Abstract
Thermogenesis in brown adipocytes, conferred by mitochondrial uncoupling protein 1 (UCP1), is receiving great attention because metabolically active brown adipose tissue may protect humans from metabolic diseases. In particular, the thermogenic function of brown-like adipocytes in white adipose tissue, known as brite (or beige) adipocytes, is currently of prime interest. A valid procedure to quantify the specific contribution of UCP1 to thermogenesis is thus of vital importance. Adrenergic stimulation of lipolysis is a common way to activate UCP1. We here report, however, that in this frequently applied setup, taking control over intracellular fatty acid levels is essential for the analysis of thermogenic function in cultured brown and brite adipocytes. By the application of these findings, we demonstrate that UCP1 is functionally thermogenic in intact brite adipocytes and adrenergic UCP1 activation is largely dependent on adipose triglyceride lipase (ATGL) rather than hormone sensitive lipase (HSL).
Keywords: brite adipocytes, brown adipocytes, thermogenesis, UCP1, uncoupled respiration
Introduction
Mammalian adipose tissues are conventionally classified into two distinct types: white adipose tissue (WAT) and brown adipose tissue (BAT). The former stores energy as triglycerides, whereas the latter directly dissipates the chemical energy of fatty acids as heat through uncoupling protein 1 (UCP1) [1,2]. Interestingly, UCP1-expressing brown adipocyte-like cells (brite, “brown-in-white”, also termed beige adipocytes) develop in typical WAT in response to cold exposure, β3-adrenergic receptor stimulation and PPARγ agonist treatment in a process termed “browning” of WAT [3–9]. Based on their energy dissipation property, brown and brite adipocytes represent putative therapeutic targets for the treatment of obesity and diabetes [10–12]. This scenario is further strengthened by the recent finding that brown and brite adipocytes are present in humans [13–17].
Non-shivering thermogenesis of brown adipocytes is conferred by mitochondrial uncoupling protein 1 (UCP1), which upon stimulation uncouples respiration from ATP synthesis and dissipates energy as heat [1,2]. UCP1 is constitutively inhibited by high concentrations of cytosolic purine nucleotides (i.e. GDP, GTP, ADP and ATP), but upon adrenergic stimulation of the cell, this inhibition is overcome by free fatty acids (FFAs) interacting directly with UCP1. Norepinephrine released from the sympathetic nervous system activates adrenergic receptors of brown adipocytes, which in turn stimulate the cAMP-dependent protein kinase PKA, leading to phosphorylation of hormone sensitive lipase (HSL) and thereby increased lipolysis. FFAs released by lipolysis serve both as activators of UCP1 and fuel for thermogenesis [18]. Adipose triglyceride lipase (ATGL) plays an essential role for the hydrolysis of triglycerides [19], and fatty acids released by phospholipases in the inner mitochondrial membrane have also been suggested to contribute to UCP1 activation [20], but the role of these lipases for the activation of UCP1 in brown and brite adipocytes has not been addressed.
Much effort has been invested into the development of assays quantifying UCP1-mediated uncoupled respiration in isolated mitochondria [21–24]. While this easily controllable system has many advantages, it is limited by artifacts associated with mitochondrial isolation, disruption of the intricate mitochondrial network integrity, lack of the native intracellular environment, and the large amount of cells or tissue needed for optimal yield and quality. A major goal of current bioenergetic research is thus the development and application of techniques to quantify mitochondrial function and cellular bioenergetics in cells [25]. One such technique, microplate-based respirometry, was developed to be used with cultured cells attached in a monolayer to a multi-well tissue culture plate and is now the preferred method to quantify UCP1-mediated leak respiration in cultured brown and brite cells [26–35]. From a historical perspective, albumin which acts as an acceptor of fatty acids must be used in the respiration medium for quantifying UCP1-mediated uncoupled respiration (for details see Supplementary Text S1). However, in the respiration buffer used by microplate-based respirometry, albumin is absent. Notably, no studies have been performed so far to validate this setup with cultured UCP1 knockout (KO) cells as the ultimate model to test the causality between uncoupled respiration and presence of UCP1. This relationship seems to have been taken for granted. It remains to be demonstrated that UCP1 is functionally thermogenic in intact brite adipocytes.
Here, we report that uncoupled respiration as measured in published protocols is not mediated by UCP1, since cultured primary adipocytes (both brown and brite) from UCP1 WT and KO have identical respiration profiles. We demonstrate that fatty acid-induced activation of UCP1 is a prerequisite for quantifying the UCP1-mediated leak respiration. In addition, when UCP1 is activated by stimulation of lipolysis, it is essential to take control over intracellular FFA levels to measure UCP1-mediated leak respiration in cells. Otherwise, an excessive rise of intracellular FFA levels released during lipolysis masks UCP1-mediated leak respiration through unspecific protonophoric action of FFAs and opening of the mitochondrial permeability transition pore (PTP) in both brown and brite adipocytes. Taken together, our studies provide critical guidelines for analyzing UCP1-mediated thermogenesis in intact brown and brite adipocytes.
Results and Discussion
Activation of UCP1 is a prerequisite for quantifying UCP1-mediated leak respiration in cultured primary adipocytes
To verify whether UCP1 is innately inactive within intact cultured primary brown and brite adipocytes, we compared the respiration profiles of UCP1 WT and KO cells without any (pre)treatment to activate UCP1. After determination of basal respiration, oligomycin, an inhibitor of adenosine triphosphate (ATP) synthase, was added to distinguish oxygen consumption used for ATP synthesis (coupled respiration) from proton leak (basal uncoupled respiration). Next, we employed the uncoupling agent carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP) to assess maximal respiratory capacity. Finally, antimycin A was added to block the electron transport chain, leaving only non-mitochondrial oxygen consumption (Fig 1A and C). We observed no differences in any of these four different respiration states between WT and KO in both brown and brite adipocytes, confirming UCP1 is inherently not leaky without stimulation (Fig 1B and D). Of note, this lack of differences was observed irrespective of the presence or absence of UCP1 in WT and KO cells, as confirmed by Western blotting (Supplementary Fig S1). Thus, without activation of UCP1, respiration measurements cannot reveal the consequences of absence or presence of UCP1 in a cell, although this setting is widely used in the literature, for example, to characterize white versus brown or brite adipocytes [26–29,35]. In these studies, the reported differential respiration between cell types is not due to UCP1 but rather reflects differences in mitochondrial content.
Figure 1. Activation of UCP1 is a prerequisite for quantifying UCP1-mediated leak respiration.
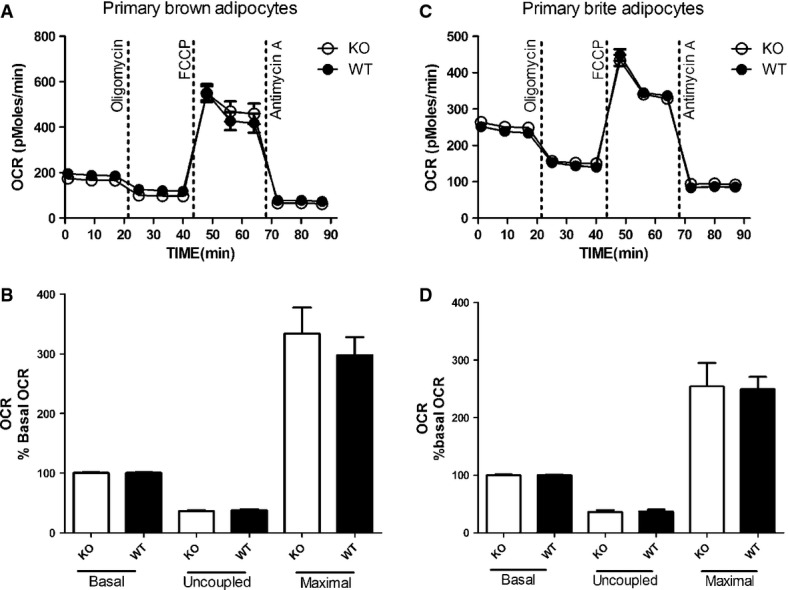
- A, B Representative time course of oxygen consumption rates (OCR) of primary brown adipocytes from UCP1 wild-type (WT) and knockout (KO) mice (A). Both genotypes display identical basal respiration, basal proton leak respiration (after oligomycin injection), maximal respiratory capacity (FCCP) and non-mitochondrial respiration (antimycin A). For comparison, mean OCR in these states was quantified (B).
- C, D Representative time course of oxygen consumption rates (OCR) of primary brite adipocytes from UCP1 wild-type (WT) and knockout (KO) mice (C). Both genotypes display identical basal respiration, basal proton leak respiration (after oligomycin injection), maximal respiratory capacity (FCCP) and non-mitochondrial respiration (antimycin A). For comparison, mean OCR in these states was quantified (D).
Data information: All data presented are mean values ± SEM (N = 3).
Free fatty acids mask UCP1-mediated leak respiration
A frequently employed method to activate UCP1 is to stimulate the intracellular signaling cascade controlling lipolysis. In our protocol, basal respiration was measured first succeeded by inhibition of coupled respiration (oligomycin) to determine basal leak respiration. Next, UCP1 was activated by FFAs released via β-adrenergic stimulation of lipolysis with isoproterenol (ISO), and the increase in UCP1-mediated leak respiration was measured. The maximum respiration rate was determined after adding the uncoupling agent FCCP. Finally, injection of antimycin A served to correct for non-mitochondrial oxygen consumption. Employing this protocol, ISO gradually increased respiration to a peak attained after 30 min (Supplementary Fig S2). Since 0.5 μM ISO was as potent as 1 μM, this concentration was chosen for further experiments.
We next evaluated our protocol with UCP1 KO primary brown and brite adipocytes. In contrast to the established model of UCP1 regulation, we found ISO-induced leak respiration to be identical in brown and brite adipocytes from both WT and KO mice (Fig 2). Thus, the ISO-induced leak respiration was completely independent of UCP1. Results were similar when we used a higher concentration of ISO (1 μM) or an alternative adrenergic agonist (CL-316,243, 1 μM) or dibutyryl cyclic AMP (db-cAMP, 0.5 and 1 mM) instead (data not shown). Notably, the rate of UCP1-independent leak respiration could not be further increased by FCCP, indicating that mitochondria were already fully uncoupled.
Figure 2. Identical isoproterenol (ISO)-induced leak respiration in primary brown and brite adipocytes from UCP1 WT and KO mice.
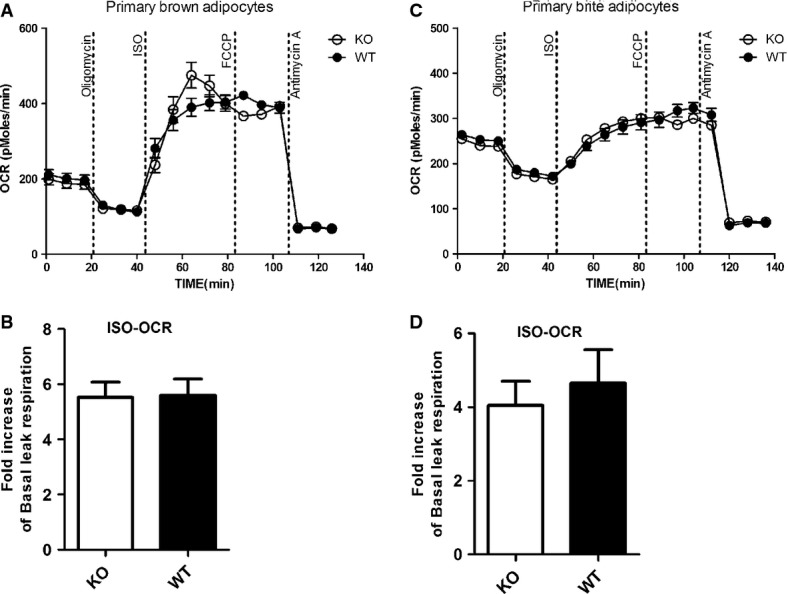
- A, B Representative time course of oxygen consumption rates (OCR) of primary brown adipocytes from UCP1 wild-type (WT) and knockout (KO) mice (A). Both genotypes display identical ISO-induced respiration, suggesting that ISO-induced leak respiration is independent of UCP1. For comparison, mean OCR in these states was quantified (B).
- C, D Representative time course of oxygen consumption rates (OCR) of primary brite adipocytes from UCP1 wild-type (WT) and knockout (KO) mice (C). Both genotypes display identical ISO-induced respiration, suggesting that ISO-induced leak respiration is independent of UCP1. For comparison, mean OCR in these states was quantified (D).
Data information: All data presented are mean values ± SEM (N = 3).
In adipocytes, FFAs liberated by lipolysis are either released into the extracellular space or accumulate in the cell serving both as substrates of β-oxidation and as activators of UCP1. In vivo, extracellular FFAs are scavenged by albumin in the blood stream. In vitro, without any extracellular fatty acid acceptor available, FFAs accumulate to high concentrations in cells and may induce unspecific uncoupled respiration as evidenced in human adipocytes [36]. Using brown adipocytes from KO mice, we tested the hypothesis that ISO-stimulated UCP1-independent leak respiration is due to FFA liberated by lipolysis. We therefore inhibited adipose triglyceride lipase (ATGL) and hormone sensitive lipase (HSL) by pretreatment of cells with Atglistatin [37] and Hi 76-0079 [19], respectively. This combination of inhibitors, known to almost completely block (−95%) lipolysis [19], abolished ISO-induced UCP1-independent leak respiration (Fig 3A and B), confirming that this phenomenon depends on lipolysis. To further explore whether the released FFAs were responsible for unspecific mitochondrial uncoupling, we mimicked lipolysis by adding 0.2 mM palmitate–BSA to the medium. Indeed, exogenous fatty acids induced leak respiration independent of UCP1 to a similar extent as treatment with ISO (Fig 3C and D). Taken together, FFAs are the effectors of UCP1-independent uncoupling.
Figure 3. Uncoupled respiration in the absence of UCP1 is dependent on lipolysis and can be mimicked by addition of exogenous free fatty acids (FFA).
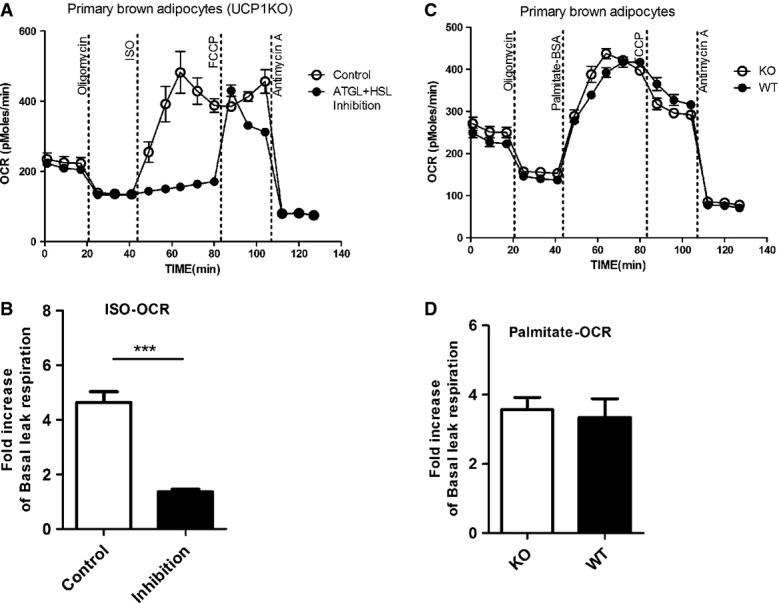
- A, BRepresentative time course of oxygen consumption rates (OCR) of primary brown adipocytes from UCP1 knockout (KO) mice pretreated with 40 μM Atglistatin (ATGL inhibitor) and 20 μM Hi76-0079 (HSL inhibitor) or vehicle (DMSO) for 1 h before bioenergetic profiling (A). Inhibition of lipolysis resulted in a significant suppression of ISO-induced leak respiration (B).
- C, D Representative time course of oxygen consumption rates (OCR) of primary brown adipocytes from UCP1 wild-type (WT) and knockout (KO) mice. Exogenous addition of free fatty acids by injection of a palmitate–BSA complex (0.2 mM) instead of ISO (C) mimics the full uncoupled respiration induced by ISO (D) in primary brown adipocytes from UCP1 WT and KO mice.
Data information: All data presented are mean values ± SEM (N = 3). ***P < 0.001.
Opening of the mitochondrial permeability transition pore (PTP) participates in ISO-induced, UCP1-independent leak respiration
The ability of FFAs to uncouple mitochondrial respiration has been known for decades [38,39]. Besides their conventional protonophoric action based on flip-flop-mediated proton translocation by FFA inserted into the inner mitochondrial membrane [40], the involvement of PTP has been suggested [36]. The PTP is a high conductance channel spanning both mitochondrial membranes and opening leads to an increase of unspecific permeability which is a common feature of apoptosis. To determine whether the mitochondrial uncoupling mediated by PTP opening is also responsible for ISO-stimulated UCP1-independent leak respiration in brown and brite adipocytes, we pretreated primary brown cells with the specific PTP inhibitor cyclosporine A (CSA). We found that a significant portion of ISO-induced leak respiration was inhibited by CSA (Fig 4), demonstrating involvement of PTP opening. In any case, the functional data on brown adipocyte thermogenesis previously published must be revisited as they most likely do not reflect UCP1 activity but rather represent unspecific FFA-induced leak respiration [8,32–34,41–43].
Figure 4. Uncoupled respiration in the absence of UCP1 is partially due to gating of the mitochondrial permeability transition pore (PTP).
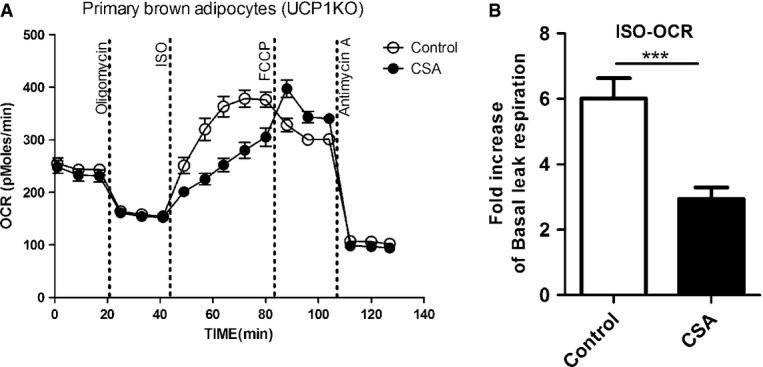
- Representative time course of oxygen consumption rates (OCR) of primary brown adipocytes from knockout (KO) mice. Adipocytes were pretreated with the PTP inhibitor cyclosporine A (CSA) (5 μg/ml, 72 h) or vehicle prior to bioenergetic profiling.
- A significant portion of ISO-induced respiration is inhibited by CSA pretreatment.
Data information: All data presented are mean values ± SEM (N = 3). ***P < 0.001.
Specific measurement of UCP1-mediated uncoupled respiration is enabled by scavenging of free fatty acids with bovine serum albumin (BSA)
To discriminate the specific contribution of UCP1 to brown adipocyte thermogenesis from other leak respiration mechanisms, we took control over the accumulation of FFAs by adding essentially fatty acid-free BSA to the respiration medium. It is known that the intracellular accumulation of FFAs can be controlled by varying the extracellular BSA concentration [44–48]. The ISO-stimulated leak respiration was significantly diminished when scavenging extracellular FFAs released by lipolysis with BSA in a dose-dependent manner in both primary brown and brite adipocytes (Supplementary Fig S3). Since 2% BSA was as effective as 3% in blocking the unspecific leak respiration induced by FFA, we conclude that these are optimal conditions to examine specific, UCP1-mediated leak respiration. Of note, the inhibitory effect of BSA on ISO-induced leak respiration is much more prominent in UCP1 KO cells, leading to significant lower levels of uncoupled respiration compared to WT cells in both brown and brite adipocytes (Fig 5). The difference in uncoupled respiration between WT and KO cells can be considered UCP1-mediated leak respiration.
Figure 5. Scavenging extracellular free fatty acids with 2% BSA allows to specifically measure UCP1-mediated leak respiration in primary brown and brite adipocytes.
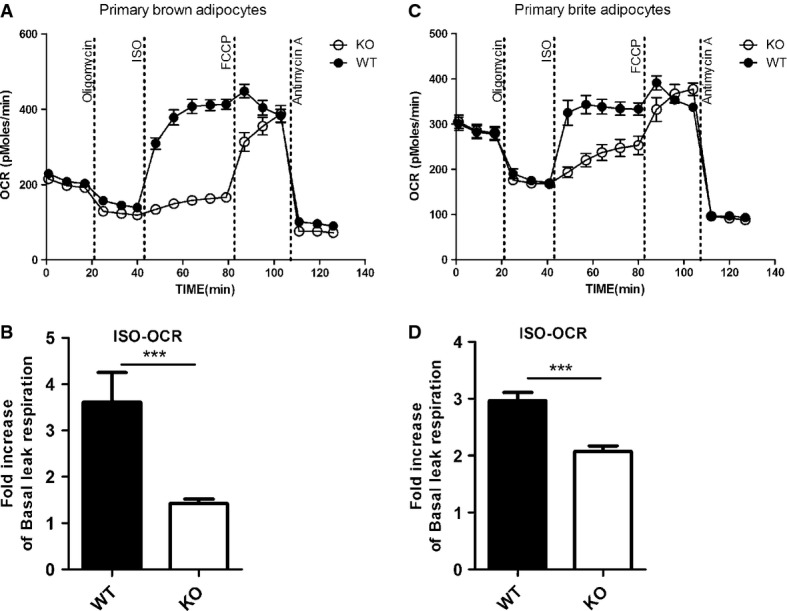
- A, B Representative time course of oxygen consumption rates (OCR) of primary brown adipocytes from UCP1 wild-type (WT) and knockout (KO) mice in the presence of 2% BSA. Comparison between WT and KO cells reveals ISO-induced leak respiration to be specific for UCP1 in brown adipocytes (B).
- C, D Representative time course of oxygen consumption rates (OCR) of primary brite adipocytes from UCP1 wild-type (WT) and knockout (KO) mice in the presence of 2% BSA. Comparison between WT and KO cells reveals ISO-induced leak respiration to be specific for UCP1 in brite adipocytes (D).
Data information: All data presented are mean values ± SEM (N = 3). ***P < 0.001.
We conclude that control over the intracellular fatty acid levels is essential for the analysis of thermogenic function in brown and brite/beige adipocytes and allows to specifically measure UCP1-mediated leak respiration. Employing this new protocol, we reproducibly quantified the UCP1-mediated component of uncoupled respiration in both brown and brite adipocytes. Importantly, brite adipocytes clearly resembled classical brown adipocytes in that they were thermogenically active in response to adrenergic stimulation. This uncoupled respiration was specific for the activity of UCP1. To our knowledge, this is the first report demonstrating that UCP1 is functionally thermogenic in intact cultured brite adipocytes. By applying this protocol, we recently revealed that strain differences in brite adipogenesis are associated with differential uncoupled respiration [49].
UCP1 activation largely depends on ATGL
It is generally accepted that lipolysis plays an important role in UCP1 activation although this concept has not been formally tested. ATGL and HSL are key enzymes involved in lipolysis. To determine the relative importance of these lipases for UCP1 activation, we compared the ISO-induced UCP1-mediated uncoupled respiration of brown adipocytes pretreated with either Atglistatin or Hi 76-0079 or both in the presence of 2% BSA. Lipase inhibition impaired ISO-induced respiration (Fig 6A). The effect of ATGL inhibition (80%), however, was much more pronounced compared with HSL inhibition (35%), demonstrating that UCP1 activity was largely dependent on ATGL rather than HSL (Fig 6B). Of note, combination of inhibitors led to an almost complete block (97%) of ISO-induced respiration, indicating a negligible contribution of phospholipases [20], previously implicated in UCP1 activation.
Figure 6. UCP1 activation largely depends on ATGL.
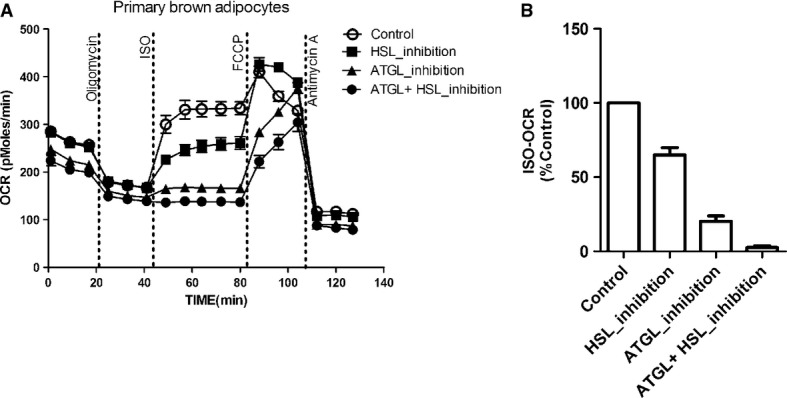
- Representative time course of oxygen consumption rates (OCR) of primary brown adipocytes from UCP1 wild-type (WT) mice in the presence of 2% BSA. Adipocytes were pretreated with 40 μM Atglistatin (ATGL inhibitor), 40 μM Hi76-0079 (HSL inhibitor) or both before bioenergetic profiling.
- The effect of ATGL inhibition on ISO-induced respiration was much larger compared to the effect of HSL inhibition.
Data information: All data presented are mean values ± SEM (N = 3).
In summary, we report that meaningful respirometric measurements of cultured brown and brite adipocytes imperatively require (i) activation of UCP1 and (ii) control over free fatty acid levels. By application of these findings, we demonstrate that UCP1 in brite adipocytes is thermogenically functional in a similar fashion as in brown adipocytes, with activation being largely dependent on adipose triglyceride lipase (ATGL).
Materials and Methods
Detailed methods can be found in Supplementary Materials and Methods.
Animals and primary cell culture
Male 129S1/SvImJ mice (UCP1-KO mice and wild-type littermates), aged 5–6 weeks, were used to prepare primary cultures of brown and brite adipocytes. Adipocyte differentiation was induced by treating confluent cells in DMEM medium (D-glucose, 25 mM) containing 10% fetal bovine serum (FBS), 0.5 mM isobutylmethylxanthine, 125 nM indomethacin, 1 mM dexamethasone, 850 nM insulin, 1 nM T3 and 1 μM rosiglitazone. After 2 days of induction, cells were maintained in differentiation media (10% FBS, 850 nM insulin, 1 nM T3 and 1 μM rosiglitazone).
Respirometry
Oxygen consumption rate (OCR) was measured at 37°C using microplate-based respirometry (XF96 extracellular flux analyzer, Seahorse Bioscience). For detailed experimental procedures, see Supplementary Materials and Methods. After the completion of an assay, the microplate was saved and protein was isolated for UCP1 phenotyping. All data presented are mean values ± SEM of three independent experiments with 8–12 replicate wells each.
Statistical analysis
Significant differences between two groups were assessed by two-tailed Student's t-test (Prism 6.0 software). A P-value < 0.05 was considered a statistically significant difference.
Acknowledgments
The Chair of Molecular Nutritional Medicine received financial support from by the Else Kröner-Fresenius Foundation. YL holds a fellowship from the Deutsche Akademische Austauschdienst (DAAD).
Funding
EU FP7 project DIABAT (HEALTH-F2-2011-278373), the Competence Network Obesity (BMBF Seed Money 01GI1325) and DFG grant KL 973/11-1 to MK.
Author contributions
YL, TF, and MK conceived the study; TS helped to establish the seahorse measurements; YL performed experiments; SS helped with the lipase inhibition experiment and Western blot analysis; YL, TF, and MK analyzed the data; YL, TF, and MK wrote the manuscript. All authors commented on the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting Information
Supplementary information for this article is available online: http://embor.embopress.org
Supplementary Figures
Supplementary Information
Supplementary Text S1
Review Process File
References
- 1.Klingenspor M, Herzig S, Pfeifer A. Brown fat develops a brite future. Obes Facts. 2012;5:890–896. doi: 10.1159/000346337. [DOI] [PubMed] [Google Scholar]
- 2.Li Y, Lasar D, Fromme T, Klingenspor M. White, brite and brown adipocytes: the evolution and function of a heater organ in mammals. Can J Zool. 2014;92:615–626. [Google Scholar]
- 3.Guerra C, Koza RA, Yamashita H, Walsh K, Kozak LP. Emergence of brown adipocytes in white fat in mice is under genetic control. Effects on body weight and adiposity. J Clin Invest. 1998;102:412–420. doi: 10.1172/JCI3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Himms-Hagen J, Melnyk A, Zingaretti MC, Ceresi E, Barbatelli G, Cinti S. Multilocular fat cells in WAT of CL-316243-treated rats derive directly from white adipocytes. Am J Physiol Cell Physiol. 2000;279:C670–C681. doi: 10.1152/ajpcell.2000.279.3.C670. [DOI] [PubMed] [Google Scholar]
- 5.Young P, Arch JRS, Ashwell M. Brown adipose tissue in the parametrial fat pad of the mouse. FEBS Lett. 1984;167:10–14. doi: 10.1016/0014-5793(84)80822-4. [DOI] [PubMed] [Google Scholar]
- 6.Cousin B, Cinti S, Morroni M, Raimbault S, Ricquier D, Penicaud L, Casteilla L. Occurrence of brown adipocytes in rat white adipose tissue: molecular and morphological characterization. J Cell Sci. 1992;103:931–942. doi: 10.1242/jcs.103.4.931. [DOI] [PubMed] [Google Scholar]
- 7.Xue B, Rim JS, Hogan JC, Coulter AA, Koza RA, Kozak LP. Genetic variability affects the development of brown adipocytes in white fat but not in interscapular brown fat. J Lipid Res. 2007;48:41–51. doi: 10.1194/jlr.M600287-JLR200. [DOI] [PubMed] [Google Scholar]
- 8.Ohno H, Shinoda K, Spiegelman BM, Kajimura S. PPARgamma agonists induce a white-to-brown fat conversion through stabilization of PRDM16 protein. Cell Metab. 2012;15:395–404. doi: 10.1016/j.cmet.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem. 2010;285:7153–7164. doi: 10.1074/jbc.M109.053942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med. 2013;19:1252–1263. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- 11.Bartelt A, Heeren J. Adipose tissue browning and metabolic health. Nat Rev Endocrinol. 2014;10:24–36. doi: 10.1038/nrendo.2013.204. [DOI] [PubMed] [Google Scholar]
- 12.Tseng YH, Cypess AM, Kahn CR. Cellular bioenergetics as a target for obesity therapy. Nat Rev Drug Discov. 2010;9:465–482. doi: 10.1038/nrd3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cypess AM, White AP, Vernochet C, Schulz TJ, Xue R, Sass CA, Huang TL, Roberts-Toler C, Weiner LS, Sze C, et al. Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nat Med. 2013;19:635–639. doi: 10.1038/nm.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lidell ME, Betz MJ, Dahlqvist Leinhard O, Heglind M, Elander L, Slawik M, Mussack T, Nilsson D, Romu T, Nuutila P, et al. Evidence for two types of brown adipose tissue in humans. Nat Med. 2013;19:631–634. doi: 10.1038/nm.3017. [DOI] [PubMed] [Google Scholar]
- 15.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 17.Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerbäck S, et al. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 18.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 19.Schweiger M, Schreiber R, Haemmerle G, Lass A, Fledelius C, Jacobsen P, Tornqvist H, Zechner R, Zimmermann R. Adipose triglyceride lipase and hormone-sensitive lipase are the major enzymes in adipose tissue triacylglycerol catabolism. J Biol Chem. 2006;281:40236–40241. doi: 10.1074/jbc.M608048200. [DOI] [PubMed] [Google Scholar]
- 20.Fedorenko A, Lishko PV, Kirichok Y. Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell. 2012;151:400–413. doi: 10.1016/j.cell.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shabalina IG, Ost M, Petrovic N, Vrbacky M, Nedergaard J, Cannon B. Uncoupling protein-1 is not leaky. Biochim Biophys Acta. 2010;1797:773–784. doi: 10.1016/j.bbabio.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 22.Shabalina IG, Petrovic N, de Jong JM, Kalinovich AV, Cannon B, Nedergaard J. UCP1 in brite/beige adipose tissue mitochondria is functionally thermogenic. Cell Rep. 2013;5:1196–1203. doi: 10.1016/j.celrep.2013.10.044. [DOI] [PubMed] [Google Scholar]
- 23.Parker N, Crichton PG, Vidal-Puig AJ, Brand MD. Uncoupling protein-1 (UCP1) contributes to the basal proton conductance of brown adipose tissue mitochondria. J Bioenerg Biomembr. 2009;41:335–342. doi: 10.1007/s10863-009-9232-8. [DOI] [PubMed] [Google Scholar]
- 24.Hirschberg V, Fromme T, Klingenspor M. Test systems to study the structure and function of uncoupling protein 1: a critical overview. Front Endocrinol (Lausanne) 2011;2:63. doi: 10.3389/fendo.2011.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brand MD, Nicholls DG. Assessing mitochondrial dysfunction in cells. Biochem J. 2011;435:297–312. doi: 10.1042/BJ20110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qiang L, Wang L, Kon N, Zhao W, Lee S, Zhang Y, Rosenbaum M, Zhao Y, Gu W, Farmer SR, et al. Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of Pparγ. Cell. 2012;150:620–632. doi: 10.1016/j.cell.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trajkovski M, Ahmed K, Esau CC, Stoffel M. MyomiR-133 regulates brown fat differentiation through Prdm16. Nat Cell Biol. 2012;14:1330–1335. doi: 10.1038/ncb2612. [DOI] [PubMed] [Google Scholar]
- 28.Sun L, Xie H, Mori MA, Alexander R, Yuan B, Hattangadi SM, Liu Q, Kahn CR, Lodish HF. Mir193b-365 is essential for brown fat differentiation. Nat Cell Biol. 2011;13:958–965. doi: 10.1038/ncb2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahfeldt T, Schinzel RT, Lee YK, Hendrickson D, Kaplan A, Lum DH, Camahort R, Xia F, Shay J, Rhee EP, et al. Programming human pluripotent stem cells into white and brown adipocytes. Nat Cell Biol. 2012;14:209–219. doi: 10.1038/ncb2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roberts LD, Boström P, O'Sullivan JF, Schinzel RT, Lewis GD, Dejam A, Lee YK, Palma MJ, Calhoun S, Georgiadi A, et al. beta-Aminoisobutyric acid induces browning of white fat and hepatic beta-oxidation and is inversely correlated with Cardiometabolic risk factors. Cell Metab. 2014;19:96–108. doi: 10.1016/j.cmet.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang J, Liu R, Wang F, Hong J, Li X, Chen M, Ke Y, Zhang X, Ma Q, Wang R, et al. Ablation of LGR4 promotes energy expenditure by driving white-to-brown fat switch. Nat Cell Biol. 2013;15:1455–1463. doi: 10.1038/ncb2867. [DOI] [PubMed] [Google Scholar]
- 32.Bordicchia M, Liu D, Amri E-Z, Ailhaud G, Dessì-Fulgheri P, Zhang C, Takahashi N, Sarzani R, Collins S. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest. 2012;122:1022–1036. doi: 10.1172/JCI59701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keipert S, Jastroch M. Brite/beige fat and UCP1 - is it thermogenesis? Biochim Biophys Acta. 2014;1837:1075–1082. doi: 10.1016/j.bbabio.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 34.Lee P, Werner CD, Kebebew E, Celi FS. Functional thermogenic beige adipogenesis is inducible in human neck fat. Int J Obes. 2014;38:170–176. doi: 10.1038/ijo.2013.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sellayah D, Bharaj P, Sikder D. Orexin is required for brown adipose tissue development, differentiation, and function. Cell Metab. 2011;14:478–490. doi: 10.1016/j.cmet.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 36.Yehuda-Shnaidman E, Buehrer B, Pi J, Kumar N, Collins S. Acute stimulation of white adipocyte respiration by PKA-induced lipolysis. Diabetes. 2010;59:2474–2483. doi: 10.2337/db10-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mayer N, Schweiger M, Romauch M, Grabner GF, Eichmann TO, Fuchs E, Ivkovic J, Heier C, Mrak I, Lass A, et al. Development of small-molecule inhibitors targeting adipose triglyceride lipase. Nat Chem Biol. 2013;9:785–787. doi: 10.1038/nchembio.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Di Paola M, Lorusso M. Interaction of free fatty acids with mitochondria: coupling, uncoupling and permeability transition. Biochim Biophys Acta. 2006;1757:1330–1337. doi: 10.1016/j.bbabio.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 39.Wojtczak L, Lehninger AL. Formation and disappearance of an endogenous uncoupling factor during swelling and contraction of mitochondria. Biochim Biophys Acta. 1961;51:442–456. doi: 10.1016/0006-3002(61)90600-x. [DOI] [PubMed] [Google Scholar]
- 40.Wojtczak L, Schonfeld P. Effect of fatty acids on energy coupling processes in mitochondria. Biochim Biophys Acta. 1993;1183:41–57. doi: 10.1016/0005-2728(93)90004-y. [DOI] [PubMed] [Google Scholar]
- 41.Lee P, Linderman JD, Smith S, Brychta RJ, Wang J, Idelson C, Perron RM, Werner CD, Phan GQ, Kammula US, et al. Irisin and FGF21 Are Cold-Induced Endocrine Activators of Brown Fat Function in Humans. Cell Metab. 2014;19:302–309. doi: 10.1016/j.cmet.2013.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu J, Boström P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tews D, Fischer-Posovszky P, Fromme T, Klingenspor M, Fischer J, Rüther U, Marienfeld R, Barth TF, Möller P, Debatin KM, et al. FTO deficiency induces UCP-1 expression and mitochondrial uncoupling in adipocytes. Endocrinology. 2013;154:3141–3151. doi: 10.1210/en.2012-1873. [DOI] [PubMed] [Google Scholar]
- 44.Vallano ML, Lee MY, Sonenberg M. Hormones modulate adipocyte membrane potential ATP and lipolysis via free fatty acids. Am J Physiol. 1983;245:E266–E272. doi: 10.1152/ajpendo.1983.245.3.E266. [DOI] [PubMed] [Google Scholar]
- 45.Kampf JP, Kleinfeld AM. Fatty acid transport in adipocytes monitored by imaging intracellular free fatty acid levels. J Biol Chem. 2004;279:35775–35780. doi: 10.1074/jbc.M403630200. [DOI] [PubMed] [Google Scholar]
- 46.Angel A, Desai KS, Halperin ML. Reduction in adipocyte ATP by lipolytic agents: relation to intracellular free fatty acid accumulation. J Lipid Res. 1971;12:203–213. [PubMed] [Google Scholar]
- 47.Cushman SW, Heindel JJ, Jeanrenaud B. Cell-associated nonesterified fatty acid levels and their alteration during lipolysis in the isolated mouse adipose cell. J Lipid Res. 1973;14:632–642. [PubMed] [Google Scholar]
- 48.Civelek VN, Hamilton JA, Tornheim K, Kelly KL, Corkey BE. Intracellular pH in adipocytes: effects of free fatty acid diffusion across the plasma membrane, lipolytic agonists, and insulin. Proc Natl Acad Sci USA. 1996;93:10139–10144. doi: 10.1073/pnas.93.19.10139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Y, Bolze F, Fromme T, Klingenspor M. Intrinsic differences in BRITE adipogenesis of primary adipocytes from two different mouse strains. Biochim Biophys Acta. 2014;1841:1345–1352. doi: 10.1016/j.bbalip.2014.06.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures
Supplementary Information
Supplementary Text S1
Review Process File


