Abstract
Children with 22q11.2 deletion syndrome (22q11DS), a copy-number variation (CNV) genetic disorder, demonstrate a great deal of variability in IQ scores and are at particular risk for cognitive difficulties, with up to 45% experiencing intellectual disability. This study explored the IQ relationship between individuals with 22q11DS, their parents and their siblings. Participants included individuals with 22q11DS, unaffected siblings and community controls, who participated in a longitudinal study of 22q11DS. Significant associations between proband and relative (parent, sibling) IQ scores were found. Results suggest that the cognitive functioning of first-degree relatives could be a useful marker of general genetic background and/or environmental effects, and can explain some of the large phenotypic variability in 22q11DS. These findings underscore the importance of including siblings and parents in studies of 22q11DS whenever possible.
Keywords: Intelligence, parent-child correlations, developmental disability, velo-cardio-facial syndrome, 22q11 deletion syndrome
1. Introduction
General cognitive ability, often referred to as intelligence, remains a highly researched construct since Galton’s work at the turn of the 20th century. Although researchers have reached a consensus that both nature and nurture play an important role in intellectual ability, much recent research has focused on the role of genetics. For example, it is widely accepted that intelligence is genetically stable, although the heritability of intelligence increases significantly with age as shared environmental influences decrease (Bouchard, 2013; Briley & Tucker-Drob, 2013; Davis, Haworth, & Plomin, 2009; Deary, Johnson, & Houlihan, 2009; DeFries, Plomin, & LaBuda, 2007; Hoekstra, Bartels, & Boomsma, 2007; Trzaskowski, Yang, Visscher, & Plomin, 2014; van Soelen et al., 2011), and heritability coefficients asymptote between the ages of 18–20 (Bouchard, 2013). As originally reported by Holzinger (1929), the mean intelligence quotients (IQs) of monozygotic twins (r = .88) are greater than those of dizygotic twins (r = .63), leading to the conclusion that genes are somewhat more influential than the environment in determining the variability of mental ability. Furthermore, the correlations between parent-child and sibling-sibling (reared together) IQ scores are also relatively high, averaging about r = .40 and r = .45, respectively (Bouchard & McGue, 1981). However, this is not to say that the environment has no effect on the heritability of intelligence, as studies continue to document the influence of environmental factors on intelligence (Kaplan, 2012; Petrill et al., 2004; Wainwright et al., 2004). A recent review of the state of genetic research on intelligence concluded that much is still unknown about the relationship between genes and cognition. For example, although the Human Genome Project is now complete, it remains unclear how many (and which) genes are involved in cognition and the specific cognitive functions that the genes subserve (Carlier & Roubertoux, 2010).
1.2. Genetics and intelligence in neurodevelopmental disorders
Despite the large number of studies examining the genetics of intelligence in typically developing populations, there has been very little research conducted with respect to understanding the relationship between genetics and intelligence among individuals with neurodevelopmental disorders. One of the few studies assessing this relationship compared parent, sibling, and proband IQ scores among children diagnosed with autism spectrum disorder (ASD) or children diagnosed with Down Syndrome (Fombonne, Bolton, Prior, Jordan, & Rutter, 1997). Fombonne et al. (1997) reported nonsignificant correlations between maternal and autism proband IQ scores, yet found significant correlations between maternal and unaffected sibling IQ scores for both the ASD and Down Syndrome groups. Fombonne et al. (1997) did not report the associations between the IQ scores of mothers and probands with Down Syndrome. An older study found significant correlations between the IQ scores of individuals with Down syndrome and their unaffected siblings and parents, when the probands were living at home. Probands living in institutions had nonsignificant IQ correlations with their unaffected siblings and fathers, but significant correlation with their mothers (Fraser & Sadovnick, 1976). The authors concluded that both additional genetic factors (beyond the trisomy 21) and environmental factors may contribute to the variability in the IQ scores of individuals with Down Syndrome, and that the IQ scores of first-degree relatives may have some predictive value when counseling the families of children with Down Syndrome.
The correlations between the IQ scores of probands and their first-degree relatives have been investigated in several other neurodevelopmental genetic disorders, including Klinefelter syndrome (47 ,XXY; Netley, 1987), Prader-Willi syndrome (PWS; Malich, Largo, Schinzel, Molinari, & Eiholzer, 2000), and Fragile X syndrome (Reiss, Freund, Baumgardner, Abrams, & Denckla, 1995). IQ scores were significantly positively correlated in boys with Klinefelter syndrome and their siblings (Netley, 1987), girls with PWS and their mothers (Malich et al., 2000), and girls with Fragile X syndrome (carrying the full mutation of the FMR1 gene) and their parents (Reiss et al., 1995). These studies suggest that genetic factors other than the specific genetic abnormality underlying the syndromes and/or environmental factors must also contribute to the intellectual functioning in children with these neurodevelopmental syndromes. Furthermore, linear equations predicting proband IQ based on the IQ scores of relatives could be derived, and may be potentially useful in clinical practice (Netley, 1987). Despite these few studies of children with developmental delays, no studies have assessed the relationship between both siblings and parents in a sample of individuals with 22q11.2 deletion syndrome (22q11DS), a genetic syndrome associated with intellectual disabilities.
Chromosome 22q11.2 deletion syndrome (22q11DS), also known as velo-cardio-facial syndrome, is a copy-number variation (CNV) genetic disorder that occurs as a result of an interstitial deletion of 40 – 50 genes on the long arm of chromosome 22 (Ryan et al., 1997). Children with 22q11DS experience various cardiac and craniofacial anomalies, as well as learning disabilities (Simon et al., 2002). Children with 22q11DS have shown a distinct neuropsychological profile, consisting of a low average Full Scale IQ (FSIQ) in the preschool years that can decrease to the borderline/intellectual disability range in the elementary and secondary school years (Golding-Kushner, Weller, & Shprintzen, 1985; Shprintzen, 2000; Swillen et al., 1997). FSIQ scores are quite variable among the 22q11DS population, with up to 45% of individuals with 22q11DS experiencing intellectual disability: mostly mild with a minority experiencing moderate to severe intellectual disability (Moss et al., 1999; Niklasson, Rasmussen, Oskarsdottir, & Gillberg, 2002; Swillen et al., 1997). In addition, a significant number of individuals with 22q11DS show a discrepancy between verbal comprehension and perceptual reasoning abilities, favoring the verbal domain (De Smedt et al., 2007; Golding-Kushner, Weller, & Shprintzen, 1985; Moss et al., 1999; Niklasson et al., 2002; Shprintzen, 2000; Swillen et al., 1997).
Several factors are associated with cognitive functioning in 22q11DS, including age (Gothelf et al., 2005; Green et al., 2009), genetic and environmental factors such as socioeconomic status (Shashi et al., 2010b), genetic variation within the 22q11.2 region (Gothelf et al., 2005; Raux et al., 2007), and origin of the deletion (De Smedt et al., 2007; Swillen et al., 1997). Familial deletions have been associated with lower IQ scores as compared to de novo deletions (De Smedt et al, 2007; Swillen et al., 1997). Age has been negatively associated with IQ (Gothelf et al., 2005; Green et al., 2009), and this effect seems to be modulated by psychiatric symptoms and/or catechol-O-methyl transferase (COMT) genotype (Gothelf et al., 2005; Vorstman et al., submitted).
1.3. The present study
While intelligence relationships between parents, siblings and youth with intellectual delays have not been a frequent topic of research interest, there are several reasons that this line of inquiry may be worthwhile to pursue. For example, research on this topic may help to provide further information regarding how best to explain the cognitive heterogeneity that is seen in youth with genetic syndromes. A greater understanding of these associations may also provide useful clinical information that can be utilized in developing effective intervention approaches. In our current longitudinal study, we evaluated the correlations between the IQ scores of individuals with 22q11DS and their parents and unaffected siblings. Based on the typically developing literature and the reports of correlations between the IQ of parents and children with developmental delays secondary to other genetic syndromes, we hypothesized that these relationships would be moderate in strength in 22q11DS, and would explain a significant portion of the variability of IQ in individuals with 22q11DS, their siblings and community controls.
2. Materials and methods
2.1. Participants
Participants were enrolled in a longitudinal study of risk factors for psychosis in 22q11DS. In addition to the 22q11DS cohort, a sibling control and a community control cohort were included. Siblings were included to account for possible environment-specific variables (e.g., socioeconomic status, home environment, etc.). Our recruitment strategy for the community control group was to oversample children with Attention-deficit/Hyperactivity Disorder (ADHD) and learning disabilities, given the high prevalence of both conditions among individuals with 22q11DS.
Children with 22q11DS and their siblings were recruited from a large academic medical center. Only children with fluorescence in situ hybridization (FISH) confirmed deletion in the q11.2 region of chromosome 22 were included in the sample. In the present analyses, we included only children for whom maternal, paternal and/or sibling IQ was available. The siblings were full siblings of the children with 22q11DS, except for one child, who was a half-sibling. We excluded youth who had a familial inheritance (n = 3) and only included youth with a de novo deletion. Our community control participants were recruited from local public schools. Children with an identifiable genetic disorder other than 22q11DS and/or children with an identifiable neurological condition (e.g., traumatic brain injury, pre-term birth) known to affect cognitive or psychiatric function were excluded from participation. Neither group of control participants received formal molecular genetic screening; 22q11DS is readily identifiable by clinical phenotype and therefore, a higher level of invasiveness (e.g., DNA analysis) was not indicated for our control participants as a measure of screening for 22q11DS.
At Time 1, 73 youth with 22q11DS (Mean age = 11.8 years, SD = 2.0), 25 siblings of youth with 22q11DS (sibling control; Mean age = 12.1 years, SD = 2.2) and an age, gender and socioeconomic status matched group of 23 non-22q11DS youth (community control; Mean age = 11.7 years, SD = 1.9) participated. No age, F (2, 121) = 0.252, p = .778, η2 = 0.004, or gender differences, χ2(2) = 2.315, p = .314, existed between the groups at Time 1. At Time 2, 29 additional participants were recruited (12 individuals with 22q11DS, 6 siblings, and 11 controls). The additional participants were matched by age to the Time 2 returnees. Thus, at Time 2, 81 youth with 22q11DS (Mean age = 14.9 years, SD = 2.1), 31 siblings of youth with 22q11DS (Mean age = 15.2 years, SD = 1.9) and 33 community controls (Mean age = 15.1 years, SD = 1.6) were included in the analyses. At Time 3, 69 youth with 22q11DS (Mean age = 18.0 years, SD = 2.2), 23 siblings of youth with 22q11DS (Mean age = 18.6 years, SD = 1.8) and 27 community controls (Mean age = 17.6 years, SD = 1.3) participated in the study. No significant age, F (2, 119) = 1.713, p = .185, η2 = 0.029, or gender differences, χ2 (2) = 0.775, p = .679, existed between the groups at Time 3. Please see Table 1 for complete participant information.
Table 1.
Participant Data
| 22q11DS | Sibling | Community Control | |
|---|---|---|---|
| Time 1 – Late Childhood | |||
| N | 73 | 25 | 23 |
| Gender (M/F) | 38/35 | 13/12 | 16/7 |
| Age | 11.8 (2.0) | 12.1 (2.2) | 11.7 (1.9) |
| FSIQ | 71.1 (13.7)a***b*** | 106.6 (15.5) b* | 95.8 (14.1) |
| Time 2 – Early Adolescence | |||
| N | 81 | 31 | 33 |
| Gender (M/F) | 40/41 | 15/16 | 18/15 |
| Age | 14.9 (2.1) | 15.2 (1.9) | 15.1 (1.6) |
| FSIQ | 69.6 (14.6)a***b*** | 103.2 (17.2) | 100.2 (12.2) |
| Time 3 – Late Adolescence | |||
| N | 69 | 23 | 27 |
| Gender (M/F) | 35/34 | 11/12 | 16/11 |
| Age | 18.0 (2.2) | 18.6 (1.8) | 17.6 (1.3) |
| FSIQ | 72.3 (14.0)a***b*** | 111.2 (17.1) | 106.0 (18.5) |
| Parental Variables | |||
| Mothers- N | 71 | 28 | 30 |
| Fathers- N | 26 | 13 | 6 |
| Maternal FSIQ- Mean (SD) | 100.6 (11.6) | 101.3 (11.4) | 99.0 (9.8) |
| Paternal FSIQ- Mean (SD) | 104.8 (13.8) | 110.0 (14.2) | 95.2 (11.2) |
Note. 22q11DS = 22q11.2 Deletion Syndrome; FSIQ = Full Scale IQ.
Compared to sibling control participants.
Compared to community control participants.
p < .05.
p < .01.
p < .001.
Chi-square tests indicated that there were no differences in attrition between our three groups, Pearson χ2(2) = 0.514, p = .773. Furthermore, participants lost to follow-up at Time 3 did not differ from those who did follow-up on any relevant Time 1 sociodemographic measures including participant age, gender, and socioeconomic status. Thus, those participants who completed Time 3 assessments appear representative of the Time 1 sample.
2.2. Procedures
Informed consent/assent was obtained from parents and children under protocols approved by the Institutional Review Board at SUNY Upstate Medical University. Each child enrolled in the study was administered a neuropsychological battery covering intelligence, academic achievement, learning/memory, attention, and executive functioning. Only intelligence scores are reported for the present study. An experienced doctoral-level examiner conducted the tests in a quiet room in the clinic. The battery took approximately three hours to complete and each participant received a 15-minute break after completing half of the battery. A licensed psychologist or a trained assistant familiar with the measures double scored all protocols. While the children completed the assessment, parents completed behavior rating scales and background information questionnaires.
Many children and adolescents with 22q11DS are prescribed psychotropic medication(s), especially methylphenidate and selective serotonin reuptake inhibitors (Gothelf et al., 2007). No attempt was made to control for medication use during the assessment; if a child was prescribed medication, parents were instructed to give the medication as prescribed on the day of the research assessment. There were no significant differences in the number of children across the three groups who were prescribed a psychotropic medication at Time 1 (22q11DS: 31.5%, Siblings: 8% and Community Controls: 30.4%; χ2(2) = 5.522, p = .063) or Time 3 (22q11DS: 26.1%, Siblings: 17.4% and Community Controls: 14.8%; χ2(2) = 4.564, p = .102).
2.3. Measures
2.3.1. Intellectual ability
We measured general intellectual functioning with the Wechsler Intelligence Scale for Children —Third edition (WISC-III; Wechsler, 1991) or Wechsler Adult Intelligence Scale – Third edition (WAIS-III; Wechsler, 1997). The WISC-III was administered to all participants at Time 1, and to participants at or under the age of 16 years, 11 months at Times 2 and 3. The WAIS-III was administered to all participants over the age of 16-11 at Times 2 and 3.
Parents were administered an abbreviated WAIS-III measure at Time 2 or Time 3. The abbreviated IQ measure consisted of the WAIS-III Vocabulary and Block Design subtests. Scores on these two subtests were utilized to estimate IQ and correlate strongly with overall FSIQ, Verbal IQ (VIQ) and Performance IQ (PIQ) performance (mean validity = 0.91; mean reliability = 0.94; Sattler, 2001). Validity coefficients for the Vocabulary and Block Design scores relative to the full form are 0.88 for VIQ and 0.83 for PIQ (Sattler, 2001).
2.4. Data analysis
Descriptive statistics and between group comparisons were computed using chi-square statistics for dichotomous variables and analyses of variance (ANOVA) for continuous variables. Holm’s (1979) sequential Bonferroni procedure to adjust p-values for multiple comparisons was employed for asserting statistical significance for omnibus tests. Eta squared (η2) is also reported for all analyses. When η2 > 0.15, effects are considered “large” in magnitude and when η2 > 0.06, effects are viewed as “medium” in magnitude (Cohen, 1988).
Following descriptive comparisons, data analyses were completed in order to best address our a priori hypotheses. Pearson r correlations between sibling and proband IQ scores, as well as parent and child IQ scores (in all three groups) were computed. R-to-z transformations were conducted on the FSIQ correlations (r at Time 1 vs. r at Time 3) for each group, in order to evaluate whether the strength of the associations increases with increasing age. In order to further characterize the relationship between variables that were significantly correlated, we conducted linear regressions with: (1) dependent variables Child IQ, and independent variables maternal or paternal IQ; and (2) dependent variables 22q11DS IQ and independent variables sibling IQ.
3. Results
3.1. Descriptive statistics
As demonstrated in Table 1, there were no significant differences between the three groups with regard to maternal FSIQ, F(2, 129) = 0.344, p = .710, η2 = .005, or paternal FSIQ, F (2, 45) = 2.430, p = .100, η2 = .104. Consistent with the extant literature, significant differences emerged at all three time points between probands with 22q11DS and both sibling and community controls. Please see Table 1 for complete descriptive analyses.
3.2. Familial IQ correlations
As demonstrated in Table 2, multiple significant associations emerged across all three groups between parent and child IQ scores. Similarly, significant associations emerged between the 22q11DS and sibling IQ scores on all three Wechsler variables (Table 3). The strength of the association between the mother and child FSIQ scores increased significantly over time in the community control group, as evidenced by a significant r-to-z transformation (Table 4). The correlation coefficients remained stable in children with 22q11DS, and did not increase significantly in unaffected siblings (Table 2 and Table 4).
Table 2.
Longitudinal Correlations Between Parent and Child IQ Scores
| Mother | Father | |||||||
|---|---|---|---|---|---|---|---|---|
| Time 1/2/3 N |
Time 1 r |
Time 2 r |
Time 3 r |
Time 1/2/3 N |
Time 1 r |
Time 2 r |
Time 3 r |
|
| 22q11DS | ||||||||
| Child VIQ-parental VIQ | 60/71/61 | .531** | .542** | .546** | 24/26/22 | .149 | .225 | .454* |
| Child PIQ-parental PIQ | 60/71/61 | .555** | .511** | .437** | 24/26/22 | .274 | .502* | .550* |
| Child FSIQ-parental FSIQ | 60/71/61 | .608** | .633** | .599** | 24/26/22 | .322 | .457* | .588* |
| Unaffected Siblings | ||||||||
| Child VIQ-parental VIQ | 22/28/21 | .444 | .386 | .490* | 12/13/10 | .013 | .286 | .139 |
| Child PIQ-parental PIQ | 22/28/21 | .482* | .623** | .723** | 12/13/10 | .139 | .567 | .146 |
| Child FSIQ-parental FSIQ | 22/28/21 | .580* | .604** | .745** | 12/13/10 | .256 | .651* | .366 |
| Community Controls | ||||||||
| Child VIQ-parental VIQ | 20/30/24 | .724** | .719** | .709** | 5/5/4 | .940* | .775 | .951 |
| Child PIQ-parental PIQ | 20/30/24 | .252 | .575** | .587** | 5/5/4 | .874 | .972* | .794 |
| Child FSIQ-parental FSIQ | 20/30/24 | .525* | .789** | .848** | 5/5/4 | .853 | .933* | .982* |
Note. 22q11DS = 22q11.2 Deletion Syndrome; VIQ = Verbal IQ; PIQ = Performance IQ; FSIQ = Full Scale IQ.
pFDR < .05.
pFDR < .01.
Table 3.
Correlation Coefficients Between Individuals with 22q11DS and Siblings
| Time 1/2/3 N |
Time 1 r |
Time 2 r |
Time 3 r |
|
|---|---|---|---|---|
| 22q11DS VIQ-sibling VIQ | 33/33/24 | .448* | .555** | .588** |
| 22q11DS PIQ-sibling PIQ | 33/33/24 | .379* | .412* | .505* |
| 22q11DS FSIQ-sibling FSIQ | 33/33/24 | .387* | .515** | .579** |
Note. 22q11DS = 22q11.2 Deletion Syndrome; VIQ = Verbal IQ; PIQ = Performance IQ; FSIQ = Full Scale IQ.
pFDR < .05.
pFDR < .01.
Table 4.
R-to-Z transformations on the Time 1 vs. Time 3 Full Scale IQ Correlation Coefficients
| Groups | Time 1 r |
Time 3 r |
z | p |
|---|---|---|---|---|
| 22q11DS-mother | .608 | .599 | 0.08 | .94 |
| 22q11DS-father | .322 | .588 | −1.08 | .28 |
| 22q11DS-sibling | .387 | .579 | −0.89 | .37 |
| Sibling-mother | .58 | .745 | −0.91 | .36 |
| Sibling-father | .256 | .366 | −0.24 | .81 |
| Control-mother | .525 | .848 | −2.04 | .04 |
| Control-father | .853 | .982 | −0.88 | .38 |
Note. 22q11DS = 22q11.2 Deletion Syndrome.
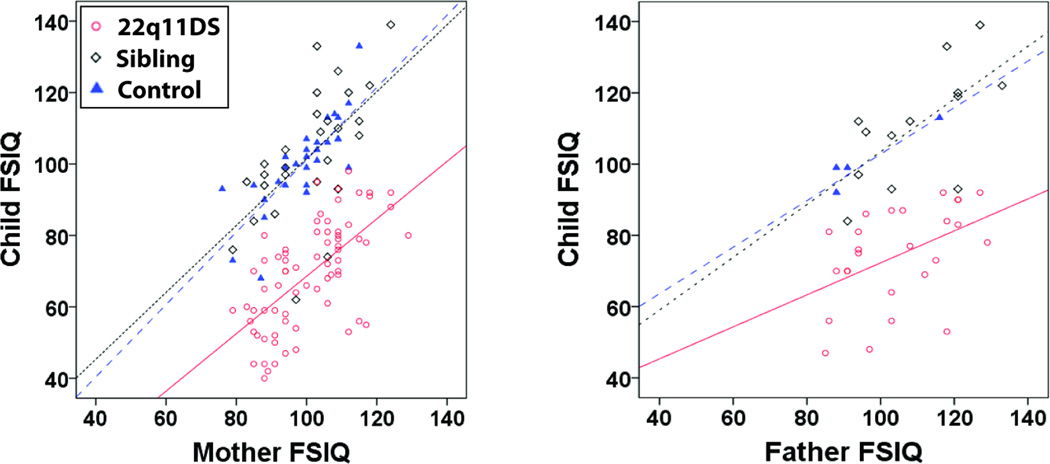
Linear regressions between parental and child IQ scores showed slopes (B) close to 1 for the siblings and community controls (Figure 1 and Table 5). In contrast, the slopes (B) of the majority of the regressions for the 22q11DS group were significantly less than 1 (Figure 1 and Table 5). Similarly, the linear regressions between sibling and 22q11DS IQ scores also had slopes less than 1 (Figure 2 and Table 5).
Figure 1.
Parent-Child FSIQ Correlations at Time 2. The lines show the linear regressions fitted to the data.
Table 5.
Linear Regressions Between Parent & Child Full Scale IQ Scores
| Time | Dependent Variable |
Independent Variable |
R^2 | B | SE B | 95% CI | p |
|---|---|---|---|---|---|---|---|
| 1 | 22q11DS FSIQ | Mother FSIQ | .370 | 0.70 | 0.12 | [0.46, 0.94] | <.001 |
| 2 | 22q11DS FSIQ | Mother FSIQ | .401 | 0.80 | 0.12 | [0.57, 1.04] | <.001 |
| 3 | 22q11DS FSIQ | Mother FSIQ | .359 | 0.73 | 0.13 | [0.48, 0.99] | <.001 |
| 2 | 22q11DS FSIQ | Father FSIQ | .209 | 0.45 | 0.18 | [0.08, 0.82] | .019 |
| 3 | 22q11DS FSIQ | Father FSIQ | .346 | 0.52 | 0.16 | [0.19, 0.85] | .004 |
| 1 | 22q11DS FSIQ | Sibling FSIQ | .150 | 0.30 | 0.13 | [0.04, 0.57] | .026 |
| 2 | 22q11DS FSIQ | Sibling FSIQ | .265 | 0.37 | 0.11 | [0.15, 0.60] | .002 |
| 3 | 22q11DS FSIQ | Sibling FSIQ | .335 | 0.44 | 0.13 | [0.17, 0.71] | .003 |
| 1 | Sibling FSIQ | Mother FSIQ | .336 | 0.79 | 0.25 | [0.27, 1.30] | .005 |
| 2 | Sibling FSIQ | Mother FSIQ | .365 | 0.94 | 0.24 | [0.44, 1.44] | .001 |
| 3 | Sibling FSIQ | Mother FSIQ | .555 | 1.23 | 0.25 | [0.70, 1.76] | <.001 |
| 2 | Sibling FSIQ | Father FSIQ | .424 | 0.74 | 0.26 | [0.17, 1.31] | .016 |
| 1 | Control FSIQ | Mother FSIQ | .276 | 0.79 | 0.30 | [0.16, 1.43] | .017 |
| 2 | Control FSIQ | Mother FSIQ | .622 | 1.01 | 0.15 | [0.71, 1.32] | <.001 |
| 3 | Control FSIQ | Mother FSIQ | .719 | 1.80 | 0.24 | [1.31, 2.30] | <.001 |
Note. 22q11DS = 22q11.2 Deletion Syndrome; FSIQ = Full Scale IQ.
Figure 2.
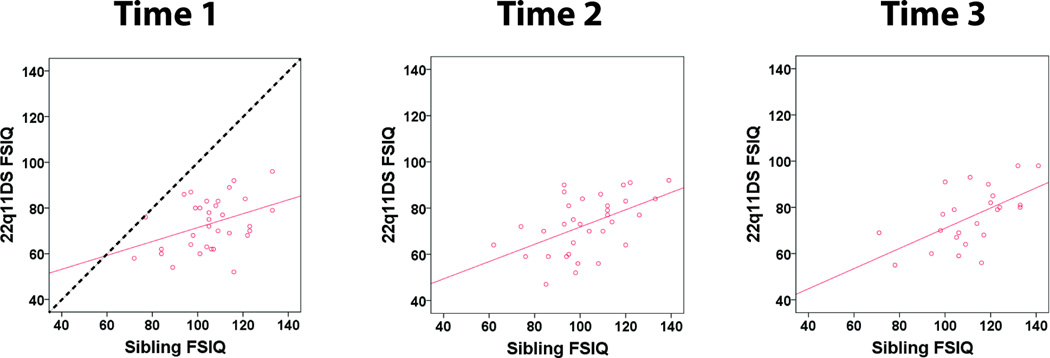
Sibling-22q11DS FSIQ Correlations. The solid lines show linear regressions fitted to the data. The dotted line has a slope of 1.
4. Discussion
The present study represents an initial attempt to explain the significant variability observed in general intellectual abilities within the 22q11DS population. Consistent with previous literature, we found that individuals with 22q11DS had an average IQ around 71. As predicted, relationships emerged between proband IQ scores and their relatives (parents and siblings). These correlations were of moderate strength, and were present across time, from late childhood to late adolescence/early adulthood.
The relationships between the IQ scores of probands with 22q11DS and their relatives are consistent with those reported in other genetic syndromes associated with developmental delays (Fombonne et al., 1997; Frazer & Sadovnick, 1976; Malich et al., 2000; Netley, 1987; Reiss et al., 1995). IQ correlations of moderate strength have been reported for daughters with PWS and their mothers (r = .58), boys with Klinefelter syndrome and their unaffected siblings (r = .633), and children with Down syndrome and their mothers, fathers, or siblings (r = .42, r = .50, and r = .59 respectively, when the proband is living at home) (Fraser & Sadovnick, 1976; Malich et al., 2000; Netley, 1987). As discussed by others, IQ is a polygenic trait, and some of the phenotypic variation of IQ in probands and their relatives is likely due to small additive effects across various genes (Malich et al., 2000). Our finding of significant correlations between probands with 22q11DS and their relatives suggests that at least some of the variability of IQ in individuals with 22q11DS must be determined by general genetic background (genes outside of the 22q11.2 region), and/or environmental factors.
The finding of significant correlations prompted us to further explore these relationships via linear regressions. Interestingly, when considering the IQ scores of probands in the context of the IQ scores of their first-degree relatives (mothers, fathers and siblings), the relative decrease in proband IQ was not uniform across the IQ range of the relatives (Figure 1). Namely, while the IQ regressions between unaffected siblings/controls and their parents had a slope close to 1 (i.e., not significantly less than 1), the majority of the slopes of the regression lines between maternal-22q11DS, paternal-22q11DS and sibling-22q11DS IQ scores were significantly less than 1 (Figure 1, Table 5). This suggests that the effect of the 22q11.2 deletion on IQ in 22q11DS may vary across individuals with 22q11DS. There may be a larger relative decrease in the IQ in individuals with 22q11DS who have first–degree relatives with higher IQ scores, though these individuals still tend to have the highest IQ scores in our 22q11DS sample. To the best of our knowledge, this relationship has not been previously described in individuals with 22q11DS or other neurodevelopmental disorders, and would benefit from replication, as well as study in other neurodevelopmental disorders.
In the current study, the strength of the association between the IQ scores of individuals with 22q11DS and their relatives remained relatively stable over time, from late childhood to early adulthood, as evidenced by the nonsignificant r-to-z transformations in Table 5. In contrast, the maternal-child FSIQ correlations increased in strength in the community controls, increasing from r = .525 (around age 11.7) to r = .848 (around age 17.6; p < .05). A similar pattern, albeit not statistically significant, was observed in the sibling group (r = .580, r = .604, and r = .745 at Times 1, 2 and 3, respectively). These findings in our controls are consistent with previous literature describing increasing heritability of IQ with age (Bouchard, 2013).
The absence of an increase in the strength of the IQ correlations in 22q11DS over time may reflect opposing influences on IQ in 22q11DS. On one hand, the strength should increase, since in general, heritability of IQ increases with age (Bouchard, 2013). On the other hand, however, negative correlations between IQ and age have been described in individuals with 22q11DS (Gothelf et al., 2005; Green et al., 2009; Niklasson, Rasmussen, Oskarsdottir, & Gillberg, 2009). Notably, a longitudinal study found that the decrease in verbal IQ over time was associated with an increase in psychotic symptoms at follow-up (at average age of 18 years; Gothelf et al., 2005). A recent large, multi-center study of the IQ trajectories in 22q11DS found that VIQ and FSIQ scores declined more steeply in individuals with 22q11DS who later developed a psychotic illness (Vorstman et al., submitted). Thus, it appears that the heterogeneity of 22q11DS IQ scores increases over time. This could result in a decrease in the strength of the association between 22q11DS and parental/sibling IQ scores with increasing age. Overall, opposing effects of increasing IQ heterogeneity in 22q11DS, coupled with increasing heritability of IQ as described in the general population, could account for the apparently stable correlations between the parental-proband and sibling-proband IQ scores found in the current study.
5. Conclusions
Our current study adds the IQ scores of first-degree relatives to the list of factors that could be associated with general cognitive function in individuals with 22q11DS. Several other variables have been previously associated with IQ variation in individuals with 22q11DS, including age (Gothelf et al., 2005; Green et al., 2009; Niklasson et al., 2009), gender (Niklasson et al., 2002; Niklasson et al., 2009), and comorbid psychiatric disorders (Gothelf et al., 2005; Green et al., 2009). Genetic factors such as origin of the deletion (familial vs. de novo; Swillen et al., 1997) and variation in 22q11.2 genes (including COMT; Gothelf et al., 2005; Raux et al., 2007; Shashi et al., 2010a), and environmental factors (socioeconomic status; Shashi et al., 2010b) have also been linked to variation in IQ in 22q11DS. Yet, some of these factors can interact with each other (Gothelf et al., 2005), and not all have been replicated independently, nor have they shown effects in the same direction (Glaser et al., 2006; Gothelf et al., 2005; Niklasson et al., 2002; Niklasson et al., 2009; Shashi et al., 2010a). Future larger prospective studies of 22q11DS could examine the relationship of multiple genetic and environmental factors to proband IQ, potential interactions between these factors, and could build comprehensive predictive models of cognitive functioning in 22q11DS. Such models could be used in personalized medicine, and help guide individualized therapeutic interventions. The importance of taking into account multiple clinical, genetic and environmental factors in personalized medicine has been highlighted recently (McEwen & Getz, 2013).
These results should be viewed in the context of our methodological constraints. We did not employ a full WAIS-III protocol for parents and administered an abbreviated intelligence battery. Given the aims of this report, ideally a full WAIS-III protocol would have been administered in order to enable a more detailed analysis of the profiles. Nonetheless, there are strong associations between the abbreviated and full Wechsler battery (Sattler, 2001). In addition, we did not measure or assess the early childhood environment in the home setting.
Despite these methodological weaknesses, these results suggest that parental IQ and unaffected sibling IQ may explain some of the heterogeneity in the 22q11DS cognitive phenotype. Such analyses can account for some of the effects of general genetic background (outside of the 22q11.2 deletion) and the environment, and help predict the phenotypes of individuals with 22q11DS. Our current findings underscore the importance of including siblings and parents in studies of 22q11DS whenever possible.
Highlights.
Familial IQ associations between individuals with 22q11.2DS and parents, siblings.
Stability of mother-child Full Scale IQ associations over time.
IQ scores of first-degree relatives related to general cognitive ability in 22q11DS.
Parent, unaffected sibling IQ as explanation for heterogeneity of 22q11DS phenotype.
Acknowledgements
This research was funded by NIH Grant MH064824 to W.R.K.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bouchard TJ. The Wilson Effect: The increase in heritability of IQ with age. Twin Research in Human Genetics. 2013;16:923–930. doi: 10.1017/thg.2013.54. [DOI] [PubMed] [Google Scholar]
- Bouchard TJ, Jr, McGue M. Familial studies of intelligence: A review. Science. 1981;212:1055–1059. doi: 10.1126/science.7195071. [DOI] [PubMed] [Google Scholar]
- Briley DA, Tucker-Drob EM. Explaining the increasing heritability of cognitive ability across development: A meta-analysis of longitudinal twin and adoption studies. Psychological Science. 2013;24:1704–1713. doi: 10.1177/0956797613478618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier M, Roubertoux P. Genetics and cognition: The impact for psychologists in applied settings. European Psychologist. 2010;15:49–57. [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. New Jersey: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- Davis OS, Haworth CM, Plomin R. Dramatic increase in heritability of cognitive development from early to middle childhood: An 8-year longitudinal study of 8,700 pairs of twins. Psychological Science. 2009;20:1301–1308. doi: 10.1111/j.1467-9280.2009.02433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deary IJ, Johnson W, Houlihan LM. Genetic foundations of human intelligence. Human Genetics. 2009;126:215–232. doi: 10.1007/s00439-009-0655-4. [DOI] [PubMed] [Google Scholar]
- DeFries JC, Plomin R, LaBuda MC. Genetic stability of cognitive development from childhood to adulthood. Developmental Psychology. 1987;23:4–12. [Google Scholar]
- De Smedt B, Devriendt K, Fryns JP, Vogels A, Gewillig M, Swillen A. Intellectual abilities in a large sample of children with Velo-Cardio-Facial Syndrome: An update. Journal of Intellectual Disability Research. 2007;51:666–670. doi: 10.1111/j.1365-2788.2007.00955.x. [DOI] [PubMed] [Google Scholar]
- Fombonne E, Bolton P, Prior J, Jordan H, Rutter M. A family study of autism: Cognitive patterns and levels in parents and siblings. Journal of Child Psychology and Psychiatry. 1997;38:667–683. doi: 10.1111/j.1469-7610.1997.tb01694.x. [DOI] [PubMed] [Google Scholar]
- Fraser FC, Sadovnick AD. Correlation of IQ in subjects with Down syndrome and their parents and sibs. Journal of Intellectual Disability Research. 1976;20:179–182. doi: 10.1111/j.1365-2788.1976.tb00942.x. [DOI] [PubMed] [Google Scholar]
- Glaser B, Debbane M, Hinard C, Morris MA, Dahoun SP, Antonarakis SE, Eliez S. No evidence for an effect of COMT Val158Met genotype on executive function in patients with 22q11 deletion syndrome. American Journal of Psychiatry. 2006;163:537–539. doi: 10.1176/appi.ajp.163.3.537. [DOI] [PubMed] [Google Scholar]
- Golding-Kushner KJ, Weller G, Shprintzen RJ. Velo-cardio-facial syndrome: Language and psychological profiles. Journal of Craniofacial Genetics and Developmental Biology. 1985;5:259–266. [PubMed] [Google Scholar]
- Gothelf D, Eliez S, Thompson T, Hinard C, Penniman L, Feinstein C, …Reiss AL. COMT genotype predicts longitudinal cognitive decline and psychosis in 22q11.2 deletion syndrome. Nature Neuroscience. 2005;8:1500–1502. doi: 10.1038/nn1572. [DOI] [PubMed] [Google Scholar]
- Gothelf D, Michaelovsky E, Frisch A, Zohar AH, Presburger G, Burg M, …Weizman A. Association of the low-activity COMT 158Met allele with ADHD and OCD in subjects with velocardiofacial syndrome. International Journal of Neuropsychopharmacology. 2007;10:301–308. doi: 10.1017/S1461145706006699. [DOI] [PubMed] [Google Scholar]
- Green T, Gothelf D, Glaser B, Debbane M, Frisch A, Kotler M, …Eliez S. Psychiatric disorders and intellectual functioning throughout development in velocardiofacial (22q11.2 deletion) syndrome. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;11:1060–1068. doi: 10.1097/CHI.0b013e3181b76683. [DOI] [PubMed] [Google Scholar]
- Hoekstra RA, Bartels M, Boomsma DI. Longitudinal genetic study of verbal and nonverbal IQ from early childhood to young adulthood. Learning and Individual Differences. 2007;17:97–114. [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics. 1979;6:65–70. [Google Scholar]
- Holzinger KJ. Relative effect of nature and nurture influences on twin differences. Journal of Educational Psychology. 1929;20:241–248. [Google Scholar]
- Kaplan JS. The effects of shared environment on adult intelligence: A critical review of adoption, twin, and MZA studies. Developmental Psychology. 2012;48:1292–1298. doi: 10.1037/a0028133. [DOI] [PubMed] [Google Scholar]
- Malich S, Largo RH, Schinzel A, Molinari L, Eiholzer U. Phenotypic heterogeneity of growth and psychometric intelligence in Prader-Willi syndrome: Variable expression of a contiguous gene syndrome or parent-child resemblance? American Journal of Medical Genetics. 2000;91:298–304. doi: 10.1002/(sici)1096-8628(20000410)91:4<298::aid-ajmg11>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Getz L. Lifetime experiences, the brain and personalized medicine: An integrative perspective. Metabolism. 2013;62:S20–S26. doi: 10.1016/j.metabol.2012.08.020. [DOI] [PubMed] [Google Scholar]
- Moss EM, Batshaw ML, Solot CB, Gerdes M, McDonald-McGinn DM, Driscoll DA, …Wang PP. Psychoeducational profile of the 22q11.2 microdeletion: A complex pattern. Journal of Pediatrics. 1999;134:193–198. doi: 10.1016/s0022-3476(99)70415-4. [DOI] [PubMed] [Google Scholar]
- Netley C. Predicting intellectual functioning in 47,XXY boys from characteristics of sibs. Clinical Genetics. 1987;32:24–27. doi: 10.1111/j.1399-0004.1987.tb03318.x. [DOI] [PubMed] [Google Scholar]
- Niklasson L, Rasmussen P, Oskarsdottir S, Gillberg C. Chromosome 22q11 deletion syndrome (CATCH 22): Neuropsychiatric and neuropsychological aspects. Developmental Medicine and Child Neurology. 2002;44:44–50. doi: 10.1017/s0012162201001645. [DOI] [PubMed] [Google Scholar]
- Niklasson L, Rasmussen P, Oskarsdottir S, Gillberg C. Autism, ADHD, mental retardation and behavior problems in 100 individuals with 22q11 deletion syndrome. Research in Developmental Disabilities. 2009;30:763–773. doi: 10.1016/j.ridd.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Petrill SA, Lipton PA, Hewitt JK, Plomin R, Cherny SS, Corley R, DeFries JC. Genetic and environmental contributions to general cognitive ability through the first 16 years of life. Developmental Psychology. 2004;40:805–812. doi: 10.1037/0012-1649.40.5.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raux G, Bumsel E, Hecketsweiler B, van Amelsvoort T, Zinkstok J, Manouvrier-Hanu S, …Campion D. Involvement of hyperprolinemia in cognitive and psychiatric features of the 22q11 deletion syndrome. Human Molecular Genetics. 2007;16:83–91. doi: 10.1093/hmg/ddl443. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Freund LS, Baumgardner TL, Abrams MT, Denckla MB. Contribution of the FMR1 gene mutation to human intellectual dysfunction. Nature Genetics. 1995;11:331–334. doi: 10.1038/ng1195-331. [DOI] [PubMed] [Google Scholar]
- Ryan AK, Goodship JA, Wilson DI, Philip N, Levy A, Seidel H, …Scambler PJ. Spectrum of clinical features associated with interstitial chromosome 22q11 deletions: A European collaborative study. Journal of Medical Genetics. 1997;34:798–804. doi: 10.1136/jmg.34.10.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler JM. Assessment of children: Cognitive applications. 4th ed. San Diego: Jerome M. Sattler, Publisher, Inc; 2001. [Google Scholar]
- Shashi V, Howard TD, Keshavan MS, Kaczorowski J, Berry MN, Schoch K, …Kwapil TR. COMT and anxiety and cognition in children with chromosome 22q11.2 deletion syndrome. Psychiatry Research. 2010a;178:433–436. doi: 10.1016/j.psychres.2010.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shashi V, Keshavan M, Kaczorowski J, Schoch K, Lewandowski KE, McConkie-Rosell A, …Kwapil TR. Socioeconomic status and psychological function in children with chromosome 22q11.2 deletion syndrome: Implications for genetic counseling. Journal of Genetic Counseling. 2010b;19:535–544. doi: 10.1007/s10897-010-9309-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shprintzen RJ. Velo-cardio-facial syndrome: A distinctive behavioral phenotype. Mental Retardation and Developmental Disabilities Research Reviews. 2000;6:142–147. doi: 10.1002/1098-2779(2000)6:2<142::AID-MRDD9>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Simon TJ, Bearden CE, Moss EM, McDonald-McGinn DM, Zackai EH, Wang PP. Cognitive development in VCFS. Progress in Pediatric Cardiology. 2002;15:109–117. [Google Scholar]
- Swillen A, Devriendt K, Legius E, Eyskens B, Dumoulin M, Gewillig M, Fryns JP. Intelligence and psychosocial adjustment in velocardiofacial syndrome: A study of 37 children and adolescents with VCFS. Journal of Medical Genetics. 1997;34:453–458. doi: 10.1136/jmg.34.6.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trzaskowski M, Yang J, Visscher PM, Plomin R. DNA evidence for strong genetic stability and increasing heritability of intelligence from age 7 to 12. Molecular Psychiatry. 2014;19:380–384. doi: 10.1038/mp.2012.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Soelen IL, Brouwer RM, van Leeuwen M, Kahn RS, Hulshoff Pol HE, Boomsma DI. Heritability of verbal and performance intelligence in a pediatric longitudinal sample. Twin Research in Human Genetics. 2011;14:119–128. doi: 10.1375/twin.14.2.119. [DOI] [PubMed] [Google Scholar]
- Vorstman JAS, Breetvelt E, Duijff SN, Jalbrzikowski M, Vogels A, Swillen A, et al. A decline in verbal intelligence precedes the onset of psychosis in patients with the 22q11.2 deletion syndrome. Manuscript submitted for publication. [Google Scholar]
- Wainwright M, Wright MJ, Geffen GM, Geffen LB, Luciano M, Martin NG. Genetic and environmental sources of covariance between reading tests used in neuropsychological assessment and IQ subtests. Behavioral Genetics. 2004;34:365–376. doi: 10.1023/B:BEGE.0000023642.34853.cb. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children - Third Edition (WISC-III) San Antonio, TX: The Psychological Corporation; 1991. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale - Third Edition (WAIS-III) San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]