Abstract
Background
Cardiopulmonary bypass subjects patients’ blood to hemodilution and nonphysiologic conditions resulting in a systemic inflammatory response. Modified ultrafiltration (MUF) counteracts hemodilution and has also been postulated to improve outcomes by pro-inflammatory cytokine removal. The objective of this study was to investigate whether the benefits of MUF include the removal of pro-inflammatory mediators, such as angiopoietin-2 (angpt-2). We hypothesize that some of the clinical benefits of MUF are related to the preferential removal of angpt-2.
Methods
We performed a prospective cohort study in children ≤ 18 years of age undergoing cardiopulmonary bypass. Serum samples were obtained from each patient: 1. Preoperative, 2. post cardiopulmonary bypass, and 3. upon intensive care unit admission. A fluid sample from the MUF effluent was also analyzed. Angpt-1, angpt-2, interleukin-8 and interleukin-10 levels were determined by enzyme-linked immunosorbent assay.
Results
Thirty-one subjects were enrolled. Angpt-1 levels significantly decreased across all time points (p<0.01). Angpt-2 concentrations were significantly elevated at intensive care unit admission when compared to both preoperative and post cardiopulmonary bypass levels (p<0.01). The angpt-2/1 ratio significantly increased post cardiopulmonary bypass to intensive care unit admission (p<0.01). There was no significant difference between the angpt-2 or angpt- 1 percent extraction within MUF effluent. Interleukin-8 and interleukin-10 significantly increased from preoperative to intensive care unit admission (both p<0.01).
Conclusions
The results of this study demonstrate that MUF removes both pro- and anti-inflammatory mediators equally. This study suggests that the clinical benefits of MUF cannot be attributed to removal of larger quantities of pro-inflammatory mediators such as angpt-2 and interleukin-8.
Keywords: Cardiopulmonary bypass, Inflammatory mediators, Perioperative care
Introduction
Cardiopulmonary Bypass (CPB) is integral in the surgical intervention of complex congenital heart disease. However, the process also subjects patient’s blood to hemodilution, hypothermia, and non-endothelialized surfaces. These factors, along with surgical trauma, ischemia-reperfusion injury and heparin-protamine interactions, act as a potent stimulus for a systemic inflammatory response. As a consequence of the resulting capillary leak, a variety of clinical corollaries ranging from post-operative edema to end organ dysfunction and death can occur [1]. The effects of capillary leak and increased total body water are especially seen in the pediatric population due to their relatively lower total blood volumes in relation to the CPB primer volume [2]. Numerous anti-inflammatory strategies have been initiated to counteract the effect from CPB [3]. The ability of modified ultrafiltration (MUF) to serve as an anti-inflammatory strategy is one that is still currently under investigation [4]. MUF was introduced in 1991 and is the process of ultrafiltration immediately following the completion of CPB [2]. In children, MUF has been shown to have clinical benefit by decreasing total body water, improving myocardial contraction, decreasing transfusion requirements and intensive care unit lengths of stay [2,4,5]. The most accepted beliefs for the clinical improvement are the removal of excess free water and its subsequent hemoconcentation effects [2,4]. There remains controversy whether MUF also removes pro-inflammatory mediators. Most of these studies have been difficult to reproduce due to small patient numbers and varying hemodilutional and hemoconcentrational effects secondary to the process of CPB and MUF, respectively [4].
Angiopoietins (angpt) are a family of vascular growth factors involved in angiogenesis. Angpt-1 and angpt-2 play opposing roles in vascular permeability through their interaction with the Tie-2 receptor [6,7,8,9]. Angpt-1 is constitutively produced by pericytes and maintains vascular quiescence, inhibiting apoptosis and stabilizing intercellular junctions. Additionally, it activates the Phosphoinositide-3-kinase/Akt cell survival signaling pathway and inhibits NFkB and Rho kinase [9]. In contrast, angpt-2 typically possesses pro-inflammatory properties by competitive inhibition of the angpt-1/Tie-2 signaling cascade. The angpt-2 interaction with the Tie-2 receptor causes widened intercellular gaps via the Rho kinase pathway, resulting in capillary leakage and transmigration of leukocytes [9]. Angpt-2 is preformed and rapidly released during periods of stimulation with interleukins, hypoxia, histamine, or thrombin [7,10]. Children undergoing CPB have elevated angpt-2/1 ratios up to twenty-four hours post operatively. In addition, the angpt-2/1 ratio correlates with intensive care unit length of stay [10]. Additionally, angpt-2 has been shown to be preferentially removed using plasma exchange [11].
Our aim was to describe the effects of MUF on plasma angiopoietins. Since angpt-2 is pre-formed and rapidly released during CPB, we hypothesize that some of the clinical benefits of MUF are related to the preferential removal of angpt-2.
Material and Methods
This study was approved by the Yale University Human Research Protection Program and registered on ClinicalTrials.gov, NCT01489475. All patients under 18 years of age undergoing CPB were offered enrollment from December 2011 to June 2013. Patient demographics, including age at surgery, diagnosis, RACHS-1 surgical severity score [12], duration of CPB, aortic cross clamp time, and intensive care unit (ICU) length of stay were collected. ICU length of stay was calculated in hours by calculating the difference between the admission vital signs and the first vital signs obtained on the inpatient floor or the last set of vital signs prior to discharge if the patient was discharged from the ICU.
Preoperative and Operative Technique
Anesthesia for all patients was performed under the supervision of a single attending anesthesiologist (DG). Most commonly, induction was performed with inhalation agents. Maintenance anesthesia was performed with a combination of inhalation agents, intermittent narcotics, intermittent benzodiazepines, and muscle relaxants.
The CPB circuit used for all patients was a roller pump with heparin primed in the circuit. The CPB circuit was primed with methylprednisolone 30 mg/kg and Amicar 75 mg/kg. It was also primed with red blood cells and fresh frozen plasma for patients weighing less than 10 kg. The degree of hypothermia for each patient was determined by the cardiothoracic surgeon and perfusionist on a case-by-case basis.
A Hemocor HPH hemoconcentrator (Minntech Corporation, Minneapolis, MN, USA) was used for MUF. Immediately following cardiopulmonary bypass, MUF was initiated. Blood traveled retrograde from the arterial cannula and was directed across the hemoconcentrator membrane. The filter pore size is rated to allow passage of particles less than 65 kD (Minntech Hemocor HPH® Hemoconcentrator, Minntech, Minneapolis, MN). The blood was then returned to the patient via the venous cannula. MUF was completed when the bypass circuit was emptied of blood for most cases. Filtration typically occurred for approximately 10 minutes or until additional plasma water was not able to be safely removed.
Sampling
Serum samples for angpt-1, angpt-2, interleukin (IL)-8, and IL-10 were taken at three different time points. The first serum sample was drawn from the arterial line in the operating room prior to surgical intervention (pre Op). The second serum sample was drawn from the CPB circuit, following surgical intervention and just prior to MUF, as the patient was being weaned off of cardiopulmonary bypass (post CPB). The last serum sample was drawn from the arterial line upon ICU admission (ICU). A final fluid sample was taken from the discarded MUF effluent following the completion of MUF. The percent MUF extraction was calculated by dividing the angiopoietin or interleukin concentration in the MUF effluent by the concentration post CPB, which was immediately prior to MUF. Serum samples were collected in tubes containing sodium citrate and were centrifuged at 4,000 × g for 10 minutes to separate the serum from the cellular components. Samples were then stored in 1 mL aliquots at −70° Celsius until analyzed.
Angpt-1 and angpt-2 levels were measured using commercially available sandwich enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN, USA). Briefly, 96-well microtiter plates were coated with the appropriate capture antibodies (100 microliters at 4 microliters/mL) in PBS for 2 hours. Plates were washed with PBS containing 0.05% Tween-20 three times and non-specific binding sites were blocked by incubation with 300 microliter of 1% bovine serum albumin in PBS for 1 hour at room temperature. After washing, 100 microliter of standard (recombinant human antibodies) or angiopoietin samples were added and incubated for 2 hours at room temperature. Plates were subsequently washed and the detection antibody was added. After 2 hours at room temperature, plates were washed and incubated with streptavidin-HRP (horseradish peroxidase, 1:200) in 1% BSA in PBS for 20 minutes at room temperature. Plates were washed and 100 microliters of substrate solution (1:1 mixture of hydrogen peroxide and tetramethylbenzidine) was added. This reaction was stopped after 20 minutes with an acidic stop solution. The optical density was measured at 450 nm with a wavelength correction of 540 nm.
IL-8 and IL-10 levels were also measured using commercially available sandwich ELISA kits (R&D Systems, Minneapolis, MN, USA). This process was similar to angiopoietins with incubation times as outlined by the manufacturer’s instructions.
Statistical Analysis
All statistical analyses were performed using SAS 9.2 software (Cary, North Carolina, USA). Pre Op, post CPB, and ICU values were compared using Friedman’s test followed by Wilcoxon Signed Rank tests for pair wise comparisons between time points. The Bonferroni method was used to correct for multiple comparisons. For MUF percent extraction, Wilcoxon Signed Rank test was used to assess differences comparing angpt-1 with angpt-2 and IL-8 with IL-10. For undetectable concentration levels the value of 1 pg/mL was given for calculations of ratios.
Results
Eighty-four patients under 18 years of age underwent cardiac surgery requiring CPB and MUF over this time period. Thirty-one patients were enrolled (37% enrollment). Patient demographic data are shown in Table 1. The median CPB times, cross clamp times and ICU lengths of stay were 98 minutes (range 28-202 minutes), 54 minutes (range 0–114 minutes), and 94 hours (range 27–860 hours), respectively. There were a variety of surgical repairs or palliations performed with ventricular septal defect repairs predominating (Table 2). The RACHS-1 risk categories are displayed in Figure 1A. Some patients underwent more than one intervention accounting for the larger number of surgeries for the number of patients. The patients included in our study had statistically greater RACHS-1 risk categories compared to the other eligible but not enrolled surgical patients (means 2.61 versus 2.23; p=0.04) (Figure 1B). All children survived to hospital discharge. All patients had adequate serum samples for angiopoietin and interleukin level analysis. However, 2 patients did not have adequate MUF effluent available for angpt or IL analysis. Three others only had enough MUF effluent available for angpt analysis.
Table 1.
Baseline Patient Characteristics
| Age, mo Median (IQR) |
6 (0.07–122) |
| Weight, Kg Median (IQR) |
7 (3.13–54.6) |
| Male, n (%) | 14 (45) |
IQR=interquartile range, Kg= kilograms, Mo=months.
Table 2.
Surgeries Performed
| N | |
|---|---|
| VSD repair | 9 |
| Secundum ASD repair | 4 |
| TOF repair | 4 |
| Glenn anastomosis | 3 |
| Subaortic membrane resection | 3 |
| TAPVR repair | 3 |
| Fontan palliation | 2 |
| Primum ASD repair | 2 |
| Pulmonary valve replacement | 2 |
| Arterial switch operation | 1 |
| Complete AVSD repair | 1 |
| Damus-Kaye Stansel procedure | 1 |
| Mitral valve repair | 1 |
| PA band takedown | 1 |
| RV-PA conduit replacement | 1 |
ASD= atrial septal defect, AVSD= atrioventricular septal defect, PA= pulmonary artery, RV= right ventricle, TOF= tetralogy of Fallot, TAPVR= total anomalous pulmonary venous return, VSD= ventricular septal defect.
Figure 1.
RACHS-1 Surgical Severity Pie Chart. A. Consented patients. B. Eligible patients who did not consent. Data expressed as percentages. RC= Risk Category.
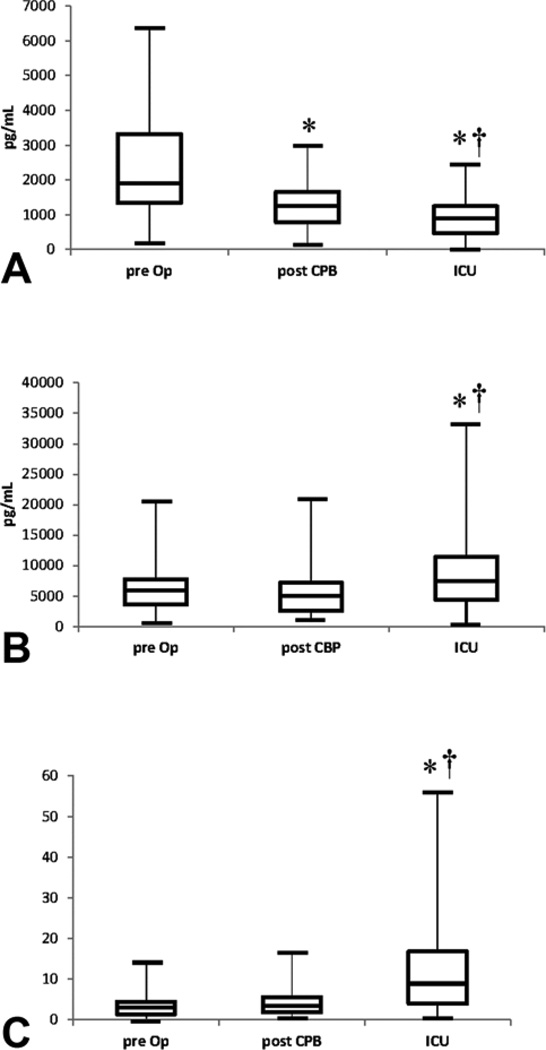
There was a statistically significant decrease in angpt-1 levels at all sampling time points (medians 1902 pg/mL, 1263 pg/mL, 902 pg/mL, respectively; p<0.01). Paired comparison also revealed significant decreases within the group (Figure 2A). Conversely, both pre Op and post CPB angpt-2 levels did not significantly change (median 5860 pg/mL versus 5059 pg/mL; p=0.11). There was a statistically significant increase in angpt-2 at ICU admission when compared to pre Op and post CPB (median 7456 pg/mL; p=0.007 and p=0.002, respectively) (Figure 2B).
Figure 2.
Angiopoietin Box plots. A) Angpt-1; B) Angpt-2; C) Angpt-2/1. The lower solid line represents the 25th percentile, the middle represents the median, and the top box represents the 75th percentile. * Statistically significant paired comparison to pre Op (p<0.01). † Statistically significant paired comparison to post CPB (p<0.01). Angpt-1= angiopoietin-1, Angpt-2= angiopoietin-2, CPB= cardiopulmonary bypass, ICU= intensive care unit, Pre Op= pre operatively.
Since both angiopoietins interact with the Tie-2 receptor with equal affinity, we also analyzed the angpt-2/1 ratio. Pair wise comparisons of angpt-2/1 ratios between pre Op to post CPB showed no significant difference (median 3.06 versus 3.49; p=0.13). Following the process of MUF, there was a statistically significant increase in the angpt-2/1 ratio at ICU admission compared to the previous two time points (median 8.92; p = 0.007 and p=0.002) (Figure 2C). There was no correlation found between the angpt-2/1 ratio post CPB or ICU admission and ICU lengths of stay, patient age, or patient weight (Table 3).
Table 3.
Angpt-2/1 Clinical Correlation
| ICU LOS | Age | Weight | |
|---|---|---|---|
| Angpt-2/1 post CPB | −0.110 | −0.093 | −0.076 |
| Angpt-2/1 ICU | −0.004 | 0.012 | −0.060 |
Data expressed as a Pearson coefficients (r).
Angpt= angiopoietin, CPB= cardiopulmonary bypass, ICU= intensive care unit, LOS= length of stay.
Angpt-1 reached detectable levels in the MUF effluent in 20 out of 29 patient samples (69%). Angpt-2 was detected in 25 out of 29 MUF effluent samples (86%). There was no statistical difference between the number of subjects with detectable MUF angpt-1 and angpt-2 levels (p=0.21). The percent MUF extraction for angpt-1 and angpt-2 was similar (medians 0.74% IQR 0.15, 1.28% and 1.02% IQR 0.6, 2.12%, respectively; p=0.54).
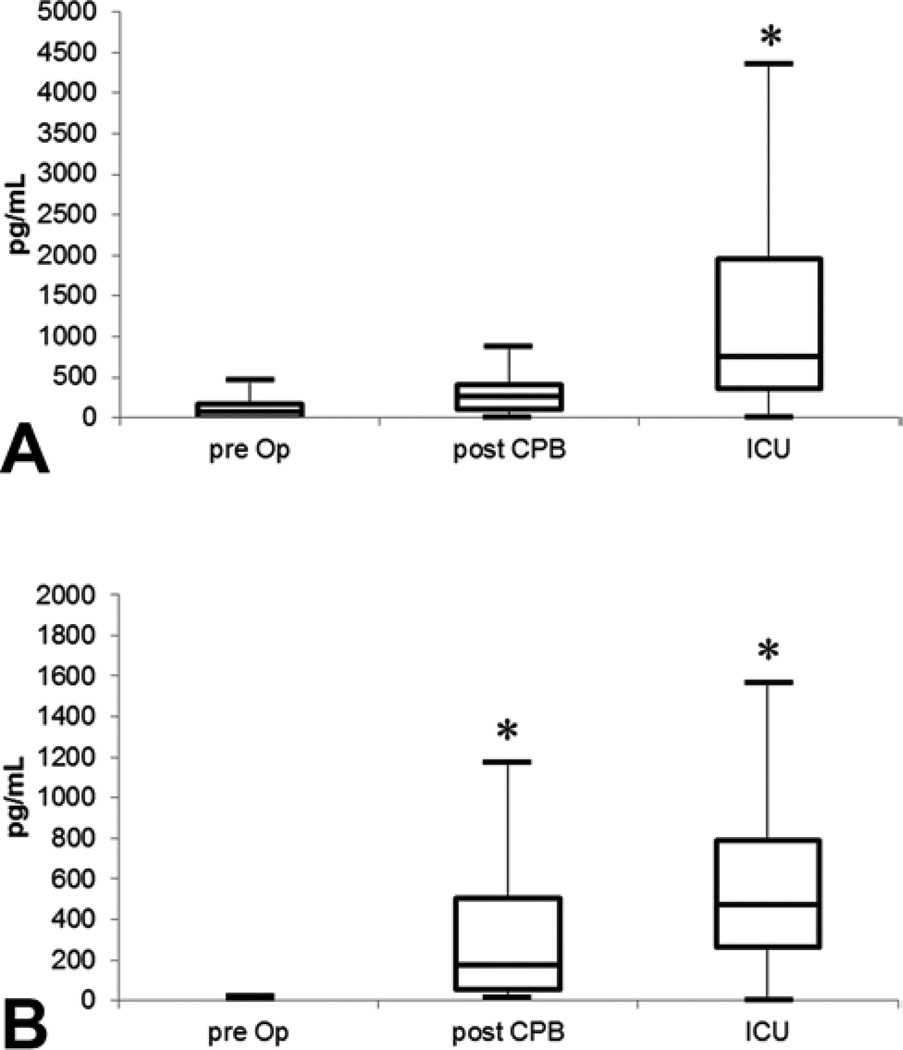
Additionally, IL-8 concentrations were detectable in 21 of 31 patients pre Op, 25 of 31 patients post CPB, and 30 of 31 patients at ICU admission. There was no statistically significant difference from pre Op to post CPB (median 80 pg/mL versus 260 pg/mL; p=0.29) or from post CPB to ICU admission (median 260 pg/mL versus 760 pg/mL; p=0.06). The increase from ICU admission was statistically significant when compared to pre Op (p =0.004) (Figure 3A).
Figure 3.
Interleukin-8 and -10 Box plots. A) IL-8; B) IL-10. The lower solid line represents the 25th percentile, the middle represents the median, and the top box represents the 75th percentile. * Statistically significant paired comparison to pre Op (p<0.01). CPB= cardiopulmonary bypass, ICU= intensive care unit, IL-8= interleukin-8, IL-10= interleukin-10, Pre Op= pre operatively.
IL-10 concentrations were detectable in 10 of 31 patients pre Op, 28 of 31 patients post CPB, and all patients upon ICU admission. There was a statistically significant increase from pre Op compared to post CPB (median 1 pg/mL versus 179 pg/mL; p<0.01). The increase from post CPB and ICU admission did not reach statistical significance (179 pg/mL versus 478 pg/mL; p=0.25). The increase from ICU admission reached statistical significance when compared to pre Op (p < 0.01) (Figure 3B).
For analysis of MUF effluent, IL-8 reached detectable levels in only 7 out of 26 patients (27%). IL-10 reached detectable levels in only 11 out of 26 patients (42%). There was no statistical significant difference in the number of subjects with detectable MUF levels of IL-8 and IL-10 (p=0.38). Due to the large number of undetectable values for these two cytokines, we were unable to perform meaningful statistics on the percent extraction.
Comment
Although the standard of care, MUF’s clinical benefits are still being elucidated. We describe here that the percent MUF extraction of angpt-2 is not statistically different from its predominantly anti-inflammatory counterpart angpt-1. In addition there is an increasing angpt- 2/1 ratio from post CPB to ICU admission. Since angpt-2 is preformed, stored in intracellular Weibel-Palade vesicles and released upon endothelial activation [7], we hypothesized that this inflammatory cytokine would be preferentially removed by MUF. Our results refute our hypothesis since the process of MUF appeared to remove angpt-2 and angpt-1 equally without an appreciable decrease in angpt-2 levels post-MUF.
The pore size of the filter used for MUF is rated to allow passage of particles less than 65 kD (Minntech Hemocor HPH® Hemoconcentrator, Minntech, Minneapolis, MN). Angpt-1 and angpt-2 have similar molecular weights of approximately 70 kD [13, 14]. Given the similar molecular weights, it is not surprising that both are filtered at equal percentages. Though angpt-2 has been shown to be removed by plasma exchange preferentially over angpt-1, this did not occur when using MUF [11]. As a preformed acute phase reactant, we were surprised that angpt-2 levels remained the same following CPB. We suspect that hemodilution affected angpt-2 levels as well as part of the decrease seen in angpt-1. Secondarily, patient rewarming, and not exposure to the CPB circuit, may be the primary inflammatory nidus that activates endothelial cell release of angpt-2. More study is needed to determine if implementing MUF after rewarming will further improve clinical outcomes. In addition, IL-8 (molecular weight of approximately 8 kD) and IL-10 (molecular weight of approximately 18 kD) had similar numbers of patients with detectable MUF levels indicating that they are also removed by the MUF filter equally [15,16,17]. It is unclear why some patients had undetectable IL-8 and IL-10 pre Op and post CPB levels but similar results have been reported [18,19].
Our study was not powered to provide clinical correlations, however prior work by others has demonstrated a correlation between vascular integrity and angpt-2 concentration. This appears to be reversible after the introduction of recombinant angpt-1 [6,8]. In addition, our group has previously shown a correlation between the angpt-2/1 ratio and ICU length of stay [10]. Since quantitatively more angpt-2 is present following endothelial activation after bypass, it is tempting to speculate that proportionally more angpt-2 is removed following MUF and that the angpt-2/1 ratio upon ICU admission is lower than if MUF were not employed. With MUF being the standard of care in most pediatric cardiothoracic programs, this speculation will not be easily tested. An alternative theory is that pro-inflammatory mediators may increase during the process of MUF, as this process exposes blood to additional non-endothelialized tubing. Again, this concept will need further investigation.
There are a few limitations of our study. These are single center data comprised of low to moderate risk surgical patients. This may have skewed the data since more complex patients may have had higher circulating angpt-2 levels. Although gross concentrations of proinflammatory mediators would likely differ, given our results, it is unlikely that this would lead to a difference in percent of pro- or anti-inflammatory MUF extraction. Second, our results are based on a single institution’s perfusion and MUF protocol, which may limit generalizability. In addition, given that MUF is provided for all patients at our institution there was no control group. Third, the middle serum sample was drawn from the bypass circuit rather than interrupting arterial line monitoring while coming off bypass. This was done in an effort to optimize patient safety and also after ample time had passed to allow for blood equilibration. We assumed in our study that the sample from the bypass circuit represents the concentration of inflammatory mediators in the patient, however a better comparison could have been made if all samples were drawn from the arterial line. Fourth, the post MUF sample was obtained at ICU admission. The time from MUF completion to ICU admission is variable and depends on many factors including hemostasis, chest wall closure, rewarming as well as ease of patient transport to the ICU. These issues could have impacted inflammatory mediator concentrations confounding MUF’s effect. However, this was done in an effort to minimize blood draws in our surgical patients, and prompted our decision to assess the discarded MUF effluent for percent protein extraction. Lastly, our sample size was small with only a 37% recruitment rate. This low recruitment rate was attributed to a single recruiter, as well as the reluctance of families to agree to research studies during the surgical process. Despite these limitations, this study is the first to investigate the percent extraction of both pro- and anti-inflammatory angiopoietins in MUF effluent following pediatric cardiothoracic surgery.
Conclusions
Angiopoietin removal following MUF is not significantly different between angpt-2 and angpt-1. The ratio of angpt-2/1 continues to increase following the process of MUF. These results call into question whether MUF’s clinical benefits are attributable to selective removal of angpt-2.
Acknowledgements
We would like to thank Dr. Dorothy Gaal (DG), Clinical Director of Pediatric Anesthesia, Yale School of Medicine. Dr. Gaal oversaw the anesthesia of each of these patients, and helped obtain the initial pre Op blood sample. We would also like to thank the Yale-New Haven Hospital perfusion team and pediatric ICU nursing staff for their invaluable help in collecting patient samples in the operating room and ICU, respectively. Lastly, we would like to thank the Yale School of Medicine Section of Pediatric Cardiology for their support and help with the project design and implementation. Research funding (JG) was provided by CTSA Grant Number UL1 RR024139 from the National Center for Research Resources and the National Center for Advancing Translational Science, components of the National Institutes of Health (NIH), and NIH roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Abbreviations and Acronyms
- Angpt
Angiopoietin
- CPB
Cardiopulmonary bypass
- ELISA
Enzyme-linked immunosorbent assay
- ICU
Intensive care unit
- IL
Interleukin
- MUF
Modified ultrafiltration
- NIH
National Institute of Health
- Pre Op
Preoperative
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to disclose
References
- 1.Warren OJ, Smith AJ, Alexiou C, Rogers PL, Jawad N, Vincent C, et al. The inflammatory response to cardiopulmonary bypass: Part 1--mechanisms of pathogenesis. Journal of cardiothoracic and vascular anesthesia. 2009;23:223–231. doi: 10.1053/j.jvca.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Naik SK, Knight A, Elliott MJ. A successful modification of ultrafiltration for cardiopulmonary bypass in children. Perfusion. 1991;6:41–50. doi: 10.1177/026765919100600106. [DOI] [PubMed] [Google Scholar]
- 3.Warren OJ, Watr AL, de Wit KL, Alexiou C, Vincent C, Darzi AW, et al. The inflammatory response to cardiopulmonary bypass: Part 2--anti-inflammatory therapeutic strategies. Journal of cardiothoracic and vascular anesthesia. 2009;23:384–393. doi: 10.1053/j.jvca.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 4.Chew MS. Does modified ultrafiltration reduce the systemic inflammatory response to cardiac surgery with cardiopulmonary bypass? Perfusion. 2004;19(Suppl 1):S57–S60. doi: 10.1191/0267659104pf719oa. [DOI] [PubMed] [Google Scholar]
- 5.Honjo O, Osaki S, Kotani Y, Akagi T, Sano S. Diagnosis-based differences in response of global ventricular performance to modified ultrafiltration in children. Circulation journal : official journal of the Japanese Circulation Society. 2010;74:86–92. doi: 10.1253/circj.cj-09-0248. [DOI] [PubMed] [Google Scholar]
- 6.Gallagher DC, Bhatt RS, Parikh SM, Patel P, Seery V, McDermott DF, et al. Angiopoietin 2 is a potential mediator of high-dose interleukin 2-induced vascular leak. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:2115–2120. doi: 10.1158/1078-0432.CCR-06-2509. [DOI] [PubMed] [Google Scholar]
- 7.Fiedler U, Scharpfenecker M, Koidl S, Hegen A, Grunow V, Schmidt JM, et al. The tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell weibel-palade bodies. Blood. 2004;103:4150–4156. doi: 10.1182/blood-2003-10-3685. [DOI] [PubMed] [Google Scholar]
- 8.Parikh SM, Mammoto T, Schultz A, Yuan HT, Christiani D, Karumanchi SA, et al. Excess circulating angiopoietin-2 may contribute to pulmonary vascular leak in sepsis in humans. PLoS medicine. 2006;3:e46. doi: 10.1371/journal.pmed.0030046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Meurs M, Kumpers P, Ligtenberg JJ, Meertens JH, Molema G, Zijlstra JG. Bench-to-bedside review: Angiopoietin signalling in critical illness - a future target? Critical care. 2009;13:207. doi: 10.1186/cc7153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giuliano JS, Jr, Lahni PM, Bigham MT, Manning PB, Nelson DP, Wong HR, et al. Plasma angiopoietin-2 levels increase in children following cardiopulmonary bypass. Intensive care medicine. 2008;34:1851–1857. doi: 10.1007/s00134-008-1174-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lovric S, Lukasz A, Hafer C, Kielstein JT, Haubitz M, Haller H, et al. Removal of elevated circulating angiopoietin-2 by plasma exchange--a pilot study in critically ill patients with thrombotic microangiopathy and anti-glomerular basement membrane disease. Thrombosis and haemostasis. 2010;104:1038–1043. doi: 10.1160/TH10-02-0138. [DOI] [PubMed] [Google Scholar]
- 12.Jenkins KJ, Gauvreau K, Newburger JW, Spray TL, Moller JH, Iezzoni LI. Consensus-based method for risk adjustment for surgery for congenital heart disease. The Journal of thoracic and cardiovascular surgery. 2002;123:110–118. doi: 10.1067/mtc.2002.119064. [DOI] [PubMed] [Google Scholar]
- 13.Xu Y, Yu Q. Angiopoietin-1, unlike angiopoietin-2, is incorporated into the extracellular matrix via its linker peptide region. The Journal of biological chemistry. 2001;276:34990–34998. doi: 10.1074/jbc.M103661200. [DOI] [PubMed] [Google Scholar]
- 14.Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, et al. Isolation of angiopoietin-1, a ligand for the tie2 receptor, by secretion-trap expression cloning. Cell. 1996;87:1161–1169. doi: 10.1016/s0092-8674(00)81812-7. [DOI] [PubMed] [Google Scholar]
- 15.Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines--cxc and cc chemokines. Advances in immunology. 1994;55:97–179. [PubMed] [Google Scholar]
- 16.Leong SR, Lowman HB, Liu J, Shire S, Deforge LE, Gillece-Castro BL, et al. Il-8 single-chain homodimers and heterodimers: Interactions with chemokine receptors cxcr1, cxcr2, and darc. Protein science : a publication of the Protein Society. 1997;6:609–617. doi: 10.1002/pro.5560060310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vieira P, de Waal-Malefyt R, Dang MN, Johnson KE, Kastelein R, Fiorentino DF, et al. Isolation and expression of human cytokine synthesis inhibitory factor cdna clones: Homology to Epstein-barr virus open reading frame BCRFI. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:1172–1176. doi: 10.1073/pnas.88.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naquib AN, Tobias JD, Hall MW, Cismowski MJ, Miao Y, Barry N, et al. The role of different anesthetic techniques in altering the stress response during cardiac surgery in children: a prospective, double-blinded, and randomized study. Pediatric Critical Care Medicine. 2013;14:481–490. doi: 10.1097/PCC.0b013e31828a742c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allan CK, Newburger JW, McGrath E, Elder J, Psoinos C, Laussen PC, et al. The relationship between inflammatory activation and clinical outcome after infant cardiopulmonary bypass. Anesthesia and Analgesia. 2010;111:1244–1251. doi: 10.1213/ANE.0b013e3181f333aa. [DOI] [PubMed] [Google Scholar]