Abstract
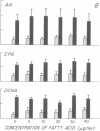
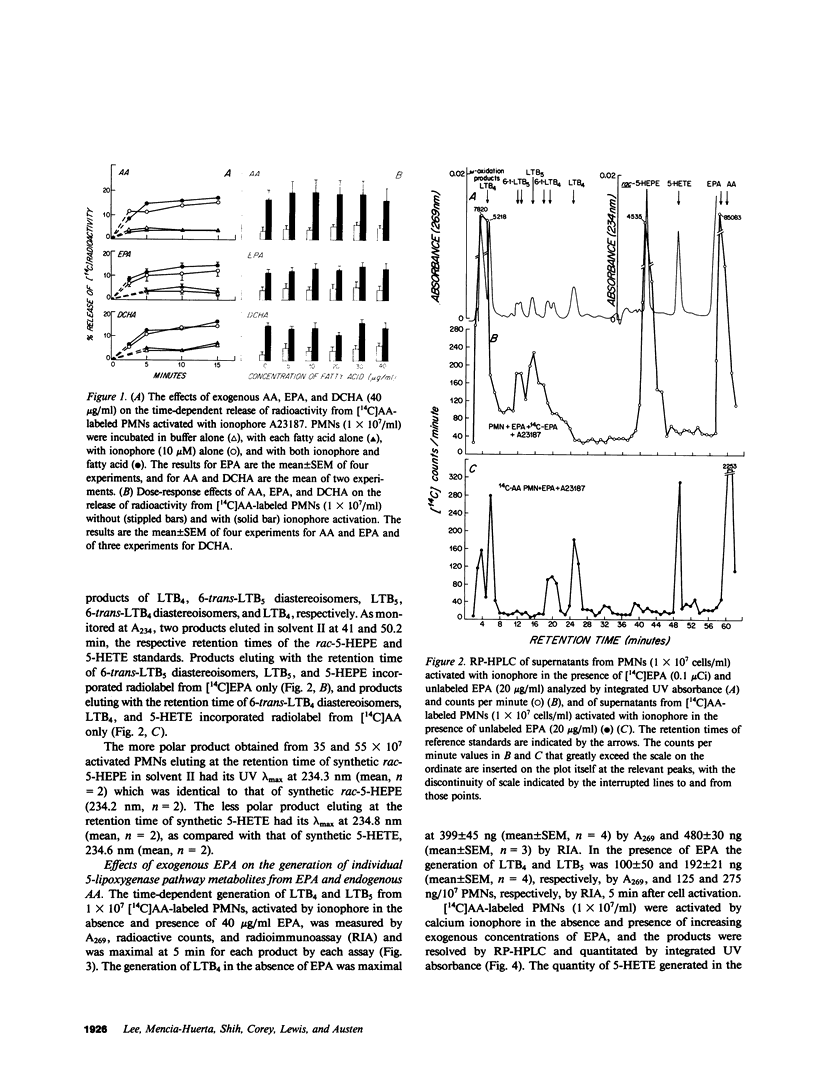
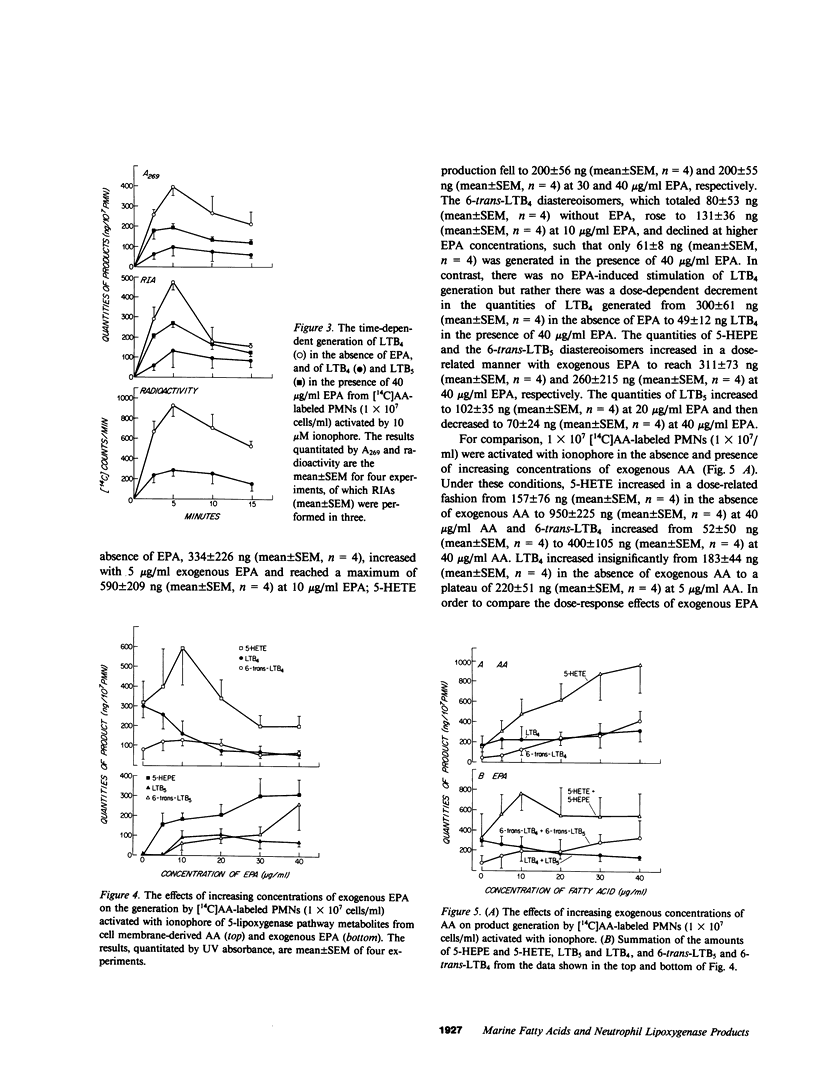
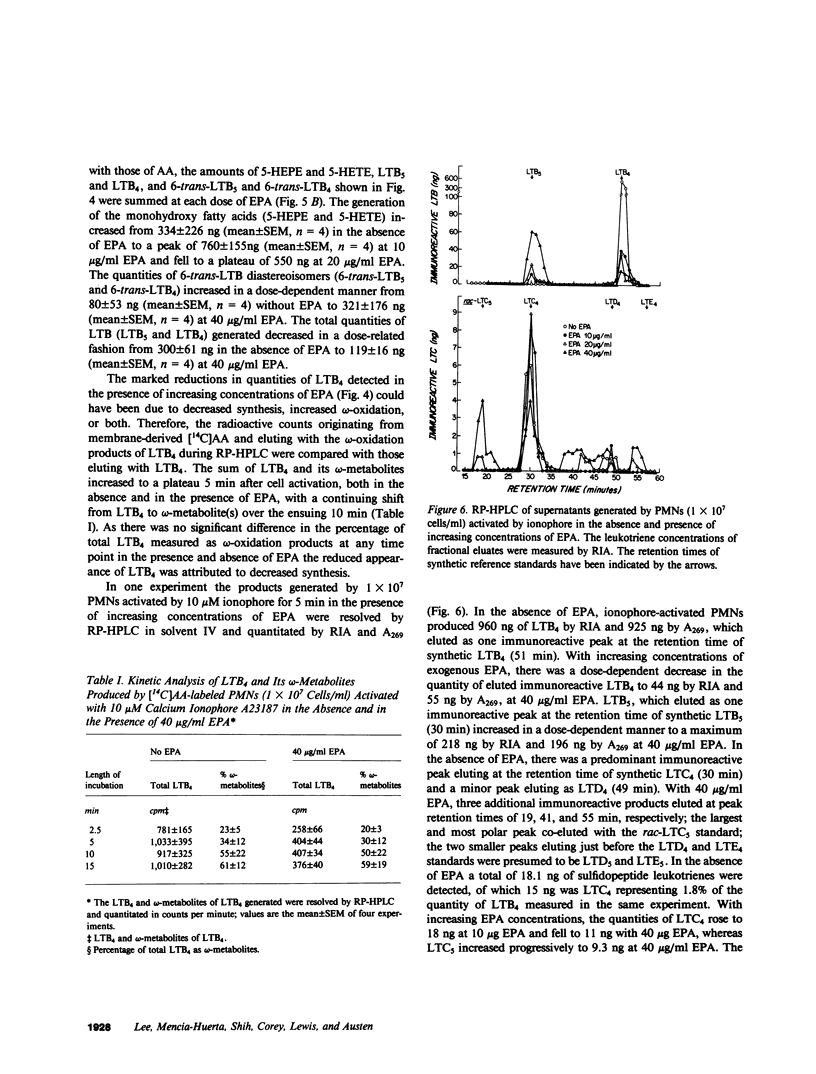
Exogenous eicosapentaenoic acid (EPA) and docosahexaenoic acid (DCHA) have been compared with exogenous arachidonic acid for their capacity to modulate the oxidative metabolism of membrane-derived arachidonic acid by the 5-lipoxygenase pathway in ionophore-activated human neutrophils and for their suitability as parallel substrates in this pathway. The products from specific 14C- or 3H-labeled substrates were isolated by reverse phase high performance liquid chromatography (RP-HPLC) and were identified by elution of radiolabel at the retention times of the appropriate synthetic standards. Each product was also characterized by its ultraviolet (UV) absorption spectrum, and 7-hydroxy-DCHA was defined in addition by analysis of its mass spectrum. The metabolites, 5-hydroxyeicosatetraenoic acid, leukotriene B4 (LTB4), 6-trans-LTB4 diastereoisomers, 5-hydroxyeicosapentaenoic acid, 6-trans-leukotriene B5 diastereoisomers, leukotriene B5 (LTB5), and 7-hydroxy-DCHA were quantitated by integrated UV absorbance during resolution by RP-HPLC. LTB4 and LTB5 were also quantitated by radioimmunoassay of the eluate fractions, and leukotrienes C4 and C5 (LTC4 and LTC5, respectively) were quantitated by radioimmunoassay alone. None of the unlabeled exogenous fatty acids (5-40 micrograms/ml) altered the release of radioactivity from [14C]arachidonic acid-labeled, ionophore-activated neutrophils. The metabolism of 5 and 10 micrograms/ml of exogenous EPA by ionophore-activated, [14C]arachidonic acid-labeled neutrophils not only generated 5-hydroxyeicosapentaenoic acid, 6-trans-LTB5, LTB5, and LTC5, but also stimulated the formation of 5-hydroxyeicosatetraenoic acid, 6-trans-LTB4 diastereoisomers, and LTC4 from membrane-derived arachidonic acid. In contrast, LTB4 production was diminished throughout the EPA dose-response, beginning at 5 micrograms/ml EPA and reaching 50% suppression at 10 micrograms/ml and 84% suppression at 40 micrograms/ml. The selective decrease in extracellular LTB4 concentrations in the presence of EPA was not due to a change in the kinetic appearance of LTB4 or to an increase in conversion to its omega-oxidation metabolites. DCHA was metabolized to 7-hydroxy-DCHA, did not stimulate metabolism of membrane-derived arachidonic acid, did not appreciably inhibit LTB4 formation, and was not a substrate for leukotriene formation. Incremental doses of exogenous arachidonic acid resulted in increased production of 5-hydroxyeicosatetraenoic acid and 6-trans-LTB4 by ionophore-activated, [14C]arachidonic acid-labeled neutrophils without any change in LTB4 production. 5-hydroxyeicosapentaenoic acid and 7-hydroxy DCHA were inactive as chemotactic factors whereas 5-hydroxyeicosatetraenoic acid exhibited 2% of the potency of LBT4. Thus, exogenous DCHA does not appreciably interfere with the metabolism of membrane-derived arachidonic acid by ionophore-activated, [14C]arachidonic acid-labeled neutrophils and is converted only to a monohydroxy derivative. In contrast, exogenous EPA attenuates the generation of LTB4 and is converted to LTB5, which is a weak and partial agonist as compared with LTB4.
Full text
PDF



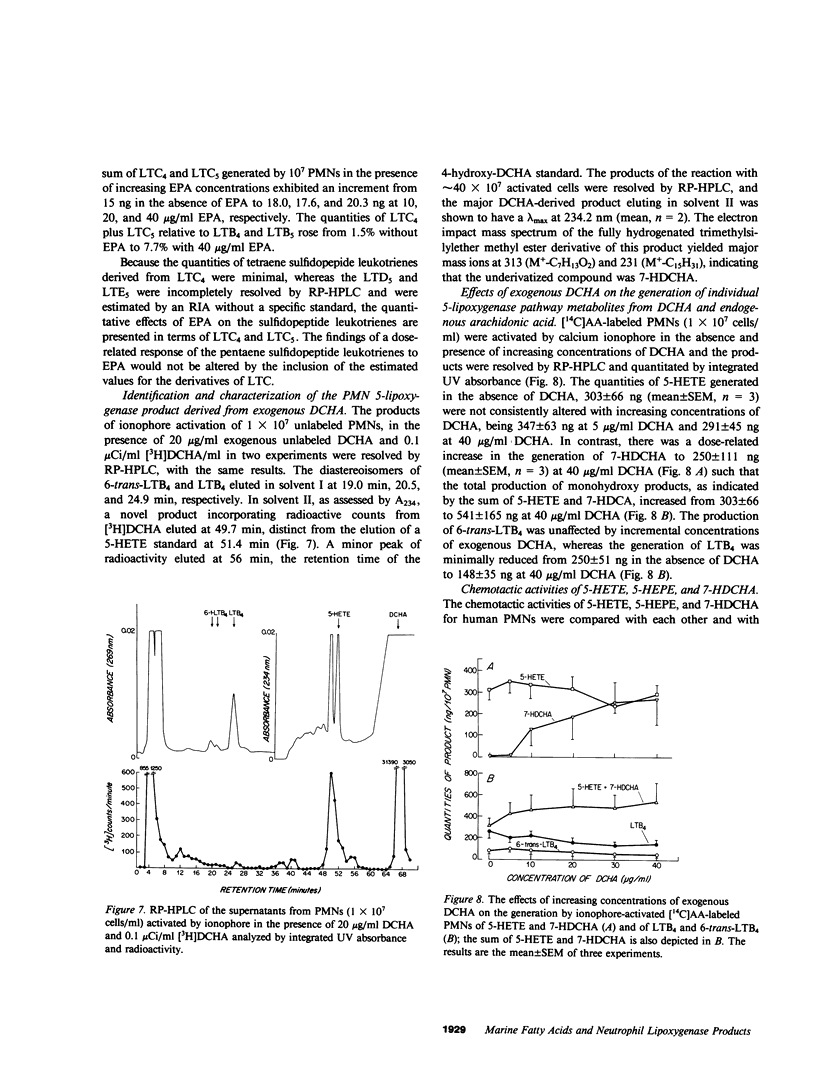
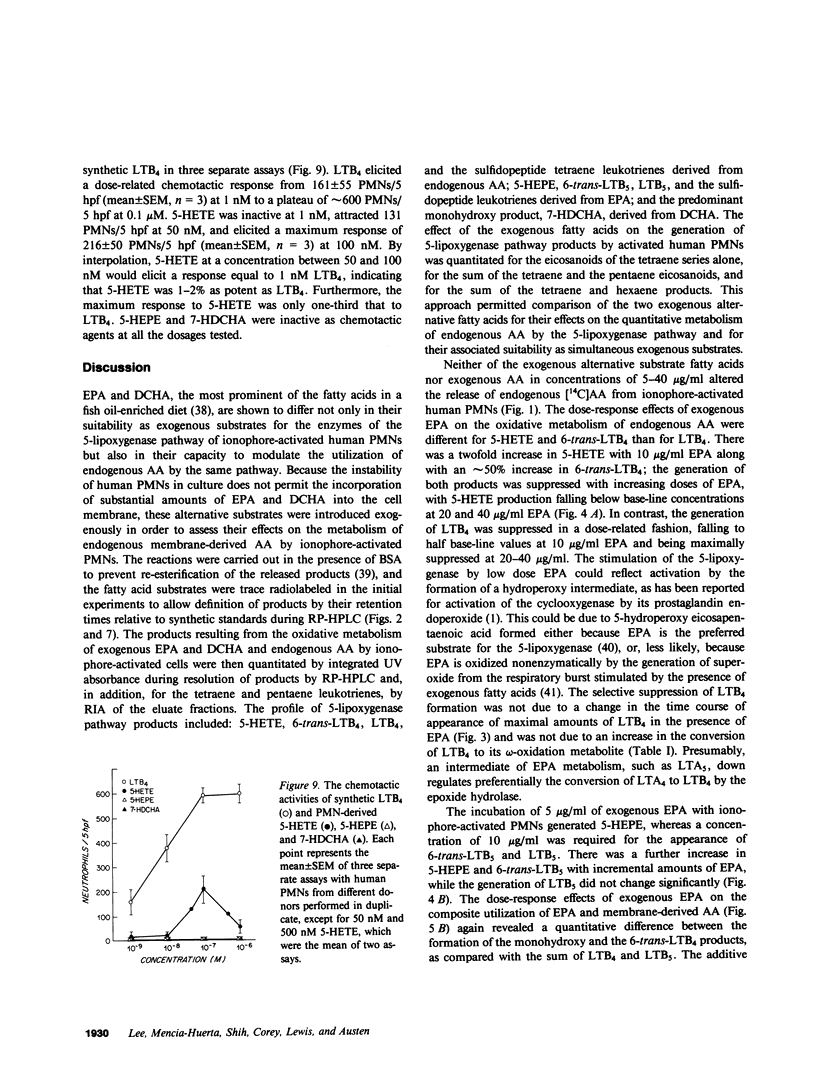



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aveldaño M. I., Sprecher H. Synthesis of hydroxy fatty acids from 4, 7, 10, 13, 16, 19-[1-14C] docosahexaenoic acid by human platelets. J Biol Chem. 1983 Aug 10;258(15):9339–9343. [PubMed] [Google Scholar]
- Badwey J. A., Curnutte J. T., Karnovsky M. L. cis-Polyunsaturated fatty acids induce high levels of superoxide production by human neutrophils. J Biol Chem. 1981 Dec 25;256(24):12640–12643. [PubMed] [Google Scholar]
- Borgeat P., Samuelsson B. Arachidonic acid metabolism in polymorphonuclear leukocytes: effects of ionophore A23187. Proc Natl Acad Sci U S A. 1979 May;76(5):2148–2152. doi: 10.1073/pnas.76.5.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgeat P., Samuelsson B. Arachidonic acid metabolism in polymorphonuclear leukocytes: unstable intermediate in formation of dihydroxy acids. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3213–3217. doi: 10.1073/pnas.76.7.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgeat P., Samuelsson B. Metabolism of arachidonic acid in polymorphonuclear leukocytes. Structural analysis of novel hydroxylated compounds. J Biol Chem. 1979 Aug 25;254(16):7865–7869. [PubMed] [Google Scholar]
- Borgeat P., Samuelsson B. Transformation of arachidonic acid by rabbit polymorphonuclear leukocytes. Formation of a novel dihydroxyeicosatetraenoic acid. J Biol Chem. 1979 Apr 25;254(8):2643–2646. [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Corey E. J., Shih C., Cashman J. R. Docosahexaenoic acid is a strong inhibitor of prostaglandin but not leukotriene biosynthesis. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3581–3584. doi: 10.1073/pnas.80.12.3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlén S. E., Hedqvist P., Hammarström S. Contractile activities of several cysteine-containing leukotrienes in the guinea-pig lung strip. Eur J Pharmacol. 1982 Dec 24;86(2):207–215. doi: 10.1016/0014-2999(82)90318-1. [DOI] [PubMed] [Google Scholar]
- Falk W., Goodwin R. H., Jr, Leonard E. J. A 48-well micro chemotaxis assembly for rapid and accurate measurement of leukocyte migration. J Immunol Methods. 1980;33(3):239–247. doi: 10.1016/0022-1759(80)90211-2. [DOI] [PubMed] [Google Scholar]
- Fels A. O., Pawlowski N. A., Cramer E. B., King T. K., Cohn Z. A., Scott W. A. Human alveolar macrophages produce leukotriene B4. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7866–7870. doi: 10.1073/pnas.79.24.7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer S., von Schacky C., Siess W., Strasser T., Weber P. C. Uptake, release and metabolism of docosahexaenoic acid (DHA, c22:6 omega 3) in human platelets and neutrophils. Biochem Biophys Res Commun. 1984 May 16;120(3):907–918. doi: 10.1016/s0006-291x(84)80193-x. [DOI] [PubMed] [Google Scholar]
- Goldman D. W., Pickett W. C., Goetzl E. J. Human neutrophil chemotactic and degranulating activities of leukotriene B5 (LTB5) derived from eicosapentaenoic acid. Biochem Biophys Res Commun. 1983 Nov 30;117(1):282–288. doi: 10.1016/0006-291x(83)91572-3. [DOI] [PubMed] [Google Scholar]
- Hammarström S. Leukotriene C5: a slow reacting substance derived from eicosapentaenoic acid. J Biol Chem. 1980 Aug 10;255(15):7093–7094. [PubMed] [Google Scholar]
- Hammerström S., Samuelsson B. Detection of leukotriene A4 as an intermediate in the biosynthesis of leukotrienes C4 and D4. FEBS Lett. 1980 Dec 15;122(1):83–86. doi: 10.1016/0014-5793(80)80407-8. [DOI] [PubMed] [Google Scholar]
- Hansson G., Lindgren J. A., Dahlén S. E., Hedqvist P., Samuelsson B. Identification and biological activity of novel omega-oxidized metabolites of leukotriene B4 from human leukocytes. FEBS Lett. 1981 Jul 20;130(1):107–112. doi: 10.1016/0014-5793(81)80676-x. [DOI] [PubMed] [Google Scholar]
- Lee T. H., Menica-Huerta J. M., Shih C., Corey E. J., Lewis R. A., Austen K. F. Characterization and biologic properties of 5,12-dihydroxy derivatives of eicosapentaenoic acid, including leukotriene B5 and the double lipoxygenase product. J Biol Chem. 1984 Feb 25;259(4):2383–2389. [PubMed] [Google Scholar]
- Leitch A. G., Lee T. H., Ringel E. W., Prickett J. D., Robinson D. R., Pyne S. G., Corey E. J., Drazen J. M., Austen K. F., Lewis R. A. Immunologically induced generation of tetraene and pentaene leukotrienes in the peritoneal cavities of menhaden-fed rats. J Immunol. 1984 May;132(5):2559–2565. [PubMed] [Google Scholar]
- Levine L., Morgan R. A., Lewis R. A., Austen K. F., Clark D. A., Marfat A., Corey E. J. Radioimmunoassay of the leukotrienes of slow reacting substance of anaphylaxis. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7692–7696. doi: 10.1073/pnas.78.12.7692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R. A., Drazen J. M., Austen K. F., Clark D. A., Corey E. J. Identification of the C(6)-S-conjugate of leukotriene A with cysteine as a naturally occurring slow reacting substance of anaphylaxis (SRS-A). Importance of the 11-cis-geometry for biological activity. Biochem Biophys Res Commun. 1980 Sep 16;96(1):271–277. doi: 10.1016/0006-291x(80)91210-3. [DOI] [PubMed] [Google Scholar]
- Lewis R. A., Mencia-Huerta J. M., Soberman R. J., Hoover D., Marfat A., Corey E. J., Austen K. F. Radioimmunoassay for leukotriene B4. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7904–7908. doi: 10.1073/pnas.79.24.7904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren J. A., Hansson G., Samuelsson B. Formation of novel hydroxylated eicosatetraenoic acids in preparations of human polymorphonuclear leukocytes. FEBS Lett. 1981 Jun 15;128(2):329–335. doi: 10.1016/0014-5793(81)80110-x. [DOI] [PubMed] [Google Scholar]
- MacGlashan D. W., Jr, Schleimer R. P., Peters S. P., Schulman E. S., Adams G. K., 3rd, Newball H. H., Lichtenstein L. M. Generation of leukotrienes by purified human lung mast cells. J Clin Invest. 1982 Oct;70(4):747–751. doi: 10.1172/JCI110670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin T. R., Altman L. C., Albert R. K., Henderson W. R. Leukotriene B4 production by the human alveolar macrophage: a potential mechanism for amplifying inflammation in the lung. Am Rev Respir Dis. 1984 Jan;129(1):106–111. doi: 10.1164/arrd.1984.129.1.106. [DOI] [PubMed] [Google Scholar]
- Murphy R. C., Hammarström S., Samuelsson B. Leukotriene C: a slow-reacting substance from murine mastocytoma cells. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4275–4279. doi: 10.1073/pnas.76.9.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needleman P., Raz A., Minkes M. S., Ferrendelli J. A., Sprecher H. Triene prostaglandins: prostacyclin and thromboxane biosynthesis and unique biological properties. Proc Natl Acad Sci U S A. 1979 Feb;76(2):944–948. doi: 10.1073/pnas.76.2.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochi K., Yoshimoto T., Yamamoto S., Taniguchi K., Miyamoto T. Arachidonate 5-lipoxygenase of guinea pig peritoneal polymorphonuclear leukocytes. Activation by adenosine 5'-triphosphate. J Biol Chem. 1983 May 10;258(9):5754–5758. [PubMed] [Google Scholar]
- Paterson N. A., Wasserman S. I., Said J. W., Austen K. F. Release of chemical mediators from partially purified human lung mast cells. J Immunol. 1976 Oct;117(4):1356–1362. [PubMed] [Google Scholar]
- Rao G. H., Radha E., White J. G. Effect of docosahexaenoic acid (DHA) on arachidonic acid metabolism and platelet function. Biochem Biophys Res Commun. 1983 Dec 16;117(2):549–555. doi: 10.1016/0006-291x(83)91235-4. [DOI] [PubMed] [Google Scholar]
- Rådmark O., Malmsten C., Samuelsson B., Goto G., Marfat A., Corey E. J. Leukotriene A. Isolation from human polymorphonuclear leukocytes. J Biol Chem. 1980 Dec 25;255(24):11828–11831. [PubMed] [Google Scholar]
- Samuelsson B., Goldyne M., Granström E., Hamberg M., Hammarström S., Malmsten C. Prostaglandins and thromboxanes. Annu Rev Biochem. 1978;47:997–1029. doi: 10.1146/annurev.bi.47.070178.005025. [DOI] [PubMed] [Google Scholar]
- Stansby M. E. Nutritional properties of fish oils. World Rev Nutr Diet. 1969;11:46–105. doi: 10.1159/000387575. [DOI] [PubMed] [Google Scholar]
- Stenson W. F., Parker C. W. Metabolism of arachidonic acid in ionophore-stimulated neutrophils. Esterification of a hydroxylated metabolite into phospholipids. J Clin Invest. 1979 Nov;64(5):1457–1465. doi: 10.1172/JCI109604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terano T., Salmon J. A., Moncada S. Biosynthesis and biological activity of leukotriene B5. Prostaglandins. 1984 Feb;27(2):217–232. doi: 10.1016/0090-6980(84)90075-3. [DOI] [PubMed] [Google Scholar]
- Weller P. F., Lee C. W., Foster D. W., Corey E. J., Austen K. F., Lewis R. A. Generation and metabolism of 5-lipoxygenase pathway leukotrienes by human eosinophils: predominant production of leukotriene C4. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7626–7630. doi: 10.1073/pnas.80.24.7626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. D., Czop J. K., Austen K. F. Release of leukotrienes by human monocytes on stimulation of their phagocytic receptor for particulate activators. J Immunol. 1984 Jun;132(6):3034–3040. [PubMed] [Google Scholar]