Abstract
CXCR3 deficient (CXCR3−/−) mice are resistant to ocular HSV-1 infection in that less mice develop encephalitis and succumb to infection in comparison to wild type (WT) animals. A region of the brain previously identified to be crucial for development of encephalitis was evaluated in HSV-1-infected CXCR3−/− and WT mice. In this region, known as the ependyma, viral titer, infiltrating leukocyte populations, and key anti-viral cytokine message levels were evaluated. We found CXCR3−/− mice possessed significantly less HSV-1 and expressed significantly more IFN-β mRNA in the brain ependyma compared to WT animals during the development of encephalitis.
Keywords: herpes simplex virus type 1, CXCR3, interferon-β, brain ependyma
1. Introduction
Herpes simplex virus type 1 (HSV-1) is a highly successful human pathogen that elicits a robust innate and adaptive immune response in mice (Egan et al., 2013). Acute infection or reactivation of the virus can result in trafficking of infectious virions along neuronal tracts to breach the central nervous system (CNS). This can lead to a productive infection in the CNS driving a disease known as herpes simplex encephalitis (HSE). The course of HSE includes cerebral edema, increased intracranial pressure, ischemia, and enlargement of the cerebral lateral ventricles in the human population and mice (Conrady et al., 2013; Lundberg et al., 2008; Sellner et al., 2006). Previously, we reported mice lacking CXCR3 (CXCR3−/−, on a C57BL/6J background) are less susceptible to HSV-1-driven mortality compared to wild type (WT) C57BL/6J mice (Wickham et al., 2005). The increased resistance was not due to changes in the recruitment of effector T cells or NK cells within the brain stem (BS) (Wickham et al., 2005; Wuest and Carr, 2008). In contrast to our findings, another group reported no difference in susceptibility comparing WT to CXCR3−/− mice both on a C57BL/6J background (Lundberg et al., 2007). While different HSV-1 strains were used to infect the mice, the report by Lundberg et al (Lundberg et al., 2007) suggested inbreeding of WT C57BL/6J mice within the institution of the investigators used in the original observation (Wickham et al., 2005) was the likely explanation for the differences observed between the two groups.
To further evaluate susceptibility of CXCR3−/− mice to HSV-1 infection and identify a mechanism to explain the increased resistance to HSE-induced mortality, we employed WT C57BL/6J mice acquired only from Jackson Laboratories over an extended period of time (2.0 years) and focused our attention within the CNS. Furthermore, CXCR3−/− mice were re-derived by Jackson Laboratories prior to experimentation in the current study as another control for genetic drift.
Previously, it has been reported HSV-1 shows a predilection for tissue surrounding the ventricular zones in organotypic brain slice cultures (Cohen et al., 2011). Consistent with this observation, we recently found HSE WT mice contain an abundance of replicating virus in the ependymal cells lining the lateral ventricles resulting in the loss of the ciliated ependyma, ventricular enlargement, and increased death in comparison to non-HSE WT mice following ocular infection (Conrady et al., 2013). Consequently, we hypothesized there was a fundamental difference in susceptibility of CXCR3−/− mice at this site that would explain the increased cumulative survival of CXCR3−/− mice compared to WT animals.
2. Materials and methods
2.1 Mice
The confirmation in the absence of the CXCR3 gene was previously described (Carr et al., 2008). Animal care and treatment was in accordance with the National Institutes of Health Guidelines on the Care and Use of Laboratory Animals and approved by the University of Oklahoma Health Sciences Center and Dean A. McGee Eye Institutes’ Institutional Animal and Care Use Committees.
2.2 HSV-1 and Murine Infections
The corneas of age (5-8 weeks old) and sex matched, anesthetized mice were scarified (Carr et al., 2006) and infected with 1,000 plaque forming units (pfu)/cornea of HSV-1 (strain McKrae). At the indicated time following infection, mice were exsanguinated and tissue extracted and assayed for viral content, leukocyte infiltration, and select cytokine and anti-viral molecule mRNA levels.
2.3 Viral Plaque Assay
At 8 days post infection (pi) brain and brain stem (BS) were removed from euthanized mice. The ependymal region was surgically removed from the brain and homogenized for subsequent viral plaque assay performed on Vero cell monolayers (Carr et al., 2008).
2.4 Flow Cytometry
Brain ependyma was removed and homogenized in 1ml of RPMI-1640 using a Wheatley Dounce homogenizer (Fisher Scientific). Suspensions were filtered through a sterile 70 μm strainer (BD Biosciences) with 4 mls of RPMI-1640 media. Samples (250 μl) were subsequently incubated with Fc block (Carr et al., 2008) diluted in 1% BSA/PBS for 15 min. Microglia were analyzed by gating mid-level CD45 (anti-mouse CD45- eFluor 450), CD11b (anti-mouse CD11b PE) and MHC class II (anti-mouse MHCII (I-A/I-E) PE) expression.
CD45Hi -expressing T cells were also labeled with anti-mouse, CD3-FITC, CD4-APC, and CD8-PE. All antibodies (eBioscience) were used at 0.4 μg and were combined with 0.8% normal rat serum (Jackson ImmunoResearch Laboratories) in a 25 μl volume of 1%BSA/PBS solution per sample. After 30 min incubation, samples were washed in 1% BSA/PBS and fixed in 1.0% paraformaldehyde overnight.
2.4 Real Time PCR
Day 6 following infection, brain ependyma and brain stem were removed and homogenized in a total of 1ml Trizol (Invitrogen) for RNA retrieval. One microgram RNA was converted to cDNA followed by use of 125ng cDNA for semi quantitative real-time PCR. Beta actin, IFN-β, and TNF-α primers are described in reference (Conrady et al., 2013). Other primer sequences are as follows: IFN-γ forward, 5’-GCCAAGTTTGAGGTCAACAA-3’, and reverse 5’GAATCAGCAGCGACTCCTTT-3’, iNOS forward, 5’ ACCTTGTTCAGCTACGCCTT-3’, and reverse 5’-TCTTCAGAGTCTGCCCATTG-3’, PGK1 forward, 5’-CTGACTTTGGACAAG CTGGACG reverse, 5’-GCAGCCTTGATCCTT TGG TTG-3’. Genes were normalized to the geometric mean of beta actin, and phosphoglycerate kinase 1 (PGK1). Downstream type-1 interferon genes were normalized to beta actin and GAPDH, all supplied by Bio-rad's PrimePCR.
3. Results
3.1 CXCR3 −/− mice contain less virus in the susceptible brain ependyma
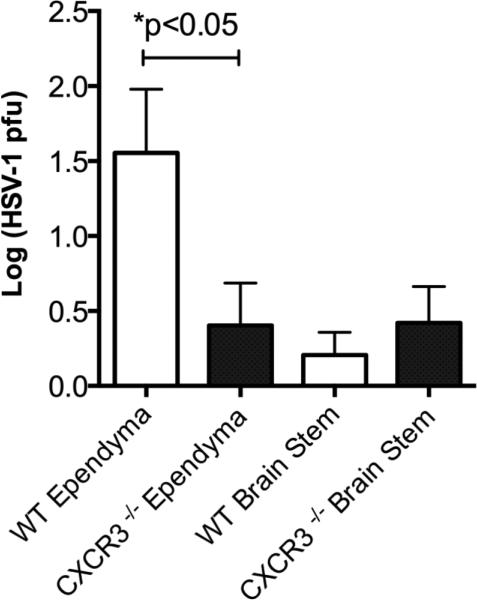
Initially, we evaluated survival of mice challenged with HSV-1 and found results similar to our previously published observation (Wickham et al., 2005). Specifically, CXCR3−/− mice were less susceptible to HSV-1 infection with an increased survival rate compared to WT mice following ocular infection with HSV-1 (91.5 ± 8.5 % compared to 49.75 ± 6.73 % cumulative survival respectively, n=14/group, p<.05). Even though both sets of mice had similar levels of infectious virus recovered in the BS, CXCR3−/− mice possessed significantly less virus in the ependyma compared to WT animals at the time when mice show significant signs of HSE at day 8 pi (Fig. 1). In mice surviving HSE, there was no difference in the clearance of infectious virus in surviving WT and CXCR3−/− mice.
Figure 1. The ependymal tissue from CXCR3−/− mice contain less infectious virus than WT controls.
Data is represented as three independent experiments with n=9/group graphed as mean with standard errors of the means (SEM). *p<0.05 comparing WT ependyma and CXCR3−/− ependyma viral titers as determined by an unpaired two-tailed t test.
3.2 A decrease in infectious virus cannot be explained by an increase in microglial activation
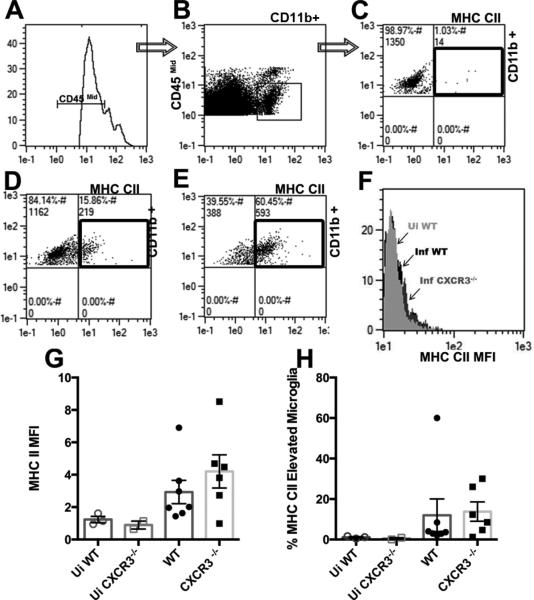
Since an experimental model utilizing mice deficient in a functional type-1 interferon receptor has demonstrated that a resident population including microglia are a major contributor to controlling HSV-1 infection (Conrady et al., 2013), we next evaluated microglial cells. Microglia are sentinels of the brain that are not only important for CNS homeostasis, but also for recovery following insults (Perry and O'Connor, 2010). Activated microglia also express MHC class II molecules in rodent brains enabling their ability to present antigen and respond to pathogens (Ousman and Kubes, 2012). Furthermore, these cells display an increase in phagocytic ability as well as an enhanced migratory capacity within the brain indicated by an increase in cell surface glycoprotein and MHC class II expression (Ousman and Kubes, 2012; Selenica et al., 2013). Therefore, one possibility for the difference in HSE manifested by WT but not CXCR3−/− mice may be at the level of microglia. Microglia isolated from the ependymal region of the brain of infected and uninfected mice revealed no change in the absolute number of cells within groups of infected or uninfected mice. However, microglia from infected mice displayed a more activated phenotype with an increase in MHC class II expression in comparison to microglia from uninfected mice (Fig. 2A-H). Microglia from WT and CXCR3−/− infected mice expressed similar levels of MHC class II suggesting that the state of activation of the microglia may not be a contributing factor in resistance to HSV-1 infection in the ependyma of the CXCR3−/− mice (Fig. 2F-H).
Figure 2. Microglia from infected mice show an increase in MHC II expression.
A-E) Gating strategy for selecting CD45 mid, CD11b, and MHC II expressing microglia. A) CD45mid-expressing microglia (via histogram) were selected from the forward and side scatter profile. C-E) CD11b+ microglia are selected from the CD45mid level population. Representative plot for up-regulated MHC II expressing microglia denoted by black box for uninfected animals (C) infected WT (D) and infected CXCR3−/− mice (E). F) Overlay of histograms representing the increase in mean fluorescence intensity of infected CXCR3−/− mice in comparison to infected and uninfected WT mice (F). G) No differences in mean fluorescence intensity (MFI) or percent of MHC class II-expressing microglia infected WT to CXCR3−/− mice (H).
3.3 An increase in IFN-βmessage expression correlates with enhanced viral control in the ependyma of CXCR3−/− animals
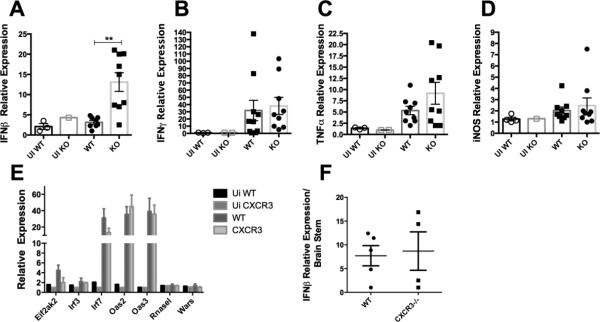
Other factors that might explain the reduction in HSE observed in HSV-1-infected CXCR3−/− mice were explored. Consistent with previous observations in the BS, there were no significant differences in the number of T cells (CD4+, CD8+, and HSV-specific CD8+), NK cells, macrophages, inflammatory monocytes, and neutrophils residing in the brain ependyma of WT and CXCR3−/− mice day 6-8 pi (data not shown). In contrast, expression of IFN-β transcript was significantly elevated in the ependyma of infected CXCR3−/− mice in comparison to WT controls (Fig. 3A). However, there was no difference observed in IFN-β expression in the brain stem (Fig. 3F). Likewise, IFN-β, inducible nitric oxide synthase (iNOS), and tumor necrosis factor (TNF)-α transcript levels were similar between infected groups (Fig. 3B-D). As a means to further explore IFN-β, we evaluated select downstream type1 interferon-inducible genes in WT and CXCR3−/− mice following infection. In contrast to our expectation, there were comparable levels of expression of these genes including Elf2Akt, IRF3, IRF7, OAS2, OAS3, RNase L, and Wars within the ependyma of CXCR3−/− mice in comparison to WT (Fig.3 E).
Figure 3. IFN-β expression is elevated in the brain ependyma of CXCR3−/− mice.
Values were determined utilizing the ΔΔCT method. (A-E). The Grubb's test was utilized to remove 1 outlier from each group (A) with results graphed and represented by mean ± SEM. **p<.01 comparing infected IFN-β levels of WT to CXCR3−/− mice as determined by an unpaired t test (A). IFN-γ (B), TNF-α (C), and iNOS (D) levels were not significantly different comparing WT to CXCR3−/− mice. Values represented from two independent experiments (A-D). E) Prime PCR of downstream type1 interferon genes (one experiment). No observable differences in IFN-ß mRNA expression within the brain stem F). All data is represented by mean ± SEM.
4. Discussion
Taken together, the reduced incidence of HSE observed in CXCR3−/− mice infected with HSV-1 correlates with a reduction in viral load and increased expression of IFN-β message in the brain ependyma. This supports previous findings that HSV-1 infection along with its elicited host response has a tropism for brain ependymal cells and signifies the importance of further investigating the host response within this region.
We did not find changes in the mobilization of leukocytes from the periphery to the CNS as might be expected in the absence of CXCR3. Using these same mice, we previously reported no changes in the recruitment of leukocytes following genital HSV-2 infection [13]. However, similar to other microbial infections of the CNS [14, 15], genital HSV-2 infected CXCR3−/− mice were highly susceptible to virus infection [13].
Currently, we are unable to explain the conundrum between susceptibility and likely anti-viral pathways that take part in resistance to HSV-1 in the CXCR3−/− mice. . Specifically, wedid not find any differences in a select list of chosen downstream anti-viral genes. However, there may be other genes not investigated that are up- regulated and critical to controlling infection. Additionally, the kinetics in downstream antiviral genes may vary with time as virus spreads through the ependyma.
Although another group (Lundberg et al., 2007) did not find C57BL/6J mice susceptible to HSE, differenttechniques in infecting the mice along with different, viral strains, might contribute to the discrepancy. Interestingly, BALB/c mice were found to succumb to HSE and CXCR3−/− mice bred on a BALB/c background were found to be resistant to morbidity (Lundberg et al., 2007). Taken together, we conclude that the deficiency in CXCR3 expression results in an inherent resistance to HSV-1 at the level of the ependymal region of the brain by, as yet, an undefined pathway.
Highlights.
CXCR3 deficient mice are resistant to herpes simplex encephalitis following ocular HSV-1 infection.
Brain ependymal tissue of CXCR3 deficient mice contain less virus compared to wild type animals.
CXCR3 deficient mice resistance to encephalitis and virus replication is correlative with a significant increase in IFN-β mRNA expression in the brain ependyma.
Acknowledgements
Principal support for the study was from NIH R01 AI053108. Additional support includes an OUHSC Presbyterian Health Foundation Presidential Professorship, Research to Prevent Blindness Senior Investigator awards (to DJJC), and NIH/NEI core grant EY021725.
List of abbreviations used
- HSV-1
herpes simplex virus type
- CNS
central nervous system
- HSE
herpes simplex encephalitis (HSE)
- WT
wild type
- NK
natural killer
- BS
brain stem
- pi
post infection
- IFN
interferon
- iNOS
inducible nitric oxide synthase
- TNF
tumor necrosis factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors contributions: Chandra Kroll and Min Zheng ordered all reagents, planned and performed experiments, acquired and interpreted data, and wrote the manuscript. Dan Carr planned experiments, interpreted data, and helped write/revise the manuscript.
Competing interests: The authors declare that they have no competing interests.
REFERENCES
- Carr DJ, Ash J, Lane TE, Kuziel WA. Abnormal immune response of CCR5-deficient mice to ocular infection with herpes simplex virus type 1. J Gen Virol. 2006;87:489–499. doi: 10.1099/vir.0.81339-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DJ, Wuest T, Ash J. An increase in herpes simplex virus type 1 in the anterior segment of the eye is linked to a deficiency in NK cell infiltration in mice deficient in CXCR3. J Interferon Cytokine Res. 2008;28:245–251. doi: 10.1089/jir.2007.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M, Braun E, Tsalenchuck Y, Panet A, Steiner I. Restrictions that control herpes simplex virus type 1 infection in mouse brain ex vivo. J Gen Virol. 2011;92:2383–2393. doi: 10.1099/vir.0.031013-0. [DOI] [PubMed] [Google Scholar]
- Conrady CD, Zheng M, van Rooijen N, Drevets DA, Royer D, Alleman A, Carr DJ. Microglia and a Functional Type I IFN Pathway Are Required To Counter HSV-1-Driven Brain Lateral Ventricle Enlargement and Encephalitis. J Immunol. 2013;190:2807–2817. doi: 10.4049/jimmunol.1203265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan KP, Wu S, Wigdahl B, Jennings SR. Immunological control of herpes simplex virus infections. J Neurovirol. 2013;19:328–345. doi: 10.1007/s13365-013-0189-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg P, Openshaw H, Wang M, Yang HJ, Cantin E. Effects of CXCR3 signaling on development of fatal encephalitis and corneal and periocular skin disease in HSV-infected mice are mouse-strain dependent. Invest Ophthalmol Vis Sci. 2007;48:4162–4170. doi: 10.1167/iovs.07-0261. [DOI] [PubMed] [Google Scholar]
- Lundberg P, Ramakrishna C, Brown J, Tyszka JM, Hamamura M, Hinton DR, Kovats S, Nalcioglu O, Weinberg K, Openshaw H, Cantin EM. The immune response to herpes simplex virus type 1 infection in susceptible mice is a major cause of central nervous system pathology resulting in fatal encephalitis. J Virol. 2008;82:7078–7088. doi: 10.1128/JVI.00619-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ousman SS, Kubes P. Immune surveillance in the central nervous system. Nat Neurosci. 2012;15:1096–1101. doi: 10.1038/nn.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH, O'Connor V. The role of microglia in synaptic stripping and synaptic degeneration: a revised perspective. ASN Neuro. 2010;2:e00047. doi: 10.1042/AN20100024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selenica ML, Alvarez JA, Nash KR, Lee DC, Cao C, Lin X, Reid P, Mouton PR, Morgan D, Gordon MN. Diverse activation of microglia by chemokine (C-C motif) ligand 2 overexpression in brain. J Neuroinflammation. 2013;10:86. doi: 10.1186/1742-2094-10-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellner J, Simon F, Meyding-Lamade U, Leib SL. Herpes-simplex virus encephalitis is characterized by an early MMP-9 increase and collagen type IV degradation. Brain Res. 2006;1125:155–162. doi: 10.1016/j.brainres.2006.09.093. [DOI] [PubMed] [Google Scholar]
- Wickham S, Lu B, Ash J, Carr DJ. Chemokine receptor deficiency is associated with increased chemokine expression in the peripheral and central nervous systems and increased resistance to herpetic encephalitis. J Neuroimmunol. 2005;162:51–59. doi: 10.1016/j.jneuroim.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Wuest TR, Carr DJ. Dysregulation of CXCR3 signaling due to CXCL10 deficiency impairs the antiviral response to herpes simplex virus 1 infection. J Immunol. 2008;181:7985–7993. doi: 10.4049/jimmunol.181.11.7985. [DOI] [PMC free article] [PubMed] [Google Scholar]