Abstract
We examined whether Cognitive-Behavioral Therapy (CBT) for social anxiety disorder (SAD) would modify self-reported negative emotion and functional magnetic resonance imaging brain responses when reacting to and reappraising social evaluation, and tested whether changes would predict treatment outcome in 59 patients with SAD who completed CBT or waitlist groups. For reactivity, compared to waitlist, CBT resulted in (a) increased brain responses in right superior frontal gyrus (SFG), inferior parietal lobule (IPL), and middle occipital gyrus (MOG) when reacting to social praise, and (b) increases in right SFG and IPL and decreases in left posterior superior temporal gyrus (pSTG) when reacting to social criticism. For reappraisal, compared to waitlist, CBT resulted in greater (c) reductions in self-reported negative emotion, and (d) increases in brain responses in right SFG and MOG, and decreases in left pSTG. A linear regression found that after controlling for CBT-induced changes in reactivity and reappraisal negative emotion ratings and brain changes in reactivity to praise and criticism, reappraisal of criticism brain response changes predicted 24% of the unique variance in CBT-related reductions in social anxiety. Thus, one mechanism underlying CBT for SAD may be changes in reappraisal-related brain responses to social criticism.
Keywords: social anxiety, emotion regulation, cognitive-behavioral therapy, reappraisal, neuroimaging, emotion
INTRODUCTION
Social anxiety disorder (SAD) is characterized by heightened fear of social evaluation in conjunction with a maladaptive pattern of emotion regulation (e.g., over-reliance on maladaptive emotion regulation strategies such as behavioral avoidance and expressive suppression (Stein & Stein, 2008; Werner, Goldin, Ball, Heimberg, & Gross, 2011), and decreased self-efficacy when implementing cognitive reappraisal (Werner et al., 2011). Cognitive-behavioral models suggest that SAD (Heimberg, Brozovich, & Rapee, 2014) involves (a) distorted appraisals of social evaluation and (b) difficulty implementing adaptive emotion regulation strategies effectively in social situations.
EMOTIONAL REACTIVITY IN SAD
Studies of emotional reactivity in SAD highlight the importance of social-evaluative contexts that trigger exaggerated patterns of emotional reactivity and activate biases in attention (Schultz & Heimberg, 2008), self-focused beliefs (Blair et al., 2011), and interpretation (Amir, Prouvost, & Kuckertz, 2012). Experimental stimuli typically used to induce emotional reactivity in patients with SAD have primarily consisted of static displays of harsh faces (Goldin, Manber, Hakimi, Canli, & Gross, 2009), social criticism and praise statements (Blair et al., 2008), valenced words (Straube, Sauer, & Miltner, 2011) and negative self-beliefs (Goldin et al., 2013).
Neuroimaging studies of emotional processing have shown that, compared to healthy controls, patients with SAD demonstrate greater activation of fear-related limbic regions (amygdala, insula, anterior cingulate cortex, parahippocampal gyrus), as well as prefrontal cortical regions (medial prefrontal cortex, orbitofrontal cortex, inferior frontal cortex), and posterior cortical regions (fusiform gyrus, posterior superior temporal gyrus) (Etkin & Wager, 2007). Most of these studies have used static stimuli to probe emotional processes. However, there is increasing interest in using potentially more powerful psychopathology-related, dynamic, ecological, and personally salient stimuli to probe emotional reactivity.
To date, three studies have used video stimuli to induce emotional reactivity in SAD patients. A positron emission tomography study (Van Ameringen et al., 2004) found lesser regional cerebral blood flow in right ventromedial frontal gyrus and right lingual gyrus in six male patients with SAD when viewing along with three confederates a video recording of themselves giving an impromptu speech (versus a socially competent stranger presenting). The results were interpreted to suggest lesser emotion regulation and greater diversion of attention away from anxiety-provoking visual stimuli. An fMRI study of 20 patients with SAD versus 20 matched healthy controls (Pujol et al., 2013) found that viewing six 30 sec video recordings of themselves performing a verbal memory task along with a clinical psychologist evaluating their performance (versus viewing unknown others performing the same task) produced no between group differences. However, a less conservative uncorrected statistical threshold that revealed neural response increases in primary visual cortex and decreases in medial frontal cortex and anterior cingulate cortex in patients versus controls was interpreted to suggest visually induced emotional arousal with insufficient recruitment of cognitive control of negative emotion. A recent study (Ziv, Goldin, Jazaieri, Hahn, & Gross, 2013) of reactivity to videotaped social criticism in 67 adults with SAD and 28 healthy controls found greater whole-brain BOLD responses in left lingual gyrus, bilateral middle temporal gyrus and right parahippocampal gyrus, but no between group differences in a priori amygdala and anterior insula regions-of-interest. These three studies show no overlap in results, most likely due to different types of dynamic evaluative stimuli, neuroimaging methods, and data analytic approaches.
EMOTION REGULATION IN SAD
Among the variety of emotion regulation strategies that influence the magnitude or duration of emotions, reappraisal is considered one of the most effective strategies for actively modulating anxiety and promoting well-being (Gross, 1998). Reappraisal is a cognitive change strategy that uses cognitive-linguistic-attention processes in an integrated manner to inhibit automatic interpretations and reframe the meaning of an emotion-eliciting stimulus to up- or down-regulate emotional responses. Reappraisal has both immediate and longer-term beneficial effects on mental and physical health (Gross & Thompson, 2007). In SAD, there is evidence of overuse of maladaptive regulation strategies (e.g., avoidance, expressive suppression) and difficulties in implementing cognitive reappraisal (D'Avanzato, Joormann, Siemer, & Gotlib, 2013; Werner et al., 2011).
Neuroimaging studies have shown that, compared to healthy controls, patients with SAD have lesser activation of cognitive control (dorsolateral prefrontal (DLPFC), medial prefrontal cortex (MPFC)) (Buhle et al., 2013) and attention (medial precuneus, posterior cingulate, bilateral dorsal parietal cortex) (Fan, McCandliss, Fossella, Flombaum, & Posner, 2005) regions when reappraising social threat (harsh facial expressions) (Goldin, Manber, et al., 2009), as well as temporally delayed activation in MPFC, DLPFC, and ventrolateral prefrontal cortex (VLPFC), and less PFC-amygdala inverse functional connectivity (Goldin, Manber-Ball, Werner, Heimberg, & Gross, 2009). Deficits in reappraisal in patients with SAD may be specific to social threat and not physical threat (Goldin, Manber, et al., 2009). These brain patterns highlight problems with brain network recruitment, timing, and connectivity in the context of disorder-specific socio-emotional probes in SAD. Less is known, however, about whether and how clinical interventions modify these aberrant brain patterns in SAD.
CHANGES IN REACTIVITY AND REGULATION DURING CBT FOR SAD
Cognitive-behavioral therapy (CBT) is the most effective psychosocial intervention for SAD (Gordon, Wong, & Heimberg, 2014), and CBT produces clinical improvements that are more enduring than those of pharmacotherapy (Canton, Scott, & Glue, 2012). Neuroimaging studies show that CBT modulates emotion processing brain networks (Clark & Beck, 2010; Porto et al., 2009). Using positron emission tomography, Furmark and colleagues (Furmark et al., 2002) were the first to demonstrate in 9 patients with SAD who responded to CBT or treatment with selective serotonin reuptake inhibitors (SSRIs) revealed post-treatment reductions in amygdala, hippocampus, and anterior/medial temporal lobe responses during a public speaking challenge. A functional magnetic resonance imaging (fMRI) study of 14 patients with SAD showed that CBT reduced elevated pre-treatment blood oxygen level dependent (BOLD) responses in right insula, medial orbitofrontal gyrus, and MPFC when reacting to angry (vs. happy) facial expressions in a forced-choice emotional face matching task relative to healthy controls (Klumpp, Fitzgerald, & Phan, 2013). In a companion to the present study using the same participants but a different fMRI experimental task (reappraising negative self-beliefs), we reported that CBT resulted in increased DLPFC and DMPFC activity, earlier temporal onset of DMPFC, and greater inverse functional connectivity between DMPFC and amygdala when reappraising patient-generated negative self-beliefs embedded in autobiographical social anxiety situations (Goldin et al., 2013). These studies provide initial evidence that CBT impacts brain responses related to emotional reactivity in limbic regions (amygdala, hippocampus, insula), social cognition (anterior temporal lobes, medial orbitofrontal gyrus, and MPFC), and cognitive control (DLPFC and DMPFC). These results suggest that CBT can modify the neural representations of affective, social, and cognitive processes that are important in SAD. The current study builds on these prior studies and extends our understanding by (a) using dynamic, ecological social evaluation videotaped stimuli (rather than static external faces or text) that have features that should more powerfully impact affective, social, and cognitive processes, (b) using a more conservative comparison of brain activity changes pre-to-post-CBT versus pre-to-post-waitlist control, (c) examining acute reactivity to both positive and negative social evaluation (rather than negative social cues only), (d) examining reappraisal to nomothetic (rather than idiographic) stimuli, and (e) directly testing whether pre-to-post treatment brain and self-rated emotional changes when reacting toand reappraising social evaluation predict CBT-related reductions in severity of social anxiety symptoms.
THE PRESENT STUDY
Our goals were to investigate the impact of CBT vs. a waitlist (WL) control group on self-reported negative emotion ratings and brain responses during reactivity to and reappraisal of dynamic social evaluation and examine whether pre-to-post-CBT changes in reactivity to and reappraisal of social evaluation would predict post-CBT social anxiety. We expected that, compared to WL, CBT would result in (1) lesser emotional reactivity as indexed by decreases in self-reported negative emotion ratings and in emotion-related limbic brain regions (amygdala, insula, anterior cingulate cortex, parahippocampal gyrus) for social praise and criticism, and (2) greater emotion regulation-related decreases in self-reported negative emotion and increases in reappraisal-related cortical regions (DMPFC, DLPFC, VLPFC, medial precuneus, posterior cingulate, bilateral dorsal parietal cortex). We also expected that (3) pre-to-post-CBT changes in brain responses when reacting to praise/criticism and reappraising criticism would predict reductions in social anxiety. Because prior studies have reported insufficient recruitment of reappraisal-related brain regions in patients with SAD in the context of BOLD signal functional connectivity, we implemented an exploratory analysis of the impact of CBT on PFC-seeded context-dependent functional connectivity for the direct contrast of reappraisal versus reacting to social criticism.
METHOD
PARTICIPANTS
After passing a telephone screen, an in-person diagnostic interview, and completing all baseline assessments, we randomly assigned 75 unmedicated patients who met DSM-IV (American Psychiatric Association [APA], 1994) criteria for a principal diagnosis of generalized SAD to either immediate CBT (n = 38) or a WL control group (n = 37) who were subsequently offered CBT (CONSORT Diagram; Supplemental Figure 1). After consideration of patient dropout (CBT n = 6, WL n = 5) and incomplete pre/post fMRI data, the final sample for this study included 31 CBT and 28 WL patients. Patients provided informed consent in compliance with the Stanford University Institutional Review Board.
EXCLUSION CRITERIA
Patients were right-handed based on the Edinburgh Handedness Inventory (Oldfield, 1971), passed a MRI safety screen, and had a principal diagnosis of generalized SAD. Further exclusion criteria included current pharmacotherapy or psychotherapy, past CBT, history of neurological disorders, and meeting diagnostic criteria for any psychiatric condition other than generalized anxiety disorder, agoraphobia without panic attacks, specific phobia, panic disorder, or dysthymia.
PROCEDURE
We recruited patients through referrals and web listings. They had to complete all baseline assessments before random assignment to CBT or WL using the Efron biased coin randomization procedure (Efron, 1971), which promotes equal sample sizes throughout the clinical trial. Patients completed fMRI and clinical measures pre- and immediately post-CBT/WL.
CLINICAL ASSESSMENT
Clinical diagnostic interviews were conducted at baseline by two Ph.D.-level trained clinical psychologists using the Anxiety Disorders Interview Schedule for DSM-IV: Lifetime Version (Di Nardo, Brown, & Barlow, 1994), which has been shown to demonstrate excellent inter-rater reliability (Brown, Di Nardo, Lehman, & Campbell, 2001). Severity of social anxiety was measured pre/post-CBT and WL using the self-report version of the Liebowitz Social Anxiety Scale (LSAS-SR; Fresco et al., 2001; Liebowitz, 1987). The LSAS-SR consists of questions that assess social interaction situations (11 items) and performance situations (13 items). A 4-point Likert-type scale is used for ratings of fear and of avoidance, with a range from 0 (none and never, respectively) to 3 (severe and usually, respectively) for situations during the past week. Ratings are summed for a total LSAS-SR score (range = 0–144). The LSAS-SR has good reliability and construct validity (Rytwinski et al., 2009). Internal consistency was excellent for the LSAS-SR (Cronbach’s alpha = .91).
COGNITIVE-BEHAVIORAL THERAPY FOR SAD
Four Ph.D.-level clinical psychologists trained by Richard G. Heimberg delivered CBT using Managing Social Anxiety: A Cognitive-Behavioral Therapy Approach, a manualized treatment protocol which included a therapist guide (Hope, Heimberg, & Turk, 2006), client workbook (Hope, Heimberg, Juster, & Turk, 2000), and consisted of 16 individual sessions administered over 4 months. CBT covered psychoeducation and orientation to CBT; cognitive restructuring skills; graduated exposure to feared social situations, within session and as homework; examination and modification of core beliefs; and relapse prevention and termination (Hope et al., 2000; Hope et al., 2006; Ledley et al., 2009).
Research team members trained in CBT treatment adherence rated every session for each client using the Cognitive-Behavioral Therapy for Social Anxiety Disorder: Therapist Adherence Scale (Hope et al., 2006) according to various criteria, using a 5-point Likert-type scale ranging from 1 (ineffective) to 5 (extremely effective). All four study therapists achieved the “in protocol” threshold for each therapy case (overall Mean = 4.61, SD = 0.24).
EXPERIMENTAL TASK
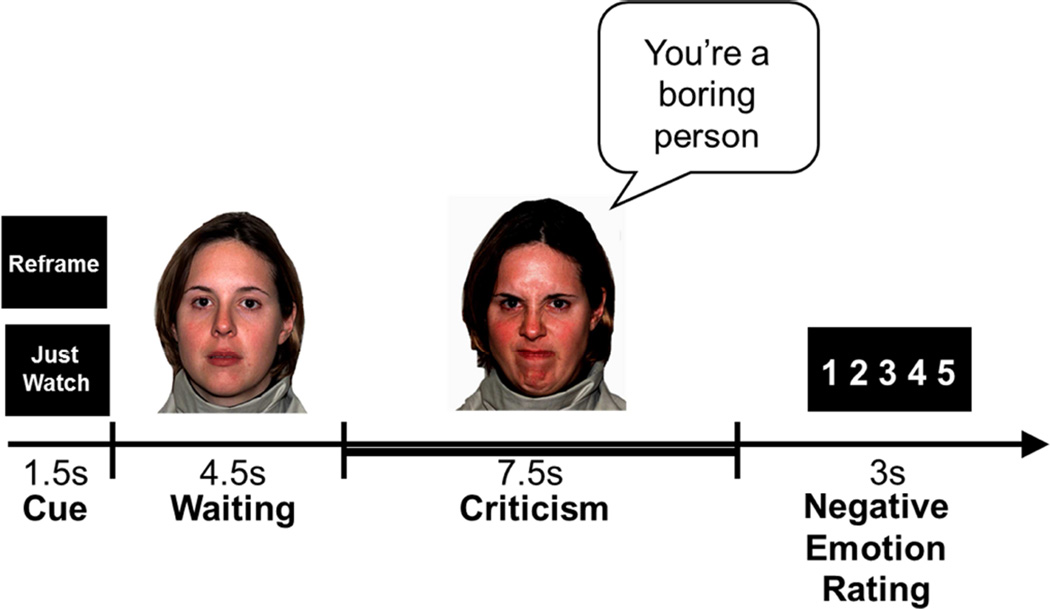
We developed a Social Evaluation Task (SET) programmed in Eprime version 1 (Psychology Software Tools, Inc.) that examined reactivity to and reappraisal of dynamic social evaluation. Stimuli consisted of 12s video clips with actors verbalizing and visually expressing social criticism or social praise. We had the actors display naturalistic dynamic happy/approving and angry/disapproving facial expressions because there is now converging evidence from experiments and meta-analyses suggesting that such dynamic facial emotion expression stimuli elicit greater brain activity than do matched static facial emotion expression stimuli in broad regions of occipital, parietal and posterior temporal cortices related to perceptual processing (e.g., posterior middle and superior temporal gyrus, fusiform gyrus, inferior and middle occipital gyrus, inferior and superior parietal lobule, cuneus), as well as regions implicated in emotional salience (e.g., amygdala, parahippocampal gyrus) and cognitive/evaluation processes (e.g., inferior frontal gyrus, orbitofrontal cortex) (Arsalidou, Morris, & Taylor, 2011; Sato, Kochiyama, Yoshikawa, Naito, & Matsumura, 2004; Trautmann, Fehr, & Herrmann, 2009). We videotaped 5 male and 5 female actors (7 Anglo-Americans and 3 Asian-Americans) with an age range of 23–50 delivering social criticism and praise statements combined with harsh or happy evaluation-congruent facial expressions (Fig. 1) to generate 16 trials of each condition (react praise, react criticism, reappraise criticism) across two runs of 342 volumes (513s) each.
Figure 1.
Experimental Design: structure of one social evaluation trial. Cued instruction, 12s video clip, emotion ratings button responses from 1=not negative to 5=extremely negative. Thick bar indicates the 7.5s period of each trial that was used for BOLD signal analysis.
Stimuli were presented in a single pseudo-randomized order with a specific condition appearing no more than twice in a row. Participants were trained prior to scanning to “Just Watch” (the video while reacting naturally without attempting to modify the situation in any way) or to “Reframe” (via reappraising or reinterpreting the situation in a way that reduces any negative response to the person delivering the social evaluation).
Each 16.5s trial consisted of a 1.5s cue (“Just Watch” or “Reframe”), a 12s video stimulus (consisting of a 4.5s wait period during which the actor silently maintained a neutral facial expression followed by a 7.5s evaluation period in which the actor verbalized a single social criticism or praise statement while displaying a harsh or happy facial expression), and a 3s emotion rating period for participants to respond to “How negative do you feel?” with a rating of 1 (not at all) to 5 (very much) using a button box. For the present study, we analyzed brain responses during the 7.5s evaluation period and self-reported negative emotion ratings. Because SAD patients may perceive neutral social stimuli as negative (Birbaumer et al., 1998; Cooney, Atlas, Joormann, Eugene, & Gotlib, 2006; Stein, Goldin, Sareen, Zorrilla, & Brown, 2002), we did not use neutral statements. Instead, we compared watch (react) and reframe (reappraise) trials to 16 trials of 12s asterisk-counting during which patients pressed a button to indicate the number of asterisks on the screen which changed every 3s and varied from 1–5 asterisks.
EMOTION RATING ANALYSIS
We used SPSS software version 20 to implement a 2 Group (CBT vs. WL) × 2 Time (Pre vs. Post-CBT/WL) repeated-measures analysis of variance to examine main and interaction effects.
MR IMAGE ACQUISITION
We used a GE 3-T Signa magnet with a T2*-weighted gradient echo spiral-in/out pulse sequence (Glover & Law, 2001) to acquire 676 functional volumes across two functional runs from 22 axial slices (repetition time=1500 milliseconds, echo time=30 milliseconds, flip angle=60°, field of view=22 cm, matrix=64×64, resolution=3.438 mm2 × 4.5 mm). We minimized head-movement with a bite-bar and foam padding. Three-dimensional high-resolution anatomical scans were acquired using fast spin-echo spoiled-grass (.85942 × 1.5 mm; field of view=22 cm, frequency encoding=256).
fMRI DATA PROCESSING
We used Analysis of Functional NeuroImages (AFNI) software (Cox, 1996) to remove outliers, register, motion correct, spatially smooth (4 mm3 isotropic Gaussian kernel), high-pass filter (.011 Hz), linear detrend, and convert into percent signal change each functional run. No volumes demonstrated motion in the x, y, or z directions in excess of ± 0.7 mm. There was no evidence of stimulus-correlated motion (all ps > .45).
fMRI STATISTICAL ANALYSIS
Using 3dDeconvolve in AFNI, we conducted a multiple-regression that included removal of mean, linear, and quadratic trends, and motion-related variance in the BOLD signal time series in each voxel. Regressors for the asterisk-counting, react, and reappraise trials were convolved with the gamma variate model (Cohen, 1997) of the hemodynamic response function. Individual brain maps were converted into Talairach atlas space (Talairach & Tournoux, 1988), and second-level group statistical parametric maps were produced according to a random-effects model. To identify differential change, we conducted whole-brain voxel-wise independent-sample t-tests on pre-to-post-CBT vs. pre-to-post-WL BOLD change scores for react (vs. asterisk) and reappraise (vs. asterisk) contrasts. We used asterisk counting as the lowest-level contrast condition that was least likely to be impacted by either CBT or WL. To correct for multiple comparisons, we used 3dFWHMx to compute the intrinsic full-width half-maximum in x, y, z directions and then 3dClustSim to compute a joint probability threshold to protect against false-positive brain activation (Forman et al., 1995). This procedure determined that a joint-probability threshold consisting of voxel-wise p < .005 threshold and cluster volume threshold of 244 mm3 (6 voxels × 3.438 mm3) resulted in a corrected protection against false-positive brain cluster detection at p < .01.
We examined whether CBT-related changes in self-reported negative emotion and BOLD brain responses when reacting to praise, reacting to criticism, and reappraising criticism predicted CBT-related reductions in social anxiety symptom severity. We conducted a single three-step hierarchical regression in SPSS version 21 with only patients randomized to CBT. In step 1, we entered negative emotion ratings for react praise, react criticism, and reappraise criticism. In step 2, we entered BOLD responses for react praise and react criticism. In step 3, we entered BOLD responses for reappraise criticism.
Because brain clusters that are derived from a thresholded t-map tend to be correlated and functionally interdependent, we used the following method to compute a single brain predictor for each of the three conditions (react praise, react criticism and reappraise criticism), thereby reducing the number of individual brain response predictors for the regression analyses. We computed the mean BOLD percentage signal change across all the voxels in each thresholded cluster identified above for each of the three conditions at baseline and post-CBT, separately. We computed pre-to-post-CBT change scores for each brain region, Z-score transformed each change score, summed the Z values to produce a single composite predictor for react praise, react criticism, and reappraise criticism, separately.
EXPLORATORY ANALYSES
For the exploratory analysis, we implemented a context-dependent functional connectivity (cdFC) analysis seeded to the right anterior medial PFC brain region that derived from the interaction of CBT vs. WL whole-brain analysis when reacting to and reappraising social criticism. We then conducted a paired t-test to identify differential cdFC for reappraise versus react criticism at post-CBT. 3dFWHMx and 3dClustSim determined that a cluster volume of 203 mm3 (5 voxels × 3.438 mm3) voxels and per voxel p < .001 resulted in clusterwise p < .05.
RESULTS
PRELIMINARY ANALYSES
CBT and WL patients did not differ significantly in gender, age, education, ethnicity, yearly income, marital status, current or past Axis I comorbidity, past psychotherapy or pharmacotherapy, age at symptom onset, or years since symptom onset (Table 1). As reported elsewhere (Goldin et al., 2012), compared to WL, CBT resulted in significantly greater reductions in social anxiety (LSAS-SR: ΔCBT = −38.3 ± 27.1 vs. WL = −9.3 ± 12.7; t(55) = 4.91, p < .001, 95% CI [−41.0, −17.0], partial eta2 (ηp2) = .34).
Table 1.
Participant Characteristics
| Variable | SAD CBT n = 31 |
SAD WL n = 28 |
|---|---|---|
| Gender (Males, n, %) | 20 (45.2%) | 20 (53.6%) |
| Age (years, M ± SD) | 33.7 ± 7.9 | 33.3 ± 10.1 |
| Education (years, M ± SD) | 16.8 ± 2.1 | 17.1 ± 2.7 |
| Ethnicity (n, % Caucasian) | 22 (57.9%) | 21 (56.8%) |
| Yearly income (M ± SD) | 79.3 ± 45.9 | 59.1 ± 44.0 |
| Marital status (n, %) | ||
| Single, never married | 17 (54.8%) | 21 (75.0%) |
| Married | 13 (41.9%) | 6 (21.4%) |
| Divorced, separated, widowed | 1 (3.3%) | 1 (3.6%) |
| Current Axis I Comorbidity (n, %) | ||
| Generalized anxiety disorder | 5 (16.1%) | 6 (21.4%) |
| Specific phobia | 3 (9.7%) | 1 (3.6%) |
| Panic disorder | 2 (6.6%) | 1 (2.7%) |
| Dysthymic disorder | 1 (3.3%) | 2 (7.2%) |
| Past non-CBT Psychotherapy (n, %) | 20 (64.5%) | 13 (46.4%) |
| Past Pharmacotherapy (n, %) | 8 (25.8%) | 11 (39.3%) |
| SSRI | 4 | 6 |
| SNRI | 0 | 1 |
| Beta Blocker | 3 | 1 |
| Ritalin | 1 | 2 |
| Buproprion | 0 | 1 |
| Age at symptom onset (years, M ± SD) | 14.0 ± 8.4 | 12.9 ± 6.1 |
| Years since symptoms onset (M ± SD) | 20.1 ± 11.7 | 20.3 ± 12.5 |
Note: CBT = Cognitive-Behavioral Therapy, M = mean, SAD = social anxiety disorder, SD = standard deviation, SNRI = serotonin and norepinephrine reuptake inhibitors, SSRI = selective serotonin reuptake inhibitor, WL = waitlist. No significant group differences (all ps > .05).
To check whether patients implemented the react and reappraise instructions on the Social Evaluation Task in a similar manner, we had patients complete a self-report assessment immediately after leaving the MR scanner both at baseline and post-CBT/WL. At baseline, there were no significant between-group differences in patients who would enter CBT or WL related to the Social Evaluation Task for (1) the number of distinct reappraisal strategies implemented (CBT vs. WL: Mean ± SD; 3.55 ± 1.50 vs. 3.80 ± 2.01; t(59) = .56, p = .58), (2) reappraisal success (0% not at all to 100% completely; CBT vs. WL: Mean ± SD; 48.15% ± 22.71 vs. 51.12% ± 18.27; t(58) = 1.01, p = .58), and (3) how worried about doing the task correctly (0% not at all to 100% completely; CBT vs. WL: Mean ± SD; 38.87% ± 26.20 vs. 42.79% ± 27.93; t(59) = .56, p = .58).
To check for response biases related to social desirability, we examined whether responses to the Marlowe-Crown Social Desirability Scale (MCSDS; Crowne & Marlowe, 1960) were associated with self-reported negative emotion ratings obtained during fMRI and reappraisal-related post-fMRI ratings. There was no association between the MCSDS and negative emotion ratings for react praise (r = .07, p > .58), react criticism (r = .14, p > .29), reappraise criticism (r = .06, p > .64), number of distinct reappraisal strategies (r = −.07, p > .61), reappraisal success (r = −.22, p > .10), and how worried the participant was about doing the task correctly (r = −.07, p > .62).
REACTIVITY TO SOCIAL PRAISE AND CRITICISM
Emotion Ratings
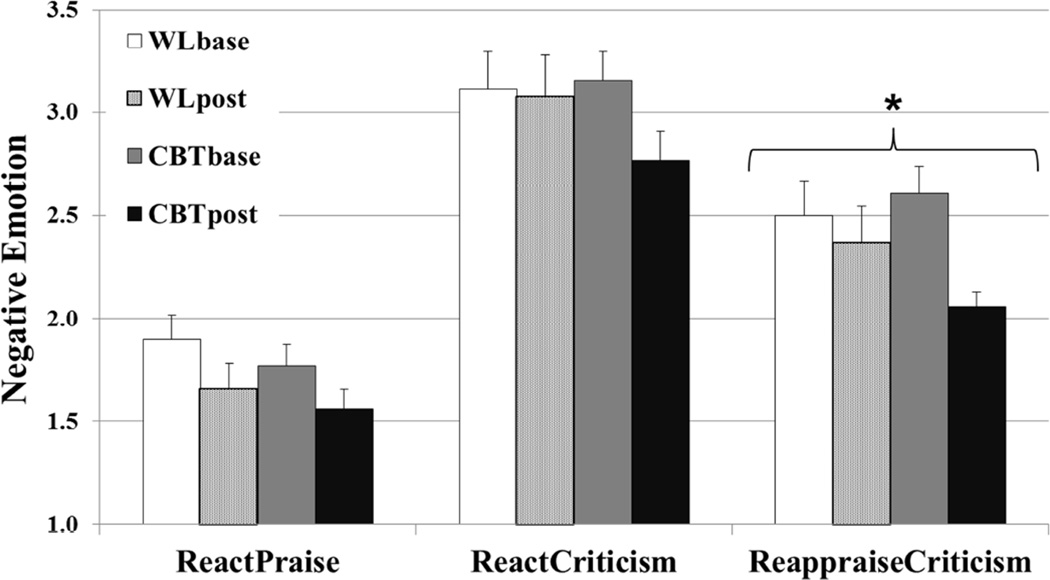
For react praise, there was no significant interaction of group × time (F(2,53) = 0.03, p = .86, ηp2 = .00) (Fig. 2), although there were significant reductions in self-reported negative emotion ratings pre-to-post-CBT (pre vs. post; 1.81 ± .63 vs. 1.53 ± .52; t(30) = 2.13, p = .043, ηp2 =.15) but not pre-to-post-WL (1.90 ± .73 vs. 1.66 ± .65; t(27) = 1.70, p = .12, ηp2 = .10). For react criticism, there was no interaction of group × time (F(2,53) = 2.15, p = .15, ηp2 = .04), although there were significant reductions in self-reported negative emotion ratings pre-to-post-CBT (3.19 ± .77 vs. 2.86 ± .77; t(30) = 2.26, p < .05, ηp2 = .16) but not pre-to-post-WL (3.07 ± .84 vs. 3.01 ± .84; t(27) = 0.17 p > .68, ηp2 = .01).
Figure 2.
Negative Emotion Ratings When Reacting to Praise or Criticism, and Reappraising Criticism Pre- and Post-Cognitive-Behavioral Therapy and Waitlist * group × time interaction p < .05
Brain Responses
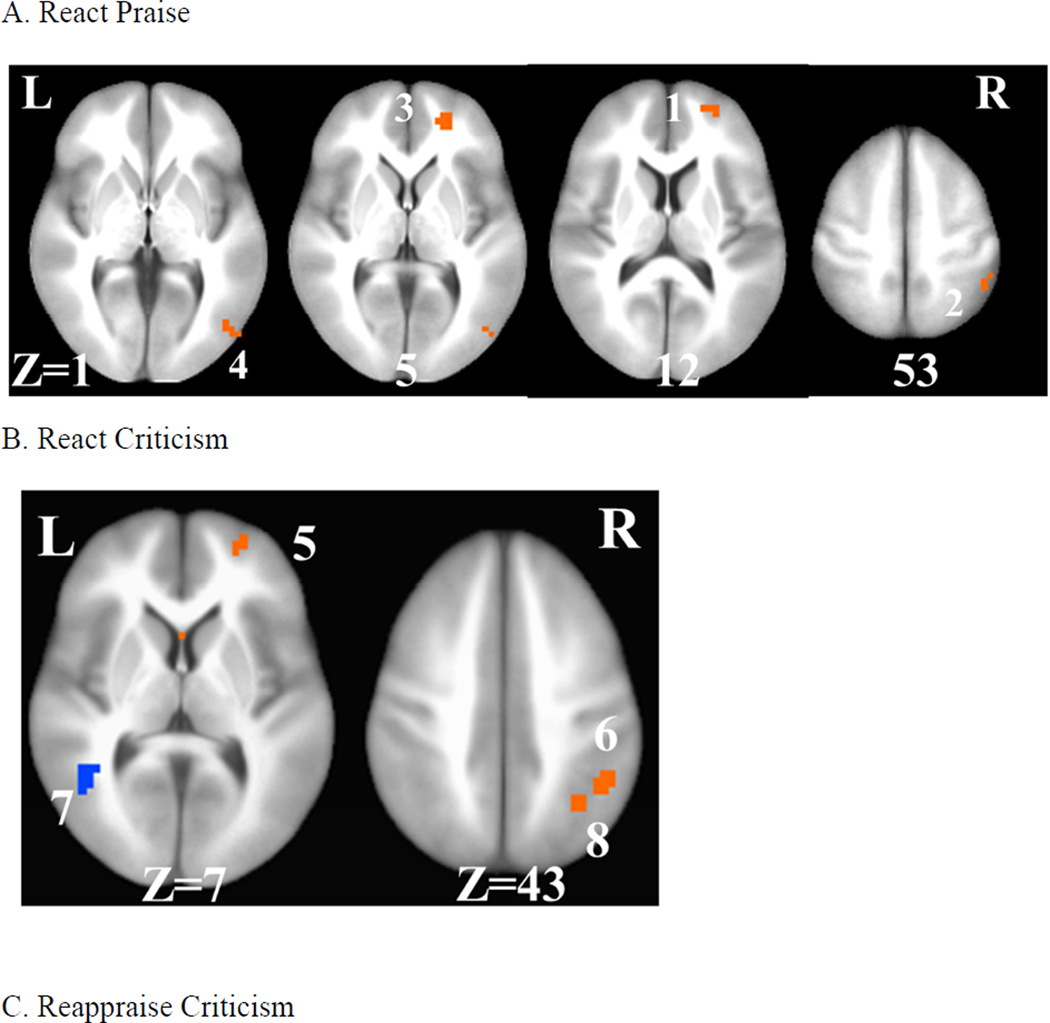
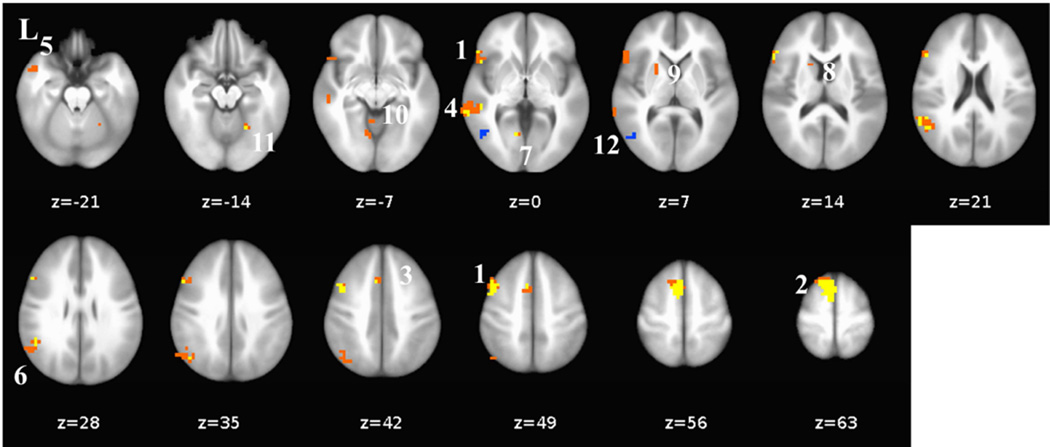
For react praise, a whole-brain between-group t-test of pre-to-post changes in BOLD signal revealed significant interactions in four distinct brain regions each characterized by increases pre-to-post-CBT and decreases pre-to-post-WL (Table 2, Fig. 3). For each brain region, compared to WL, CBT resulted in significantly greater increases in right superior frontal gyrus (ΔCBT vs. ΔWL; cluster#1: .033 vs. −.078; ηp2 = .37), inferior parietal lobule (cluster#2: .064 vs. −.096; ηp2 = .28), superior frontal gyrus (cluster#3: .038 vs. −.059; ηp2 = .37), and middle occipital gyrus (cluster#4: .020 vs. −.191; ηp2 = .26).
Table 2.
Differential Pre-to-Post-Cognitive-Behavioral Therapy vs. Waitlist Changes in BOLD Responses for React Praise/Criticism and Reappraise Criticism
| # | Brain Region | BA | Pre-to-Post CBT vs. WL |
xyz | Vol | % | t-test |
|---|---|---|---|---|---|---|---|
|
React Praise (vs. Asterisk-Counting) |
|||||||
| 1 | R Superior Frontal Gyrus | 10 | CBT > WL | 24 55 12 | 958 | .15 | 4.69 |
| 2 | R Inferior Parietal Lobule | 40 | CBT > WL | 45 −48 53 | 958 | .29 | 2.95 |
| 3 | R Superior Frontal Gyrus | 10 | CBT > WL | 24 52 5 | 479 | .13 | 3.68 |
| 4 | R Middle Occipital Cortex | 19 | CBT > WL | 48 −75 1 | 372 | .26 | 3.80 |
|
React Criticism (vs. Asterisk-Counting) |
|||||||
| 5 | R Superior Frontal Gyrus | 10 | CBT > WL | 24 56 12 | 851 | .16 | 3.66 |
| 6 | R Inferior Parietal Lobule | 40 | CBT > WL | 45 −48 46 | 638 | .18 | 3.06 |
| 7 | L Posterior Superior Temporal Gyrus |
39 | WL > CBT | −45 −48 8 | 532 | −.11 | −3.42 |
| 8 | R Inferior Parietal Lobule | 7 | CBT > WL | 34 −58 43 | 372 | .12 | 3.81 |
|
Reappraise Criticism (vs. Asterisk-Counting) |
|||||||
| 9 | R Superior/Middle Frontal Gyrus |
10 | CBT > WL | 21 56 19 | 692 | .15 | 3.53 |
| 10 | L Posterior Superior Temporal Gyrus |
39 | WL > CBT | −45 −48 8 | 638 | −.12 | −3.51 |
| 11 | R Middle Occipital Gyrus | 19 | CBT > WL | 48 −72 1 | 532 | .26 | 4.12 |
Note. # = cluster number, CBT = cognitive-behavioral therapy, WL = waitlist, BOLD = blood oxygen dependent signal, BA = Brodmann area, xyz = Talairach coordinates at voxel with peak BOLD signal, Vol = volume in mm3, % = peak percent signal change, R = right, L = left, t-value ≥ 2.92, voxel p < .005, minimum cluster volume threshold ≥ 244 mm3 (6 voxels × 3.438 mm3), cluster p < .01.
Figure 3.
Differential Pre-to-Post-CBT vs. Waitlist Changes in Whole-Brain BOLD
Responses for React Praise, React Criticism, and Reappraise Criticism
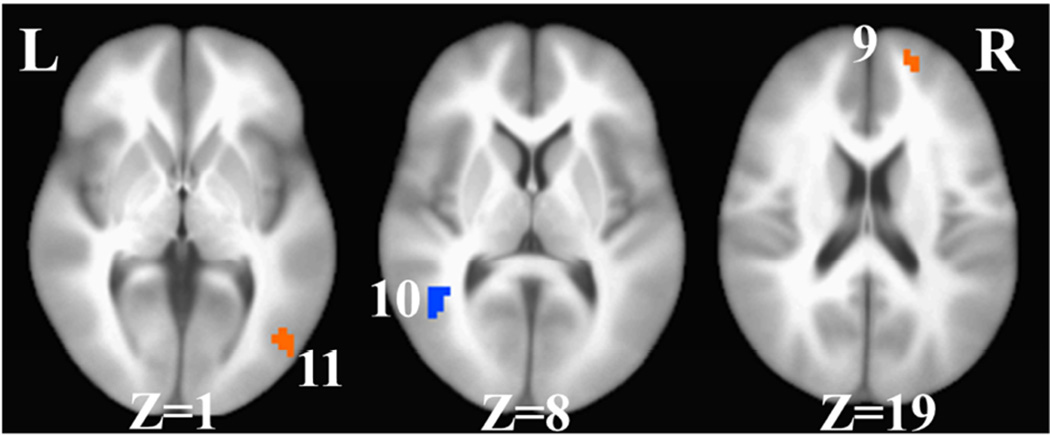
A. React praise: 1= right anterior medial prefrontal cortex, 2=right inferior parietal lobule, 3=right medial prefrontal cortex, 4=right middle occipital cortex, B. React criticism: 5= right anterior medial prefrontal cortex, 6= right inferior parietal lobule, 7= left posterior superior temporal gyrus, 8 = right inferior parietal lobule, and C. Reappraise criticism: 9 = right medial prefrontal cortex, 10 = left posterior superior temporal gyrus, 11 = right inferior temporal gyrus/middle occipital gyrus. L = left, R = right, Z = superior (+) to inferior (−).
For react criticism, a whole-brain between-group t-test of pre-to-post changes in BOLD signal also identified significant interactions in four distinct brain regions (Table 2, Fig. 3). Interaction effects were characterized by increases pre-to-post-CBT and decreases pre-to-post-WL in three brain regions: right superior frontal gyrus (ΔCBT vs. ΔWL; cluster#5: .040 vs. −.085; ηp2 = .30), inferior parietal lobule (cluster#6: .049 vs. −.069; ηp2 = .24), and inferior parietal lobule (cluster#8: .033 vs. −.064; ηp2 = .20). Only the left posterior superior temporal gyrus (cluster#7: −.058 vs. .032; ηp2 = .28) demonstrated an opposite interaction pattern characterized by increases pre-to-post-WL and decreases pre-to-post-CBT.
REAPPRAISAL OF SOCIAL CRITICISM
Emotion Ratings
For reappraise criticism, there was a significant group × time interaction (F(2,53) = 4.15, p = .047, ηp2 = .07) derived from significant reductions pre-to-post-CBT (pre vs. post; 2.63 ± .66 vs. 2.01 ± .37; t(30) = 5.35, p < .001, ηp2 = .48), but not pre-to-post-WL (2.53 ± .79 vs. 2.33 ± .79; t(27) = 1.55, p = .13, ηp2 = .08) (Fig. 2).
Brain Responses
For reappraise criticism, a whole-brain between-group t-test of pre-to-post changes in BOLD signal revealed significant interaction effects in three distinct brain regions (Table 2, Fig. 3). The two brain regions characterized by increases pre-to-post-CBT and decreases pre-to-post-WL included right superior/middle frontal gyrus (ΔCBT vs. ΔWL; cluster#9: .037 vs. −.082; ηp2 = .26) and right middle occipital gyrus (cluster#11: .011 vs. −.193; ηp2 = .27). The single brain region that yielded the opposite pattern was the left posterior superior temporal gyrus (cluster#10: −.064 vs. .024; ηp2 = .28).
PREDICTING CBT-RELATED CHANGES IN CLINICAL SYMPTOMS
A three-step hierarchical regression (Table 3) showed that in step 1 negative emotion rating changes pre-to-post-CBT for react praise, react criticism, and reappraise criticism did not predict pre-to-post-CBT decreases in social anxiety symptoms (ΔLSAS-SR) (R2 = .097, F(3,28) = 0.68, p = .57) and none of the three predictors was significant (all ps > .40). When BOLD brain response changes pre-to-post-CBT for react praise and react criticism were entered in step 2, they did not explain additional variance in pre-to-post-CBT decreases in social anxiety symptoms (ΔR2 = .009, F(5,26) = 0.08, p = .92). However, when reappraise criticism brain responses were entered in step 3, they accounted for significant additional variance in social anxiety symptoms (ΔR2 = .24, F(6,25) = 5.76, p = .029, zero-order correlation = −.28, partial correlation = −.52).
Table 3.
Summary of Hierarchical Regression Analysis for Variables Predicting Pre-to-Post Cognitive Behavioral Therapy Reduction in Social Anxiety Symptom Severity
| Model 1 | Model 2 | Model 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | B | SE B | β | B | SE B | β | B | SE B | β | |
| Negative Emotion Ratings | ||||||||||
| - ΔCBT React Praise | 9.11 | 12.48 | 0.19 | 8.73 | 13.17 | 0.18 | 8.23 | 11.64 | 0.17 | |
| - ΔCBT React Criticism | −5.51 | 8.35 | −0.19 | −6.63 | 9.27 | −0.23 | −7.20 | 8.20 | −0.25 | |
| - ΔCBT Reappraise Criticism | −8.53 | 10.10 | −0.22 | −7.76 | 10.90 | −0.20 | −6.19 | 9.65 | −0.16 | |
| BOLD Brain Responses | ||||||||||
| - ΔCBT React Praise | −1.47 | 3.79 | −0.12 | 3.37 | 3.91 | 0.28 | ||||
| - ΔCBT React Criticism | 1.06 | 3.25 | 0.10 | 3.74 | 3.08 | 0.34 | ||||
| - ΔCBT Reappraise Criticism | −12.78 | 5.32 | −0.76* | |||||||
| R2 | .10 | .11 | .34 | |||||||
| F for change in R2 | 0.68 | 0.08 | 5.76* | |||||||
Note. B = unstandardized beta weight, SE = standard error, β = standardized beta weight
p < .05
EXPLORATORY ANALYSES OF FUNCTIONAL CONNECTIVITY
We used the right anterior medial PFC region that resulted from the interaction of group by time for react criticism (cluster#5) and reappraise criticism (cluster#9) as the seed in a context-dependent functional connectivity analysis for react criticism vs. asterisk counting, reappraise criticism vs. asterisk counting, and reappraise vs. react criticism. As shown in Supplemental Figure 2, post-CBT react criticism and reappraise criticism had a very similar pattern of positive connectivity between the right anterior medial PFC seed and medial, dorsomedial, bilateral dorsolateral and bilateral ventrolateral PFC, precuneus, bilateral anterior temporal pole, bilateral posterior temporal lobe, thalamus, and lingual gyrus.
However, a paired-samples t-test that directly contrasted reappraise (vs. react) criticism showed greater positive connectivity between the anterior medial PFC seed and dorsomedial PFC, left dorsal anterior cingulate cortex, left dorsolateral PFC, left ventrolateral PFC, left posterior superior temporal gyrus, left supramarginal gyrus, left anterior temporal pole, left lingual gyrus, left caudate, putamen, and right cerebellar culmen. There was positive connectivity for react (vs. reappraise) criticism in the left posterior middle temporal gyrus (Fig. 4, Table 4).
Figure 4.
Right Anterior Medial Prefrontal Cortex-Seeded Differential Context-Dependent Functional Connectivity for Reappraise vs. React Social Criticism Post-CBT
Reappraise > React: 1 = left dorsolateral and ventrolateral prefrontal cortex, 2 = left medial prefrontal cortex, 3 = left dorsal anterior cingulate cortex, 4 = left posterior superior temporal cortex, 5 = left anterior temporal pole, 6 = left supramarginal gyrus, 7 = left lingual gyrus, 8 = left caudate, 9 = left lentiform / putamen, 10 = left culmen, 11 = right culmen, 12 = left posterior middle temporal gyrus.
Table 4.
Differential Right Anterior Medial Prefrontal Cortex-Seeded Context-Dependent Functional Connectivity When Reappraising vs. Reacting to Social Criticism Post-CBT
| # | Brain Region | xyz | Vol | t-test |
|---|---|---|---|---|
| Reappraise > React | ||||
| Frontal Lobe | ||||
| 1 | L Dorsolateral PFC / L Ventrolateral PFC |
−45 7 46 | 5,405 | 5.08 |
| 2 | L Medial PFC | −7 11 60 | 4,511 | 5.68 |
| 3 | L Dorsal ACC | −7 18 43 | 325 | 4.30 |
| Temporal Lobe | ||||
| 4 | L Superior Temporal Gyrus | −48 −34 1 | 2,072 | 5.27 |
| 5 | L Anterior Temporal Pole | −45 11 −23 | 284 | 3.99 |
| Parietal Lobe | ||||
| 6 | L Supramarginal Gyrus | −48 −51 26 | 3,495 | 5.90 |
| Occipital Lobe | ||||
| 7 | L Lingual Gyrus | −10 −58 −2 | 528 | 4.54 |
| Sub-Cortical | ||||
| 8 | L Caudate | −17 14 12 | 203 | 3.87 |
| 9 | L Lentiform / Putamen | −21 11 8 | 244 | 4.11 |
| Cerebellum | ||||
| 10 | L Culmen | −7 −48 −9 | 203 | 4.14 |
| 11 | R Culmen | 24 −55 −19 | 406 | 4.21 |
| Reappraise < React | ||||
| 12 | L Posterior Middle Temporal Gyrus |
−55 −65 5 | 569 | −4.32 |
Note. # = cluster number, BOLD=blood oxygen level dependent, BA=Brodmann Area, xyz = Talairach coordinates at voxel with peak BOLD signal, Vol = volume in mm3, R=right, L=left, t-value ≥3. 63, voxel p<.001, minimum cluster volume threshold ≥203 mm3 (5 voxels × 3.438 mm3), cluster p < .05.
DISCUSSION
This randomized controlled trial showed that in patients with generalized SAD, compared to WL, CBT reduced self-reported negative emotions when reappraising social criticism and modified BOLD signal responses when reacting to social praise and criticism, as well as when reappraising criticism. Furthermore, CBT-related increases in brain responses when reappraising criticism (after controlling for negative emotion ratings and brain responses when reacting to praise or criticism) predicted CBT-related reductions in social anxiety symptoms.
REACTIVITY TO SOCIAL PRAISE AND CRITICISM
For negative emotion ratings, there were significant pre-to-post-CBT reductions when reacting to criticism (ηp2 = .16) or praise (ηp2 = .15), but no significant reductions in the WL group for criticism (ηp2 = .01) or praise (ηp2 = .10). However, there was no interaction of group by time, which means that we cannot be certain that the apparent effects of CBT on reactivity to praise and criticism were not the result of habituation due to the repetition of the social evaluation task.
Neurally, for react praise, group by time interactions revealed greater activation for CBT (vs. WL) in right superior frontal gyrus (SFG), inferior parietal lobule (IPL), and middle occipital gyrus (MOG). The two SFG activation clusters are located in the frontopolar sub-region of Brodmann Area 10 that has input/output connections with other PFC higher-order association cortex areas and have been implicated in executive functions (Petrides & Pandya, 2007), most prominently monitoring and mentalizing.
In the posterior part of the brain, there were CBT-related BOLD response increases in two distinct visual processing brain regions. The right IPL is part of the dorsal “where” stream of visual attention (Ungerleider & Haxby, 1994) and has been implicated in maintaining attention on current task goals and alerting to salient stimuli in the environment. The right lateral MOG is implicated in initial feature analysis of object and face stimuli, as well as both allocentric (other-centered) and egocentric (self-centered) spatial perception (Boccia, Nemmi, & Guariglia, 2014). Furthermore, the finding of only right hemisphere activation clusters converges with research showing that the right hemisphere has a dominant role in face perception (Sergent, Ohta, & MacDonald, 1992). While speculative, these results suggest CBT might enhance visual attention towards (approach) rather than away from (avoidance) dynamic social stimuli, perhaps because they are perceived as less threatening.
For react criticism, group by time interactions showed CBT-related increases in right SFG and IPL similar to react praise. The single difference was a CBT-related decrease in left posterior superior temporal gyrus (pSTG), a region known as Werneke’s area that is implicated in auditory/linguistic processing (Price, 2012). A recent meta-analysis of brain changes in psychotherapy for anxiety and depressive disorders found similar reductions in left posterior temporal gyrus (Messina, Sambin, Palmieri, & Viviani, 2013). However, the exploratory anterior medial PFC seeded functional connectivity analysis shows that react criticism post-CBT activates a network of brain regions implicated in reappraisal component processes (cognitive control, linguistic, and attention processes). Considered together, these brain results reflect spontaneous (i.e., uncued) emotion regulation of social criticism, which may be related to extensive training in cognitive restructuring during CBT.
REAPPRAISAL OF SOCIAL CRITICISM
For negative emotion ratings, a group by time interaction demonstrated CBT-related down-regulation of negative emotion when reappraising criticism. The effect of CBT on reappraisal (ηp2 = .42) was nearly three times larger than its effect on reactivity. This suggests that patients with SAD were able to implement reappraisal when cued post-CBT. Other studies have also found pre-to-post-CBT increases in the general use of reappraisal (Kocovski, Fleming, Hawley, Huta, & Antony, 2013; Moscovitch et al., 2012), reappraisal of negative self-beliefs (Goldin et al., 2013), and reappraisal self-efficacy (Goldin et al., 2012) among patients with SAD.
Neurally, for reappraise criticism, a group by time interaction revealed CBT-related increases in right SFG and right MOG, and decreases in left pSTG. Similar to our study, a recent meta-analysis of psychotherapy interventions for anxiety and depression disorders found no evidence of changes in limbic regions such as amygdala and insula (Messina et al., 2013). More specifically, fMRI studies of CBT for SAD have not found reductions in amygdala BOLD responses to threatening facial stimuli (Klumpp et al., 2013) or negative self-beliefs (Goldin et al., 2013). However, a PET study of public speaking found reductions in amygdala, hippocampus and temporal gyrus in CBT or SSRI treatment responders (Furmark et al., 2002). Thus the impact of CBT on amygdala and insula responses in adults with SAD remains unclear.
The reappraisal-related right SFG region found in this study overlaps with the medial PFC and has been implicated in multiple executive cognitive functions, including monitoring, mentalizing, introspection, self-referential meta-cognitive evaluations, and reappraisal of emotional reactivity (Goldberg, Harel, & Malach, 2006; Goldin, Manber-Ball, et al., 2009; Gusnard, Akbudak, Shulman, & Raichle, 2001; Petrides & Pandya, 2007; Schmitz, Kawahara-Baccus, & Johnson, 2004). One fMRI study (Goldin et al., 2013) showed CBT-related increases in DMPFC and left DLPFC BOLD activation, and DMPFC-seeded functional connectivity to a SFG region when reappraising negative self-beliefs in patients with generalized SAD similar to the one observed in the current study. The exploratory analysis of right SFG seeded functional connectivity for reappraise vs. react criticism yielded greater positive connectivity in reappraisal-related brain regions. Compared to healthy controls, patients with SAD showed temporally delayed activation of this region when attempting to reappraise negative self-beliefs which may be related to deficits in emotion regulation (Goldin, Manber-Ball, et al., 2009). In conjunction with the functions attributed to increased right MOG and decreased left pSTG responses, as well as the right SFG activation found across the react and reappraise conditions, CBT appears to enhance both volitional (i.e., cued) and automatic (i.e., spontaneous) emotion regulation.
PREDICTING CBT-RELATED CHANGES IN CLINICAL SYMPTOMS
CBT-related changes in self-reported negative emotion ratings, and react praise and react criticism brain changes were not predictive of social anxiety symptoms. Reappraise criticism brain changes, however, significantly predicted 24% of unique variance in social anxiety symptom reduction. This finding provides support for cognitive models that propose emotion regulation enhancement is one mechanism by which CBT reduces social anxiety symptoms (Campbell-Sills & Barlow, 2007), and highlights the utility of neuroimaging in refining our understanding of how treatments work.
LIMITATIONS AND FUTURE DIRECTIONS
The current study is limited to inferences about CBT-related changes in reactivity to criticism and praise and reappraisal of criticism. Future studies might consider reappraisal of other types of social stimuli that vary in intensity (e.g., high vs. low) and type (e.g., social vs. non-social; experimenter-selected vs. participant-selected). Investigating the differential impact of clinical treatments with different mechanisms of change (e.g., CBT vs. mindfulness-based interventions) on reactivity and reappraisal systems could determine whether there are common targets of change that generalize across treatment modalities. To determine whether reactivity and reappraisal are consistent transdiagnostic mechanisms, it will be important to examine brain changes across multiple anxiety and depression diagnoses. Although this study detected pre-to-post-CBT brain changes, it might be useful to investigate whether those brain changes persist following CBT training and how they relate to longer-term treatment outcome.
Supplementary Material
Highlights.
-
*
Similar CBT-related brain changes when reacting to social praise and criticism.
-
*
No significant CBT-related decreases in negative emotion to praise and criticism.
-
*
CBT-related negative emotion decreases when reappraising social criticism.
-
*
Only CBT-related reappraisal brain changes predicted decreases in social anxiety.
ACKNOWLEDGMENTS
This research was supported by an NIMH Grant R01 MH076074, awarded to James Gross, Ph.D. Richard G. Heimberg, Ph.D., is one of the authors of the commercially available CBT protocol which was utilized in this study. We wish to thank Gary Glover, Ph.D., for his technical assistance with magnetic resonance imaging. Philippe Goldin, who is independent of any commercial funder, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
None of the authors of this manuscript have any biomedical financial interests or potential conflicts of interest.
REFERENCES
- Amir N, Prouvost C, Kuckertz JM. Lack of a benign interpretation bias in social anxiety disorder. Cognitive Behaviour Therapy. 2012;41(2):119–129. doi: 10.1080/16506073.2012.662655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsalidou M, Morris D, Taylor MJ. Converging evidence for the advantage of dynamic facial expressions. Brain Topography. 2011;24(2):149–163. doi: 10.1007/s10548-011-0171-4. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4 ed. Washington, DC: Author; 1994. [Google Scholar]
- Birbaumer N, Grodd W, Diedrich O, Klose U, Erb M, Lotze M, Flor H. fMRI reveals amygdala activation to human faces in social phobics. Neuroreport. 1998;9(6):1223–1226. doi: 10.1097/00001756-199804200-00048. [DOI] [PubMed] [Google Scholar]
- Blair K, Geraci M, Devido J, McCaffrey D, Chen G, Vythilingam M, Pine DS. Neural response to self- and other referential praise and criticism in generalized social phobia. Archives of General Psychiatry. 2008;65(10):1176–1184. doi: 10.1001/archpsyc.65.10.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair KS, Geraci M, Otero M, Majestic C, Odenheimer S, Jacobs M, Pine DS. Atypical modulation of medial prefrontal cortex to self-referential comments in generalized social phobia. Psychiatry Research - Neuroimaging. 2011;193(1):38–45. doi: 10.1016/j.pscychresns.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccia M, Nemmi F, Guariglia C. Neuropsychology of environmental navigation in humans: Review and meta-analysis of fMRI studies in healthy participants. Neuropsychology Review. 2014:1–16. doi: 10.1007/s11065-014-9247-8. volume number? [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TA, Di Nardo PA, Lehman CL, Campbell LA. Reliability of DSM-IV anxiety and mood disorders: Implications for the classification of emotional disorders. Journal of Abnormal Psychology. 2001;110(1):49–58. doi: 10.1037//0021-843x.110.1.49. [DOI] [PubMed] [Google Scholar]
- Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, Ochsner KN. Cognitive reappraisal of emotion: A meta-analysis of human neuroimaging studies. Cerebral Cortex. 2013 doi: 10.1093/cercor/bht154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell-Sills L, Barlow DH. Incorporating emotion regulation into conceptualizations and treatments of anxiety and mood disorders. In: Gross JJ, editor. Handbook of emotion regulation. New York: Guilford; 2007. pp. 542–559. [Google Scholar]
- Canton J, Scott KM, Glue P. Optimal treatment of social phobia: Systematic review and meta-analysis. Neuropsychiatric Disease and Treatment. 2012;8:203–215. doi: 10.2147/NDT.S23317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DA, Beck AT. Cognitive theory and therapy of anxiety and depression: Convergence with neurobiological findings. Trends in Cognitive Sciences. 2010;14(9):418–424. doi: 10.1016/j.tics.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Cohen MS. Parametric analysis of fMRI data using linear systems methods. Neuroimage. 1997;6(2):93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- Cooney RE, Atlas LY, Joormann J, Eugene F, Gotlib IH. Amygdala activation in the processing of neutral faces in social anxiety disorder: Is neutral really neutral? Psychiatry Research. 2006;148(1):55–59. doi: 10.1016/j.pscychresns.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Crowne D, Marlowe D. A new scale of social desirability independent of psychopathology. Journal of Consulting Psychology. 1960;24(4):349–354. doi: 10.1037/h0047358. [DOI] [PubMed] [Google Scholar]
- D'Avanzato C, Joormann J, Siemer M, Gotlib IH. Emotion regulation in depression and anxiety: Examining diagnostic specificity and stability of strategy use. Cognitive Therapy and Research. 2013;37(5):968–980. [Google Scholar]
- Di Nardo PA, Brown TA, Barlow DH. Anxiety Disorders Interview Schedule for DSM-IV: Lifetime version (ADIS-IV-L) New York, NY: Oxford University Press; 1994. [Google Scholar]
- Efron B. Forcing a sequential experiment to be balanced. Biometrika. 1971;58(3):403–417. [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: A meta-analysis of emotional rrocessing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry. 2007;164(10):1476. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI. The activation of attentional networks. Neuroimage. 2005;26(2):471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Fresco DM, Coles ME, Heimberg RG, Liebowitz MR, Hami S, Stein MB, Goetz D. The Liebowitz Social Anxiety Scale: A comparison of the psychometric properties of self-report and clinician-administered formats. Psychological Medicine. 2001;31(6):1025–1035. doi: 10.1017/s0033291701004056. [DOI] [PubMed] [Google Scholar]
- Furmark T, Tillfors M, Marteinsdottir I, Fischer H, Pissiota A, Langstrom B, Fredrikson M. Common changes in cerebral blood flow in patients with social phobia treated with citalopram or cognitive-behavioral therapy. Archives of General Psychiatry. 2002;59(5):425–433. doi: 10.1001/archpsyc.59.5.425. [DOI] [PubMed] [Google Scholar]
- Glover GH, Law CS. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magnetic Resonance in Medicine. 2001;46:515–522. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- Goldberg II, Harel M, Malach R. When the brain loses its self: Prefrontal inactivation during sensorimotor processing. Neuron. 2006;50(2):329–339. doi: 10.1016/j.neuron.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Goldin PR, Manber T, Hakimi S, Canli T, Gross JJ. Neural bases of social anxiety disorder: Emotional reactivity and cognitive regulation during social and physical threat. Archives of General Psychiatry. 2009;66(2):170–180. doi: 10.1001/archgenpsychiatry.2008.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, Manber-Ball T, Werner K, Heimberg R, Gross JJ. Neural mechanisms of cognitive reappraisal of negative self-beliefs in social anxiety disorder. Biological Psychiatry. 2009;66(12):1091–1099. doi: 10.1016/j.biopsych.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, Ziv M, Jazaieri H, Hahn K, Heimberg R, Gross JJ. Impact of cognitive behavioral therapy for social anxiety disorder on the neural dynamics of cognitive reappraisal of negative self-beliefs: Randomized clinical trial. JAMA Psychiatry. 2013;70(10):1048–1056. doi: 10.1001/jamapsychiatry.2013.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, Ziv M, Jazaieri H, Werner K, Kraemer H, Heimberg RG, Gross JJ. Cognitive reappraisal self-efficacy mediates the effects of individual cognitive-behavioral therapy for social anxiety disorder. Journal of Consulting and Clinical Psychology. 2012;80(6):1034–1040. doi: 10.1037/a0028555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D, Wong J, Heimberg R. Cognitive-behavioral therapy for social anxiety disorder: The state of the science. In: Weeks J, editor. The Wiley-Blackwell handbook of social anxiety disorder. New York: Wiley-Blackwell; 2014. pp. 477–497. [Google Scholar]
- Gross JJ. The emerging field of emotion regulation: An integrative review. Review of General Psychology. 1998;2(3):271–299. [Google Scholar]
- Gross JJ, Thompson RA. Emotion regulation: Conceptual foundations. In: Gross JJ, editor. Handbook of emotion regulation. New York: Guilford; 2007. pp. 3–24. [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. Proceedings of the National Academy of Science U S A. 2001;98(7):4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimberg RG, Brozovich FA, Rapee RM. A cognitive-behavioral model of social anxiety disorder. In: Hofmann SG, DiBartolo PM, editors. Social anxiety: Clinical, developmental, and social perspectives. 3rd edition. Waltham, MA: Academic Press; 2014. pp. 705–728. [Google Scholar]
- Hope DA, Heimberg RG, Juster HR, Turk CL. Managing social anxiety: A cognitive-behavioral approach. San Antonio, TX: The Psychological Corp; 2000. [Google Scholar]
- Hope DA, Heimberg RG, Turk CL. Therapist guide for managing social anxiety: A cognitive-behavioral therapy approach. New York: Oxford University Press; 2006. [Google Scholar]
- Klumpp H, Fitzgerald DA, Phan KL. Neural predictors and mechanisms of cognitive behavioral therapy on threat processing in social anxiety disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry (0) 2013 doi: 10.1016/j.pnpbp.2013.05.004. What is (0)? [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocovski NL, Fleming JE, Hawley LL, Huta V, Antony MM. Mindfulness and acceptance-based group therapy versus traditional cognitive behavioral group therapy for social anxiety disorder: A randomized controlled trial. Behaviour Research and Therapy. 2013;51(12):889–898. doi: 10.1016/j.brat.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Ledley DR, Heimberg RG, Hope DA, Hayes SA, Zaider TI, Dyke MV, Fresco DM. Efficacy of a manualized and workbook-driven individual treatment for social anxiety disorder. Behavior Therapy. 2009;40(4):414–424. doi: 10.1016/j.beth.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Liebowitz MR. Social phobia. Modern Problems of Pharmacopsychiatry. 1987;22:141–173. doi: 10.1159/000414022. [DOI] [PubMed] [Google Scholar]
- Messina I, Sambin M, Palmieri A, Viviani R. Neural correlates of psychotherapy in anxiety and depression: A meta-analysis. PLoS ONE. 2013;8(9):e74657. doi: 10.1371/journal.pone.0074657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovitch DA, Gavric DL, Senn JM, Santesso DL, Miskovic V, Schmidt LA, Antony MM. Changes in judgment biases and use of emotion regulation strategies during cognitive-behavioral therapy for social anxiety disorder: Distinguishing treatment responders from nonresponders. Cognitive Therapy and Research. 2012;36(4):261–271. [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Efferent association pathways from the rostral prefrontal cortex in the macaque monkey. Journal of Neuroscience. 2007;27(43):11573–11586. doi: 10.1523/JNEUROSCI.2419-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porto PR, Oliveira L, Mari J, Volchan E, Figueira I, Ventura P. Does cognitive behavioral therapy change the brain? A systematic review of neuroimaging in anxiety disorders. Journal of Neuropsychiatry and Clinical Neuroscience. 2009;21(2):114–125. doi: 10.1176/jnp.2009.21.2.114. [DOI] [PubMed] [Google Scholar]
- Price CJ. A review and synthesis of the first 20years of PET and fMRI studies of heard speech, spoken language and reading. NeuroImage. 2012;62(2):816–847. doi: 10.1016/j.neuroimage.2012.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol J, Giménez M, Ortiz H, Soriano-Mas C, López-Sola M, Farré M, Martín-Santos R. Neural response to the observable self in social anxiety disorder. Psychological Medicine. 2013;43(4):721–731. doi: 10.1017/S0033291712001857. [DOI] [PubMed] [Google Scholar]
- Rytwinski NK, Fresco DM, Heimberg RG, Coles ME, Liebowitz MR, Cissell S, Hofmann SG. Screening for social anxiety disorder with the self-report version of the Liebowitz Social Anxiety Scale. Depression and Anxiety. 2009;26:34–38. doi: 10.1002/da.20503. [DOI] [PubMed] [Google Scholar]
- Sato W, Kochiyama T, Yoshikawa S, Naito E, Matsumura M. Enhanced neural activity in response to dynamic facial expressions of emotion: An fMRI study. Brain Research Cogn Brain Res. 2004;20(1):81–91. doi: 10.1016/j.cogbrainres.2004.01.008. write out full journal name. [DOI] [PubMed] [Google Scholar]
- Schmitz TW, Kawahara-Baccus TN, Johnson SC. Metacognitive evaluation, self-relevance, and the right prefrontal cortex. NeuroImage. 2004;22(2):941–947. doi: 10.1016/j.neuroimage.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Schultz LT, Heimberg RG. Attentional focus in social anxiety disorder: Potential for interactive processes. Clinical Psychology Review. 2008;28(7):1206–1221. doi: 10.1016/j.cpr.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Sergent J, Ohta S, MacDonald B. Functional neuroanatomy of face and object processing: A positron emission tomography study. Brain. 1992;115(1):15–36. doi: 10.1093/brain/115.1.15. [DOI] [PubMed] [Google Scholar]
- Stein MB, Goldin PR, Sareen J, Zorrilla LT, Brown GG. Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Archives of General Psychiatry. 2002;59:1027–1034. doi: 10.1001/archpsyc.59.11.1027. [DOI] [PubMed] [Google Scholar]
- Stein MB, Stein DJ. Social anxiety disorder. Lancet. 2008;371(9618):1115–1125. doi: 10.1016/S0140-6736(08)60488-2. [DOI] [PubMed] [Google Scholar]
- Straube T, Sauer A, Miltner WHR. Brain activation during direct and indirect processing of positive and negative words. Behavioural Brain Research. 2011;222(1):66–72. doi: 10.1016/j.bbr.2011.03.037. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme; 1988. [Google Scholar]
- Trautmann SA, Fehr T, Herrmann M. Emotions in motion: dynamic compared to static facial expressions of disgust and happiness reveal more widespread emotion-specific activations. Brain Research. 2009;1284:100–115. doi: 10.1016/j.brainres.2009.05.075. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Haxby JV. 'What' and 'where' in the human brain. Current Opinion in Neurobiology. 1994;4(2):157–165. doi: 10.1016/0959-4388(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Van Ameringen M, Mancini C, Szechtman H, Nahmias C, Oakman JM, Hall GBC, Farvolden P. A PET provocation study of generalized social phobia. Psychiatry Research - Neuroimaging. 2004;132(1):13–18. doi: 10.1016/j.pscychresns.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Werner KH, Goldin PR, Ball TM, Heimberg RG, Gross JJ. Assessing emotion regulation in social anxiety disorder: The emotion regulation interview. Journal of Psychopathology and Behavioral Assessment. 2011;33(3):346–354. [Google Scholar]
- Ziv M, Goldin PR, Jazaieri H, Hahn KS, Gross JJ. Is there less to social anxiety than meets the eye? Behavioral and neural responses to three socio-emotional tasks. Biology of Mood & Anxiety Disorders. 2013;3(5):681–691. doi: 10.1186/2045-5380-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.