Abstract
Background
The OX40/OX40L interaction contributes to an optimal T cell response following allergic stimuli and plays an important role in the maintenance and reactivation of memory T effector cells.
Objective
We tested whether treatment with an anti-OX40L monoclonal antibody (MAb) would inhibit allergen-induced responses in subjects with asthma.
Methods
Twenty-eight mild, atopic asthmatic subjects were recruited for a double-blind, randomized, placebo-controlled, parallel-group trial (ClinicalTrials.gov identifier NCT00983658) to compare blockade of OX40L using a humanized anti-OX40L MAb to placebo-administered intravenously in 4 doses over 3 months. Allergen inhalation challenges were carried out 56 and 113 days after the first dose of study drug. The primary outcome variable was the late-phase asthmatic response. Other outcomes included the early-phase asthmatic response, airway hyperresponsiveness, serum IgE levels, blood and sputum eosinophils, safety and tolerability.
Results
Treatment with anti-OX40L MAb did not attenuate the early- or late-phase asthmatic responses at days 56 or 113 compared with placebo. In the anti-OX40L MAb treatment group, total IgE was reduced 17% from pre-dosing levels, and sputum eosinophils decreased 75% by day 113 (both P = 0.04). There was no effect of anti-OX40L MAb on airway hyperresponsiveness or blood eosinophils. The frequency of AEs was similar in both groups.
Conclusion and Clinical Relevance
Pharmacological activity of anti-OX40L MAb was observed by decreases in serum total IgE and airway eosinophils at 16 weeks post-dosing, but there was no effect on allergen-induced airway responses. It is possible that the treatment duration or dose of antibody was insufficient to impact the airway responses.
Keywords: allergen inhalation challenge, anti-OX40L, mild atopic asthma, proof-of-activity
Introduction
Asthma is a complex chronic inflammatory disorder of the airways. While its cause is multifactorial, one factor predisposing to the development of the disease appears to be atopy, a propensity to make IgE, which is associated with T helper type 2 (Th2) CD4+ T cells.
OX40 (CD134) is a costimulatory receptor transiently expressed on activated T cells [1,2]. Its ligand, OX40L, is expressed on a broad range of cell types, including dendritic, vascular endothelial, natural killer, mast and B cells. OX40L is up-regulated on dendritic cells following stimulation with the IL-7-like cytokine thymic stromal lymphopoietin (TSLP), CD40L or toll-like receptor ligands [3]. The OX40–OX40L interaction contributes to an optimal T cell response and plays an important role in the maintenance and reactivation of memory T effector cells. OX40–OX40L interactions have also been implicated in the suppression of regulatory T cell function [4]. Importantly, OX40–OX40L expression is increased in the bronchial submucosa in mild asthma, but not in moderate-to-severe disease, and is related to the degree of tissue eosinophilia [5]. Interactions between OX40 and its ligand, OX40L therefore may play an important role in the generation and maintenance of Th2-type responses and in the development of allergic lung inflammation. Thus, blocking OX40–OX40L interactions potentially could down-modulate the allergic inflammation observed in asthmatic subjects.
Anti-OX40L is a recombinant, human monoclonal antibody of the IgG1 subclass which binds OX40L with high affinity and blocks OX40–OX40L interactions, resulting in inhibition of events downstream from OX40 signalling in T cells [6]. Anti-OX40L monoclonal antibody (MAb) shows in vitro effector function; thus, in addition to blockade of signalling, it is also capable of lysing OX40L-expressing cells by antibody-dependent cell cytotoxicity and complement-dependent cytotoxicity. Anti-OX40L MAb was shown to inhibit Th2 inflammation in a non-human primate model of asthma in vivo [6].
In the current study, pharmacological activity of inhibition of the OX40L–OX40 pathway was evaluated in subjects with mild allergic asthma using a model of allergen inhalation challenge.
Materials and methods
Subjects
Twenty-four mild atopic stable asthmatic subjects with a history of episodic wheeze and shortness of breath were recruited for a clinical trial (ClinicalTrials.gov identifier NCT00983658). Subjects were screened for the following inclusion and exclusion criteria; non-smoking, 18–65 years old, with body weight between 50 and 125 kg, forced expiratory volume in 1 s (FEV1) ≥70% of predicted and the provocative concentration of methacholine causing a 20% fall in FEV1 (methacholine PC20) ≤16 mg/mL (Table 1). Reversibility was not tested, as lung function was nearly normal. Subjects had no other lung disease, no self-reported lower respiratory tract infection or worsening of asthma for 8 weeks before screening. Sensitizing allergens were avoided with the exception of house dust mite. Subjects allergic to pollens were tested out of season. Subjects were steroid naïve and did not use any asthma medications with the exception of infrequently inhaled short-acting beta2-agonist, which was withheld for at least 8 h before spirometry. Rigorous exercise and caffeinated beverages were avoided before laboratory visits.
Table 1.
Subject characteristics. Data are shown as mean (standard deviation). FEV1; forced expiratory volume in one second. PC20 – provocative concentration causing a 20% fall in FEV1. EAR – maximum percentage change in FEV1 during the early asthmatic response 0–2 h post-allergen and LAR – maximum percentage change in FEV1 during the late asthmatic response 3–7 h post-allergen
| Group | Placebo | Anti-OX40L MAb |
|---|---|---|
| Age (years) | 33.9 (12.0) | 33.4 (13.3) |
| Gender (male/female) | 7/7 | 8/6 |
| FEV1 (% predicted) | 84.9 (14.7) | 91.7 (11.4) |
| Methacholine PC20 (mg/mL) | 0.79 (0.05–13.5) | 1.62 (0.3–11.6) |
| Inhaled Allergen | House Dust Mite – 5 | House Dust Mite – 6 |
| Cat – 4 | Cat – 6 | |
| Grass – 5 | Ragweed – 2 | |
| Screening EAR (% change) | −36.1 (7.58) | −39.28 (6.45) |
| Screening LAR (% change) | −27.23 (10.05) | −22.7 (7.06) |
Study design
This Phase II, double-blind, placebo-controlled, randomized, parallel-group study, conducted in 5 sites in Canada, enrolled subjects from October 2009 to April 2010. The study was approved by the Research Ethics Board of each institution, and signed informed consent was obtained from all subjects. The primary outcome variable was the late-phase allergen-induced asthmatic response, and the secondary outcome variables were the early-phase allergen-induced asthmatic response and airway hyperresponsiveness. Sputum eosinophils, serum IgE, pharmacokinetics and safety and tolerability of anti-OX40L MAb were also measured.
Screening of subjects occurred over 1–2 weeks with assessments of airway hyperresponsiveness, skin prick test, blood chemistry and haematology, and responses to inhaled allergen challenge. Subjects who developed an early-phase asthmatic response (a fall in FEV1 of at least 20% within 2 h post-allergen), followed by a late-phase asthmatic response (a fall in FEV1 of at least 15% between 3 and 7 h post-allergen) were randomized to receive anti-OX40L MAb or placebo in a 1:1 ratio.
The dosing regime was previously tested in Phase 1 studies in atopic allergic rhinitis with no safety concerns. Subjects received a loading dose of 8 mg/kg intravenously on Day 1 followed by monthly 4 mg/kg doses on Days 29, 57 and 85. Allergen challenges were conducted at Days 56 and 113 (2 and 4 months after the first dose, respectively). Methacholine PC20 was measured 24 h before and 24 h after allergen challenges, and sputum samples were induced at 24 h before, and again at 7 and 24 h after allergen challenges. Blood was collected for pharmacokinetic analysis at Days 1, 29, 56, 85, 113, 169 and 253. All subjects were followed for adverse events during the study and for 6 months after the last dose of study drug (Fig. 1). All subjects received a tetanus booster during the screening period.
Figure 1.
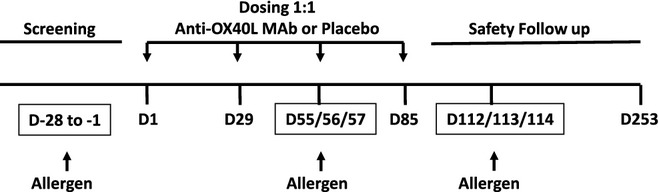
Study schematic.
Laboratory procedures
Study medication
The antibody was supplied as a sterile preservative-free, lyophilized powder for IV infusion and placebo contained the same excipients. Drug and placebo were prepared by an unblinded pharmacist who had no other involvement with the study. Subjects and study site personnel remained blinded to treatment assignment until the end of the follow-up visit at Day 197, and the sponsor remained blinded to treatment assignment until Day 113.
Methacholine inhalation test
Methacholine inhalation challenge was performed as described by Cockcroft [7] using tidal breathing from a Wright nebulizer. The test was terminated when a fall in FEV1 of at least 20% of the lowest postsaline value occurred, and the methacholine PC20 was calculated.
Allergen inhalation challenge
Allergen inhalation was performed as described by O'Byrne and colleagues [8]. The concentration of allergen extract for inhalation was determined from a formula described by Cockcroft and co-workers [9]. During a screening period, doubling concentrations of allergen were given until a ≥ 20% fall in FEV1 at 10 minutes post-allergen was reached. The FEV1 was then measured at regular intervals until 7 h after allergen inhalation. The same dose of allergen was to be administered for the two post-dosing allergen challenges at Day 56 and Day 113. The early and late asthmatic responses (EAR and LAR) were defined as the largest fall in FEV1 between 0 and 3 h, and 3 h to 7 h post-challenge, respectively.
Sputum analysis
Sputum was induced and processed using the method described by Pizzichini and co-workers [10]. Cells were prepared on glass slides for differential counts and stained with Diff Quik (American Scientific Products, McGaw Park, IL, USA). Cell counting was performed at one investigative site and included inflammatory cells and airway epithelial cells only.
Peripheral blood analyses
Whole blood was collected for measurements of eosinophils. Serum samples from all subjects were analysed for anti-OX40L MAb levels, total IgE and allergen-specific IgE. Peripheral blood mononuclear cells were isolated for frequency of antigen-specific IFNγ-secreting T cells (Supporting Information Data S1). Circulating DCs were phenotyped and measured in blood.
Statistical analysis
Activity analyses were based on a modified ITT population, consisting of randomized subjects who received at least one dose of study drug and had at least one post-baseline primary efficacy measurement. Data are presented as mean (SD) unless stated otherwise. The primary analysis was the treatment comparison of the area under the curve (AUC) for the late-phase asthmatic response on Day 113 between active drug and placebo. Treatment arms were compared using unpaired t-tests or Wilcoxon rank sum tests, as applicable. Methacholine PC20 values were log-transformed to fit a normal distribution before statistical analysis and presented as doubling dose change. ELIspot data were analysed by Wilcoxon signed-rank test and corrected for multiple comparisons.
Results
Sixty-three subjects were screened for the study and 28 subjects were enrolled. Fourteen subjects were randomized to anti-OX40L MAb and 14 subjects were randomized to placebo treatment. All subjects in the study received 4 doses of study treatment except for one patient in the placebo cohort who received 2 doses of placebo treatment. Four discontinued from the study; one subject randomized to anti-OX40L MAb (lost to follow-up) and 3 subjects randomized to placebo (one subject who received 2 doses of placebo developed moderate cellulitis; one subject withdrew from the study, and one was lost to follow-up after receiving 4 doses of placebo). Subject demographics were matched for age and gender, and baseline spirometry was similar (Table 1).
Allergen-induced asthmatic responses
Twenty-six subjects completed the Day 113 visit (12 placebo, 14 anti-OX40L MAb) and were included in the efficacy analyses. 23 subjects completed the Day 56 visit (9 placebo, 14 anti-OX40L MAb). Reasons for not completing allergen challenges included discontinuation from the study, serious adverse events (SAE) and unstable asthma.
The LAR AUC3–7 h measured during the late asthmatic responses in the anti-OX40L MAb, and placebo groups were comparable at screening, being 54.5 (SD 21.8)% × time and 58.9 (SD 22.2)% × time, respectively. There was no effect of anti-OX40L MAb on the LAR AUC3–7 h, with Day 56 means in the anti-OX40L MAb and placebo groups being 46.7 (32.6)% × time and 16.6 (20.0)% × time, respectively, (P >; 0.05) and the Day 113 means in the anti-OX40L MAb and placebo groups being 38.3 (SD 30.7)% × time and 23.6 (SD 31.8)% × time, respectively (P >; 0.05). There was no treatment effect on the EAR or LAR (Fig. 2) (P >; 0.05).
Figure 2.
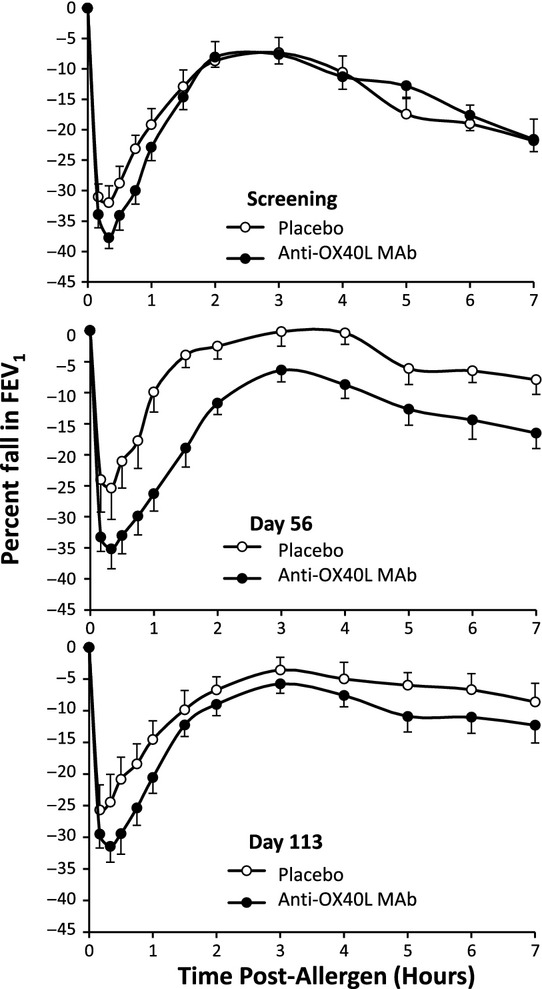
Mean (SEM) allergen-induced change in FEV1 during the screening period, and 56 and 113 days post-dosing with placebo and anti-OX40L MAb.
The EAR AUC0–2 h during the early asthmatic response in the anti-OX40L MAb and placebo groups were comparable at the screening visit, being 48.5 (SD 13.0)% × time and 41.5 (SD 13.0)% × time, respectively. There was no improvement by anti-OX40L MAb treatment on AUC0–2 h measured on Day 56, being 51.5 (SD 18.3)% × time vs. 26.3 (SD 19.7)% × time for placebo (P >; 0.05) or on Day 113, being 41.8 (SD 15.3)% × time vs. 32.2 (SD 19.1)% × time for placebo (P >; 0.05).
Airway hyperresponsiveness
There was no significant effect of anti-OX40L MAb treatment on measurements of baseline (pre-allergen) or allergen-induced airway hyperresponsiveness (P >; 0.05). The Day 56 allergen challenge reduced the methacholine PC20 by 1.08 (SD 1.20) doubling doses in the anti-OX40L MAb group and by 0.71 (SD 1.04) doubling doses in the placebo group, with a mean difference of 0.37 (95% CI − 0.66, 1.4). The allergen challenge conducted on Day 113 reduced the methacholine PC20 by 0.78 (SD 0.64) doubling doses in the anti-OX40L MAb group and by 0.02 (SD 1.01) doubling doses in the placebo group, with the mean difference (anti-OX40L MAb mean – placebo mean) of 0.76 (95% CI 0.05, 1.47).
Serum IgE
The level of total IgE measured at baseline was similar between groups. A significant reduction in median total IgE of 16.5% from baseline was observed on Day 113 in the anti-OX40L MAb-treated group versus a 14% increase in the placebo group (P = 0.04) (Fig. 3). Levels of serum total IgE showed trends of sustained reduction up to Day 253 but data were limited by a small sample size due to subjects lost to follow-up. Median levels of allergen-specific IgE showed trends for reduction in the anti-OX40L MAb group, being 8% and 20% on Days 169 and 253, respectively.
Figure 3.
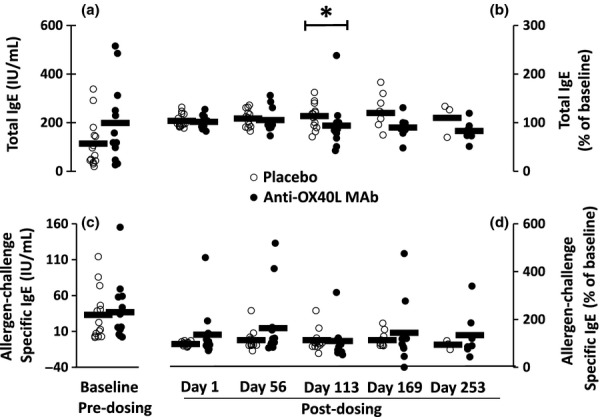
Mean (bars) and individual subject (circles) total IgE (a and b) and allergen-challenge-specific IgE (c and d) measured at baseline (a and c), and post-dosing, expressed as a percentage of baseline (b and d) with placebo (open circles) and anti-OX40L MAb (closed circles). *P < 0.05
Eosinophils
The baseline level of peripheral blood eosinophils was similar between anti-OX40L MAb and placebo groups with no difference between the 2 groups post-dosing (Fig. 4). Sputum was collected from subjects who could produce samples. Eosinophils in sputum were low in both groups at baseline. With anti-OX40L MAb treatment, there was a 75% median reduction in sputum eosinophils measured 112 days post-dosing (pre-allergen challenge), compared with 14% reduction in placebo (P = 0.04) (Fig. 5). There was an allergen-induced increase in the level of eosinophils measured in sputum; however, there were no differences in the level of sputum eosinophils between anti-OX40L MAb and placebo when measured at Days 56 and 113 (7 h post-allergen), or at Days 57 and 114 (24 h post-allergen) (data not shown).
Figure 4.
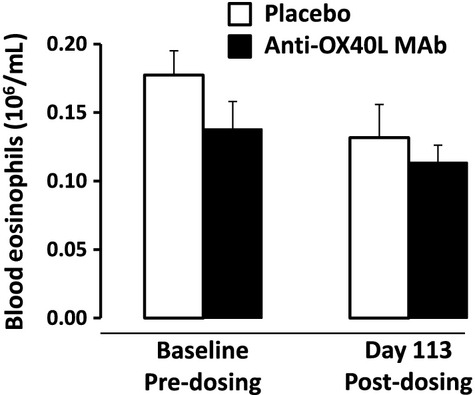
The mean (SEM) level of peripheral blood eosinophils measured at baseline and 113 days post-dosing.
Figure 5.
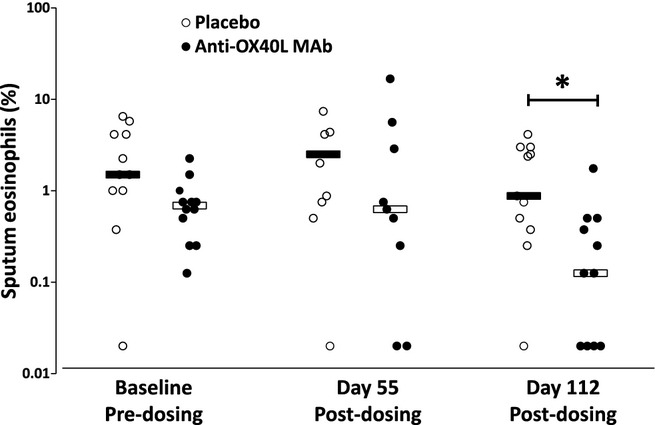
Median (bars) and individual (circles) sputum eosinophils measured at baseline and 56 and 112 days post-dosing with placebo (open circles) and anti-OX40L MAb (closed circles) treatment. *P < 0.05.
Pharmacokinetics
The mean elimination half-life of anti-OX40L MAb from serum was 28.5 (SD 6.01) days. (Supporting Information Data S1).
Frequency of T cells specific for sensitizing allergen and recall antigens
Frequencies of T cells were similar between placebo and active treatment groups at baseline (Supporting Information Figure S1).
Absolute numbers of circulating dendritic cells (DC)
Numbers of circulating myeloid DC type 1 (mDC1), myeloid DC type 2 (mDC2) and plasmacytoid DC (pDC) were similar between placebo and anti-OX40L MAb groups during screening, and no significant changes were observed between these groups during the treatment period (Supporting Information Figure S2).
Safety
Eight subjects (57.1%) who received anti-OX40L MAb experienced AEs and 13 (92.9%) subjects who received placebo experienced AEs. No severe adverse events were reported among subjects who received anti-OX40L MAb, and all subjects tested negative to antitherapeutic antibodies (Supporting Information Data S1).
Discussion
We conducted a proof-of-activity study using allergen inhalation challenge in mild atopic asthmatic subjects to evaluate the effects of blocking anti-OX40L on allergen-induced airway responses. There was no effect of anti-OX40L MAb treatment on allergen-induced early- and late-phase asthmatic responses, allergen-induced sputum eosinophils or methacholine airway hyperresponsiveness. In keeping with the proposed role of OX40–OX40L signalling, we observed a small but statistically significant reduction in serum total IgE with anti-OX40L MAb treatment, with a corresponding decrease in pre-allergen sputum eosinophils, indicating pharmacological activity of the anti-OX40L MAb. No changes in the frequency of T cells responding to sensitizing allergens or recall antigens were observed indicating that the treatment did not induce global suppression of T cell immunity.
In human disease, there is good evidence that TSLP is associated with asthma [11]. OX40L is expressed by dendritic cells following activation with TSLP. Ligation of OX40 on naïve CD4+ T cells plays a key role in allergic disease by inducing their differentiation into effector Th2 cells, which produce high levels of the Th2 cytokines IL-4, IL-5 and IL-13, [12,13], and thus can trigger allergic inflammatory Th2 immune responses. Expression of OX40 and OX40L has been shown to be increased in the bronchial submucosa in mild but not moderate-to-severe asthma, and its expression positively correlates with IL-4 and tissue eosinophils [5]. OX40L is also expressed on airway smooth muscle cells from asthmatic subjects and is up-regulated by TNF-alpha [14], suggesting that inflammatory cell interactions with OX40L on airway smooth muscle may induce functional consequences. In vitro experiments have shown that blocking OX40–OX40L interactions in cocultures of CD4+ T cells and TSLP-activated dendritic cells inhibits the production of Th2 cytokines [13]. Blockade of OX40–OX40L interactions also limits proliferation of Th2 memory cells by arresting these cells at the G0 phase of the cell cycle [15] highlighting the roles of OX40L in the induction of inflammatory Th2 cells and the maintenance of the Th2 memory cell pool. The current study evaluated several cytokines/chemokines and T cell subsets, including memory and effector T cells in the blood and saw no effect (data not shown). The lack of effect seen may be due to differences in the sampling site because most animal studies are able to assess airway samples and lymph tissue.
Animal models of allergic airways disease have been instrumental to identify TSLP and OX40–OX40L as critical elements for development of airway inflammation and hyper-reactivity. In murine models of asthma, OX40-deficient mice challenged with ovalbumin showed significantly lower levels of Th2 cytokines, total serum IgE, mucus secretion, decreased lung inflammation, reduced eosinophilia and goblet cell hyperplasia, and significantly attenuated airway hyper-reactivity compared with wild-type control mice [16–19].
An anti-OX40L-MAb has also been evaluated in a mouse model of asthma [17–20] with beneficial results. Administration of anti-OX40L MAb resulted in significant decreases in Th2 cytokines and antigen-specific serum IgE and IgG1 levels, and attenuated Th2 cytokines and infiltration of CD4+ T cells and eosinophils in BAL fluid of mouse and non-human primate models of asthma, demonstrating OX40L is a critical mediator in TSLP-induced allergic inflammation [6]. Of direct relevance to the current study where asthmatic subjects were challenged with an antigen to which they were already sensitized, administration of anti-OX40L Ab in mouse and non-human primate lead to a significant decrease in serum IgE levels, release of Th2 cytokines and reactivation and infiltration of memory CD4+ T cells during the recall response to antigen [6]. In the current study, we observed a reduction in total serum IgE 113 days after the first dose of anti-OX40L MAb, concurrent with lower sputum eosinophil counts. These findings are in keeping with the observed effects of OX40L blockade in animal models of asthma. Furthermore, the OX40 signalling pathway has been shown to play an important role in regulating CD4+ T cells in other TSLP-mediated inflammatory diseases, such as autoimmune encephalomyelitis [20,21].
Positive findings from preclinical studies have suggested that blockade of OX40 and OX40L in vivo could provide new therapeutic targets for inflammatory immunological disorders such as allergic asthma; however, investigations into the role of OX40–OX40L in humans is very limited, and mouse models cannot predict success of clinical trials in human asthma. Subjects enrolled in the study had mild asthma and thus had low eosinophil levels in blood and sputum. Our findings of reduced IgE and airway eosinophils in subjects treated with anti-OX40L MAb supports the notion that OX40–OX40L signalling contributes to the development of allergic inflammation, even though the effect that we observed is limited both in magnitude and duration. The effect of anti-OX40L MAb on IgE and airway eosinophils is not as marked as observations in murine models, and this can be attributed to the inherent large biological variation within research subjects with different duration of disease and sensitizing antigens, versus mice with the same background strain. Of note, blockade of costimulation by CD28, which leads to suboptimal activation of T cells, is effective in preventing antigen-induced airway inflammation and hyperresponsiveness in mouse models of asthma, but had no effect on BAL eosinophil levels in asthmatic subjects following segmental allergen challenge [22]. Collectively, these results suggest targeting T cell function and expansion in humans may not be as effective as in murine models and/or that recently discovered sources of Th2 cytokines, such as type 2 innate lymphocytes [23] are not affected by OX40L blockade. That we did not observe an effect of anti-OX40L MAb on measures of airway hyperresponsiveness at baseline or following allergen challenge, or an effect on allergen-induced bronchoconstriction suggests that the pathways downstream from OX40–OX40L signalling do not play a major role in the development of these physiological changes.
We acknowledge that there are several limitations when interpreting the data from this study. The dose regimen of anti-OX40L MAb in this study was chosen based on safety and efficacy considerations. The safety of this dose regimen was supported by previous testing of anti-OX40L MAb in single and multiple ascending dose Phase I studies in atopic allergic rhinitis patients, together with the safety profile provided by a 6-month non-clinical chronic toxicity study. The dose of anti-OX40L MAb in this study was also expected to result in exposures sufficient to inhibit OX40–OX40L interactions in the airways. The overall exposure in this study is predicted to maintain more than 25-fold higher serum concentration than the IC90 demonstrated in in vitro assays of efficacy. An excess of anti-OX40L MAb in the serum allowed for the unknown difference between the amount of drug required to show efficacy in the lung and the observed effective concentration based on in vitro assays. A loading dose of 8 mg/kg was chosen to allow rapid achievement of steady-state serum antibody concentrations. Anti-OX40L MAb is postulated to have a relatively slow onset because the mechanism of action relies on the effects of OX40L on dendritic cells, which are resident cells in lung tissue and lymph nodes in the lung. As such, the exposure was given over 4 months as a rapid on-set of action was not expected. We were able to provide a high exposure of antibody in this small proof-of-activity study based on safety exposures in humans but the exposures in the non-human primate studies were at approximately twofold to threefold higher than given in this study [6]. Although we reached the target anti-OX40L MAb levels in serum which were based on efficacy in in vitro assays, and we were likely to have fully blocked OX40L pathway in both this human study and the non-human primate study, we have no indication that this concentration is actually effective in humans. Furthermore, there are no proven biomarkers for OX40L regulation, and because the recognition of allergen presented by dendritic cells is situated in local lymph nodes, the development of assays to demonstrate target engagement or downstream pharmacodynamic activity are challenging. We were surprised by the placebo response seen particularly in the LAR response for both Day 56 and Day 113. Typically, the allergen challenge model has not shown placebo responses such as we saw in this study [24,25]. We do not think it impacts the interpretation of the results as the small sample size would not have been able to control for Type I error.
Targeting TSLP or OX40–OX40L has the potential to be an effective therapeutic strategy, and perhaps a disease modifier, given the role of the OX40–OX40L axis in allergic sensitization and the development of Th2 lymphocytes. Similar to the effects of mepolizumab, anti-OX40L MAb reduced eosinophil levels with an absence of effects upon LAR or airway hyperresponsiveness [26]. It is possible that in addition to its effects on allergic sensitization, OX40L blockade might also reduce exacerbation frequency as observed with mepolizumab [27]. While it is entirely possible that this intervention is most effective when administered prior to the onset of established allergic sensitization, the timing of intervention in the current study was long after the establishment of allergic sensitization. Furthermore, we do not know whether subjects selected for development of allergen-induced LAR have the clinically relevant ongoing activation of the OX40–OX40L axis. There is evidence in murine models of asthma that OX40L blockade inhibits reactivation and infiltration of memory CD4+ T cells during the recall response to antigen, [6]; however, it is not well understood whether anti-OX40L MAb should be an effective treatment in established human asthma. Neither OX40 or its ligand has emerged in unbiased screens of gene expression in asthma, and furthermore, redundancy in the regulation of Th2 inflammation may diminish the impact of targeting OX40 signalling alone.
Treatment of subjects with mild allergic asthma with anti-OX40L MAb had no effect on allergen-induced airway responses or on airway hyperresponsiveness to methacholine, but had a small though significantly reduced serum total IgE and airway eosinophils at 16 weeks post-dosing. It is possible that the duration of dosing or the dose of drug administered in the current study was insufficient for impacting airway responses in these subjects.
Funding
Genentech Inc., a member of the Roche group and the AllerGen Network of Centers of Excellence, Clinical Investigator Collaborative.
Acknowledgments
The authors would like to thank Ignacio Rodriguez, Maria Fuentes, Peng Lu, Mirella Lazarov and Judith Jaegar and Mike Derby for their review of the manuscript, and Francine Deschesnes, Linda Hui and Richard Watson for co-ordinating the study visits.
Conflict of Interests
GMG, LPB, DWC, JMF, IM and POB have received Grants-in-Aid from Genentech. SM, WP, YZ, HS, DM and JM are employees at Genentech. CC, ML, KK, BED, ML CK and BD have no conflicts of interest to declare.
Supporting Information
Additional Supporting Information may be found in the online version of this article
Figure S1. The mean (background subtracted) frequency of antigen-specific IFNγ-secreting T cells before and after treatment with anti-OX40L MAb. PBMC were isolated before (Day 5) and after (Day 112) treatment with placebo or anti-OX40L MAb, and cultured with sensitizing allergen and recall antigen.
Figure S2. The mean (SEM) number of mDC1 (top panel), mDC2 (middle panel) and pDCs (bottom panel) following treatment with placebo (open bars) and anti-OX40L MAb (solid bars) measured post-treatment Days 55 and 112 (pre-allergen) and 57 and 114 (24 hrs after allergen challenge).
Data S1. Additional methods and results.
References
- 1.Croft M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat Rev Immunol. 2003;3:609–20. doi: 10.1038/nri1148. [DOI] [PubMed] [Google Scholar]
- 2.Kaur D, Brightling C. OX40/OX40 ligand interactions in T-Cell regulation and asthma. Chest. 2012;141:494–9. doi: 10.1378/chest.11-1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sugamura K, Ishii N, Weinberg AD. Therapeutic targeting of the effector T-cell co-stimulatory molecule OX40. Nat Rev Immunol. 2004;4:420–31. doi: 10.1038/nri1371. [DOI] [PubMed] [Google Scholar]
- 4.Liu Y-J. Thymic stromal lymphopoietin: master switch for allergic inflammation. J Exp Med. 2006;203:269–73. doi: 10.1084/jem.20051745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siddiqui S, Mistry V, Doe C, Stinson S, Foster M, Brightling C. Airway wall expression of OX40/OX40L and interleukin-4 in asthma. Chest. 2010;137:797–804. doi: 10.1378/chest.09-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seshasayee D, Lee WP, Zhou M, et al. In vivo blockade of OX40 ligand inhibits thymic stromal lymphopoietin driven atopic inflammation. J Clin Invest. 2007;117:3868–78. doi: 10.1172/JCI33559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cockcroft DW. Measure of airway responsiveness to inhaled histamine or methacholine; method of continuous aerosol generation and tidal breathing inhalation. In: Hargreave FE, Woolcock AJ, editors. Airway responsiveness: measurement and interpretation. Mississauga: Astra Pharmaceuticals Canada Ltd; 1985. pp. 22–8. [Google Scholar]
- 8.O'Byrne PM, Dolovich J, Hargreave FE. Late asthmatic responses. Am Rev Respir Dis. 1987;136:740–51. doi: 10.1164/ajrccm/136.3.740. [DOI] [PubMed] [Google Scholar]
- 9.Cockcroft DW, Murdock KY, Kirby J, Hargreave F. Prediction of airway responsiveness to allergen from skin sensitivity to allergen and airway responsiveness to histamine. Am Rev Respir Dis. 1987;135:264–7. doi: 10.1164/arrd.1987.135.1.264. [DOI] [PubMed] [Google Scholar]
- 10.Pizzichini E, Pizzichini MM, Efthimiadis A, et al. Indices of airway inflammation in induced sputum: reproducibility and validity of cell and fluid-phase measurements. Am J Respir Crit Care Med. 1996;154(2 Pt 1):308–17. doi: 10.1164/ajrccm.154.2.8756799. [DOI] [PubMed] [Google Scholar]
- 11.Harada M, Hirota T, Jodo AI, et al. Thymic stromal lymphopoietin gene promoter polymorphisms are associated with susceptibility to bronchial asthma. Am J Respir Cell Mol Biol. 2011;44:787–93. doi: 10.1165/rcmb.2009-0418OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohshima Y, Yang LP, Uchiyama T, et al. OX40 costimulation enhances interleukin- 4 (IL-4) expression at priming and promotes the differentiation of naive human CD4 (+) T cells into high IL-4-producing effectors. Blood. 1998;1:3338–45. [PubMed] [Google Scholar]
- 13.Ito T, Wang YH, Duramad O, et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005;202:1213–23. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burgess JK, Blake AE, Boustany S, et al. CD40 and OX40 ligand are increased on stimulated asthmatic airway smooth muscle. J Allergy Clin Immunol. 2005;115:302–8. doi: 10.1016/j.jaci.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Wang YH, Ito T, Wang YH, et al. Maintenance and polarization of human Th2 central memory T cells by thymic stromal lymphopoietin-activated dendritic cells. Immunity. 2006;24:827–38. doi: 10.1016/j.immuni.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 16.Jember AG, Zuberi R, Liu FT, Croft M. Development of allergic inflammation in a murine model of asthma is dependent on the costimulatory receptor OX40. J Exp Med. 2001;5:387–92. doi: 10.1084/jem.193.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arestides RS, He H, Westlake RM, et al. Costimulatory molecule OX40L is critical for both Th1 and Th2 responses in allergic inflammation. Eur J Immunol. 2002;32:2874–80. doi: 10.1002/1521-4141(2002010)32:10<2874::AID-IMMU2874>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 18.Hoshino A, Tanaka Y, Akiba H, et al. Critical role for OX40 ligand in the development of pathogenic Th2 cells in a murine model of asthma. Eur J Immunol. 2003;33:861–9. doi: 10.1002/eji.200323455. [DOI] [PubMed] [Google Scholar]
- 19.Salek-Ardakani S, Song J, Halteman BS, et al. OX40 (CD134) controls memory T helper 2 cells that drive lung inflammation. J Exp Med. 2003;198:315–24. doi: 10.1084/jem.20021937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weinberg AD, Wegmann KW, Funatake C, Whitham RH. Blocking OX-40/OX-40 ligand interaction in vitro and in vivo leads to decreased T cell function and amelioration of experimental allergic encephalomyelitis. J. Immunol. 1999;62:1818–26. [PubMed] [Google Scholar]
- 21.Weinberg AD, Bourdette DN, Sullivan TJ, et al. Selective depletion of myelin-reactive T cells with the anti-OX-40 antibody ameliorates autoimmune encephalomyelitis. Nat Med. 1996;2:183–9. doi: 10.1038/nm0296-183. [DOI] [PubMed] [Google Scholar]
- 22.Parulekar A, Boomer JS, Patterson BM, et al. A randomized, controlled trial to evaluate inhibition of T cell costimulation in allergen induced airway inflammation. Am J Respir Crit Care Med. 2013;187:494–501. doi: 10.1164/rccm.201207-1205OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bartemes KR, Iijima K, Kobayashi T, Kephart GM, McKenzie AN, Kita H. IL-33-responsive lineage-CD25+CD44(hi) lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs. J Immunol. 2012;188:1503–13. doi: 10.4049/jimmunol.1102832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fahy JV, Fleming HE, Wong HH, et al. The effect of an anti-IgE monoclonal antibody on the early- and late-phase responses to allergen inhalation in asthmatic subjects. Am J Respir Crit Care Med. 1997;155:1828–34. doi: 10.1164/ajrccm.155.6.9196082. [DOI] [PubMed] [Google Scholar]
- 25.Gauvreau GM, Becker AB, Boulet LP, et al. The effects of an anti-CD11a MAb, efalizumab on allergen-induced airway responses and airway inflammation in subjects with atopic asthma. J Allergy Clin Immunol. 2003;112:331–8. doi: 10.1067/mai.2003.1689. [DOI] [PubMed] [Google Scholar]
- 26.Leckie MJ, ten Brinke A, Khan J, et al. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet. 2000;356:2144–8. doi: 10.1016/s0140-6736(00)03496-6. [DOI] [PubMed] [Google Scholar]
- 27.Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe asthma (DREAM): a multicenter, double-blind, placebo-controlled trial. Lancet. 2012;18:651–9. doi: 10.1016/S0140-6736(12)60988-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. The mean (background subtracted) frequency of antigen-specific IFNγ-secreting T cells before and after treatment with anti-OX40L MAb. PBMC were isolated before (Day 5) and after (Day 112) treatment with placebo or anti-OX40L MAb, and cultured with sensitizing allergen and recall antigen.
Figure S2. The mean (SEM) number of mDC1 (top panel), mDC2 (middle panel) and pDCs (bottom panel) following treatment with placebo (open bars) and anti-OX40L MAb (solid bars) measured post-treatment Days 55 and 112 (pre-allergen) and 57 and 114 (24 hrs after allergen challenge).
Data S1. Additional methods and results.


