Abstract
Based upon age and type of farming exposures, a wide range of studies demonstrate either protective or deleterious effects of the farming environment on asthma. In this review, we highlight key studies supporting the concept that farming exposure protects children from asthma and atopy based on studies performed largely in European pediatric cohorts. Various types of farming in certain regions appear to have a greater effect on asthma protection, as does the consumption of unpasteurized milk. In the United State, where concentrated animal feeding operations (CAFOs) are more common, asthma is increased in children exposed especially to swine CAFOs; whereas, rates of atopy and allergy are lower in these children. We also review studies evaluating the role of farming exposures both as a child and/or as an adult on asthma seen in adults. The importance of microbes in farming environments and the contribution of various components of the innate immune system including toll-like receptors to the underlying mechanisms of asthma related to farming exposures are also reviewed.
Keywords: asthma, atopy, farming, innate immunity
Introduction
Asthma is a chronic inflammatory disease process involving the airways, which is characterized by recurrent airway hyper-responsiveness, bronchospasm, and reversible obstruction. Asthma is a heterogeneous group of conditions of potential genetic pre-disposition with environmental influence.[1] The heterogeneity of asthma results in a disease process that is variable in its severity and phenotype. Whereas asthma has historically been broadly categorized as IgE (allergic)-mediated allergic asthma or non-IgE mediated asthma, studies are now focusing on accurately capturing the multiple clinical phenotypes of this heterogeneous disorder.[2] The phenotypes of importance continue to include atopic status, but also factor in disease onset, clinical features, biomarkers such as eosinophilic inflammation and nitric oxide generation, genetics, and response to therapies.[3–7] Although it remains unclear how farming exposures affect these new clusters of asthma phenotypes, there have been recent advances into the understanding of how farming exposures might differentially affect various asthmatic phenotypes, which will be highlighted in this review. Based upon age and type of farming exposures, a wide range of studies have demonstrated protective or deleterious effects of the farming environment on asthma conditions. The evidence supporting the theory of farming exposures as variably protective against the development of asthma and in support of the “hygiene hypothesis” is strongest in several European pediatric cohorts. The impact of farming exposures in the development of “non-atopic” asthma remains present in children, but is less well described. In adult-focused studies, farming exposures are important risk factors in occupational and workplace-exacerbated asthma. The aim of this article is to review the recent literature to better appreciate current knowledge of the effects of farming and its influence upon asthma in both children and adults.
Farming Exposures in Children is Protective: The evidence
Asthma and atopy have been shown to occur both independently and jointly in patient populations. In specific countries such as the United Kingdom and Australia, the prevalence of asthma and atopic skin reactivity has increased, while in other countries such as Hong Kong, Germany, and Italy there has been an increase in atopy, but not asthma.[8] Further, numerous studies report an inverse correlation for atopy for children who were raised on a farm versus nonfarm children, suggesting that farming is protective of atopy.[9–11] Interestingly, asthma and farming exposures do not consistently follow a similar trend, which is likely explained by the multi-factorial clinical phenotypes of asthma and the heterogeneity of farming exposures.[10]
In Europe, multiple large studies have investigated the effect of the farming environment with allergic disorders. The Allergy and Endotoxin Study (ALEX) conducted from 1999–2004 and the Prevention of Allergy Risk factors for Sensitization In children related to Farming and Anthroposophic Lifestyle (PARSIFAL) project conducted from 2001–2004 have been important studies demonstrating farming exposures as protective against atopic and asthmatic conditions. We will highlight here the more recent GABRIELA (A multidisciplinary study to identify the Genetic and Environmental causes of Asthma In the European Community) advanced surveys, which were conducted 2006–2010.
ALEX Study
The ALEX study team reported the protective nature of early exposure of children to stables and unpasteurized milk. Those being exposed to multiple factors including stables, farm life and unpasteurized milk had the most protection against development of asthma (OR: 0.14; 95% CI 0.14–0.48).[12] Early life (prior to age 1 year) or increased frequency of farming exposure conferred greater protection from the development of asthma by age 5 years. For example, those who were exposed to farming environments and unpasteurized milk in their first year of life displayed protection from asthma; however, if children did not receive both of the exposures until after the first year of life, protection from development of asthma by age 5 years was not apparent (aOR 0.88 95% CI 0.42–1.86).[12] Non-allergic asthma prevalence was 1% in those who frequently visited stables in the first year as compared with 4% in those who did not (p=0.034).[12] In allergic children, the incidence of non-allergic asthma in those who frequently visited stables in the first year of life was 8% compared with those who did not frequently visit stables, who had an incidence of asthma of 25% (p=0.029).[12]
Strengths and Weaknesses of the ALEX study
The ALEX study was retrospectively performed, which introduces potential for recall bias. This issue might have influenced the recall of the amount of time spent as infants (or as young children) in stables on the rural farms. The sample size of the ALEX study was quite large, with initially over 2500 parents completing a questionnaire on asthma and atopy from several European countries. Moreover, parents of nearly 1000 children consented to allowing their children to be tested for IgE antibodies to common local allergens, providing objective data. A potential weakness was that the diagnosis of asthma was elucidated by questionnaire data without complementary pulmonary function testing. However, core questions from the International Study of Asthma and Allergies in Childhood (ISAAC) questionnaire were utilized.
PARSIFAL study
The PARSIFAL studies sought to further define explanations for the possible protective effect of farming on asthma development. The PARSIFAL gathered its data on children from both rural and suburban areas of Europe, including Austria, Germany, Sweden, Switzerland, and the Netherlands. In this study, IgE levels to specific allergens were measured, and survey responses for atopy and asthma symptoms were expanded in comparison to the ALEX study. Similar to the ALEX study, a significantly lower prevalence of asthma for children exposed to farming environments was found (aOR, 0.49; 95% CI 0.35–0.69), but the protection with farming conferred for atopy was greater (aOR 0.24; 95% CI, 0.18–0.34).[13–15] Consistent with the ALEX Study, the PARSIFAL study found that unpasteurized farm milk consumption in the first year of life or ever in life was also associated with reduced risk for childhood atopic diseases.[12–16]
Strengths and Weakness of the PARSIFAL study
The PARSIFAL study expanded upon information gained in the ALEX study because the study population included comparisons among rural and farm children to suburban children. Similar to the ALEX study, the PARSIFAL study relied upon questionnaire data for the diagnosis of asthma as opposed to pulmonary function testing. However, the diagnosis of asthma included a previous doctor’s diagnosis of asthma and wheezing in addition to the ISAAC questionnaire data. A strength of the PARSIFAL study included collection of allergen-specific IgE levels. A doctor’s diagnosis of eczema or rhinoconjunctivitis was also included to help identify history of allergic symptoms for all participants in the study. Like the ALEX study, the PARSIFAL study limited questionnaires to children age 5–13 years. Another strength of the PARSIFAL study is that mattress dust in the German areas of the study was collected to provide exposure data on potential microbial influences (discussed later in this review).
GABRIELA study
The more recent GABRIELA studies demonstrate similar findings in relation to asthma and allergy as the ALEX and PARSIFAL studies. The GABRIELA study was a large European study, including 8,334 school children in Germany, Switzerland, and Austria, and demonstrated decreased prevalence of asthma (aOR 0.76; 95%, CI 0.65–0.89) and atopy (aOR 0.51; 95% CI, 0.46–0.57) in children exposed to farming environments compared to children not exposed to farming environments. The GABRIELA study findings to date have also highlighted other factors which may be important in understanding the correlations with atopy, asthma, and degree of protection.[9, 11, 14, 17, 18] The GABRIELA study group has found that the protective nature of farm exposures in Europe is not universal. A subset of Polish farm children within the GABRIELA studies did not appear to gain as much protection against the development of asthma compared to those children who lived on a farm in the Alpine areas.[9, 17] While some protection was conferred to those farmers’ children in the Alpine regions, protection did not consistently reach significance, and it appeared to be counter-balanced by factors conferring increased risk for asthma, such as fruit cultivation or grain shred use.[9, 17] These observations demonstrate that depending on farm location and farming practice type in Europe, the protective and deleterious effects of farms on the prevalence and risk of development of asthma are not universal.
Further evidence strengthening the role of unpasteurized milk consumption in the protection against atopy and asthma as found in the PARSIFAL and GABRIELA study groups was found by Sozańska, et al.[19] The group found that regular consumption of unpasteurized milk in the first year of life was protective of atopy in non-farming children and adults compared to those who did not consume unpasteurized farm milk (aOR 0.22 95% CI 0.07–0.65 and aOR 0.52 95% CI 0.30–0.81, respectively).[19] Regular consumption of unpasteurized milk in the first year of life was also protective against the diagnosis of asthma “ever” in both farmers and non-farmers (aOR 0.30 95% CI 0.19–0.44 and aOR 0.72 95% CI 0.53–0.87, respectively).[19] These studies highlight an important role for unpasteurized milk, regardless of farming status, and suggest that unpasteurized milk consumption may be a bigger player than the farming environment alone.
As reported by MacNeill et al., children within the GABRIELA Survey were found to have decreased prevalence of asthma on farms in Poland if they were exposed to cattle farming compared to those who were not (aOR 0.76; 95% CI 0.65–.089; p<0.01). [9] In the more cattle predominant Alpine farming areas of Europe, contact with cattle from pregnancy to age 3 years was protective as compared to those unexposed children (aOR 0.74; CI 0.62–0.89, p=0.002). [17] Protection against development of asthma was also observed when children were exposed to cows and straw (aOR 0.79; CI 0.66–0.95, p =0.01) or cows and unpasteurized cow’s milk (aOR 0.77; CI 0.66–0.90, p=.001). [17] The same compounded protective effect was not seen, however, with atopy. These observations suggest that asthma is likely more heterogeneous than an allergic-mediated process. [17] In the Polish regions, fruit cultivation and use of grain shred as animal feed was shown to potentially negate some of the protective effect otherwise exerted by cattle (aOR 1.55 CI 1.14–2.11, p =0.01 and aOR 1.33 CI 1.14–1.55 p=<0.01, respectively). [9] MacNeill et al. also noted that, as compared to Alpine areas, exposure to unpasteurized farm milk was not as protective against development of atopy and did not reach statistical significance in Polish areas (aOR 0.88, 95% CI 0.69–1.12; p=0.30) versus the Alpine areas where unpasteurized cow’s milk was protective of atopy (aOR 0.73, 95% CI 0.64–0.84; p<0.001). The diminished protective effect conferred in the Polish regions is theorized to be secondary to differences in farming practices between the two regions whereby there is a higher prevalence of cattle on farms in Alpine areas (>55%) versus farms in Polish areas (<20%).
A consistent association with these European cohort studies is the finding that unpasteurized farm milk consumption appears to be protective against atopic and asthmatic disease, but the explanation for these observations has not fully been determined. Loss, et al. investigated the role of unpasteurized farm milk consumption as part of the GABRIELA study.[20] The authors hypothesized that increased microbial burden of raw farm milk would explain its protective effects. In a sample of 222 children who consumed milk that was available for culture, consumption of any raw farm milk was significantly protective of development of asthma (aOR 0.59, 95% CI 0.46–0.74; p<0.001), independent of other farm exposures, and that boiled farm milk was not associated with a protective effect (aOR 1.11, 95% CI 0.86–1.44). Although unboiled, raw farm milk had much higher rate and number of total viable bacteria than boiled farm, pasteurized, or high heated shop milk, the specific bacterial content or bacterial type was not significantly associated with asthma protection after adjustment for consumption of raw farm milk. [20] However, the high microbial burden of the raw farm milk made this correlation difficult to measure. For example, the Geometric Mean Ratio (GMR) of raw farm milk was exceptionally high (9500, 95% CI of 6200–14600) in comparison with high heated shop milk (GMR 4.55, 95% CI 3.60–5.76). Common identifiable microbes were much higher within raw farm milk as compared to any of the other types of milk (raw farm, pasteurized, or heated shop), which further complicated the ability to find an association of asthma protection attributed to an unique microbe within the milk.[20] A hypothesis might be made that the individual types of bacteria within these samples could affect asthma and atopy development; however, finding a specific microbe responsible for disease association was not feasible by the authors because of the remarkably high geometric mean ratios found in raw farm milk. Alternatively, investigating the viable microbes (instead of total number of cultured bacteria) within these samples may lead to interesting associations between asthma and atopy. The group did find, however, that the presence of specific whey proteins were inversely associated with asthma, but not atopy: Bovine Serum Albumin (high levels; aOR 0.53 95% CI 0.30–0.97), α-lactalbumin (aOR 0.71 95% CI 0.52–0.97), and β-lactoglobulin (aOR 0.62 95% CI 0.39–0.97).[20] Further work could focus on the effect of denaturing these proteins and their effect on asthma and atopy.
Strengths and Weakness of the GABRIELA studies
The data obtained from the GABRIELA Surveys are one of the largest to date. The initial study population consisted of over 100,000 children, which has led to multiple secondary and tertiary surveys and objective data collections. These investigations have expanded knowledge of how specific farm exposures, particularly raw milk consumption, effect asthma and atopy. As with previous studies, exposure collection is subjected to recall bias. In addition, the farming exposures represent typically small, family run farms. The size and type of farming prevent direct comparisons with the larger corporate style farms that are more prevalent in other regions in the world.
Farming exposures increase risk of pediatric non-atopic asthma: The Evidence
Farming practices differ depending on geographical location. For example, large animal confined feeding operations are increasingly dominating the United States agricultural industry. The United States has noticed a marked decline in the number of swine producers, while the number of hogs produced has remained constant, which indicates an increase in the number of hogs per farmer and density per square foot that these animals reside.[21] Information regarding the effect of these industrial animals farming environmental exposures on the development of childhood asthma is not clear. These types of operations typically have a run off of animal waste to a large central lagoon, and depending on size of feeding operation, these facilities will have varying types of waste management systems.[21] Pavilonis et al. reported a significant association between relative exposure to these types of facilities (based on proximity and wind) and diagnosis of childhood asthma (OR 1.51; 95% CI 1.08–2.09, p=0.014) and medication use for wheeze or asthma (OR 1.38; 95% CI 1.04–1.81, p=0.023) in a rural county in Iowa, United States.[22] Moreover, with increasing relative exposure to these large animal feeding operations, a linear increased risk for asthma was noted.[22] Furthermore, Merchant, et al. in 2005 investigated atopic and asthma prevalence in rural Iowa as part of the Keokuk County Rural Health Study. Reported asthma was more common in children who lived on farms that raised swine as compared to rural children who did not live on farms. The highest prevalence for asthma was found in children who lived on farms who raised any swine and also added antibiotics to their feed (55.8% reported asthma; OR 2.47; CI 1.29–4.74 p=0.01), in comparison with those who did not live on a farm with swine. Despite the increased prevalence of asthma, the rates of atopy and allergy (serum IgE levels) were lower in this same group of swine-exposed farm children, further highlighting the heterogeneity of asthma.[23] A Danish study of farming children reported similar findings. While they also found being raised on a farm to be protective against the development of allergic asthma, it was shown that being exposed to swine and dairy confinements increased the risk of non-allergic asthma development later in life (OR 3.37 95% CI 1.63–6.97 and OR 2.47 95% CI 1.14–5.34, respectively).[24] These findings differ from the findings of the PARSIFAL study team in Europe where pig farming was notably protective of asthma and atopy.[25] These differences in conferred protection from asthma between the European and United States studies may be secondary to the differences in farming practices in Europe and the United States. The major differences being large-scale hog confinements in the United States versus the smaller, family run farms in Europe. Of those farms that raised hogs in the PARSIFAL study, 80% had less than 10 hogs each.[25] In the Iowa study, of those farms raising hogs, 46% had over 500 hogs each.[23] As farming practices continue to evolve, further research is necessary to understand the risk of respiratory diseases and long-term consequences in potentially exposed children as these later studies suggest a high rate of asthma and respiratory disease in children raised around these large animal farming environments.
Farming Exposures Influence Asthma in Adults
Information regarding the effect of farming on the asthma phenotype in the adult population is less well-studied. As part of the PARSIFAL studies in Europe, Douwes, et al. investigated the timing of farming exposures and their effect on adult (age 25–49 years) asthma. [26] The group found that adults were protected from “asthma ever” regardless of when they lived in a farming environment, including: current and childhood exposure (OR 0.56, CI 0.46–0.68), current exposure only (OR 0.69, CI 0.56–0.85), and childhood-only exposure (OR 0.87, CI 0.63–1.19).[26] Protection from development of asthma was greatest with childhood and current farming exposure and least protective with childhood-only exposure, suggesting that benefits from early in life exposure to farming environments do not necessarily confer life-long protection for asthma. In addition, after a significant time period of farming exposures (40–50 years), there was a gradual increase in asthma diagnosis, reported as an increase in “shortness of breath.” The increase in asthma diagnosis over increasing time exposed to farming areas supports prior work that prolonged occupational agricultural exposures leads to the development of chronic obstructive pulmonary disease and other pulmonary diseases.[27, 28] Increased diagnosis of asthma over time also raises the question of whether the diagnosis of asthma is accurate or whether other respiratory disorders are present. The distinction of whether the diagnosis of asthma in this setting was non-allergic asthma versus atopic (or allergic asthmatic) disease is also important to distinguish. Work in large animal confinement feeding operations is associated with increased exposures to gases, organic dust, animal byproducts, and pesticides. These exposures not only increase the risk of non-atopic asthma over time, but also increase the risk of occupational asthma, organic dust toxic syndrome, and hypersensitivity pneumonia. [15, 16, 28]
Eduard et al. studied 8482 farmers and their spouses in Norway in 1991 via questionnaire and blood work to identify the effect of endotoxin exposure on atopy and asthma in adult farmers.[29] They demonstrated a prevalence of asthma of 3.7% with 80% of those asthmatics being non-atopic. Asthma was nearly twice as prevalent in non-atopic farmers with >1 type of livestock than in non-atopic farmers without livestock (aOR 1.8, 95% CI 1.1 – 3.2). Paradoxically, asthma was three times less prevalent in atopic farmers with more than 1 type of livestock than in atopic farmers without livestock (aOR 0.32, 95% CI 0.11 – 0.97).[29] The correlation between decreased prevalence of atopy in farmers with increasing numbers of different types of livestock likely indicates that the adult farmer with asthma is less allergic. Adult farmers with respiratory disease demonstrate neutrophilic, as opposed to eosinophilic, airway inflammation, frequently in response to bacterial endotoxin.[30] Moreover, analysis of annual exposures noted a significant dose-response correlation between amount of dust, fungal spore, bacteria, endotoxin, and ammonia with development of non-atopic asthma. [29] The dose-response correlation between endotoxin and development of non-atopic asthma does not appear unique to adult farmers. Smit et al. demonstrated increased prevalence of respiratory symptoms including wheeze (aOR 1.41 95% CI 1.16–1.72), wheezing with shortness of breath (aOR 1.50, 95% CI 1.18–1.90), and daily cough (aOR 1.29, 95% CI 1.03–1.62), with continued gram negative bacterial cell wall endotoxin exposure in farming environments.[31] The detrimental effect of endotoxin was present in all study subjects, including those workers who lived on farms from childhood. These findings demonstrate that endotoxin was not protective for symptoms of wheeze or cough despite workers being exposed to farms from childhood. [31] These farmers, however, did have a lower prevalence of hay fever, an IgE mediated process. [31] Furthermore, this group reported a dose-response relationship with symptomatic wheeze over 12 month time related to increasing amounts of endotoxin in the farm setting.[31] In addition, endotoxin exposure, in a dose-dependent nature has been associated with decreased pulmonary function and increased airway hyper-responsiveness in pig farmers sensitized to common allergens.[32] However, several other studies have demonstrated that endotoxin levels do not consistently explain the airway inflammatory consequences, suggesting that other microbial species or particulate agents may be important.[33–36]
A Portuguese study investigating effects of particulate matter also noted that adult swine farmers were at increased risk of asthma if exposed to high levels of particulate matter contamination in the swine farms where they worked.[37] Those who were exposed to high concentrations of larger sized particulate matter (2.5, 5, and 10) had an increased trend toward diagnosis of asthma, and those with high exposures to high concentration of smaller sized particulate matter (0.5 and 1) had an increased risk of respiratory symptoms such as sneezing or runny nose.[37]
Pesticides (including herbicides, insecticides, and fungicides) might also represent an important farming exposure that could contribute to the development of both allergic and nonallergic asthma.[38] In the United States Agricultural Health Study (AHS) of 19,263 farmers, a sub-study of male farmers revealed that either pesticide poisoning or high pesticide exposure event (HPEE) history was associated with increases in allergic asthma (aOR 1.98, 95% CI 1.30 – 2.99) and non-allergic asthma diagnosis (aOR 1.96, 95% CI 1.49–2.56).[38] In another AHS study by Hoppin et al., women (n=702) who were diagnosed with adult onset asthma had statistically significant increased risk of development of atopic asthma if ever exposed to pesticide (OR 1.46, 95% CI 1.14–187) but not atopic asthma (OR 1.00, 95% CI 0.82–1.22).[39] However, growing up on the farm was slightly protective against atopic asthma in these women who also applied pesticides (OR 0.73, 95% CI 0.53–1.01) versus those who did not grow up on the farm but did apply pesticides (OR 1.26, 95% CI 0.91–1.74).[39]
Farming Exposure Agents: Microbial burden and Innate Immune Signaling
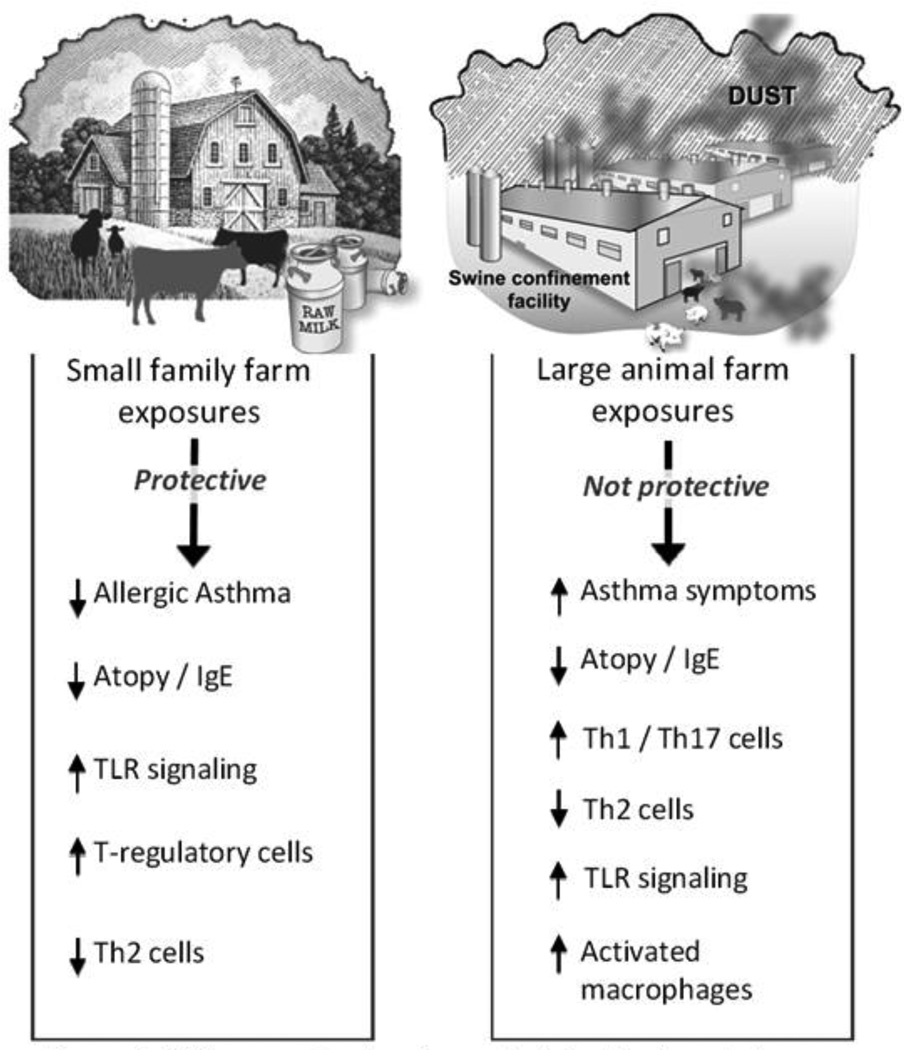
Numerous studies report significant increases in microbial and fungal burden associated with various farming exposures, and the role of these microbial agents in asthma development and airway inflammation is complex, related to the farm setting, and might help explain the immune modulation or effect on various asthma phenotypes. A schematic summary figure of the differential impact of these farming exposures and their influence on asthma consequences is shown in Figure 1.
Figure 1.
Differences in signaling and clinical indings between small and large farm exposures.
Both the PARFISAL and GABRIELA studies found correlation between farming environments and increased environmental microbial burden, which was associated with decreased asthma outcomes. [14, 40] By testing mattress dust samples, a widely diverse microbial biome was identified. Specifically, living on a farm had a positive association with more frequent findings of gram-positive and gram-negative microbes in mattresses and number of detectable single strand polymorphism bands for bacterial taxa (odds ratio per additional 10 bands 1.47; 95% CI 1.20to 1.80; p<0.0001) and fungal taxa (odds ratio for each additional taxon, 2.38; 95% CI, 1.89 to 3.00; p<0.001) as compared to reference group.[13–15] One of the GABRIELA studies looked at the settled dust from children’s rooms and evaluated it for culture of bacterial and fungal organisms. Gram negative bacterial rods in the study homes were noted to confer protection against atopy. This finding reinforces the theory of endotoxin burden protecting against the development of atopy in children. [7, 14, 40, 41] The general role of endotoxin in asthmatic diseases is discussed further in the review series by Alexis, et al. [14, 25, 28, 40, 41]
Potential explanations for the immune-protective effects of these various farming exposures have focused on innate immune signaling pathways, particularly the highly conserved Toll-like receptor (TLR) recognition signaling pathways. In general, activation of TLRs enhance T-helper (Th)1 immune responses as opposed to the allergic, Th2 polarized immune responses. In the ALEX study, blood cells from farmers’ children expressed significantly higher amounts of the TLR co-receptor CD14 mRNA versus non-farmers’ children (0.96 vs. 0.43, p= 0.0013) and also increased amount of TLR2 mRNA (0.11 vs. 0.04, p<0.0001).[42, 43] Work previously done as part of the PARSIFAL study also identified that TLR2, TLR4, and CD14 genes were all up-regulated in children ages 5–13 years with mothers who were exposed during pregnancy to stables.(aGMR 1.44 95% CI 1.04–1.98 p=0.027; aGMR 1.4 95% CI 1.07–1.83 p=0.015; and aGMR 1.66 95% CI 1.18–2.33 p=0.003, respectively). The reference population of children was a Swiss sub-set of the children from the PARSIFAL study that demonstrated decreased asthma diagnosis in children exposed to farming environments (as previously discussed).[13, 25, 43, 44] The authors suggested that these findings contribute to the theory that early farm exposure (including in utero) modulate the innate immune system.[43, 44] Interestingly, increased expression of TLR2, TLR4, and CD14 was also observed in children consuming unpasteurized farm milk.[43, 44] Moreover, the association was strengthened with increasing number of animal species the mother had contact with during pregnancy.[44]
PASTURE/EFRAIM study
In the Prevention Against Allergy: Study in Rural Environments/Mechanisms of Early Protective Exposures on Allergy Development (PASTURE/EFRAIM) study, 500 rural pregnant women from Austria, Finland, France, Germany, and Switzerland who lived on farms, and 500 pregnant women who did not live on farms, were recruited and cord blood from healthy neonates was ex vivo stimulated to investigate T-cell cytokine specific marker responsiveness. [43, 45, 46] These studies demonstrated significant impact of maternal farming environment and development of IgE responses from stimulated cord blood.[43, 47–49]Specifically, noted was maternal farm exposure to animal sheds resulted in higher allergen-specific protection, especially for seasonal allergies (aOR 0.47, 95% CI 0.25 – 0.86).[49] In addition to allergen protection, non-farm children had increased levels of cord blood IgE and less ex vivo stimulated interferongamma (IFN-γ).[48] IFN-γ is a known modulator of allergic disease in that decreased expression in ex vivo stimulation studies at birth has been associated with increased risk for development of allergic symptoms and disease later in life, including respiratory diseases.[43, 50, 51] In contrast to these ex vivo stimulation studies, Frei et al. investigated in vivo, serum IFN-γ levels, and demonstrated significantly decreased IFN-γ levels in school-aged farm children in comparison to non-farm exposed children, suggesting that the immunoprotection effect might not be explained only by a Th1/Th2 paradigm shift.[52] Additional work from this study group has focused on the role of T-regulatory T cells (Tregs). In ex vivo lipopolysaccharide (LPS)-stimulated cellular assays, doctor-diagnosed asthma was associated with decreased T-regulatory cell numbers stimulation (aOR 0.26; 95% CI 0.08-0-88 p=.30). Moreover, Treg cell numbers were increased in those who consumed farm milk (geometric mean ratio = 1.57 (95% CI 1.27–1.95 p<0.001), independent of farm exposures, suggesting a driving role of unpasteurized milk in modulating disease outcomes.[45] T regulatory cell numbers remained present until age 4.5 years (age of survey) and future studies following these children to assess Treg number and asthma development could support the importance of this finding. [45]
Moreover, the PASTURE/EFRAIM study group found that Th17 lineage markers in ex vivo stimulated cord blood were not influenced by maternal farming exposure, but that polymorphisms for Th17 did influence Th17/Treg cell marker expression.[53] Treg and Th17 lineage cell markers were positively correlated with each other and influenced by maternal farm exposure history, highlighting the role of genetics combined with specific maternal and childhood environmental exposures (particularly unpasteurized milk consumption) in influencing the allergic asthma development.[45, 53] Th17 polarized T cells have been linked with subset phenotypes of asthma, and moreover, Th17 lineage cells correlate to neutrophilic influx.[3] Although Th17 lineage markers were not up-regulated in studies conducted on maternal blood in the PASTURE study, there has been evidence of a possible Th17-skewed response in other studies.[53]
Strengths and weakness of the PASTURE/EFRAIM study
The PASTURE/EFRAIM study was unique in that it prospectively recruited pregnant females actively living in farming environments and compared these women to women living in non-farming environments. The investigators prospectively followed the infants and children with several lines of objective data collectively prospectively. This is of incredible value and will continue to provide knowledge into the importance of farming exposures, especially in pregnant mothers. A potential weakness is that the non-farm participants in this study were all from communities of less than 30,000 citizens, and those communities with urban industry were also excluded, potentially limiting its extrapolation to urban and industrial settings. [54]
Animal Modeling studies
In a rodent model, repetitive swine confinement facility organic dust extract exposures promoted a Th1/Th17 lung microenvironment with associated airway neutrophils. [41, 55] A significant increase of Th17 (IL-17A) has also been noted in mouse lung tissue after exposure to settled dust from flower bulb, onion, cattle, and pig farms in the Netherlands, with an associated decrease in Th2 response. [56] In the same study, farm workers from the same locations as the dust collection sites were also noted to have higher amounts of circulating Th17 and Th1 as compared with control groups and an overall protection against Th2 responses. [56] IL-17 expression was also shown to increase in bronchoalveolar lavage fluid cells in healthy human volunteers following a swine barn exposure challenge. [57] Collectively, these studies suggest that various farming exposures might influence the adaptive immune response through regulating T lymphocyte differentiation, which could possibly explain the various asthma phenotype consequences.
Laboratory work has been done showing correlates with organic dust/microbial exposure influencing the airway inflammatory response. Animal models have demonstrated important roles for signaling pathways utilized in mediating dust induced airway responses. For example, the common adaptor protein, MyD88 has been shown to be a central pathway in mediating inflammatory consequences with further functional roles described for some of the pattern recognition receptors previously discussed (TLR2 and TLR4) and also others: TLR9, cytosolic nucleotide oligomerization domain2 (NOD2), and scavenger receptor A (SRA/CD206). [58–62] Laboratory based studies also support a role for protein kinase C activation in mediating inflammation caused by organic-dust. [63] In human studies, genetic polymorphisms in TLR signaling pathways have been demonstrated to impact clinical disease manifestations. In particular, Gao et al. looked at different polymorphisms of TLR2 on lung function of swine operation workers.[64] The group found that workers with TLR2-16933T/A polymorphism had statistically significant greater mean values of lung function than the wild-type sequence (160ml in FEV1 and 390 mL/s in FEF25%–75% after exposure to swine confinement work. [64] A significant difference was also noted in FEF25%–75% with polymorphism of TLR2 Arg753Gln of +670 mL/s versus the wild type TLR2. [64] Previous work by Eder and the ALEX Study Team showed that farmers’ children who carried a T allele on the TLR2/-16934 gene were significantly less likely to have a diagnosis of asthma as compared to those farmers’ children who had the AA genotype (3% vs. 13% p=0.012). [65] Asthma symptoms, atopic sensitization, and current hay fever symptoms were also significantly lower in the group with the TLR2 polymorphism, but protection against asthma itself was independent of atopy. [65] Similar findings have been found for TLR4 where drops in FEV1 were less in those with TLR4 polymorphism 299/399. After exposure to swine confinement facility, those with the polymorphism had a significantly less decline in FEV1 after exposure to hog confinement facility with high levels of endotoxin, suggesting a protective effect of these polymorphisms. [66] Variations in the CD14 gene have also been shown to alter response to endotoxin-exposed farmers, with those being homozygous for CD14/-159T or CD14/-1619G having less wheezing in comparison with the CC or AA genotype, respectively, in a study of farmers with high-endotoxin exposure from the Keokuk County Rural Health Cohort Study in rural Iowa. [67] Each of these studies demonstrates that polymorphisms in genotype are also highly important in the development of the asthma phenotype, both in children and adults, highlighting the importance of gene-environment interaction.
While the studies performed thus far examining asthma and the hygiene hypothesis have generated a host of new knowledge on the heterogeneity of asthma, the how and why microbial exposures, farm life, and unpasteurized milk effect both allergic and non-allergic asthma requires further study. Most of the current data supporting a protective effect of farming has come from European farm studies. These farms were primarily small, family-run farms with a much different structure than the industrial farm which has become much more prevalent in the United States. Creating these studies in United States farms (both small and large) would be important to ascertain key environmental factors that might be positively or negatively influencing asthma development and disease severity. In addition, prospective longitudinal studies complemented with exposure data, while difficult to perform, would further add to our current knowledge of farming factors impacting respiratory disease. Certainly the PASTURE/EFRAIM study will continue to produce great new avenues for research and enhanced clarity to existing theories. In addition, studies such as AGRICOH, a consortium of agricultural cohort studies involving 22 cohorts from nine countries in five continents, while not focused specifically on asthma and respiratory disease may provide valuable insights across multiple cultures and over time. [68] Further investigations into not only how these specific exposures modulate disease, but also enhancement of tools to conduct “farm” field research is needed. Collectively, these insights could identify potential methods of preventing and treating asthma. As farming practices continue to evolve into industrial systems to meet world-wide consumer demand, collecting data on exposed adult workers is necessary, particularly as immigrant working populations in this occupational field are growing. Another important area of future research includes understanding the critical host defense signaling pathways that might provide insights into host genetic factors to aid in the development of novel therapies. Further identifying how both innate and adaptive immunity play into the development of the disease process and whether certain microbial or specific protein exposures are potentially affecting both the development and modification of the immune system will continue to be a source of investigation. Finally, as global farming practices change, it is possible that a shift in the theories behind farming and its influence on the asthma phenotype and classification could potentially also change, providing new opportunities for investigation and understanding of how the farm environment is linked to various phenotypes of asthma.
Highlights.
Recent major studies of farming and asthma in children are reviewed.
Farming has both protective and detrimental influences on childhood asthma.
Farm exposures in adults often have negative influences on asthma.
Microbial components of farm exposures are key in mechanisms influencing asthma.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of all sources of funding for the research reported in the manuscript: none
References
- 1.Bel EH. Mild Asthma. N Engl J Med. 2013;396:549. doi: 10.1056/NEJMcp1214826. [DOI] [PubMed] [Google Scholar]
- 2.Tattersfield AE, Knox AJ, Britton JR, Hall IP. Asthma. Lancet. 2002;360:1313–1322. doi: 10.1016/s0140-6736(02)11312-2. [DOI] [PubMed] [Google Scholar]
- 3.Mckinley L, Alcorn JF, Peterson A, Dupont RB, Kapadia S, Logar A, et al. TH17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J Immunol. 2008;181:4089–4097. doi: 10.4049/jimmunol.181.6.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haldar P, Pavord ID, Shaw DE, Berry MA, Thomas M, Brightling CE, et al. Cluster Analysis and Clinical Asthma Phenotypes. American Journal of Respiratory and Critical Care Medicine. 2008;178:218–224. doi: 10.1164/rccm.200711-1754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woodruff PG, Barmak M, Choy DF, Jia G, Abbas AR, Ellwanger A, et al. T-helper Type 2-driven Inflammation Defines Major Subphenotypes of Asthma. American Journal of Respiratory and Critical Care Medicine. 2009;180:388–395. doi: 10.1164/rccm.200903-0392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wenzel S. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012;18:716–725. doi: 10.1038/nm.2678. [DOI] [PubMed] [Google Scholar]
- 7.Brooks C, Pearce N, Douwes J. The hygiene hypothesis in allergy and asthma: an update. Curr Opin Allergy Clin Immunol. 2013;13:70–77. doi: 10.1097/ACI.0b013e32835ad0d2. [DOI] [PubMed] [Google Scholar]
- 8.Waltraud E, Markus J, von Mutius E. The Asthma Epidemic. The New England Journal of Medicine. 2006;355:2226–2235. doi: 10.1056/NEJMra054308. [DOI] [PubMed] [Google Scholar]
- 9.Macneill SJ, Sozanska B, Danielewicz H, Debinska A, Kosmeda A, Boznanski A, et al. Asthma and allergies: is the farming environment (still) protective in Poland? The GABRIEL Advanced Studies. Allergy. 2013;68:771–779. doi: 10.1111/all.12141. [DOI] [PubMed] [Google Scholar]
- 10.Martinez FD, Vercelli D. Asthma. Lancet. 2013;382:1360–1372. doi: 10.1016/S0140-6736(13)61536-6. [DOI] [PubMed] [Google Scholar]
- 11.Fuchs O, Genuneit J, Latzin P, Buchele G, Horak E, Loss G, et al. Farming environments and childhood atopy, wheeze, lung function, and exhaled nitric oxide. Journal of Allergy and Clinical Immunology. 2012;130:382–288. doi: 10.1016/j.jaci.2012.04.049. [DOI] [PubMed] [Google Scholar]
- 12.Riedler J, Braun-Fahrlander C, Waltraud E, Schreuer M, Waser M, Maisch S, et al. Exposure to farming in early life and development of asthma and allergy: a cross-sectional survey. Lancet. 2001;358:1129–1133. doi: 10.1016/S0140-6736(01)06252-3. [DOI] [PubMed] [Google Scholar]
- 13.Alfven T, Braun-Fahrlander C, von Mutius E, Riedler J, Scheynius A, van Hage M, et al. Allergic diseases and atopic sensitization in children related to farming and anthropoophic lifestyle - the PARSIFAL study. Allergy. 2006;61:414–421. doi: 10.1111/j.1398-9995.2005.00939.x. [DOI] [PubMed] [Google Scholar]
- 14.Ege MJ, Mayer M, Normand A, Genuneit J, Cookson WOCM, Braun-Fahrlander C, et al. Exposure to Environmental Microorganisms and Childhood Asthma. N Engl J Med. 2011;364:701–709. doi: 10.1056/NEJMoa1007302. [DOI] [PubMed] [Google Scholar]
- 15.von Mutius E, Radon K. Living on a Farm: Impact on Asthma Induction and Clinical Course. Immunology and Allergy Clinics of North America. 2008;28:631–647. doi: 10.1016/j.iac.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 16.von Mutius E. 99th Dahlem Conference on Infection, Inflammation and Chronic Inflammatory Disorders: Farm lifestyles and the hygiene hypothesis. Clinical and experimental immunology. 2010;160:130–135. doi: 10.1111/j.1365-2249.2010.04138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Illi S, Depner M, Genuneit J, Horak E, Loss G, Strunz-Lehner C, et al. Protection from childhood asthma and allergy in Alpine farm environments - the GABRIEL Advanced Studies. J Allergy Clin Immunol. 2012;129:1470. doi: 10.1016/j.jaci.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 18.Genuneit J, Buchele G, Waser M, Kovacs K, Debinska A, Boznanski A, et al. The GABRIEL Advanced Surveys: study design, participation, and evaluation of bias. Paediatr Perinat Epidemiol. 2001;25:436–437. doi: 10.1111/j.1365-3016.2011.01223.x. [DOI] [PubMed] [Google Scholar]
- 19.Sozanska B, Pearce N, Dudek K, Cullinan P. Consumption of unpasteurized milk and its effects on atopy and asthma in children and adult inhabitants in rural Poland. Allergy. 2013;68:644–650. doi: 10.1111/all.12147. [DOI] [PubMed] [Google Scholar]
- 20.Loss G, Apprich S, Waser M, Kneifel W, Genuneit J, Buchele G, et al. The Protective effect of farm milk consumption on childhood asthma and atopy: The GABRIELA study. J Allergy Clin Immunol. 2011;128:766. doi: 10.1016/j.jaci.2011.07.048. [DOI] [PubMed] [Google Scholar]
- 21.Key ND, Mcbride WD. The changing economics of U.S. hog production. US Department of Agriculture, Economic Research Service. 2008;52 [Google Scholar]
- 22.Pavilonis BT, Sanderson WT, Merchant JA. Relative exposure to swine animal feeding operations and childhood asthma prevalence in an agricultural cohort. Environ Res. 2013;122:74–80. doi: 10.1016/j.envres.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Merchant JA, Naleway AL, Svendsen ER, Kelly KM, Burmeister LF, Stromquist AM, et al. Asthma and Farm Exposures in a Cohort of Rural Iowa Children. Environ Health Perspect. 2005;113:350–356. doi: 10.1289/ehp.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Omland O, Hjort C, Pedersen OF, Miller MR, Sigsgaard T. New-onset asthma and the effect of environment and occupation among farming and nonfarming rural subjects. J Allergy Clin Immunol. 2011;128:761. doi: 10.1016/j.jaci.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 25.Ege MJ, Frei R, Bieli C, Schram-Bijerk D, Waser M, Benz MR, et al. Not all farming environments protect the development of asthma and wheeze in children. Journal of Allergy and Clinical Immunology. 2007;119:1140–1147. doi: 10.1016/j.jaci.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 26.Douwes J, Travier N, Huang K, Cheng S, McKenzie J, Le Gros G, et al. Lifelong farm exposure may strongly reduce the risk of asthma in adults. Allergy. 2007;62:1158–1165. doi: 10.1111/j.1398-9995.2007.01490.x. [DOI] [PubMed] [Google Scholar]
- 27.Eduard W, Pearce N, Douwes J. Chronic Bronchitis, COPD, and Lung Function in Farmers: The Role of Biological Agents<br /><br />. Chest. 2009;146:716. doi: 10.1378/chest.08-2192. [DOI] [PubMed] [Google Scholar]
- 28.May S, Romberger D, Poole J. Respiratory Health Effects of Large Animal Farming Environments. Journal of Toxicology and Environmental Health, Part B: Critical Reviews. 2012;15:524–541. doi: 10.1080/10937404.2012.744288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eduard W, Douwes J, Omenaas E, Heederik D. Do farming exposures cause or prevent asthma? Results from a study of adult Norwegian farmers. Thorax. 2004;59:381. doi: 10.1136/thx.2004.013326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Douwes J, Gibson P, Pekkanen J, Pearce N. Non-eosinophilic asthma: importance and possible mechanisms. Thorax. 2002;57:643. doi: 10.1136/thorax.57.7.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smit LAM, Heederik D, Doekes G, Blom C, van Zweden I, Wouter IM. Exposure-response analysis of allergy and respiratory symptoms in endotoxin-exposed adults. Eu Respir J. 2008;31:1241–1248. doi: 10.1183/09031936.00090607. [DOI] [PubMed] [Google Scholar]
- 32.Portengen L, Preller L, Tielen M, Doekes G, Heederik D. Endotoxin exposure and atopic sensitization in pig farmers. J Allergy Clin Immunol. 2005;115:797. doi: 10.1016/j.jaci.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 33.Reynolds SJ, Donham KJ, Whitten P, Merchant JA, Burmeister LF, Popendorf WJ. Longitudinal evaluation of dose-response relationships with environmental exposures and pulmonary function in swine production workers. Am J Ind Med. 1996;29:33. doi: 10.1002/(SICI)1097-0274(199601)29:1<33::AID-AJIM5>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz DA, Thorne PS, Yagla SJ, Burmeister LF, Olenchock SA, Watt JL, et al. The role of endotoxin in grain dust-induced lung disease. Am J Respir Crit Care Med. 1995;152:603. doi: 10.1164/ajrccm.152.2.7633714. [DOI] [PubMed] [Google Scholar]
- 35.Donham KJ, Cumro D, Reynolds SJ, Merchant JA. Dose-response relationships between occupational aerosol exposures and cross-shift declines of lung function in poultry workers: recommendations for exposure limits. J Occup Environ Med. 2000;42:260. doi: 10.1097/00043764-200003000-00006. [DOI] [PubMed] [Google Scholar]
- 36.Vogelzang PF, van der Gulden JW, Folgering H, Kolk JJ, Heederik D, Preller L, et al. Endotoxin exposure as a major determinant of lung function decline in pig farmers. Am J Respir Crit Care Med. 1998;157:15. doi: 10.1164/ajrccm.157.1.9703087. [DOI] [PubMed] [Google Scholar]
- 37.Viegas S, Mateus V, Almeida-Silva M, Carolino E, Viegas C. Occupational exposure to particulate matter and respiratory symptoms in Portuguese swine barn workers. J Toxicol Environ Health A. 2013;76:1007. doi: 10.1080/15287394.2013.831720. [DOI] [PubMed] [Google Scholar]
- 38.Hoppin JA, Umbach DM, London SJ, Henneberger PK, Kullman GJ, Coble J, et al. Pesticide use and adult-onset asthma among male farmers in the Agricultural Health Study. Eu Respir J. 2009;34:1296. doi: 10.1183/09031936.00005509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoppin JA, Umbach DM, London SJ, Henneberger PK, Kullman GJ, Alavanja MCR, et al. Pesticides and Atopic and Onatopic Asthma among Farm Women in the Agricultural Health Study. Am J Respir Crit Care Med. 2008;177:11. doi: 10.1164/rccm.200706-821OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Braun-Fahrlander C, Riedler J, Herz U, Eder W, Waser M, Grize L, et al. Environmental exposure to endotoxin and its relation to asthma in school-age children. N Engl J Med. 2002;347:869. doi: 10.1056/NEJMoa020057. [DOI] [PubMed] [Google Scholar]
- 41.Poole JA. Farming-associated environmental exposures and effect on atopic diseases. Ann Allergy Asthma Immunol. 2012;109:93–98. doi: 10.1016/j.anai.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lauener RP, Birchler T, Adamski J, Braun-Fahrlander C, Bufe A, Herz U, et al. Expression of CD14 and Toll-like receptor 2 in farmers' and non farmers' children. Lancet. 2002;360:456. doi: 10.1016/S0140-6736(02)09641-1. [DOI] [PubMed] [Google Scholar]
- 43.von Mutius E, Vercelli D. Farm living: effects on childhood asthma and allergy. Nat Rev Immunol. 2010;10:861. doi: 10.1038/nri2871. [DOI] [PubMed] [Google Scholar]
- 44.Ege MJ, Bieli C, Frei R, van Strien RT, Riedler J, Ublagger E, et al. Prenatal farm exposure is related to the expression of receptors of the innate immunity and to atopic sensitization in school age children. J Allergy Clin Immunol. 2006;117:817. doi: 10.1016/j.jaci.2005.12.1307. [DOI] [PubMed] [Google Scholar]
- 45.Lluis A, Depner M, Gaugler B, Saas P, Casaca VI, Raedler D, et al. Increased regulatory T-cell numbers are associated with farm milk exposure and lower atopic sensitization and asthma in childhood. J Allergy Clin Immunol. 2014;133:551. doi: 10.1016/j.jaci.2013.06.034. [DOI] [PubMed] [Google Scholar]
- 46.von Mutius E, Schmid S the PASTURE Study Group. The PASTURE project: EU support for the improvement of knowledge about risk factors and preventitive factors for atopy in Europe. Allergy. 2006;61:407. doi: 10.1111/j.1398-9995.2006.01009.x. [DOI] [PubMed] [Google Scholar]
- 47.Pfefferle P, Buchele G, Roponen M, Ege MJ, Krauss-Etschmann S, Benuneit J, et al. Cord blood cytokines are modulated by maternal farming activities and consumption of farm dairy products during pregnancy: the PASTURE Study. J Allergy Clin Immunol. 2010;125:108. doi: 10.1016/j.jaci.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 48.Pfefferle P, Sel S, Ege MJ, Buchele G, Blumer N, Krauss-Etschmann S, et al. Cord blood allergen-specific IgE is associated with reduced IFN-gamma production by cord blood cells: the Protection against Allergy-Study in Rural Environments (PASTURE) Study. J Allergy Clin Immunol. 2008;122:711. doi: 10.1016/j.jaci.2008.06.035. [DOI] [PubMed] [Google Scholar]
- 49.Ege MJ, Herzum I, Buchele G, Krauss-Etschmann S, Lauener R, Roponen M, et al. Prenatal exposure to a farm environment modifies atopic sensitization at birth. J Allergy Clin Immunol. 2008;122:407. doi: 10.1016/j.jaci.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 50.Vuillermin PJ, Ponsonby AL, Sffery R, Tang ML, Ellis JA, Sly P, et al. Microbial exposure, interferon gamma gene demethylation in naive T-cells, and the risk of allergic disease. Allergy. 2009;64:348. doi: 10.1111/j.1398-9995.2009.01970.x. [DOI] [PubMed] [Google Scholar]
- 51.Stern DA, Guerra S, Halonen M, Wright AL, Martinez FD. Low IFN-gamma production in the first year of life as a predictor of wheeze during childhood. J Allergy Clin Immunol. 2007;120:835. doi: 10.1016/j.jaci.2007.05.050. [DOI] [PubMed] [Google Scholar]
- 52.Frei R, Roduit C, Bieli C, Loeliger S, Waser M, Scheynius A, et al. Expression of Genes Related to Anti-Inflammatory Pathways Are Modified Among Farmers' Children. PLoS One. 2014;9 doi: 10.1371/journal.pone.0091097. 3/23/14, http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0091097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lluis A, Ballenberger N, Illi S, Kabesch M, Ilig T, Schleich I, et al. Regulation of TH17 markers early in life through maternal farm exposure. J Allergy Clin Immunol. 2014;133:864. doi: 10.1016/j.jaci.2013.09.030. [DOI] [PubMed] [Google Scholar]
- 54.Loss G, Bitter S, Wohlgensinger J, Frei R, Roduit C, Genuneit J, et al. Prenatal and early-life exposures alter expression of innate immunity genes: The PASTURE cohort study. J Allergy Clin Immunol. 2012;130:523. doi: 10.1016/j.jaci.2012.05.049. [DOI] [PubMed] [Google Scholar]
- 55.Poole JA, Gleason AM, Bauer C, West WW, Alexis N, Reynolds SJ, et al. αβ T cells and a mixed Th1/Th17 response are important in organic dust-induced airway disease. Ann Allergy Asthma Immunol. 2012;109:266. doi: 10.1016/j.anai.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Robbe P, Spierenburg E, Draijer C, Brandsma C, Telenga E, van Oosterhout A, et al. Shifted T-cell polarisation after agricultural dust exposure in mice and men. Thorax. 2014 Mar 25; doi: 10.1136/thoraxjnl-2013-204295. 2014. http://thorax.bmj.com/content/early/2014/02/17/thoraxjnl-2013-204295. [DOI] [PubMed] [Google Scholar]
- 57.Ivanov S, Palmberg L, Venge P, Larsson K, Linden A. Interleukin-17A mRNA and protein expression within cells from the human bronchoalveolar space after exposure to organic dust. Respir Res. 2005;6:44. doi: 10.1186/1465-9921-6-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Poole JA, Wyatt TA, Kielian T, Oldenburg P, Gleason AM, Bauer A, et al. Toll-like receptor 2 regulates organic dust-induced airway inflammation. Am J Respir Cell Mol Biol. 2011;45:711. doi: 10.1165/rcmb.2010-0427OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bauer C, Kielian T, Wyatt TA, Romberger DJ, West WW, Gleason AM, et al. Myeloid differentiation factor 88-dependent signaling is critical for acute organic dust induced airway inflammation in mice. Am J Respir Cell Mol Biol. 2013;48:781. doi: 10.1165/rcmb.2012-0479OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Poole JA, Anderson L, Gleason AM, West WW, Romberger DJ, Wyatt TA. Pattern recognition scavenger receptor A/C204 regulates airway inflammatory homeostasis following organic dust extract exposures. J Immunotoxicol. 2014 doi: 10.3109/1547691X.2014.882449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Poole JA, Kielian T, Wyatt TA, Gleason AM, Stone J, Palm K, et al. Organic dust augments nucelotide binding oligomerization domain expression via an NF-{kappa}B pathway to negatively regulate inflammatory responses. Am J Physiol Lung Cell Mol Physiol. 2011;301:296. doi: 10.1152/ajplung.00086.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Charavaryamath C, Juneau V, Suri SS, Janardhan KS, Townsend H, Sing B. Role of Toll-like receptor 4 in lung inflammation following exposure to swine barn air. Exp Lung Res. 2008;34:19. doi: 10.1080/01902140701807779. [DOI] [PubMed] [Google Scholar]
- 63.Poole JA, Romberger DJ, Bauer C, Gleason AM, Sisson JH, Oldenburg PJ, et al. Protein kinase C epsilon is important in modulating organic-dust-induced airway inflamation. Exp Lung Res. 2012;38:383. doi: 10.3109/01902148.2012.714841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gao Z, Dosman JA, Rennie DC, Schwartz DA, Yange IV, Beach J, et al. Association of Toll-like receptor 2 gene polymorphisms with lung function in workers in swine operations. Ann Allergy Asthma Immunol. 2013;110:44. doi: 10.1016/j.anai.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 65.Eder W, Kilmecki W, Yu L, von Mutius E, Riedler J, Braun-Fahrlander C, et al. Toll-like receptor 2 as a major gene for asthma in children of European farmers. J Allergy Clin Immunol. 2004;113:432. doi: 10.1016/j.jaci.2003.12.374. [DOI] [PubMed] [Google Scholar]
- 66.Senthilselvan A, Dosman JA, Chenard L, Burch LH, Predicala BZ, Sorowski R, et al. Toll-like receptor 4 variants reduce airway response in human subjects at high endotoxin levels in a swine facility. J Allergy Clin Immunol. 2009;123:1034. doi: 10.1016/j.jaci.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 67.LeVan TD, Von Essen S, Romberger DJ, Lambert GP, Martinez FD, Vasquez MM, et al. Polymorphisms in the CD14 gene associated with pulmonary function in farmers. Am J Respir Crit Care Med. 2005;171:773. doi: 10.1164/rccm.200404-530OC. [DOI] [PubMed] [Google Scholar]
- 68.Leon ME, Beane Freeman LE, Douwes J, Hoppin JA, Kromhout H, Lebailly P, et al. AGRICOH: A consortium of agricultural cohorts. Int J Environ Res Public Health. 2011;8:1341–1357. doi: 10.3390/ijerph8051341. [DOI] [PMC free article] [PubMed] [Google Scholar]