Abstract
Inflammation is a complicated biological process in response to harmful stimuli, which involves the cooperation of immune system and vascular system. Upon pathogen invasion or tissue injury, resident innate immune cells such as macrophages and dendritic cells are activated and release inflammatory mediators, which result in the vasodilation and recruitment of leukocytes, mainly monocytes and neutrophils. As two of the most important inflammation-mediating immune cells, macrophages and neutrophils have long been regarded to have a pro-inflammatory effect. However, increasing evidences suggest the role of macrophage and neutrophil in inflammation is more complicated and diversified than we thought. Differently activated macrophages and neutrophils lead to diverse even opposite activities. Precise understanding of the role of different subpopulations is critical to achieve the effective treatment for inflammatory diseases. In this review, we discuss the two potentially distinct activation routes of macrophages and neutrophils in obesity and diabetes.
Keywords: Alternative activation, Classical activation, Inflammation, Macrophage, Neutrophil
Introduction
Constantly, human beings are assaulted by foreign bodies while our bodies defend against them through the immune system. One type of the immune responses is anti-inflammation, in which cells primarily from the monocyte and neutrophil cell lineages participate. Traditionally, inflammatory response is believed to start with neutrophils leaving vasculature toward an injured location as the initial responders[1, 2]. After neutrophils are recruited to that area, these cells actively kill or phagocytose foreign bodies and release cytokines[2]. It is also believed that as the inflammatory response continues, monocytes would then be recruited following neutrophils to the site of injury and differentiate into macrophages[1]. After neutrophils and monocytes enter that area, various cytokines are released to aid either pro-inflammatory or anti-inflammatory responses[1, 3]. Thus, neutrophils were originally thought to be intimately related with acute inflammation while monocytes/macrophages to be with chronic inflammation. However, in 2003, monocytes were found to travel independently of neutrophils toward injured sites[1]. This finding has been supported by several other studies, and now points toward neutrophils and monocytes act freely during injury responses[1, 3]. Nevertheless, there are still conflicting findings that point one way or another[4–6]. Here, we summarize the two potentially distinct properties of monocytes/macrophages and neutrophils in inflammatory responses.
Monocyte Differentiation
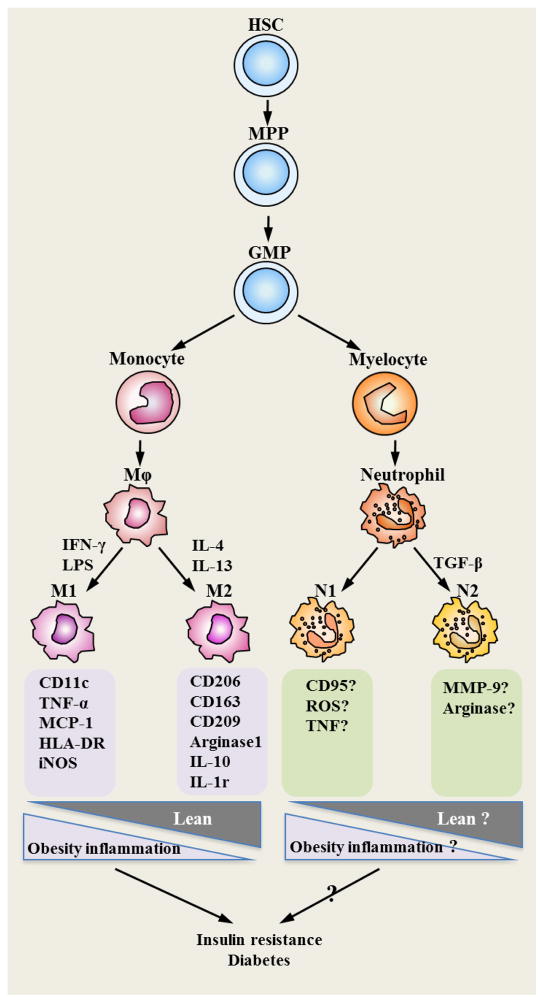
Monocytes are known to originate in the bone marrow from myeloid progenitor cells and then released into the peripheral blood (Figure 1)[7]. Monocytes have a relatively short lifetime in the blood circulation. They only stay for an average of 10 to 20 hours in the blood and then enter into the tissues so as to be activated and differentiated into macrophages [8]. As early as 1939, monocytes were reported to be able to emigrate from the blood and differentiate into macrophages in the tissues[9]. The recruitment of monocytes to the peripheral was found to be enhanced by inflammatory stimuli[10].
Figure 1. Heterogeneity of monocyte/macrophage and neutrophil.
Monocyte and neutrophil are originated from the same progenitor cell (GMP) that is differentiated from hematopoietic stem cell (HSC). GMP is able to differentiate into monocyte or myelocyte. When enter into tissues, monocytes become macrophages. Macrophages acquire distinct features when activated through different ways. “Classically” activated macrophages (M1) express CD11c, HLA-DR, and iNOS, secret TNF-α and MCP-1, while “alternatively” activated macrophages (M2) express CD206, CD163, CD209, IL-1r, arginase 1 and IL-10. In obesity/diabetes, polarization of macrophages skews towards to “M1”, which contributes to the maintenance of chronic inflammation and insulin resistance in various tissues. Similar to macrophages, neutrophils differentiated from myelocytes display different phenotype when activated in different environment. “N1” possesses a pro-inflammatory or anti-tumor phenotype, whereas “N2” driven by TGF-β displays anti-inflammatory or pro-tumor phenotype. Increased levels of CD95, TNF and ROS are responsible for the anti-tumor phenotype of “N1”, while higher levels of MMP-9 and arginase might contribute to the pro-tumor phenotype of “N2”. However, as the classification of “N1” and “N2” was not proposed until recently, it requires further studies to identify the “N1”- or “N2”-specific protein expression profile and their functional role in obesity/diabetes. Abbreviations: HSC: hematopoietic stem cell; GMP: Granulocyte-monocyte Progenitor; MPP: multiple potent progenitor; TNF: tumour necrosis factor; ROS: Reactive oxygen species; MMP-9: Matrix metallopeptidase 9; HLD-DR: human leukocyte antigens; iNOS: inducible nitric oxide synthase; MCP-1: monocyte chemoattractant protein-1; IL-1r: interleukin-1 receptor; IL-10: interleukin-10.
As one of the first lines in immune defense, macrophages are essential to antigen recognition, initiation of inflammatory response and tissue repair[11]. Macrophages reside in almost every tissue and are responsible for monitoring signs of tissue injury and infection. They also coordinate with adaptive immune responses to clear pathogens and participate in tissue homeostasis[12]. By using a transgenic mouse model in which depletion of macrophage can be induced by the administration of diphtheria toxin, Goren and coworkers proved that depletion of macrophages severely impaired wound inflammation, angiogenesis and tissue remodeling[13].
Human subpopulations of monocytes can be identified by the expression of various levels of CD14, CD16 or CD64[8, 14]. While murine monocytes do not have an exclusive marker, they can be identified based on high Ly-6C expression and low CD31 expression[8].
Monocytes vary in size and have different degrees of granularity and varied nuclear morphology. They are capable of differentiating into dendritic cells or macrophages. Due to the heterogeneity of the monocytes, macrophages are also highly heterogenous[14]. Monocytes express distinct chemokine receptors and adhesion molecules. They are preferentially recruited into different tissues and differentiated into distinct subtype of macrophages. For instance, monocytes that emigrated into the liver differentiate into Kupffer cells in the liver, microglia in the brain and spinal cord, and alveolar macrophages in the lung.
Monocyte/macrophages in tissue injury: promoting tissue repair or enhancing tissue destruction?
During host defense, the infiltration of monocytes into inflammatory site usually helps the clearance of foreign antigens and tissue repair. By releasing pro-inflammatory, angiogenic, fibrogenic or mitogenic cytokines, macrophages cooperate with other immune and progenitor cells to limit tissue injury and repair tissue damage[15]. When recruited to injured tissue, macrophages remove debris by phagocytosis and release various cytokines to attract fibroblasts, lymphocytes and endothelial cells to repair the tissue damage[16–20]. The infiltration of macrophages often precedes the formation of new vessel sprouts and alters the microenvironment to facilitate vascularization[19]. They release matrix metalloproteases (MMPs) and plasmin to regulate angiogenesis by modifying the microenvironment of extracellular matrix (ECM)[16]. Therefore, exposure to macrophages could enchance vascularization in the injured tissue [18, 20]. In addition, the depletion of macrophages and circulating monocytes was reported to be able to reduce the recruitment of fibroblasts and fibrosis[17, 21–24].
Monocyte hyperresponsiveness, however, may also exacerbate tissue injury by uncontrolled release of cytokines. Macrophages have long been documented as being capable of causing tissue damage from severe inflammatory response, and depletion of macrophages during these responses appears to reduce the tissue damage[3]. Tissue damage appears when the tissue loss occurs at a faster rate than the proliferation of new tissue[3]. While apoptosis is needed to create a balance between old cells and newly proliferated cells, the presence of macrophages greatly increases the apoptosis rate of tissue resident cells, such as parenchymal and stromal cells, and mesenchymal stem cells[3]. Duffield reported in 2005 that distinct macrophages play different role in tissue injury, either injury-inducing or repair-promoting[25]. Taking advantage of a murine model with conditional macrophage depletion, they demonstrated that depletion of macrophages during tissue injury enhanced the loss of myofibroblast-like hepatic stellate cells and matrix, while depletion of macrophages during recovery phase attenuated matrix degradation[25].
Macrophage polarization: “M1” versus “M2”
Immediately after its discovery, macrophages are perceived to possess a strong inflammation promoting ability[26]. However, increasing evidence suggests not all macrophage subtypes promotes inflammatory response. Other than promoting inflammation, certain macrophages are found to be able to attenuate inflammation. Based on this finding, macrophages are roughly divided into 2 subgroups: “M1” (classically activated macrophage) and “M2” (alternatively activated macrophage) (Table 1). In terms of “M1” versus “M2” macrophage, in the late 20th century, CD4+ T cells were divided into 2 major subgroups: Th1 and Th2 [27]. Th1 cells are characterized by the secretion of IFN-γ and are responsible for the clearance of intracellular pathogens, whereas Th2 cells secrete IL-4 and IL-13 and mediate humoral immune response. IFN-γ was found to be able to increase antigen presenting capacity, pro-inflammatory cytokine synthesis, and phagocytosis in macrophages[28]. Macrophages that are activated by microbial products and interferon (IFN)-γ, a Th1-derived cytokine, are called “M1” or classically activated macrophages While IL-4, a Th2-derived cytokine, was able to activate macrophages in a manner characterized by induction of major histocompatibility complex (MHC) class II antigens, upregulation of mannose receptor (MRC1) and enhanced capacity to eliminate pathogens[29–31]. Distinct from M1 (classically activated macrophages), IL-4 induced macrophages displayed an entirely different phagocytic receptor and cytokine secretory profile. The initial study regarding the effect of IL-4 on macrophage activation revealed that IL-4 suppressed IL-1β and IL-8 secretions as well as respiratory burst on macrophages. Macrophages that are activated by Th2-derived cytokines IL-4 and/or IL-13 are called “M2” or alternatively activated macrophages. “M1” cells are potent effector cells to clear foreign pathogens and initiate adaptive immunity, while “M2” cells moderate inflammation and adaptive immune responses, promote tissue repairing and angiogenesis. Therefore, macrophages are regarded as having diverse biological functions. They may promote or hinder inflammatory response, and may either enhance tissue repair or induce tissue destruction.
Table I.
macrophage subpopulations in diseases
| M1 or M2 | Disease Model | Subjects | Key Findings | References |
|---|---|---|---|---|
| M1 | NA | Human macrophages | IFN-γ can activate macrophages by increasing its antigen presenting capacity, pro-inflammatory cytokine production and phagocytosis. | Nathan et al., 1983 [28] |
| M1 | Pneumonia | Mouse | IKKβ suppresses M1 macrophage activation in response to LPS and infection. | Fong et al., 2008 [48] |
| M1 | Obesity, Type 2 diabetes | Human | Endotoxin induces resistin production by macrophages, which contributes to the insulin resistance in type 2 diabetes. | Lehrke et al., 2004 [41] |
| not specified | Liver ischaemia- reperfusion injury | Rat | Simvastatin provide protection against the adverse effects of I/R injury by suppressing TNF-a, LDH, and serum aminotransferase activity (This phenotype is probably related to “M1” cells). | Dibazar et al., 2008 [32] |
| not specified | Experimental cardiac injury | Rat | A subset of macrophages infiltrating necrotic myocardium expresses osteopontin during cardiac injury (This phenotype is probably related to “M1” cells). | Murry et al., 1994 [35] |
| not specified | Obesity | Mouse | Resistin is mainly produced by macrophages in human. It exacerbates adipose inflammation and insulin resistance in mice (This phenotype is probably related to “M1” cells). | Qatanani et al., 2009 [45] |
|
| ||||
| M2 | NA | Mouse | IL-4 enhances macrophage mannose receptor expression and activity. L-4 induces alternative activation of macrophages, characterized by an elevated endocytic capacity of mannosylated ligands, increased MHC-II expression, and reduced proinflammatory cytokine production. | Stein et al., 1992 [30] |
| M2 | Parasite infection | Mouse | IL-4 promotes the uptake and parasite killing activity of macrophages. | Wirth et al., 1989 [31] |
| M2 | NA | Human | IL-4 suppresses the production of superoxide by macrophages. | Abramson and Gallin, 1990 [78] |
| M2 | Obesity, Leishmaniasis infection | Mouse (Mac-PPARγ KO and PPARδ−/− mouse) | PPARγ is required for the alternative activation of macrophages and regulates insulin resistance. | Odegaard et al., 2007 [55] |
| M2 | Obesity | Mouse (PPARδ−/−) | PPARδ regulates the expression of arginase 1, costimulatory molecules, and pattern recognition receptors during the alternative activation of macrophages. | Odegaard et al., 2008 [58] |
| M2 | Atherosclerosis | Human | The activation of PPARγ skews monocytes toward an anti-inflammatory M2 phenotype. | Bouhlel et al., 2007 [57] |
| M2 | NA | Human | M2 enhances regulatory properties of Treg cells by inducing membrane bound TGF-β1. | Savage et al., 2008 [39] |
| M2 | Obesity | Mouse | Adipoctyes and hepatocytes can produce IL-4 and IL-13 cytokines to promote M2 activation and limit inflammation. PPARδ is required for the alternative activation of macrophages. | Kang et al., 2008 [49] |
| not specified | Pneumococcal pneumonia | Mouse | Alveolar macrophages reduce mortality of pneumococcal pneumonia by suppressing polymorphonuclear cells mediated inflammation (This phenotype is probably related to “M2” cells). | Knapp et al., 2003 [36] |
| not specified | Multiple sclerosis | Human | Macophages express a series of anti-inflammatory molecules and are unable to response to inflammatory stimuli after myelin-ingestion (This phenotype is probably related to “M2” cells). | Boven et al., 2006 [37] |
| not specified | NA | Mouse | Macrophages obtain an anti-inflammatory phenotype after S1P or S1P1 receptor-specific agonist treatment (This phenotype is probably related to “M2” cells). | Hughes et al., 2008 [38] |
| not specified | Insulin resistance | Mouse | Deficiency of PPARγ in macrophages results in insulin resistance and poor responses to antidiabetic thiazolidinediones (This phenotype is probably associated with lack of “M2”). | Hevener et al., 2007 [56] |
| not specified | Balloon injury | Rat, Rabbit | Depletion of macrophages by clodronate-containing liposomes decreased neointimal formation after mechanical arterial injury (This phenotype is probably related to “M2” cells). | Danenberg et al., 2002[17] |
| not specified | Hypoxia-induced pulmonary remodeling | Rat, Calve | Precursors of a monocyte/macrophage lineage are essential contributors to hypoxia-induced pulmonary vascular remodeling (This phenotype is probably related to “M2” cells). | Frid et al., 2006 [24] |
| not specified | Wound healing | Mouse (lysM-Cre/DTR transgenic mouse) | Depletion of macrophages severely impaired wound inflammation, angiogenesis and tissue remodeling (This phenotype is probably related to “M2” cells). | Goren et al., 2009 [13] |
| not specified | Ischemic cardiomyopathy | Mouse (MCP-1 transgenic mouse) | Macrophage infiltration facilitates vascularization by proceding the formation of new vessel sprouts and altering the microenvironment (This phenotype is probably related to “M2” cells). | Moldovan et al., 2000 [19] |
|
| ||||
| M1/M2 | Obesity, Air pollution | Mouse | M1/M2 balance is associated with air pollution induced insulin resistance. | Sun et al., 2009 [46] |
| M1/M2 | Obesity, Air pollution | Mouse | When exposed to air pollution, M1/M2 balance is altered and associated with insulin resistance. | Xu et al., 2010 [47] |
| M1/M2 | Hepatic fibrosis | Mouse (CD11b-DTR transgenic mouse), Rat | Macrophages have distinct effect on liver injury and repair. Depletion of macrophages when liver fibrosis reduced scarring, while depletion during recovery led to a failure of matrix degradation. | Duffield, 2010 [25] |
| M1/M2 | NA | Mouse | Both IFN-γ and IL-4 can increase MHC-II on macrophages. | Cao et al., 1989 [29] |
NA, not available
Characteristics of Macrophage polarization: pro- and anti-inflammatory
Macrophages demonstrate the ability to either promote or suppress inflammation [3, 14]. Pro-inflammatory stimuli-induced macrophages (“M1”) possess pro-inflammatory features, while anti-inflammatory cytokines such as IL-4 or transforming growth factor (TGF)-β-induced macrophages (“M2”) display a more healing phenotype, helping to develop connective/scar tissue and reduce cellular apoptosis[3].
In order to create a pro-inflammatory response, “M1” macrophages release a series of cytokines and free radicals[3]. These cytokines can serve multiple functions, such as causing apoptosis, or causing a further influx of macrophages. Tumor necrosis factor (TNF)-α, for example, causes cell death in the tissues, such as kidney, lung or liver [3, 32]. Soluble Fas ligand, released by macrophages, cause neutrophil apoptosis [3]. Other cytokines, such as monocyte chemotactic protein (MCP)-1, attract more macrophages to the injury site[33]. In in vitro studies, pro-inflammatory macrophages cultured together with myofibroblasts produce collagenases, which reduce tissue integrity[3].
Inflammation mediated by “classically” activated macrophage is indispensable for the protection against invasion. However, excessive inflammation might also be harmful to the host. Several mechanisms have been employed to limit excessive inflammation. IL-4-mediated “alternative” activation of macrophages was reported to be one of the most important mechanisms to attenuate excessive inflammation. While macrophages have the ability to clear away cells at the injury sites, sometimes with too much vigor, they can also aid in wound healing, help to ameliorate inflammation that macrophages are generally associated with.
One function of these inflammation-resolving macrophages is to create new ECM[3]. In in vitro studies, the co-culturing of anti-inflammatory macrophages and myofibroblasts resulted in greater production of fibronectin, collagen I (if stimulated by IL-4), and TGF-β[3]. Additionally, anti-inflammatory macrophages significantly increase the expression of tissue transglutaminase, which is vital toward the protection of matrix proteins from breaking down by proteases[34]. Rodent models have demonstrated that under injury to the cardiovascular and dermal systems, macrophages can also produce certain matrix protein[35]. One type of matrix protein, osteopontin, produced by macrophages during the initial stage of tissue injury, helps recruit more macrophages while down-regulating enzymes that break down the matrix[3]. In addition to mediating host defense against infection by phagocytosis and inflammatory cytokine secretion, alveolar macrophages have been reported to be able to attenuate polymorphonuclear cells (PMN)-mediated inflammation and thus reduce mortality in murine pneumococcal pneumonia model[36]. Boven and coworkers reported that macrophages acquired anti-inflammatory function after ingestion of myelin in multiple sclerosis patients [37]. Myelin-containing macrophages expressed a series of anti-inflammatory molecules and were unable to respond to inflammatory stimuli[37]. It’s also reported that sphingosine-1-phosphate (S1P) confers anti-inflammatory function on macrophages via S1P1 receptor. Treatment with S1P or S1P1 receptor-specific agonist suppressed inflammatory response of macrophages to LPS[38].
Other than their direct anti-inflammatory activity, “M2” macrophages were also reported to be able to induce other anti-inflammatory cells. Savage reported that “M2” macrophages co-cultured with T cells have regulatory properties[39]. “M2” macrophages also induced T regulatory (Treg) cells expressing membrane bound TGF-β1 that regulates T cell activation[39].
M1/M2 imbalance in Diabetes
Macrophage-mediated inflammation plays an important role in the development of insulin resistance. A much higher level of macrophage infiltration in adipose tissue was found in obese subjects compared with that of lean subjects and appears to be the major mediator of inflammation[40]. Nevertheless, different activation of macrophages has distinct effect on insulin resistance.
“M1” promotes adipose inflammation and results in insulin resistance. In obesity, adipocytes release pro-inflammatory cytokines and fatty acids to induce classical activation of macrophages. “M1” macrophages secrete more pro-inflammatory cytokines such as TNFα and MCP-1 to induce a chronic low grade inflammation in tissues (such as liver, muscle, and adipose tissue). In addition to pro-inflammatory cytokines that enhance adipose inflammation and thus result in insulin resistance, “M1” macrophages also release resistin, an adipokine that contributes to insulin resistance[41]. The adipocyte-derived resistin, which was initially discovered as an adipokine released by adipocyte[42], was confirmed to be able to cause insulin resistance in rodent models[42–44]. Resistin was then reported to be secreted by LPS-induced macrophages and macrophage-derived resistin that can exacerbate adipose inflammation and insulin resistance in mice[41, 45]. We also found that “M1”/“M2” imbalance was associated with air pollution-induced insulin resistance[46, 47]. Air pollution could increase the infiltration of macrophages with “M1” phenotype in adipose tissue and promote insulin resistance[46, 47].
On the contrary, “M2” macrophages antagonize “M1” macrophages and tune adipose inflammation to reduce insulin resistance. “M2” macrophages express very low concentrations of Nos2, MHC class and IL-12[48]. Not only do “M2” macrophages express lower levels of pro-inflammatory cytokines which are typically seen in “M1” macrophages, but also they express higher level of IL-10[48]. Anti-inflammatory macrophages increase migration when attracted by Th2 cytokines, such as IL-4 and IL-13[48]. Under normal circumstances, adipocytes and hepatocytes produce IL-4 and IL-13 and induce alternative activation of macrophages to limit inflammation and arrest insulin resistance[49]. Engagement of IL-4 to its receptor (IL-4R) induces a signaling cascade of Janus kinase (JAK) family/signal transducer and activator of transcription 6 (STAT6), insulin receptor substrate (IRS) family/phosphoinositide 3-kinase (PI3K) pathway[50, 51]. After binding to IL-4, IL-4Rα formed a dimer with either common γc chain or IL-13Rα, followed by activation of JAK or IRS. Phosphorylated JAK activates STAT6 and initiates the transcription of target genes, whereas phosphorylated IRS activates PI3K[52–54]. In addition to JAK/STAT and PI3K pathways, nuclear receptors peroxisome proliferator-activated receptors (PPARs) are also involved in the “alternative” activation of macrophages. PPARγ controls the metabolic processes in macrophages induced by IL-4. PPARγ deficiency shifted macrophages from “M2” to “M1” and thus promoted insulin resistance and glucose intolerance in mice[55–57]. Alternative activation of macrophages also can be promoted by PPARδ and thus modulate obesity-induced insulin resistance[49, 58]. PPARδ regulates the expression of arginase 1, costimulatory molecules, and pattern recognition receptors such as Mrc1 and Clec7a[58]. IL-4/STAT6 activation could also inhibits PPARα and attenuates adipose inflammation[59]. Disruption of STAT6 enhanced PPARα-driven program of oxidative metabolism and decreased insulin action[59]. Cintra et al. reported that suppression of M2 cytokine IL-10 by either neutralizing Ab or antisense oligonucleotide increased hepatic inflammation and reduced insulin signal transduction[60], suggesting M2 plays a role in modulating inflammation and insulin resistance.
Taken together, M1 and M2 play distinct roles in the pathogenesis of diabetes. The imbalance of M1/M2 results in the dysregulation of adipose inflammation and metabolic dysfunction, which subsequently promote insulin resistance.
Neutrophil differentiation
Neutrophils are short-lived leukocytes that share the same bone marrow myeloid progenitor/precursor with monocytes. Precursor cells can be stimulated to differentiate into mature neutrophils through signals from granulocyte macrophage colony stimulating factor (GM-CSF)[61]. Neutrophils are thought to be very important to early immune response. As the first leukocyte population to be recruited to the sites of inflammation upon inflammatory stimuli, neutrophils constitute the first line of defense against foreign antigens and insults. Other functions of neutrophils include the release of cytokines to attract other immune cells[2].
Recently, Fridlender and co-workers reported that neutrophils can differentiate into two distinct subsets of “N1” and “N2” under different environments[62]. This classification has been proposed for only a short time period and only a few studies have focused on the anti-inflammatory “N2” neutrophils (Table 2). Pro-inflammatory neutrophil phenotyped as “N1” seems to occur only in areas close to the stimulus. Isolation of the spleen indicated no statistical difference between the SM-16 (N1) fed mice and control (not N1) fed mice, which signifies that “N1” neutrophils are produced triggered by the stimulus, rather than systemically[62]. Conversely, when the K-ras mutation was activated in adenocarcinoma in the lung of mice, neutrophil count was raised to 43%, confirming that neutrophil “N1” activation is a localized event[62].
Table II.
neutrophil subpopulations in diseases
| N1/N2 | Disease Model | Subjects | Key Findings | References |
|---|---|---|---|---|
| N1 | Tuberculous pleurisy | Guinea pigs | TGF-β neutralization increases neutrophils in pleural exudate. | Allen et al., 2008 [69] |
| N1 | Tumor | Mouse | TGF-β blockade reduces tumor metastasis. | Kazemfar et al., 2009 [70] |
| N2 | NA | Human | Regulatory T cells inhibit neutrophils survival and their ROS and cytokine production, which is confirmed at least partially mediated by and IL-10. | Lewkowicz et al., 2006 [65] |
| N2 | NA | Human | TGF-β1 suppresses neutrophil degranulation to prevent them from initiating an inflammatory response. | Shen et al., 2007 [64] |
| N2 | Stroke | Mouse | PPAR-γ activation with rosiglitazone induces “N2” neutrophils. | Cuartero et al. 2013 [63] |
| N/N2 | Tumor | Mouse | Blockade of TGF-β promotes tumor-associated neutrophils with an anti-tumor phenotype, which is referred as N1. TGF-β in tumor microenvironment induces a pro-tumor neutrophils, N2. | Fridlender et al., 2009 [62] |
NA, not available
In the presence of TGF-β, however, neutrophils adopt a different role in chronic inflammation and tumors. TGF-β stimulation is largely responsible for the neutrophils to differentiation into the “N2”, a pro-tumor phenotype. This phenotype not only has a different function but also physiologically different in response to other cytokines. First, upon differentiation these neutrophils have a circular polynuclear chain. Second, “N2” phenotype neutrophils seem to respond to arginase, MCP-1 and RANTES (regulated upon activation, normal T cell expressed and secreted)[62]. A recent study by Cuartero suggested that PPARγ activation by rosiglitazone could also lead to the differentiation of “N2” with expression of M2 marker such as Ym1 and CD206[63]. While the cellular physiology and cytokine response has been elucidated, it is currently impossible to determine whether these “N2” phenotype cells originate from the spleen and then attracted to the area, or whether local differentiation occurred due to the lack of obvious markers[62]. So while some information is known about the characterization of pro-tumor neutrophils, little is known about their origins.
Neutrophil “N1” versus “N2”
Recently, the multifunctional abilities of neutrophils have drawn attention. It has become apparent that neutrophils also have the ability to aid or hinder the progression of inflammation. Other than its classical function of anti-tumor, neutrophils acquired protumorigenic phenotype after treated by TGF-β. Mirroring the nomenclature of “M1”/”M2” macrophages, Fridlender classified neutrophils into 2 subtype: “N1” (similar to “M1”) and “N2” (similar to “M2”) [62].
Fridlender reported that “N2” driven by TGF-β acquired pro-tumor phenotype[62]. Functionally, “N2” neutrophils promote the growth of tumors and chronic inflammation. These neutrophils produce high levels of arginase, which inhibits T cell activity and thus prevents binding to the FAS ligand[62]. Shen and coworkers reported that as low as 1 pg/ml TGF-β pretreatment can inhibit neutrophil degranulation, but does not affect their chemotaxis in response to IL-8. They postulated that was the reason why increased infiltrating neutrophils in the endometrium during menses do not initiate inflammatory response or release degradative enzymes[64]. CD4+CD25+ regulatory T cell, a major TGF-β-producing cell, suppresses ROS and cytokine production by neutrophils. Treg also abolishes LPS-induced neutrophil survival and promotes their death. Those anti-inflammatory effects on neutrophils are confirmed at least partially mediated by TGF-β and IL-10[65]. Depletion of “N2” neutrophils by blockade of TGF-β increased activated CD8+ T cells[62]. This suggests that pro-tumor neutrophils are also immunosuppressive[62]. With “N2” strongly promoting tumors, neutrophils demonstrate their ability to carry out two opposing roles in immunity, much like macrophages.
Therefore, neutrophils may also have the ability to be differentiated into two distinct subsets: N1 and N2, and exert distinct functions in inflammation.
Neutrophil responses: pro- and anti-inflammatory
Neutrophils provide a key initial immune response to infection, including releasing various inflammatory cytokines and eicosanoids, and generating of reactive oxygen species (ROS) via a respiratory burst[66]. They can also induce endothelial damage and regulate vascular permeability at the site of infection[67]. The primary role of the neutrophil in the immune response is to inhibit bacterial growth until adaptive immune responses can be initiated. However, in some case, such as immune complex tissue deposition, can promote abnormal neutrophil activation, leading to enhanced inflammation and result in tissue damage[68].
Prior menses, neutrophils infiltrated into the endometrium of uterus significantly increased. However, increased neutrophils in endometrium do not initiate inflammatory responses. TGF-β that is produced by endometrial stromal and epithelial cells was reported to be able to induce a low degranulation phenotype on neutrophils, demonstrating not all neutrophils possess a high pro-inflammatory capability[64]. It has been suggested that blocking TGF-β pathway increases the proportion of neutrophils in serum of chronic diseases, such as inflammation[69]. With this in mind, Fridlender administered type I TGF-β receptor (Alk-5/Ak-4) kinase inhibitor (SM16) to mice to investigate changes in myeloid cells[62]. Administration of SM16 increased the percentage of CD11b+ Ly6G+ cells (neutrophils) in the tumors. Furthermore, neutrophil depletion (80–90%) by 1A8 antibody in SM-16 mice lead to dramatically increased tumor growth[62]. However, after systemic neutrophils reloaded, the SM-16 mice, once again, saw stunted tumor growth. This treatment was then tested on local tumor growth. Again, the same result occurred: tumor growth increased[62]. Consistent with this study, another group also found that TGF-β blockage, which was proposed to be able to induce “N1” neutrophils, suppressed tumor metastases[70]. In vitro experiment demonstrated that CD11b+ cells (neutrophils) from mice treated with SM16 are capable of releasing cytotoxins to cancer cells at higher concentrations control neutrophils isolated from untreated mice [62]. The mechanism by which these neutrophils adopt to destroy cells appears to be through ROS. When the cells are subject to superoxide blocking, the percentage of cell death in the tumor significantly dropped below that of the control group[62]. Moreover, mice fed with SM16 were found to have increased levels of iNOS and decreased levels of arginase. In total, the mRNA expressions of 5 neutrophil chemoattractants were upregulated (MIP-2a/CXCL2, LIX/CXCL5, MIP1a/CCL3, KC/CXCL1 and GM-CSF). Physiologically, these neutrophils are characterized by being more lobulated and hypersegmented[62]. In addition, these neutrophils have lost the circular nuclei shape, unlike the majority of other types of leukocytes.
N1/N2 imbalance in type 2 Diabetes
It has been considered that the primary cell type responsible for inflammation in type 2 diabetes is the inflammatory macrophage in adipose tissues[40]. However, early during the course of diet-induced obesity in mice, neutrophils have also been shown to be recruited to adipose tissues[71] (Elgazar-Carmon et al., 2008). Recent studies reported that neutrophils[72–74] and elastase[75] secreted by neutrophils dramatically increased in adipose tissue of high-fat diet induced obese mice (Talukdar et al., 2012). Furthermore, the pharmacological inhibition or genetic deletion of elastase led to improved insulin sensitivity and inflammation[75, 76](Talukdar et al., 2012). Based on these studies, neutrophils also play an important role in diabetes. Given the fact that high levels of TGF-β-producing Tregs were found in adipose tissue of lean subjects and obesity significantly reduced Treg numbers in adipose tissue[77], it is possible that TGF-β-induced N2 is dominant in adipose tissue of lean individuals while obesity may skew the polarization of neutrophils towards N1 (Figure 1). However, does N1/N2 imbalance indeed occur in obesity/diabetes? What’s the role of N1 and N2 neutrophil in diabetes? Further studies are required to address these questions.
Conclusions
As the major innate immune cells, monocytes and neutrophils are not only essential to inflammatory responses against invading pathogens and tumor cells but also responsible for tuning excessive inflammation, tissue remodeling and tissue repair. Those diverse effects are mediated by distinct subpopulations of monocytes and neutrophils. “Classically” activated macrophages “M1” and neutrophils “N1” are capable of mediating inflammation and tissue destruction, while “alternatively” activated macrophages “M2” and neutrophils “N2” function to tune inflammatory response and initiate tissue repair. Understanding the diverse subpopulations and activities of those cells is required to reveal the nature of various inflammatory diseases and develop more efficacious therapeutic approaches for human diseases.
Acknowledgments
This work was supported by NIH grants ES016588, ES017412, and ES018900 to Dr. Sun.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Henderson RB, et al. Rapid recruitment of inflammatory monocytes is independent of neutrophil migration. Blood. 2003;102(1):328–35. doi: 10.1182/blood-2002-10-3228. [DOI] [PubMed] [Google Scholar]
- 2.Petrofsky M, Bermudez LE. Neutrophils from Mycobacterium avium-infected mice produce TNF-alpha, IL-12, and IL-1 beta and have a putative role in early host response. Clinical Immunology. 1999;91(3):354–8. doi: 10.1006/clim.1999.4709. [DOI] [PubMed] [Google Scholar]
- 3.Duffield JS. The inflammatory macrophage: a story of Jekyll and Hyde. Clin Sci (Lond) 2003;104(1):27–38. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 4.Soehnlein O, et al. Neutrophil secretion products pave the way for inflammatory monocytes. Blood. 2008;112(4):1461–71. doi: 10.1182/blood-2008-02-139634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soehnlein O, et al. Neutrophil secretion products regulate anti-bacterial activity in monocytes and macrophages. Clinical and Experimental Immunology. 2008;151(1):139–45. doi: 10.1111/j.1365-2249.2007.03532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soehnlein O, et al. Neutrophil primary granule proteins HBP and HNP1–3 boost bacterial phagocytosis by human and murine macrophages. J Clin Invest. 2008;118(10):3491–502. doi: 10.1172/JCI35740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Volkman A, Gowans JL. The Origin of Macrophages from Bone Marrow in the Rat. Br J Exp Pathol. 1965;46:62–70. [PMC free article] [PubMed] [Google Scholar]
- 8.Sunderkotter C, et al. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. Journal of Immunology. 2004;172(7):4410–7. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- 9.Ebert RH, Florey HW. The extravascular development of the monocyte observed in vivo. Brit J Exp Pathol. 1939;20:342–56. [Google Scholar]
- 10.Van Furth R, et al. Quantitative study on the production and kinetics of mononuclear phagocytes during an acute inflammatory reaction. Journal of Experimental Medicine. 1973;138(6):1314–30. doi: 10.1084/jem.138.6.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laskin DL, et al. Macrophages and tissue injury: agents of defense or destruction? Annu Rev Pharmacol Toxicol. 2011;51:267–88. doi: 10.1146/annurev.pharmtox.010909.105812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenberger CM, Finlay BB. Phagocyte sabotage: disruption of macrophage signalling by bacterial pathogens. Nat Rev Mol Cell Biol. 2003;4(5):385–96. doi: 10.1038/nrm1104. [DOI] [PubMed] [Google Scholar]
- 13.Goren I, et al. A transgenic mouse model of inducible macrophage depletion: effects of diphtheria toxin-driven lysozyme M-specific cell lineage ablation on wound inflammatory, angiogenic, and contractive processes. Am J Pathol. 2009;175(1):132–47. doi: 10.2353/ajpath.2009.081002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5(12):953–64. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 15.Laskin DL, Pendino KJ. Macrophages and Inflammatory Mediators in Tissue-Injury. Annual Review of Pharmacology and Toxicology. 1995;35:655–77. doi: 10.1146/annurev.pa.35.040195.003255. [DOI] [PubMed] [Google Scholar]
- 16.Crowther M, et al. Microenvironmental influence on macrophage regulation of angiogenesis in wounds and malignant tumors. J Leukoc Biol. 2001;70(4):478–90. [PubMed] [Google Scholar]
- 17.Danenberg HD, et al. Macrophage depletion by clodronate-containing liposomes reduces neointimal formation after balloon injury in rats and rabbits. Circulation. 2002;106(5):599–605. doi: 10.1161/01.cir.0000023532.98469.48. [DOI] [PubMed] [Google Scholar]
- 18.Greenburg GB, Hunt TK. The proliferative response in vitro of vascular endothelial and smooth muscle cells exposed to wound fluids and macrophages. J Cell Physiol. 1978;97(3 Pt 1):353–60. doi: 10.1002/jcp.1040970310. [DOI] [PubMed] [Google Scholar]
- 19.Moldovan NI, et al. Contribution of monocytes/macrophages to compensatory neovascularization: the drilling of metalloelastase-positive tunnels in ischemic myocardium. Circ Res. 2000;87(5):378–84. doi: 10.1161/01.res.87.5.378. [DOI] [PubMed] [Google Scholar]
- 20.Polverini PJ, et al. Activated macrophages induce vascular proliferation. Nature. 1977;269(5631):804–6. doi: 10.1038/269804a0. [DOI] [PubMed] [Google Scholar]
- 21.Duffield JS. Macrophages and immunologic inflammation of the kidney. Semin Nephrol. 2010;30(3):234–54. doi: 10.1016/j.semnephrol.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murray LA, et al. TGF-beta driven lung fibrosis is macrophage dependent and blocked by Serum amyloid P. Int J Biochem Cell Biol. 2011;43(1):154–62. doi: 10.1016/j.biocel.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 23.Sung SA, et al. Reduction of renal fibrosis as a result of liposome encapsulated clodronate induced macrophage depletion after unilateral ureteral obstruction in rats. Nephron Exp Nephrol. 2007;105(1):e1–9. doi: 10.1159/000096859. [DOI] [PubMed] [Google Scholar]
- 24.Frid MG, et al. Hypoxia-induced pulmonary vascular remodeling requires recruitment of circulating mesenchymal precursors of a monocyte/macrophage lineage. Am J Pathol. 2006;168(2):659–69. doi: 10.2353/ajpath.2006.050599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duffield JS, et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115(1):56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Metchnikoff E. Immunity in the Infectious Diseases. New York: Macmillan; 1905. [Google Scholar]
- 27.Mosmann TR, Coffman RL. Th1-Cell and Th2-Cell - Different Patterns of Lymphokine Secretion Lead to Different Functional-Properties. Annual Review of Immunology. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 28.Nathan CF, et al. Identification of Interferon-Gamma as the Lymphokine That Activates Human Macrophage Oxidative-Metabolism and Anti-Microbial Activity. Journal of Experimental Medicine. 1983;158(3):670–89. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao H, et al. Differential Regulation of Class-Ii Mhc Determinants on Macrophages by Ifn-Gamma and Il-4. Journal of Immunology. 1989;143(11):3524–31. [PubMed] [Google Scholar]
- 30.Stein M, et al. Interleukin-4 Potently Enhances Murine Macrophage Mannose Receptor Activity - a Marker of Alternative Immunological Macrophage Activation. Journal of Experimental Medicine. 1992;176(1):287–92. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wirth JJ, et al. Effects of Il-4 on Macrophage Functions - Increased Uptake and Killing of a Protozoan Parasite (Trypanosoma-Cruzi) Immunology. 1989;66(2):296–301. [PMC free article] [PubMed] [Google Scholar]
- 32.Dibazar F, et al. Simvastatin decreases hepatic ischaemia/reperfusion-induced liver and lung injury in rats. Folia Morphol (Warsz) 2008;67(4):231–5. [PubMed] [Google Scholar]
- 33.Yadav A, et al. MCP-1: chemoattractant with a role beyond immunity: a review. Clin Chim Acta. 2010;411(21–22):1570–9. doi: 10.1016/j.cca.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 34.Murtaugh MP, et al. Induction of tissue transglutaminase in mouse peritoneal macrophages. Journal of Biological Chemistry. 1983;258(18):11074–81. [PubMed] [Google Scholar]
- 35.Murry CE, et al. Macrophages express osteopontin during repair of myocardial necrosis. Am J Pathol. 1994;145(6):1450–62. [PMC free article] [PubMed] [Google Scholar]
- 36.Knapp S, et al. Alveolar macrophages have a protective antiinflammatory role during murine pneumococcal pneumonia. Am J Respir Crit Care Med. 2003;167(2):171–9. doi: 10.1164/rccm.200207-698OC. [DOI] [PubMed] [Google Scholar]
- 37.Boven LA, et al. Myelin-laden macrophages are anti-inflammatory, consistent with foam cells in multiple sclerosis. Brain. 2006;129(Pt 2):517–26. doi: 10.1093/brain/awh707. [DOI] [PubMed] [Google Scholar]
- 38.Hughes JE, et al. Sphingosine-1-phosphate induces an antiinflammatory phenotype in macrophages. Circ Res. 2008;102(8):950–8. doi: 10.1161/CIRCRESAHA.107.170779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Savage ND, et al. Human anti-inflammatory macrophages induce Foxp3+ GITR+ CD25+ regulatory T cells, which suppress via membrane-bound TGFbeta-1. Journal of Immunology. 2008;181(3):2220–6. doi: 10.4049/jimmunol.181.3.2220. [DOI] [PubMed] [Google Scholar]
- 40.Olefsky JM, Glass CK. Macrophages, Inflammation, and Insulin Resistance. Annual Review of Physiology. 2010;72:219–46. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 41.Lehrke M, et al. An inflammatory cascade leading to hyperresistinemia in humans. Plos Medicine. 2004;1(2):e45. doi: 10.1371/journal.pmed.0010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steppan CM, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409(6818):307–12. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 43.Qi Y, et al. Loss of resistin improves glucose homeostasis in leptin deficiency. Diabetes. 2006;55(11):3083–90. doi: 10.2337/db05-0615. [DOI] [PubMed] [Google Scholar]
- 44.Banerjee RR, et al. Regulation of fasted blood glucose by resistin. Science. 2004;303(5661):1195–8. doi: 10.1126/science.1092341. [DOI] [PubMed] [Google Scholar]
- 45.Qatanani M, et al. Macrophage-derived human resistin exacerbates adipose tissue inflammation and insulin resistance in mice. J Clin Invest. 2009 doi: 10.1172/JCI37273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun Q, et al. Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity. Circulation. 2009;119(4):538–46. doi: 10.1161/CIRCULATIONAHA.108.799015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu X, et al. Effect of early particulate air pollution exposure on obesity in mice: role of p47phox. Arterioscler Thromb Vasc Biol. 2010;30(12):2518–27. doi: 10.1161/ATVBAHA.110.215350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fong CH, et al. An antiinflammatory role for IKKbeta through the inhibition of “classical” macrophage activation. Journal of Experimental Medicine. 2008;205(6):1269–76. doi: 10.1084/jem.20080124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kang K, et al. Adipocyte-derived Th2 cytokines and myeloid PPARdelta regulate macrophage polarization and insulin sensitivity. Cell Metabolism. 2008;7(6):485–95. doi: 10.1016/j.cmet.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kelly-Welch AE, et al. Interleukin-4 and interleukin-13 signaling connections maps. Science. 2003;300(5625):1527–8. doi: 10.1126/science.1085458. [DOI] [PubMed] [Google Scholar]
- 51.Leonard WJ, O’Shea JJ. JAKS AND STATS: Biological implications. Annual Review of Immunology. 1998;16:293–322. doi: 10.1146/annurev.immunol.16.1.293. [DOI] [PubMed] [Google Scholar]
- 52.Chomarat P, Banchereau J. Interleukin-4 and interleukin-13: their similarities and discrepancies. Int Rev Immunol. 1998;17(1–4):1–52. doi: 10.3109/08830189809084486. [DOI] [PubMed] [Google Scholar]
- 53.Nelms K, et al. The IL-4 receptor: Signaling mechanisms and biologic functions. Annual Review of Immunology. 1999;17:701–38. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- 54.Martinez FO, et al. Alternative Activation of Macrophages: An Immunologic Functional Perspective. Annual Review of Immunology. 2009;27:451–83. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 55.Odegaard JI, et al. Macrophage-specific PPAR gamma controls alternative activation and improves insulin resistance. Nature. 2007;447(7148):1116–U12. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hevener AL, et al. Macrophage PPAR gamma is required for normal skeletal muscle and hepatic insulin sensitivity and full antidiabetic effects of thiazolidinediones. J Clin Invest. 2007;117(6):1658–69. doi: 10.1172/JCI31561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bouhlel MA, et al. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metabolism. 2007;6(2):137–43. doi: 10.1016/j.cmet.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 58.Odegaard JI, et al. Alternative M2 activation of Kupffer cells by PPAR delta ameliorates obesity-induced insulin resistance. Cell Metabolism. 2008;7(6):496–507. doi: 10.1016/j.cmet.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ricardo-Gonzalez RR, et al. IL-4/STAT6 immune axis regulates peripheral nutrient metabolism and insulin sensitivity. Proc Natl Acad Sci U S A. 2010;107(52):22617–22. doi: 10.1073/pnas.1009152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cintra DE, et al. Interleukin-10 is a protective factor against diet-induced insulin resistance in liver. J Hepatol. 2008;48(4):628–37. doi: 10.1016/j.jhep.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 61.Marturana F, et al. Short-term exposure of umbilical cord blood CD34(+) cells to granulocyte-macrophage colony-stimulating factor early in culture improves ex vivo expansion of neutrophils. Cytotherapy. 2010 doi: 10.3109/14653249.2010.518610. [DOI] [PubMed] [Google Scholar]
- 62.Fridlender ZG, et al. Polarization of Tumor-Associated Neutrophil Phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16(3):183–94. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cuartero MI, et al. N2 neutrophils, novel players in brain inflammation after stroke: modulation by the PPARgamma agonist rosiglitazone. Stroke. 2013;44(12):3498–508. doi: 10.1161/STROKEAHA.113.002470. [DOI] [PubMed] [Google Scholar]
- 64.Shen L, et al. Inhibition of human neutrophil degranulation by transforming growth factor-beta1. Clinical and Experimental Immunology. 2007;149(1):155–61. doi: 10.1111/j.1365-2249.2007.03376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lewkowicz P, et al. Lipopolysaccharide-activated CD4+CD25+ T regulatory cells inhibit neutrophil function and promote their apoptosis and death. Journal of Immunology. 2006;177(10):7155–63. doi: 10.4049/jimmunol.177.10.7155. [DOI] [PubMed] [Google Scholar]
- 66.Malech HL. The role of neutrophils in the immune system: an overview. Methods Mol Biol. 2007;412:3–11. doi: 10.1007/978-1-59745-467-4_1. [DOI] [PubMed] [Google Scholar]
- 67.Weiss SJ, et al. Role of hydrogen peroxide in neutrophil-mediated destruction of cultured endothelial cells. J Clin Invest. 1981;68(3):714–21. doi: 10.1172/JCI110307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marzocchi-Machado CM, et al. Fcgamma and complement receptors: expression, role and co-operation in mediating the oxidative burst and degranulation of neutrophils of Brazilian systemic lupus erythematosus patients. Lupus. 2002;11(4):240–8. doi: 10.1191/0961203302lu172oa. [DOI] [PubMed] [Google Scholar]
- 69.Allen SS, et al. Altered inflammatory responses following transforming growth factor-beta neutralization in experimental guinea pig tuberculous pleurisy. Tuberculosis (Edinb) 2008;88(5):430–6. doi: 10.1016/j.tube.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 70.Kazemfar K, et al. Combined IL-8 and TGF-beta blockade efficiently prevents neutrophil infiltrates into an A549-cell tumor. Immunol Lett. 2009;122(1):26–9. doi: 10.1016/j.imlet.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 71.Elgazar-Carmon V, et al. Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J Lipid Res. 2008;49(9):1894–903. doi: 10.1194/jlr.M800132-JLR200. [DOI] [PubMed] [Google Scholar]
- 72.Nijhuis J, et al. Neutrophil activation in morbid obesity, chronic activation of acute inflammation. Obesity (Silver Spring) 2009;17(11):2014–8. doi: 10.1038/oby.2009.113. [DOI] [PubMed] [Google Scholar]
- 73.Andrade VL, et al. Evaluation of plasmatic MMP-8, MMP-9, TIMP-1 and MPO levels in obese and lean women. Clin Biochem. 2012;45(6):412–5. doi: 10.1016/j.clinbiochem.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 74.Trellakis S, et al. Low adiponectin, high levels of apoptosis and increased peripheral blood neutrophil activity in healthy obese subjects. Obes Facts. 2012;5(3):305–18. doi: 10.1159/000339452. [DOI] [PubMed] [Google Scholar]
- 75.Talukdar S, et al. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat Med. 2012;18(9):1407–12. doi: 10.1038/nm.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mansuy-Aubert V, et al. Imbalance between neutrophil elastase and its inhibitor alpha1-antitrypsin in obesity alters insulin sensitivity, inflammation, and energy expenditure. Cell Metab. 2013;17(4):534–48. doi: 10.1016/j.cmet.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Feuerer M, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15(8):930–9. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Abramson SL, Gallin JI. IL-4 inhibits superoxide production by human mononuclear phagocytes. J Immunol. 1990;144(2):625–30. [PubMed] [Google Scholar]