITD AML patients relapsed with the ITD.12 In another report, 16 of 17 (94%) FLT3/ITD AML patients who relapsed had the ITD mutation at relapse,26 which is identical to what we found in the present study.
Our findings are similar to those of prior studies using patient-specific PCR for detection of FLT3/ITD mutational status.19–22 A significant advantage of FLT3 TD-PCR, however, is that it uses standardized primers rather than patient specific primers, so that it can be performed routinely in a clinical laboratory. Our results confirm that post-transplant MRD by FLT3 mutational status predicts relapse in FLT3/ITD AML patients. Since all seven patients who were negative for MRD before transplant remained negative at day 60 following transplant and did not relapse, a negative pre-transplant MRD assay appears to be quite meaningful.
Of the 21 patients who were negative for MRD at day 60, the two who ultimately relapsed both had informative courses. Patient 24 relapsed with FLT3 wild type AML. Given that this patient had an ITD allelic burden of less than 5% at diagnosis, it is likely that the FLT3/ITD mutation represented a non-dominant sub-clone in this patient. While this patient’s case might be interpreted as corroborating the warnings of those who are wary of using FLT3 mutational status as a test for MRD, the changing of a patient’s FLT3 status appears to interfere with MRD detection in only a small proportion of patients. The “instability” of FLT3 mutations therefore represents a relatively uncommon limitation of using FLT3 status to determine MRD. Patient 27, the other patient who was negative for MRD at day 60 but relapsed, had converted to positive by the 12 month mark and ultimately relapsed with FLT3/ITD AML. This patient’s relapse at 28 months was the latest relapse among our patients. Thus, based on a limited sample, it appears that a negative day 60 post-transplant MRD assay suggests that relapse is unlikely. Moreover, the case of Patient 27 suggests that it may be useful to continue monitoring for MRD after day 60, since it is possible that this patient would have benefitted from therapy with a FLT3 inhibitor at the time of conversion from MRD negative to MRD positive.
There are also instructive cases of patients who were positive for the FLT3/ITD mutation on day 60. Patient 28 had a positive day 60 MRD assay and remains in a durable remission. This individual’s case provides evidence that a graft versus leukemia effect can occur after day 60. Patient 2 also was positive for the FLT3/ITD mutation at day 60. While treated with sorafenib, this patient remained in remission until approximately 12 months following transplant and relapsed quickly upon discontinuation of this drug, suggesting that FLT3 inhibition may have a role in maintenance therapy for some patients with FLT3/ITD AML following allogeneic transplant, much in the same way that BCR-ABL inhibition is used in Philadelphia chromosome positive acute lymphoblastic leukemia (Ph+ ALL).
Sustained remissions have been reported with the FLT3 inhibitor sorafenib administered as monotherapy following transplant.27 However, using sorafenib following post-transplant relapse does not always appear to be an effective strategy.28 Currently, investigators are working to identify patients early who may be at high risk for relapse and likely to benefit from posttransplant FLT3 inhibition. Studies have suggested that sorafenib, in combination with the allogeneic immune effects of transplant, may induce durable remissions in some patients.27 It is possible that MRD by FLT3/ITD mutational status will identify patients who will benefit from post-transplant therapy such as FLT3 inhibition, withdrawal of GVHD prevention, or donor lymphocyte infusion. We are currently examining the use of TD-PCR prospectively in an ongoing clinical trial using post-transplant sorafenib in FLT3/ITD AML patients.
One significant limitation of the TD-PCR technique is the inability of the assay to be informative in FLT3/ITD AML patient with shorter ITDs, which account for approximately 25% of FLT3/ITD AML cases. Moreover, in our study, bone marrow DNA specimens were not available for 17 patients in our cohort of FLT3/ITD AML patients undergoing transplant. Furthermore, the TD-PCR technique does not overcome instability of some patients’ FLT3 mutational status, which remains an important challenge to finding an optimal MRD marker. In spite of these limitations, our findings reveal that FLT3 mutational status can be useful in detecting MRD. In addition, to the authors’ knowledge, this study represents the largest consecutive series of FLT3/ITD AML patients undergoing transplant at a single institution.
In summary, our results support the use of FLT3/ITD mutational status by TD-PCR as a marker for MRD. The FLT3 TD-PCR technique, with defined primer sets, would be relatively simple to adopt as a standard assay in clinical laboratories. Given that the present study has been performed using bone marrow samples, one future direction of this work will be to determine whether the TD-PCR assay is predictive using peripheral blood, which would render the assay more convenient to perform. Prospective studies will likely be necessary to validate MRD assessed by FLT3 TD-PCR as a prognostic instrument and as a means of guiding therapy.
A new technique, TD-PCR, can detect the FLT3/ITD mutation with high sensitivity.
Using TD-PCR, FLT3 mutational status can be used as a marker of MRD.
TD-PCR predicts relapse in FLT3/ITD AML patients undergoing allogeneic transplant.
Median relapse-free survival in transplanted FLT3/ITD AML patients was 15.0 months.
Median overall survival in transplanted FLT3/ITD AML patients was 27.7 months.
Acknowledgements
This work was supported in part by R21HG004315 (CDG) and R21HG005745 (CDG) from the National Institutes of Health. This work was also supported by the MD Anderson leukemia SPORE P50 CA100632 (MJL) and by the Johns Hopkins oncology institutional training grant from the National Institutes of Health, 2T32CA009071-32 (MRG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures: The authors have no relevant financial conflicts of interest.
References
- 1.Marcucci G, Maharry K, Radmacher MD, et al. Prognostic significance of, and gene and microRNA expression signatures associated with, CEBPA mutations in cytogenetically normal acute myeloid leukemia with high-risk molecular features: a Cancer and Leukemia Group B Study. J Clin Oncol. 2008;26:5078–5087. doi: 10.1200/JCO.2008.17.5554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schlenk RF, Dohner K, Krauter J, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358:1909–1918. doi: 10.1056/NEJMoa074306. [DOI] [PubMed] [Google Scholar]
- 3.Levis M, Small D. FLT3: ITDoes matter in leukemia. Leukemia. 2003;17:1738–1752. doi: 10.1038/sj.leu.2403099. [DOI] [PubMed] [Google Scholar]
- 4.Nakao M, Yokota S, Iwai T, et al. Internal tandem duplication of the flt3 gene found in acute myeloid leukemia. Leukemia. 1996;10:1911–1918. [PubMed] [Google Scholar]
- 5.DeZern AE, Sung A, Kim S, et al. Role of allogeneic transplantation for FLT3/ITD acute myeloid leukemia: outcomes from 133 consecutive newly diagnosed patients from a single institution. Biol Blood Marrow Transplant. 2011;17:1404–1409. doi: 10.1016/j.bbmt.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kayser S, Döhner K, Krauter J, et al. Impact of allogeneic transplantation from matched related and unrelated donors on clinical outcome in younger adult AML patients with FLT3 internal tandem duplications; ASH Annual Meeting; 2010. Abstract 909. [Google Scholar]
- 7.Bornhauser M, Illmer T, Schaich M, et al. Improved outcome after stem-cell transplantation in FLT3/ITD-positive AML. Blood. 2007;109:2264–2265. doi: 10.1182/blood-2006-09-047225. [DOI] [PubMed] [Google Scholar]
- 8.Sengsayadeth SM, Jagasia M, Engelhardt BG, et al. Allo-SCT for high-risk AML-CR1 in the molecular era: impact of FLT3/ITD outweighs the conventional markers. Bone marrow transplant. 2012;47:1535–1537. doi: 10.1038/bmt.2012.88. [DOI] [PubMed] [Google Scholar]
- 9.Gale RE, Hills R, Kottaridis PD, et al. No evidence that FLT3 status should be considered as an indicator for transplantation in acute myeloid leukemia (AML): an analysis of 1135 patients, excluding acute promyelocytic leukemia, from the UK MRC AML10 and 12 trials. Blood. 2005;106:3658–3665. doi: 10.1182/blood-2005-03-1323. [DOI] [PubMed] [Google Scholar]
- 10.Brunet S, Labopin M, Esteve J, et al. Impact of FLT3 internal tandem duplication on the outcome of related and unrelated hematopoietic transplantation for adult acute myeloid leukemia in first remission: a retrospective analysis. J Clin Oncol. 2012;30:735–741. doi: 10.1200/JCO.2011.36.9868. [DOI] [PubMed] [Google Scholar]
- 11.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 12.Nazha A, Cortes J, Faderl S, et al. Activating internal tandem duplication mutations of the fms-like tyrosine kinase-3 (FLT3-ITD) at complete response and relapse in patients with acute myeloid leukemia. Haematologica. 2012;97:1242–1245. doi: 10.3324/haematol.2012.062638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murphy KM, Levis M, Hafez MJ, et al. Detection of FLT3 internal tandem duplication and D835 mutations by a multiplex polymerase chain reaction and capillary electrophoresis assay. J Mol Diagn. 2003;5:96–102. doi: 10.1016/S1525-1578(10)60458-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thiede C, Steudel C, Mohr B, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99:4326–4335. doi: 10.1182/blood.v99.12.4326. [DOI] [PubMed] [Google Scholar]
- 15.Kiyoi H, Towatari M, Yokota S, et al. Internal tandem duplication of the FLT3 gene is a novel modality of elongation mutation which causes constitutive activation of the product. Leukemia. 1998;12:1333–1337. doi: 10.1038/sj.leu.2401130. [DOI] [PubMed] [Google Scholar]
- 16.Walter RB, Gooley TA, Wood BL, et al. Impact of pre-transplantation minimal residual disease, as detected by multiparametric flow cytometry, on outcome of myeloablative hematopoietic cell transplantation for acute myeloid leukemia. J Clin Oncol. 2011;29:1190–1197. doi: 10.1200/JCO.2010.31.8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yin JA, O'Brien MA, Hills RK, et al. Minimal residual disease monitoring by quantitative RT-PCR in core binding factor AML allows risk stratification and predicts relapse: results of the United Kingdom MRC AML-15 trial. Blood. 2012;120:2826–2835. doi: 10.1182/blood-2012-06-435669. [DOI] [PubMed] [Google Scholar]
- 18.Kronke J, Schlenk RF, Jensen KO, et al. Monitoring of minimal residual disease in NPM1-mutated acute myeloid leukemia: a study from the German-Austrian acute myeloid leukemia study group. J Clin Oncol. 2011;29:2709–2716. doi: 10.1200/JCO.2011.35.0371. [DOI] [PubMed] [Google Scholar]
- 19.Schiller J, Praulich I, Krings Rocha C, Kreuzer KA. Patient-specific analysis of FLT3 internal tandem duplications for the prognostication and monitoring of acute myeloid leukemia. Eur J Haematol. 2012;89:53–62. doi: 10.1111/j.1600-0609.2012.01785.x. [DOI] [PubMed] [Google Scholar]
- 20.Abdelhamid E, Preudhomme C, Helevaut N, et al. Minimal residual disease monitoring based on FLT3 internal tandem duplication in adult acute myeloid leukemia. Leuk Res. 2012;36:316–323. doi: 10.1016/j.leukres.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Scholl S, Loncarevic IF, Krause C, Clement JH, Hoffken K, Sayer HG. Analyses of minimal residual disease based on Flt3 mutations in allogeneic peripheral blood stem cell transplantation. J Cancer Res Clin Oncol. 2005;131:279–283. doi: 10.1007/s00432-004-0660-x. [DOI] [PubMed] [Google Scholar]
- 22.Scholl S, Loncarevic IF, Krause C, Kunert C, Clement JH, Hoffken K. Minimal residual disease based on patient specific Flt3-ITD and -ITT mutations in acute myeloid leukemia. Leuk Res. 2005;29:849–853. doi: 10.1016/j.leukres.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 23.Lin MT, Tseng LH, Beierl K, et al. Tandem duplication PCR: An ultrasensitive assay for the detection of internal tandem duplications of the FLT3 gene. Diagn Mol Pathol. 2013;22:149–155. doi: 10.1097/PDM.0b013e31828308a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakano Y, Kiyoi H, Miyawaki S, et al. Molecular evolution of acute myeloid leukaemia in relapse: unstable N-ras and FLT3 genes compared with p53 gene. Br J Haematol. 1999;104:659–664. doi: 10.1046/j.1365-2141.1999.01256.x. [DOI] [PubMed] [Google Scholar]
- 25.Kottaridis PD, Gale RE, Langabeer SE, Frew ME, Bowen DT, Linch DC. Studies of FLT3 mutations in paired presentation and relapse samples from patients with acute myeloid leukemia: implications for the role of FLT3 mutations in leukemogenesis, minimal residual disease detection, and possible therapy with FLT3 inhibitors. Blood. 2002;100:2393–2398. doi: 10.1182/blood-2002-02-0420. [DOI] [PubMed] [Google Scholar]
- 26.Shih LY, Huang CF, Wu JH, et al. Internal tandem duplication of FLT3 in relapsed acute myeloid leukemia: a comparative analysis of bone marrow samples from 108 adult patients at diagnosis and relapse. Blood. 2002;100:2387–2392. doi: 10.1182/blood-2002-01-0195. [DOI] [PubMed] [Google Scholar]
- 27.Metzelder SK, Schroeder T, Finck A, et al. High activity of sorafenib in FLT3-ITD-positive acute myeloid leukemia synergizes with allo-immune effects to induce sustained responses. Leukemia. 2012;26:2353–2359. doi: 10.1038/leu.2012.105. [DOI] [PubMed] [Google Scholar]
- 28.Sharma M, Ravandi F, Bayraktar UD, et al. Treatment of FLT3-ITD-positive acute myeloid leukemia relapsing after allogeneic stem cell transplantation with sorafenib. Biol Blood Marrow Transplant. 2011;17:1874–1877. doi: 10.1016/j.bbmt.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]