Abstract
Context. Primary leiomyoma of the liver is a rare tumour with uncertain pathogenesis with similar presentation with other tumours of the liver. Little is known about its clinical course. Objectives. To review the literature for case reports of primary leiomyoma of the liver. Methods. Extensive literature search was carried out for case reports of primary leiomyoma of the liver. Results. A total of 36 cases of primary leiomyoma of the liver were reviewed. The mean age of presentation is 43 years with slight female sex affectation; females accounted for 55.6% of the cases reported in the literature. The average size of the tumour is 8.7 cm. 34.4% of the cases reviewed were incidental finding with the mean follow-up time of 33 months with most cases reporting no evidence of disease. Conclusions. Primary leiomyoma of the liver is very rare tumour with complex pathogenesis which remains largely unknown. Imaging of the tumour does not allow for a tissue specific diagnosis; hence histological review of the tissue specimen and immunohistochemical stains are imperative for diagnosis. Surgical resection is both diagnostic and curative. The diagnosis of primary leiomyoma of the liver should be considered as a differential in the management of liver tumours.
1. Introduction
Leiomyoma is a benign smooth muscle neoplasm of mesenchymal origin which commonly occurs in the genitourinary system and the gastrointestinal tract of the body but which rarely occurs in the liver [1, 2]. The first case report of primary leiomyoma of the liver was first described in a 42-year-old woman by Demel in 1926 [3].
This paper seeks to review primary leiomyoma of the liver in the literature because of its rarity, unclear pathogenesis, and the diagnostic challenges it poses in clinical practice.
2. Methods
Case reports and case series of primary leiomyoma of the liver were retrieved by extensive literature search of PubMed, Ovid SP, Cochrane database of systematic reviews, Embase, and Clinical Evidence Online. Further search of the literature was carried out by manually searching the relevant references of the studies retrieved. The inclusive criteria include relevant publications of primary leiomyoma of the liver and hence studies with coexisting leiomyoma in other parts of the body were excluded.
Epidemiologic, pathologic, clinical, imaging, and prognostic data were retrieved and assessed for all studies. The search keywords include primary hepatic leiomyoma, primary leiomyoma of the liver, primary benign lesions of the liver, and primary tumours of the liver.
3. Results
The clinical and pathologic characteristics of the 35 cases reviewed with the treatment and the clinical outcome are outlined in Table 1.
Table 1.
Clinical and pathologic features of the reviewed cases of primary leiomyoma of the liver.
| Cases | Age | Sex | Clinical features | Size (cm) | Location | EBV status | Mitosis | Immunosuppression | Necrosis | Tx | F/U (Mths) | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Perini et al. [19] | 45 | M | Epigastric pain | 4.3 | LL | Positive | Nr | Yes | nr | sectionectomy | 4 | ned |
| Davidoff et al. [18] | 5 | M | Incidental | 15 | RL | Positive | Low | Yes | nr | R trisegmentectomy | 10 | ned |
| Cheuk et al. [22] | 37 | M | Abdominal discomfort | 3.5 | LL | Positive | Nr | Yes | nr | conservative | nr | nr |
| Prevot et al. [10] | 33 | M | Incidental (autopsy) | 2 | RL | Positive | None | Yes | None | no surgical tx | 0 | D |
| Sclabas et al. [20] | 30 | F | Epigastric pain | 4.4, 0.6 | LL | Positive | Low | Yes | nr | LL hepatectomy | 30 | ned |
| Luo et al. [2] | 48 | M | RUQ pain | 4.9 | LL | Negative | Low | Yes | nr | LL hepatectomy | 24 | ned |
| Raber et al. [23] | 46 | F | Incidental | 2.8 | RL | Nr | None | Yes | nr | conservative | 84 | ned |
| Doyle et al. [24] | 5 | F | Incidental | 3 | LL | Nr | Low | Yes | Yes | LL segmentectomy | 8 | ned |
| Ha et al. [25] | 9 | M | Incidental | 5.6 | LL | Nr | Nr | Yes | Yes | LL hepatectomy | nr | ned |
| Yoon et al. [34] | 41 | F | RUQ mass | 19 | RL | Nr | Nr | None | nr | RL hepatectomy | nr | nr |
| Yanase et al. [6] | 59 | F | Liver dysfunction | 13 | RL | Nr | Low | None | None | RL hepatectomy | 21 | ned |
| Beuzen et al. [5] | 36 | F | RUQ pain | 5 | LL | Nr | None | None | None | Bi segmentectomy | 108 | ned |
| Santos et al. [13] | 28 | F | RUQ pain | 5.5 | RL | None | None | None | None | segmentectomy | 36 | ned |
| Urizono et al. [8] | 71 | M | Incidental | 3 | RL | Nr | None | None | nr | caudate lobectomy | nr | ned |
| Perini et al. [19] | 45 | F | RUQ pain | 20 | RL | Nr | None | None | Yes | segmentectomy | 72 | ned |
| Marin et al. [39] | 64 | F | Incidental | 3 | RL | Nr | None | None | nr | R hepatectomy | 12 | ned |
| Belli et al. [12] | 67 | F | Abdominal mass | 30 | RL | Nr | Low | None | nr | ER hepatectomy | 48 | ned |
| Sousa et al. [36] | 61 | F | Dyspepsia | 9.5 | LL | Nr | None | None | nr | LL hepatectomy | 16 | ned |
| Hollands et al. [11] | 17 | M | Epigastric pain | 9 | LL | Nr | None | None | nr | LL hepatectomy | 12 | ned |
| Kanazawa et al. [37] | 31 | M | Incidental | 3.5 | LL | Nr | None | None | nr | LL segmentectomy | nr | nr |
| Reinertson et al. [1] | 32 | F | RUQ pain | 10 | LL | Nr | None | None | nr | LL hepatectomy | 24 | ned |
| kalil et al. [7] | 44 | F | RUQ mass | 7 | RL | Nr | Nr | None | nr | atypical resection | nr | nr |
| Imasato et al. [9] | 61 | F | Incidental | 4.5 | RL | Nr | Nr | None | nr | RL hepatectomy | nr | nr |
| Hawkins et al. [4] | 66 | M | Abdominal mass | 13 | LL | Nr | Low | Nr | nr | LL hepatectomy | 48 | ned |
| Herzberg et al. [31] | 30 | F | RUQ pain | 19 | RL | Nr | Nr | Nr | nr | R hepatectomy | nr | nr |
| Ishak and Rabin [35] | 64 | M | Abdominal mass | Nr | RL | Nr | Nr | Nr | nr | laparotomy | nr | nr |
| Demel [3] | 42 | F | RUQ pain | 12 | RL | Nr | Nr | Nr | nr | laparotomy | nr | nr |
| Mesenas et al. [48] | 59 | M | Incidental | 3.6 | RL | Nr | Nr | Nr | nr | segmentectomy | nr | nr |
| Rummeny et al. [33] | 46 | F | RUQ pain | Nr | Nr | Nr | Nr | Nr | nr | nr | nr | nr |
| Tan et al. [38] | 31 | F | Nr | Nr | Nr | Nr | None | None | nr | hepatic resection | nr | nr |
| Tan et al. [38] | 42 | M | Nr | Nr | Nr | Nr | None | None | nr | hepatic resection | nr | nr |
| Tan et al. [38] | 69 | M | Nr | Nr | Nr | Nr | None | None | nr | hepatic resection | nr | nr |
| Sadler et al. [21] | 36 | M | Abdominal pain | Nr | LL | Nr | Low | Yes | None | hepatic resection | nr | nr |
| Sadler et al. [21] | 36 | M | Abdominal pain | Nr | LL | Nr | Nr | Yes | nr | conservative | nr | nr |
| Bartoli et al. [49] | 34 | F | Incidental | Nr | RL | Nr | Nr | Nr | nr | RL hepatectomy | nr | nr |
| Rios-Dalenz [32] | 87 | F | RUQ pain | Nr | LL | Nr | Nr | Nr | nr | no surgical tx | nr | nr |
Nr: not reported; ned: no evidence of disease.
4. Discussion
Primary leiomyomas of the liver are very rare tumours. Eighty-seven years after the first case of primary leiomyoma of the liver was reported, to the best knowledge of the author, 36 cases of primary leiomyoma of the liver have so far been reported in the literature.
Hawkins et al. [4] in 1980 proposed criteria that must be met for the diagnosis of primary liver leiomyoma. The tumour must be composed of leiomyocytes. Secondly, the presence of leiomyoma in other sites of the body like uterus and the gastrointestinal tract must be excluded. If the uterus is surgically absent, the diagnosis of primary leiomyoma of the liver must not be made without the review of the report and sections from the hysterectomy.
4.1. Epidemiology
Primary leiomyomas of the liver have been reported in both paediatric and adult populations. There are reports in the literature to suggest the incidence of the tumour in both immunocompetent and immunosuppressed patients. The mean age of presentation is 43 years (range 4.6–87). Primary leiomyomas of the liver have been reported to have female sex predilection [2, 5–7]. Luo et al. suggested that the observed female preponderance may be partly due to the activity of the smooth muscle cells in female urogenital tissue in carcinogenesis [2]. However this view seems to contradict one of the main diagnostic criteria for primary leiomyoma of the liver as proposed by Hawkins et al. [4] which seeks to exclude leiomyoma in other parts of the body especially in the urogenital tissue.
This review of 36 cases however demonstrates slight female sex affectation with females accounting for 55.6% of the cases. Familial predispositions have not been reported. The distribution of the lesion is equal in both right and left lobes of the liver with two cases involving the caudate lobe of the liver [8, 9].
4.2. Pathogenesis
The pathogenesis of primary leiomyoma of the liver is not clear and largely unknown. Some theories have emerged as to the possible pathogenesis of these tumours. Proliferations of smooth muscle of the hepatic vessels or the biliary tree have been suggested as a possible origin [2, 10–13]. However the argument against origin from the bile ducts is that large extra hepatic ducts have very few smooth cells [11, 14].
Immunosuppressive states which include either posttransplant patients on immunosuppressive therapy or patients with human immunodeficiency virus (HIV) have been suggested as a possible causal factor in primary leiomyoma of the liver. Increased risks of de novo neoplasia after transplantation are well documented in the literature [15–17].
Possible explanations for the susceptibility of immunocompromised patients to neoplasms include the disruption of the immunosurveillance ability of the host with the subsequent development of the tumours that would otherwise have been suppressed by a normal immune system [18]. The incidence of primary leiomyoma of the liver within the setting of immunosuppression in this review which accounts for 39.3% of the cases appears to be in support of this theory [2, 10, 18–25]. Immunosuppression alone does not totally explain the pathogenesis of this tumour because of the incidence of the tumour in immunocompetent individuals.
The theory of the possible role of viral induced oncogenesis has been suggested. This is because of the evidence that implicates some DNA viruses in the aetiology of some neoplasms particularly Epstein-Barr virus in smooth muscle tumours [16, 18]. This theory is further supported by the observation that patients with immunosuppression are at high risks of developing virus-associated neoplasms although the exact mechanism is not known [18, 26].
A possible explanation is a multistep theory of viral oncogenesis which suggests that virus infected cells undergo an uncontrolled polyclonal proliferation in the setting of the reduced immune surveillance of the viral transformed cells. Further cytogenetic events alter the growth regulation of a subset of cells, leading to a monoclonal expansion of tumour cells [18]. In support of this theory is the observation that 5 cases were reported to be positive for EBV in this review and the 5 cases were also in the setting of immunosuppression [10, 18–20, 22]. The fact that other case reports in this review were not associated with Epstein-Barr virus suggests a rather complex pathogenesis for primary leiomyoma of the liver. Epstein-Barr viral oncogenesis alone does not explain the pathogenesis. Virus associated tumours have been observed to exhibit different range of differentiation from well differentiated to poorly differentiated and some may show features suggestive of leiomyosarcomas [27]. The risk of other cancers in patients with immunosuppression not linked to viruses is also increased [28–30].
4.3. Clinical Features
The clinical presentation of primary leiomyoma of the liver is similar to the presentation of other liver neoplasms. The most common clinical symptom in this review is abdominal, epigastric, or right upper quadrant pain which accounts for 42.4% of cases reported [1–3, 5, 11, 13, 19–21, 31–33]. 33.3% of the cases were incidental with one of them an incidental finding at autopsy [10]. Other clinical features include abdominal mass [4, 7, 12, 34, 35], abdominal discomfort [22], dyspepsia [36], and liver dysfunction [6].
Primary leiomyoma of the liver may rarely present as a composite tumour. Yanase et al. reported the case of a 59-year-old lady with 13 × 10 × 9 cm firm tumour with mainly a solid tissue portion and interconnected multilocular cystic lesions. Histologic diagnosis of primary leiomyoma of the liver encasing hepatobiliary cystadenoma was made [6].
Screening for tumour markers alpha fetoprotein, carbohydrate antigen 19-9 and carcinoembryonic antigen are usually negative [11, 12, 19]. Serological testing for EBV combined with in situ hybridization indicates the tumour cells positive for EBV encoded small RNA (Figure 1) [2, 10, 18, 20]. In situ hybridization is the gold standard for the detection and localization of latent EBV in tissues [2].
Figure 1.
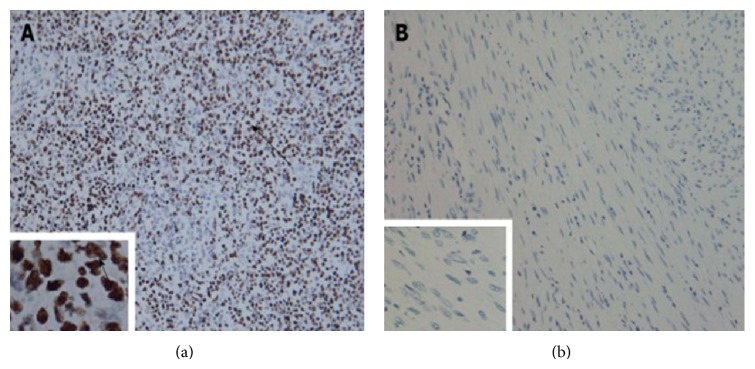
Tumour cells stained positive by in situ hybridization with Epstein-Barr virus-encoded small RNA. (a) Positive control staining ×200, ×1000; (b) tumour cell staining ×200, ×1000. Arrows indicate positive staining of the nuclei. Reprinted from [2] with the permission of the Director, Editorial Office of the World Journal of Gastroenterology.
4.4. Imaging
Imaging alone does not show tissue specific diagnosis and cannot reliably differentiate between primary leiomyoma and other differential diagnosis like leiomyosarcoma, hepatocellular adenoma, hepatocellular carcinoma, angiomyolipoma, and hypervascular metastatic lesions [36, 37]. There is no notable difference in the imaging of patients with or without immunosuppression [19].
4.5. CT
CT findings in leiomyoma of the liver have been variously reported in the cases reviewed as hypodense lesions with strong enhancement in both arterial and portal phase [5, 11, 13, 21, 23, 36, 38] with some reports describing peripheral rim enhancement [8, 37]. An increased enhancement in the arterial phase and a sustained homogeneous enhancement in both hepatic venous and equilibrium phases have also been reported [39] (Figure 2).
Figure 2.
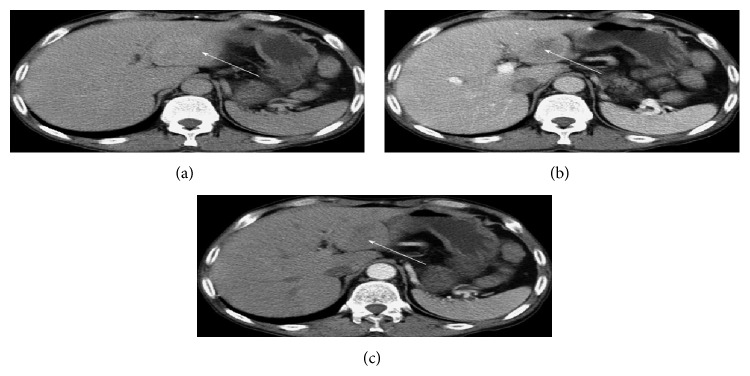
CT Abdomen with a mass in segment III of the liver in the hepatic equilibrium (a), portal venous (b), and hepatic arterial phase (c). Reprinted from [2] with the permission of the Director, Editorial Office of the World Journal of Gastroenterology.
4.6. Ultrasound
Ultrasound findings in primary leiomyoma of the liver in the literature have been described as hypoechoic lesions with varying degrees of heterogeneity [5, 8, 11, 36, 37]. Perini et al. reported heterogeneous mass displacing the inferior vena cava (IVC) and the right kidney medially across the midline [19].
4.7. MRI
MRI findings from several studies suggest hypointense lesions on T1-weighted MRI images with hyperintense lesions on T2-weighted sequences with inhomogeneous contrast uptake [8, 19, 20, 37]. However hypointense lesions in the T2-weighted MRI images have also been reported which the authors associated with the dense fusocellular nature of the tumour [36].
The development of liver specific MR contrast agents which includes reticuloendothelial system specific contrast agents and hepatocytes specific contrast agents have been shown to potentially improve the detection and characterization of liver lesions by providing functional and morphologic information of the liver simultaneously [39–41]. Gadobenate dimeglumine is a gadolinium-based contrast agent that is partially taken up by functioning liver cells and excreted without biotransformation through the biliary duct system. Gadobenate dimeglumine shows a vascular-interstitial distribution in the first minutes after bolus injection. Normal liver and benign liver lesions show increased signal intensity on T1-weighted MR images during the delayed liver-specific phase because of active contrast uptake by functioning hepatocytes. The absence of contrast retention during the liver-specific phase is believed to be indicative of malignant liver lesions [39].
It has also been suggested that liver specific MR contrast agents may be misleading in the diagnosis of primary leiomyoma of the liver. The absence of contrast retention during the liver specific contrast enhanced MRI, in the case report, led them to suspect a malignant lesion of the liver but it turned out to be a primary leiomyoma on histology after surgical resection [39]. This finding is consistent with earlier reports in the literature that demonstrated equivocal appearance of primary leiomyoma of the liver after the administration of liver specific contrast agents [36, 41].
4.8. Angiography
Angiography has been reported to demonstrate irregular [37], marginal [8], or diffuse [5] hypervascularity. Hawkins also reported a selective angiogram through the left hepatic artery which demonstrated abnormal mass effect, stretching of the feeding vessels, and scattered pooling throughout the tumour. The authors concluded that the angiography study was nondiagnostic [4].
4.9. Preoperative Diagnosis
Attempts have been made to make a preoperative diagnosis of this tumour so as to prevent unwarranted diagnostic surgical procedures. CT guided fine needle biopsy had reportedly failed to determine the nature of the mass despite the fact that the primary leiomyoma of the liver in this case was the largest ever reported in the literature with a size of 30 cm. The patient underwent extended right hepatectomy. No extra-hepatic lesions were seen at surgery and the uterus was normal [12]. Percutaneous biopsy was attempted with mixed outcomes in the two cases reported by Sadler et al. [21]. One reported “well differentiated smooth muscle neoplasm consistent with hepatic leiomyoma” while multiple attempts at percutaneous biopsy were not successful in the second case [21].
Sousa et al. initially performed a US-guided fine needle aspiration (FNA) which was inconclusive because of because of insufficient sample which included only a small group of normal looking hepatocytes [36]. The histological review of the 18G Trucut biopsy sample taken by Sousa et al. proved to be accurate in the diagnosis of leiomyoma of the liver which was further confirmed after surgical resection [36]. This accurate preoperative diagnosis from biopsy sample is consistent with other reports in the literature [24, 25].
The inconclusive FNA report from the case reported by Sousa et al. is consistent with the series reported by Guy et al. who further reiterated the difficulty of getting adequate sample in 10% of cases where FNA was used in the diagnosis of spindle cell lesions of the liver [42]. Hence FNA does not seem appropriate and adequate for the diagnosis of primary leiomyoma of the liver.
4.10. Macroscopic Features
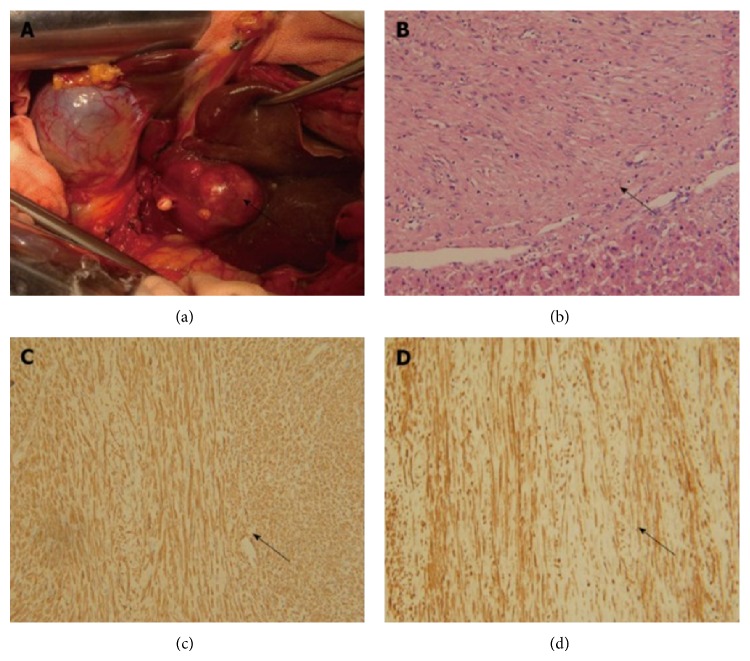
The average size of the tumour in this review is 8.7 cm (range 2–30). The largest size of this tumour in the literature, 30 cm, was reported by Belli et al. in a 67-year-old woman who presented with abdominal mass [12]. Primary Leiomyoma of the liver has been described in the literature as a solitary firm, white, fasciculate, and well demarcated tumour which is consistent with the findings of this review [4, 10, 19, 39]. One author reported a case of primary leiomyoma of the liver with two sharply delineated tumours [20] but other cases in the review have been reported as solitary tumours. The shape has been reported to be roughly spherical [20] to oval [19] (Figure 3(a)).
Figure 3.
Pathologic features. (a) Intraoperative feature of primary leiomyoma of the liver. (b) Tumour (arrow) and normal liver tissue, H&E staining, ×200; (c) α-smooth muscle actin staining (arrow) of tumour tissues, immunohistochemical staining, ×200; (d) Desmin staining (arrow) of tumour tissues, immunohistochemical staining, ×200. Reprinted from [2] with the permission of the Director, Editorial Office of the World Journal of Gastroenterology.
4.11. Microscopic Features
Histological review of tissue sections and specimens is absolutely important because the distinction between benign and malignant smooth muscle tumours of the gastrointestinal tract on imaging is not very clear [12].
The cellular architecture has been variably described as multiple interlacing bundles of uniform spindle cells [39] homogeneous pattern of interlacing bundles of uniform elongate cells with a plump spindle shaped [4], whirling bundles of well differentiated regular spindle shaped smooth muscle cells [2, 12], and highly cellular population of spindle cells arranged in interwoven fascicles (Figure 3(b)). Cells have slightly eosinophilic [20] to abundant eosinophilic cytoplasm [10, 24]. High density reticular fibres with a peripheral collagen rich zone which indicates expanding growth have been reported [20, 24]. Electron microscopy findings suggest tumour cells with well-defined basement membrane, scattered electron dense condensations in the plasma membrane, abundant glycogen, and pinocytotic vesicles and cytoplasmic filaments [24].
Central [19, 25] and focal [24] areas of necrosis have been described in the case reports. However it was not stated in the case reports if the necrosis were coagulative in nature.
Primary leiomyomas of the liver have been largely reported without evidence of mitotic changes except for few case reports which variously reported scarce, low, and rare mitoses [2, 4, 6, 12, 18, 20, 21, 24]. Histological features suggestive of malignancy include prominent cellular atypia with nuclear pleomorphism, large size, presence of infiltration, dense cellularity, degenerative changes, areas of coagulative necrosis, and increased mitotic rates (more than 1/10 HPF) [1, 12, 18, 19].
Mitotic index as defined by the number of mitoses per specified high power field was not documented for some of the case reports [2, 12, 21]. Doyle et al. [24] and Hawkins et al. [4] reported mitotic count of less than 1/10 HPF (high power field). Davidoff et al. [18] and Sclabas et al. [20] reported a mitotic count of 1/50 HPF and less than 1/20 HPF, respectively. Various mitotic indexes have been suggested as a cut off criteria for leiomyosarcoma in nonuterine smooth muscle tumours. Ranchod and Kempson [43] suggested five or more mitoses/10 HPF while between 5–10/50 HPF has been suggested by some authors [44–46]. The use of mitotic index in a uniform manner for all cases reported in the future is needful to ensure unequivocal diagnosis especially in a case reported with mild cellular atypia in the presence of mitoses [21] which may suggest a distinct possibility of malignancy.
4.12. Immunohistochemistry
The cells in the case reports reviewed have been reported to be positive to alpha smooth muscle actin (Figure 3(c)). Some of the cells also stained positive to Desmin (Figure 3(d)) [2, 24] and Vimentin [12]. There is lack of expression of CD 34 [20, 39], CD 68, Vimentin [10] HMB-45, S100 [19, 20], CD 117, and DOG1 [2]. Ultrastructural studies have been reported to show filaments, some dense bodies, and a few pinocytic vesicles [4]. The immunohistochemical stains are useful in ruling out possible differential diagnosis. CD 117, CD34, and DOG1 are markers of gastrointestinal stromal tumours (GIST) and HMB-45 reactivity suggests angiomyolipoma [5, 36, 47].
4.13. Treatment
Primary leiomyoma of the liver is amenable to surgery. Surgical resection of the tumour appears to be both diagnostic and curative in this review of the literature. The prognosis of this tumour appears to be excellent without evidence of disease during the follow-up of the cases. The average follow-up of the cases in this review is 33 months (range 4–108).
5. Conclusion
This paper to the best of the knowledge of the author is the largest review of case reports of primary leiomyoma of the liver in the literature. Primary leiomyoma of the liver is a very rare tumour with a complex pathogenesis which remains largely unknown. The diagnosis of the primary leiomyoma of the liver must meet a set of diagnostic criteria proposed by Hawkins et al. which ensures the cells are leiomyocytes and the exclusion of coexisting leiomyoma from other sites of the body. Metastatic workup to exclude occult leiomyoma elsewhere should be undertaken. This should include investigations like oesophagogastroduodenoscopy, colonoscopy, imaging techniques like CT scans and MRI, and a thorough exploration during surgery.
Primary leiomyoma of the liver should be considered as a differential diagnosis of liver lesions with or without immunosuppression. Multiple imaging techniques do not allow for a tissue specific diagnosis; hence histological review of the tissue specimen and immunohistochemical stains are imperative for diagnosis. Surgical resection is both diagnostic and curative.
Acknowledgment
Many thanks to the Director, Editorial Office of the World Journal of Gastroenterology for the kind permission to use the slides reprinted from [2].
Conflict of Interests
The author declares that there is no conflict of interests regarding the publication of this paper.
References
- 1.Reinertson T. E., Fortune J. B., Peters J. C., Pagnotta I., Balint J. A. Primary leiomyoma of the liver. A case report and review of the literature. Digestive Diseases and Sciences. 1992;37(4):622–627. doi: 10.1007/BF01307591. [DOI] [PubMed] [Google Scholar]
- 2.Luo X.-Z., Ming C.-S., Chen X.-P., Gong N.-Q. Epstein-Barr virus negative primary hepatic leiomyoma: case report and literature review. World Journal of Gastroenterology. 2013;19(25):4094–4098. doi: 10.3748/wjg.v19.i25.4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Demel R. Ein operierter fall von leber-myom. Virchows Archiv für Pathologische Anatomie und Physiologie und für Klinische Medizin. 1926;261(3):881–884. doi: 10.1007/BF01892215. [DOI] [Google Scholar]
- 4.Hawkins E. P., Jordan G. L., McGavran M. H. Primary leiomyoma of the liver. Successful treatment by lobectomy and presentation of criteria for diagnosis. The American Journal of Surgical Pathology. 1980;4(3):301–304. doi: 10.1097/00000478-198006000-00014. [DOI] [PubMed] [Google Scholar]
- 5.Beuzen F., Roudie J., Moali I., Maitre S., Barthelemy P., Smadja C. Primary leiomyoma of the liver: a rare benign tumor. Gastroenterologie Clinique et Biologique. 2004;28(11):1169–1172. doi: 10.1016/S0399-8320(04)95200-1. [DOI] [PubMed] [Google Scholar]
- 6.Yanase M., Ikeda H., Ogata I., Ohno A., Moriya A., Miura N., Kimura S., Mori M., Oka T., Ohtomo K., Mori K., Matsuura A., Harihara Y., Takayama T., Makuuchi M. Primary smooth muscle tumor of the liver encasing hepatobiliary cystadenoma without mesenchymal stroma. The American Journal of Surgical Pathology. 1999;23(7):854–859. doi: 10.1097/00000478-199907000-00016. [DOI] [PubMed] [Google Scholar]
- 7.Kalil A. N., Ferreira M. T., Ressler F., Zettler C. Hepatic leyomioma in an immunocompetent patient. Revista do Colegio Brasileiro de Cirurgioes. 2009;36(4):362–363. doi: 10.1590/S0100-69912009000400017. [DOI] [PubMed] [Google Scholar]
- 8.Urizono Y., Ko S., Kanehiro H., et al. Primary leiomyoma of the liver: report of a case. Surgery Today. 2006;36(7):629–632. doi: 10.1007/s00595-006-3204-y. [DOI] [PubMed] [Google Scholar]
- 9.Imasato M., Tono T., Kano T., Kimura Y., Iwazawa T., Ohnishi T., Nakano Y., Yano H., Okamoto S., Monden T. Primary leiomyoma of the liver: a case report. Nippon Geka Gakkai Zasshi. 2005;106(11):725–729. [PubMed] [Google Scholar]
- 10.Prevot S., Neris J., de Saint Maur P. P. Detection of Epstein Barr virus in an hepatic leiomyomatous neoplasm in an adult human immunodeficiency virus 1-infected patient. Virchows Archiv. 1994;425(3):321–325. doi: 10.1007/BF00196156. [DOI] [PubMed] [Google Scholar]
- 11.Hollands M. J., Jaworski R., Wong K. P., Little J. M. A leiomyoma of the liver. HPB Surgery. 1989;1(4):337–343. doi: 10.1155/1989/45978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belli G., Ciciliano F., Iannelli A., Marano I. Hepatic resection for primary giant leiomyoma of the liver. HPB. 2001;3(1):11–12. doi: 10.1080/136518201753173692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santos I., Valls C., Leiva D., Serrano T., Martinez L., Ruiz S. Primary hepatic leiomyoma: case report. Abdominal Imaging. 2011;36(3):315–317. doi: 10.1007/s00261-010-9648-y. [DOI] [PubMed] [Google Scholar]
- 14.Mahour G. H., Wakim K. G., Soule E. H., Ferris D. O. Structure of the common bile duct in man: presence or absence of smooth muscle. Annals of Surgery. 1967;166(1):91–94. doi: 10.1097/00000658-196707000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Penn I. Malignancies associated with immunosuppressive or cytotoxic therapy. Surgery. 1978;83(5):492–502. [PubMed] [Google Scholar]
- 16.Lee E. S., Locker J., Nalesnik M., Reyes J., Jaffe R., Alashari M., Nour B., Tzakis A., Dickman P. S. The association of Epstein-Barr virus with smooth-muscle tumors occurring after organ transplantation. The New England Journal of Medicine. 1995;332(1):19–25. doi: 10.1056/NEJM199501053320104. [DOI] [PubMed] [Google Scholar]
- 17.Starzl T. E., Nalesnik M. A., Porter K. A., Ho M., Iwatsuki S., Griffith B. P., Rosenthal J. T., Hakala T. R., Shaw B. W., Jr., Hardesty R. L. Reversibility of lymphomas and lymphoproliferative lesions developing under cyclosporin-steroid therapy. The Lancet. 1984;1(8377):583–587. doi: 10.1016/S0140-6736(84)90994-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davidoff A. M., Hebra A., Clark B. J., III, Tomaszewski J. E., Montone K. T., Ruchelli E., Lau H. T. Epstein-Barr virus-associated hepatic smooth muscle neoplasm in a cardiac transplant recipient. Transplantation. 1996;61(3):515–517. doi: 10.1097/00007890-199602150-00036. [DOI] [PubMed] [Google Scholar]
- 19.Perini M. V., Fink M. A., Yeo D. A., Carvalho C. A., Morais C. F., Jones R. M., Christophi C. Primary liver leiomyoma: a review of this unusual tumour. ANZ Journal of Surgery. 2013;83(4):230–233. doi: 10.1111/j.1445-2197.2012.06257.x. [DOI] [PubMed] [Google Scholar]
- 20.Sclabas G. M., Maurer C. A., Wente M. N., Zimmermann A., Büchler M. W. Case report: hepatic leiomyoma in a renal transplant recipient. Transplantation Proceedings. 2002;34(8):3200–3202. doi: 10.1016/S0041-1345(02)03563-7. [DOI] [PubMed] [Google Scholar]
- 21.Sadler M., Mays W. L., Albert P., Javors B. Hepatic leiomyomas in two adult patients with aids: intravenous contrast-enhanced CT and MR imaging. Emergency Radiology. 2002;9(3):175–177. doi: 10.1007/s10140-002-0214-y. [DOI] [PubMed] [Google Scholar]
- 22.Cheuk W., Li P. C. K., Chan J. K. C. Epstein-Barr virus-associated smooth muscle tumour: a distinctive mesenchymal tumour of immunocompromised individuals. Pathology. 2002;34(3):245–249. doi: 10.1080/00313020220131309. [DOI] [PubMed] [Google Scholar]
- 23.Raber E. L., Cheng A.-L., Dong W.-F., Sutherland F. Primary hepatic leiomyoma in a transplant patient: characterization with magnetic resonance imaging. Transplantation. 2012;93(2):e4–e5. doi: 10.1097/TP.0b013e31823ec0cf. [DOI] [PubMed] [Google Scholar]
- 24.Doyle H., Tzakis A. G., Yunis E., Starzl T. E. Smooth muscle tumor arising de novo in a liver allograft: a case report. Clinical Transplantation. 1991;5(1):60–62. [PMC free article] [PubMed] [Google Scholar]
- 25.Ha C., Haller J. O., Rollins N. K. Smooth muscle tumors in immunocompromised (HIV negative) children. Pediatric Radiology. 1993;23(5):413–414. doi: 10.1007/BF02011979. [DOI] [PubMed] [Google Scholar]
- 26.Safai B., Diaz B., Schwartz J. Malignant neoplasms associated with human immunodeficiency virus infection. A Cancer Journal for Clinicians. 1992;42(2):74–95. doi: 10.3322/canjclin.42.2.74. [DOI] [PubMed] [Google Scholar]
- 27.Chelimilla H., Badipatla K., Ihimoyan A., Niazi M. A rare occurrence of primary hepatic leiomyosarcoma associated with Epstein Barr virus infection in an AIDs patient. Case Reports in Gastrointestinal Medicine. 2013;2013 doi: 10.1155/2013/691862.691862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.London N. J., Farmery S. M., Will E. J., Davison A. M., Lodge J. P. A. Risk of neoplasia in renal transplant patients. The Lancet. 1995;346(8972):403–406. doi: 10.1016/S0140-6736(95)92780-8. [DOI] [PubMed] [Google Scholar]
- 29.Buccianti G., Ravasi B., Cresseri D., Maisonneuve P., Boyle P., Locatelli F. Cancer in patients on renal replacement therapy in Lombardy, Italy. The Lancet. 1996;347(8993):59–60. doi: 10.1016/S0140-6736(96)91591-3. [DOI] [PubMed] [Google Scholar]
- 30.Dantal J., Hourmant M., Cantarovich D., Giral M., Blancho G., Dreno B., Soulillou J.-P. Effect of long-term immunosuppression in kidney-graft recipients on cancer incidence: randomised comparison of two cyclosporin regimens. The Lancet. 1998;351(9103):623–628. doi: 10.1016/S0140-6736(97)08496-1. [DOI] [PubMed] [Google Scholar]
- 31.Herzberg A. J., MacDonald J. A., Tucker J. A., Humphrey P. A., Meyers W. C. Primary leiomyoma of the liver. American Journal of Gastroenterology. 1990;85(12):1642–1645. [PubMed] [Google Scholar]
- 32.Rios-Dalenz J. L. Leiomyoma of the liver. Archives of Pathology. 1965;79:54–56. [PubMed] [Google Scholar]
- 33.Rummeny E., Weissleder R., Stark D. D., Saini S., Compton C. C., Bennett W., Hahn P. F., Wittenberg J., Malt R. A., Ferrucci J. T. Primary liver tumors: diagnosis by MR imaging. The American Journal of Roentgenology. 1989;152(1):63–72. doi: 10.2214/ajr.152.1.63. [DOI] [PubMed] [Google Scholar]
- 34.Yoon G. S., Kang G. H., Kim O. J. Primary myxoid leiomyoma of the liver. Archives of Pathology and Laboratory Medicine. 1998;122(12):1112–1115. [PubMed] [Google Scholar]
- 35.Ishak K. G., Rabin L. Benign tumors of the liver. Medical Clinics of North America. 1975;59(4):995–1013. doi: 10.1016/s0025-7125(16)31998-8. [DOI] [PubMed] [Google Scholar]
- 36.Sousa H. T., Portela F., Semedo L., et al. Primary leiomyoma of the liver: accurate preoperative diagnosis on liver biopsy. BMJ Case Reports. 2009 doi: 10.1136/bcr.09.2008.0898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kanazawa N., Izumi N., Tsuchiya K., Sakurai K., Hamano K., Itakura J., Asahina Y., Noguchi O., Uchihara M., Miyake S., Miyakawa N., Kawachi Y., Shiotsu H., Taki K., Himeno Y., Enomoto N., Watanabe M., Sakai T. A case of primary leiomyoma of the liver in a patient without evidence of immunosuppression. Hepatology Research. 2002;24(1):80–88. doi: 10.1016/S1386-6346(02)00014-1. [DOI] [PubMed] [Google Scholar]
- 38.Tan W., Wu G., Zheng C. Imaging findings of primary hepatic leiomyoma. Chinese-German Journal of Clinical Oncology. 2009;8(3):134–136. doi: 10.1007/s10330-008-0163-3. [DOI] [Google Scholar]
- 39.Marin D., Catalano C., Rossi M., Guerrisi A., Di Martino M., Berloco P., Passariello R. Gadobenate dimeglumine-enhanced magnetic resonance imaging of primary leiomyoma of the liver. Journal of Magnetic Resonance Imaging. 2008;28(3):755–758. doi: 10.1002/jmri.21519. [DOI] [PubMed] [Google Scholar]
- 40.Semelka R. C., Helmberger T. K. G. Contrast agents for mr imaging of the liver. Radiology. 2001;218(1):27–38. doi: 10.1148/radiology.218.1.r01ja2427. [DOI] [PubMed] [Google Scholar]
- 41.Gandhi S. N., Brown M. A., Wong J. G., Aguirre D. A., Sirlin C. B. MR contrast agents for liver imaging: what, when, how? Radiographics. 2006;26(6):1621–1636. doi: 10.1148/rg.266065014. [DOI] [PubMed] [Google Scholar]
- 42.Guy C. D., Yuan S., Ballo M. S. Spindle-cell lesions of the liver: diagnosis by fine-needle aspiration biopsy. Diagnostic Cytopathology. 2001;25(2):94–100. doi: 10.1002/dc.2011. [DOI] [PubMed] [Google Scholar]
- 43.Ranchod M., Kempson R. L. Smooth muscle tumors of the gastrointestinal tract and retroperitoneum: a pathologic analysis of 100 cases. Cancer. 1977;39(1):255–262. doi: 10.1002/1097-0142(197701)39:1<255::aid-cncr2820390139>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 44.Morgan B. K., Compton C., Talbert M., Gallagher W. J., Wood W. C. Benign smooth muscle tumors of the gastrointestinal tract. A 24-year experience. Annals of Surgery. 1990;211(1):63–66. doi: 10.1097/00000658-199001000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Appelman H. D. Smooth muscle tumours of the GI Tract: what we know that Stout didn’t know. The American Journal of Surgical Pathology. 1986;10:83–99. [PubMed] [Google Scholar]
- 46.Akwari O. E., Dozois R. R., Weiland L. H., Beahrs O. H. Leiomyosarcoma of the small and large bowel. Cancer. 1978;42(3):1375–1384. doi: 10.1002/1097-0142(197809)42:3<1375::aid-cncr2820420348>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 47.Makhlouf H. R., Remotti H. E., Ishak K. G. Expression of KIT (CD117) in angiomyolipoma. The American Journal of Surgical Pathology. 2002;26(4):493–497. doi: 10.1097/00000478-200204000-00012. [DOI] [PubMed] [Google Scholar]
- 48.Mesenas S. J., Ng K. Y., Raj P., Ho J. M. S., Ng H. S. Primary leiomyoma of the liver. Singapore Medical Journal. 2000;41(3):129–131. [PubMed] [Google Scholar]
- 49.Bartoli S., Alo P., Leporelli P., Puce E., Di Tondo U., Thau A. Primary leiomyoma of the liveer. Minerva Chirurgica. 1991;46(13-14):777–779. [PubMed] [Google Scholar]