Abstract
Juvenile hormones (JHs) are synthesized by the corpora allata (CA) and play a key role in insect development. A decrease of JH titer in the last instar larvae allows pupation and metamorphosis to proceed. As the anti-metamorphic role of JH comes to an end, the CA of the late pupa (or pharate adult) becomes again “competent” to synthesize JH, which would play an essential role orchestrating reproductive maturation. In the present study, we provide evidence that ecdysis triggering hormone (ETH), a key endocrine factor involved in ecdysis control, acts as an allatotropic regulator of JH biosynthesis, controlling the exact timing of CA activation in the pharate adult mosquito. Analysis of the expression of Aedes aegypti ETH receptors (AeaETHRs) revealed that they are present in the CA and the corpora cardiaca (CC), and their expression peaks 4 h before eclosion. In vitro stimulation of the pupal CA glands with ETH resulted in an increase in JH synthesis. Consistent with this finding, silencing AeaETHRs by RNA interference (RNAi) in pupa resulted in reduced JH synthesis by the CA of one day-old adult females. Stimulation with ETH resulted in increases in the activity of juvenile hormone acid methyltransferase (JHAMT), a key JH biosynthetic enzyme. Furthermore, inhibition of IP3R-operated mobilization of endoplasmic reticulum Ca2+ stores prevented the ETH-dependent increases of JH biosynthesis and JHAMT activity. All together these findings provide compelling evidence that ETH acts as a regulatory peptide that ensures proper developmental timing of JH synthesis in pharate adult mosquitoes.
Keywords: Juvenile hormone, mosquito, biosynthesis, corpora allata, ecdysis triggering hormone
INTRODUCTION
Juvenile hormones (JHs) play a key role in insect development (Riddiford, 2012; Goodman and Cusson, 2012). JH postpones metamorphosis of insect larvae until they have attained an appropriate stage and size. Then, during the final larval instar, a drop in JH secretion permits a metamorphic molt (Smykal et al., 2014). JHs are synthesized by the corpora allata (CA), a pair of endocrine glands with neural connections to the brain (Tobe and Stay, 1985). In Aedes aegypti mosquitoes the CA is seemingly inactive for most of the duration of the pupal stage (Nouzova et al., 2011; Rivera-Perez et al, 2014). As the anti-metamorphic role of JH comes to an end, the CA of the late pupa (or pharate adult) is reactivated and becomes “competent” to synthesize JH. This is a period of dramatic developmental changes as the female mosquito prepares itself for its adult life, where JH would have an essential role orchestrating reproductive maturation (Klowden, 1997).
After adult eclosion, female mosquitoes must mature before they can produce eggs in response to a blood meal. The posteclosion (PE) development of midgut, fat body and ovaries, three critical tissues that are involved in blood meal digestion and reproduction, are JH-dependent processes (Klowden et al., 1997; Noriega, 2004). JH-dependent ovarian previtellogenic maturation involves changes in primary follicles, nurse cells and follicular epithelium (Gwadz and Spielman, 1973; Raikhel and Lea, 1985, 1991). Extensive JH-dependent cellular remodeling has also been described in the fat body (Raikhel and Lea, 1983, 1990) and midgut (Rossignol et al., 1982; Noriega et al., 1997). JH controls the PE maturation of mosquito tissues in time-dependent and titer-dependent manner by regulating three major gene clusters named early-, mid- and late-posteclosion JH-dependent clusters (Riddiford, 2013). Transcription of genes in these clusters is controlled by low, intermediate and high JH titers respectively. In the fat body, many of early-PE and mid-PE genes are primarily involved in carbohydrate and lipid metabolism, whereas the late genes are primarily important for the buildup of the protein-synthesizing machinery (Zou et al., 2013; Riddiford, 2013).
The activation of JH synthesis during the transition from pupa to adult is a two-step process. An initial developmentally-regulated moderate increase occurs at eclosion (Rivera-Perez et al., 2014). Only if teneral nutrient are above a particular threshold, a nutritionally-regulated major increase follows around 12h post-ecdysis (Caroci et al., 2004; Rivera et al., 2014). For pharate adult mosquitoes the exact timing of CA activation is critical and it has to be developmentally coordinated with eclosion. Neuroendocrine factors involved in the regulation of ecdysis, such as ecdysis triggering hormone (ETH), would be perfect molecules to synchronize CA activation with molting to adult.
ETH is a small C-terminally amidated peptide that is released into the hemolymph to activate pre-ecdysis and ecdysis motor programs in the central nervous system (Žitňan et al., 1999; Žitňan and Adams, 2012). Specialized endocrine cells called Inka cells synthesize ETH (Adams et al., 2013; Žitňan et al., 2007). The genes encoding ETH and ETH receptors (ETHR) appear to be under tight regulation by 20-hydroxyecdysone (20E) (Žitňan and Adams, 2012). In A. aegypti larvae levels of ETHR transcripts rise in synchronism with steroid levels (Dai and Adams, 2009; Margam et al., 2006). 20E regulates not only ETHR expression, but also synthesis of ETHs as well as release of these peptides from Inka cells. A sharp increase in ETH levels in Inka cells of the moth Manduca sexta occurs immediately after steroids rise at molt initiation, and injection of ecdysteroids into freshly molted animals induces production of ETHs (Žitňanová et al., 2001). The presence of an ecdysteroid response element (EcRE) in the promoter region of several ETH genes suggests direct regulation of the ETH gene by 20E (Park et al., 1999; Žitňan et al., 2002, 2003). On the other hand, declining steroid levels are required for secretory competence of Inka cells (Kingan et al., 1997; Kingan and Adams, 2000). ETH receptors are highly expressed in the CA of the moths Bombyx mori and Manduca sexta (Yamanaka et al., 2008); so it has been suggested that ETH could play a role in the regulation of JH biosynthesis (Yamanaka et al., 2008).
In the present study, we provide evidence that ETH acts as an allatotropic regulator of JH III biosynthesis, controlling the exact timing of CA activation in the pharate adult mosquito. Analysis of the expression of A. aegypti ETH receptors (AeaETHRs) revealed that they are present in the CA and the corpora cardiaca (CC), and their expression peaks 4 h before adult ecdysis. In vitro stimulation of the pupal CA glands with ETH resulted in an increase in JH synthesis. Consistent with this finding, silencing AeaETHRs by RNA interference (RNAi) in pupa resulted in reduced JH synthesis by the CA of one day-old adult females. Stimulation with ETH resulted in an increase in the activity of juvenile hormone acid methyltransferase, a key JH biosynthetic enzyme. Furthermore, we provide evidence that ETH increases JH synthesis by mobilizing calcium from intracellular stores, since inhibition of IP3R-operated mobilization of endoplasmic reticulum Ca2+ stores prevented the ETH-dependent increase of JH synthesis, as well as the increase of JHAMT activity. All together these findings provide compelling evidence that ETH acts as a regulatory peptide ensuring proper timing of JH biosynthesis in pharate adult mosquitoes.
MATERIALS AND METHODS
Insects
A. aegypti of the Rockefeller strain were reared at 28 °C and 80% humidity as previously described (Nouzova et al., 2011). Adult mosquitos were offered a cotton pad soaked in a 3% sucrose solution.
Chemicals
Custom made peptide A. aegypti ETH1 (Aea-ETH1) (DETPGFFIKLSKSVPRI-NH2) was provided by Biopeptide Co. (San Diego, CA), purified by reverse phase liquid chromatography and assessed to be 98% pure by analytical mass spectrometry and amino acid analysis. Stock of aqueous solutions of Aea-ETH1 were prepared at a concentration of 10−5 M and stored in aliquots at −80 °C. Injection of AeaETH1 induced premature ecdysis behavior in pharate adult confirming the biological activity of our synthetic peptide. 2-amino-ethoxydiphenyl borate (2-APB) was from Sigma (St. Louis, MO).
Dissections of corpora allata complexes
Pharate adult female mosquitos were cold-anesthetized and dissected in Aedes physiological saline (APS) (138 mM NaCl, 8.4 mM KCl, 4 mM CaCl2, 2 mM MgCl2, 12 mM NaH2PO4, 12 mM Na2HPO4 and 42.5 mM sucrose) as previously described (Li et al., 2003). Unless otherwise noted, preparations were of intact corpora allata-corpora cardiaca (CA-CC) complexes connected to the brain and head capsule and are denoted as BR-CA-CC complexes. Two additional preparations of CA complexes for in vitro experiments were used: (1) “denervated” CA-CC complexes, in which the CA-CC were separated from the brain; and (2) “isolated” CA, in which the CA was isolated from both the brain and the CC (Nouzova et al., 2012).
Quantitative Real-Time PCR (q-PCR)
Total RNA was isolated using RNA-binding glass powder as previously described (Noriega and Wells, 1993). Contaminating genomic DNA was removed using the DNA-free™ kit (Ambion, Austin, TX). Reverse transcription of RNA was carried out using Qscript (Quanta BioSciences, Gaithersburg, MD), according to the manufacturer’s recommendations. Relative expression of selected genes was quantified by real-time PCR performed in a 7300 Real-Time PCR System using TaqMan® Gene Expression Assays together with TaqMan® Universal PCR Master Mix (Applied Biosystems, Foster City, CA). Reactions were run in triplicate in a 20 μl volume and normalized to the house keeping gene 60S ribosomal protein L32 (rpL32) mRNA expression for each sample. Primer probes sequences for L32, adipokinetic hormone (AKH), crustacean cardioacceleratory peptide (CCAP), juvenile hormone acid methyltransferase (JHAMT), epoxidase (EPOX) and ecdysis triggering hormone receptor (AeaETHRs) are provided in the Supplemental table 1.
Testing the effect of ETH on JH synthesis
BR-CA-CC complexes were dissected and incubated in the presence of different concentrations of ETH (10−6 to 10−12 M) at 32°C for 4 h in 150 μl of tissue culture media M-199 (Lavallette, NJ, USA) containing 2% Ficoll, 25 mM HEPES (pH 6.5) and 50 μM methionine. Controls were not treated with the peptide. Biosynthesized JH III was labelled with a fluorescent tag and analyzed by reverse phase high performance liquid chromatography coupled to a fluorescent detector (HPLC-FD) as previously described (Rivera-Perez et al., 2012).
Juvenile hormone acid methyltransferase activity assay
Five CA were dissected in APS and transferred to 100 μl of Tris-HCl buffer (50 mM, pH 7.4), sonicated in a water bath sonicator for 3 min, placed on ice for 1 min and centrifuged (13, 000 g, 10 min, 4 °C). Supernatants (crude extract) were collected. Juvenile hormone acid methyltransferase activity in crude extracts was tested by adding farnesoic acid (FA) to a final concentration of 50 μM, and S-adenosyl methionine (SAM) to a final concentration of 10 μM. Samples were incubated for 2 hours in a water bath at 37 °C (Rivera-Perez et al., 2014). Reactions were stopped by adding 500 μl of hexane. Samples were vortexed for 1 min and centrifuged (13, 000 g, 10 min, 4°C). The organic phase was recovered and filtered into a new Eppendorf tube. Lastly, samples were dried with N2 and stored at −20 °C until analyzed. Conversion of FA into methyl farnesoate (MF) was determined by HPLC (Rivera-Perez et al., 2014).
Synthesis and microinjection of dsRNA
Target sequences for double-stranded RNA (dsRNA) were designed against a common region of both splice variants of ETHR (AeaETHR-A and AeaETHR-B), as well as against the Yellow Fluorescent Protein (YFP). Targeted regions were amplified by PCR using the primers included in Supplemental table 1. The dsRNAs were produced using T7 RNA polymerase and the MEGAscript RNAi kit (Ambion, Austin, TX) as previously described (Perez-Hedo et al., 2013). Newly pupated females (2 hours after pupation) were cold-anesthetized and injected intrathoracically with 0.96 μg of dsRNA.
Immunohistochemistry
Immunohistochemical detection of ETH in tissues of pharate-adult and newly emerged female mosquitoes were performed using an antiserum against Manduca sexta pre-ecdysis-triggering hormone (PETH) as described by Žitňan et al. (2003). Abdomens and thoraxes were fixed overnight in 4% paraformaldehyde in phosphate buffered saline (PBS, pH 7.4). Tissues were washed with PBS + 0.3% Triton X-100 (PBST), blocked with 5% milk in PBS and incubated for 48 h with rabbit antiserum against PETH (1:1000). Afterwards, tissues were washed with PBST and incubated overnight with goat anti-rabbit immunoglobulin G (DyLight 594 IgG; Thermo Scientific) diluted 1:1000. Labelled tissues were washed in PBST and mounted in glycerol containing 4′,6′-diamino-2-phenylindole (DAPI; 2 μg/ml) (Sigma, St Louis, MO). Photographs were taken using a DM 5500 B Leica fluorescence microscope with a Leica DFC 310 FX mounted camera and Leica LAS imaging software.
Statistical analysis
Statistical analyses were performed using the GraphPad Prism Software (San Diego, CA, USA). The results are expressed as means ± S.E.M. Significant differences (p< 0.05) were determined with a one tailed students t-test performed in a pair wise manner or by one-way ANOVA followed by Tukey’s test.
RESULTS
The pupal stage last 50 h in females
Female pupae were collected at 30 min intervals as they molted from fourth instar larvae into the pupal stage. The duration of the pupal stage was determined by scoring at hourly intervals the number of eclosing adults. The eclosion to adult stage began 46 h after pupation (1%), with the majority of insects emerging at 50 h (40%) and the last at 53 h (1.5%) (Supplemental Fig. 1).
PETH-immunoreactivity in Inka cells decreases at eclosion
Inka cells exhibited strong PETH-like staining in pupa 2 h before ecdysis (Fig. 1A). On the other hand the immunoreactivity disappeared when tissues were stained immediately after ecdysis (0 h) (Fig. 1B); indicating that Inka cells had released most of their ETH contents during ecdysis. The PETH antibody has cross-reactivity with peptides containing a conserved amidated sequence motif (RXamide or PRX amide; X = I, L, M, V), and therefore stains cells in the abdominal ganglia of mosquitoes. Samples from 0 h and 2 h were always processed simultaneously, so we were able to verify that the antibody staining was always working well; still the staining of the ventral ganglia was used as a positive control for the immunohistochemical studies in samples from newly eclosed females (Fig. 1C).
Fig. 1. PETH-immunoreactivity in Inka cells.
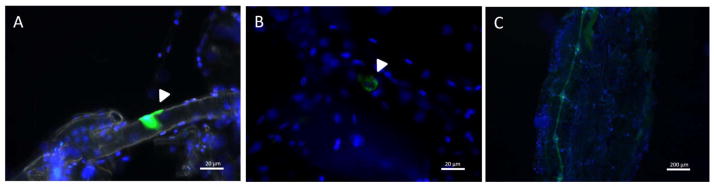
(A) Intense immunoreactivity was observed in Inka cells from −2 h pharate adult. Scale bar, 20 μm. (B) Staining almost disappeared in Inka cell just after ecdysis (0h). Scale bar, 20 μm. (C) Intense immunoreactivity was observed in ventral ganglia just after ecdysis (0h); this is due to antibody cross reactivity and does not represent PETH staining. Scale bar, 200 μm. These are representative images of 3 independent experiments evaluating 5 insects of each stage (−2 and 0 h).
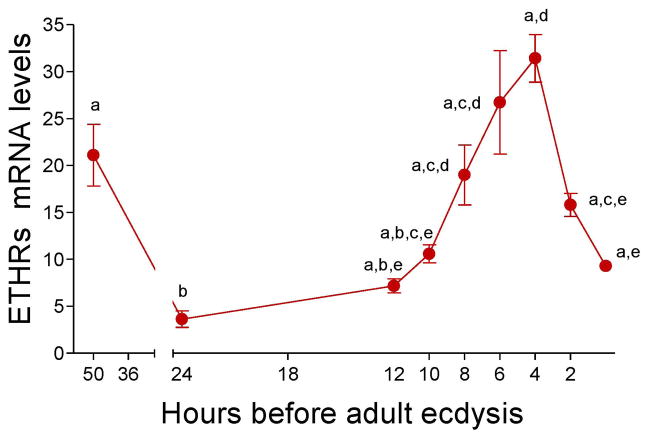
Expression of AeaETH receptor mRNAs in the CA peak 4 hours before adult eclosion
Real-time PCR was used to analyze the combined expression of the two AeaETH receptors (AeaETHR-A and AeaETHR-B) in CA-CC complexes during the development of female pupa (Fig. 2). AeaETHR mRNA levels exhibited two peaks, one shortly after pupation at −50 h and the second before adult eclosion at −4 h.
Fig. 2. Developmental expression of AeaETH-receptors.
ETHR transcripts in CC-CA complexes during the pupal stage were measured using q-PCR. Levels of ETHR mRNA are expressed as copy number of AeaETHR mRNA/10,000 copies of rpL32 mRNA. Each q-PCR data point is the mean ± S.E.M. of two independent biological replicates of 20 CA-CC complexes. Significant differences (p< 0.05) were determined by one-way ANOVA followed by Tukey’s test.
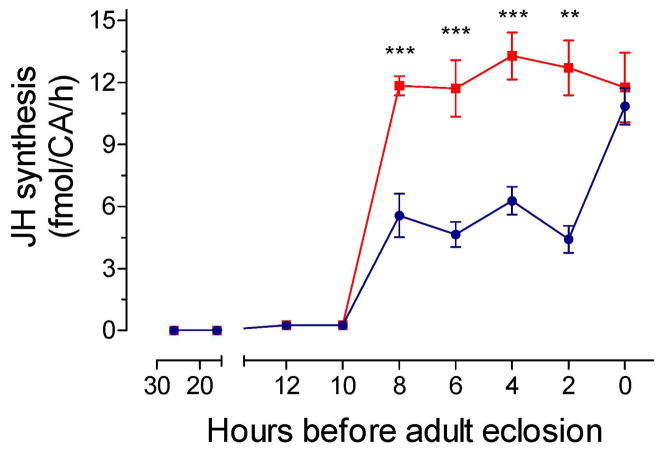
AeaETH1 stimulates JH synthesis by BR-CA-CC complexes dissected from pharate adults
The effect of increasing concentrations of AeaETH1 on JH synthesis was analyzed on BR-CA-CC complexes dissected from −2 h pupa. A series of ETH concentrations from 10−6 to 10−12 M were tested using the in vitro JH biosynthesis assay. A concentration of 10−9 M was selected for all future assays since it significantly increased JH biosynthesis (Supplemental Fig. 2), and it is consistent with the previously described EC50 of AeaETHRs for AeaETH1 and AeaETH2 (Dai and Adams, 2009). Pupal BR-CA-CC complexes dissected from −50 h to −10 h prior to adult eclosion did not synthesize detectable amounts of JH III and were insensitive to AeaETH1 stimulation. On the contrary, addition of AeaETH1 to BR-CA-CC complexes dissected from late pupa ranging from −8 h to −2 h before eclosion showed significantly higher levels of JH biosynthesis in response to AeaETH1 treatments when compared to controls (Fig. 3). Finally, ETH1 was unable to increase JH synthesis when applied to BR-CA-CC complexes dissected from newly emerged adult females (0 h) because synthesis already is at the maximum levels normally observed during the first hours after adult eclosion (Rivera-Perez et al., 2014).
Fig. 3. Effect of ETH on JH synthesis during pupal stage.
JH biosynthesis by Br-CA-CC complexes with and without addition of ETH (10−9 M) was assayed in vitro using HPLC-FD detection method. Each data point represents the mean ± S.E.M. of 3–8 independent determinations of individual Br-CA-CC complexes. Asterisks denote significant differences (unpaired t-test; ** P ≤ 0.01; *** P ≤ 0.001)
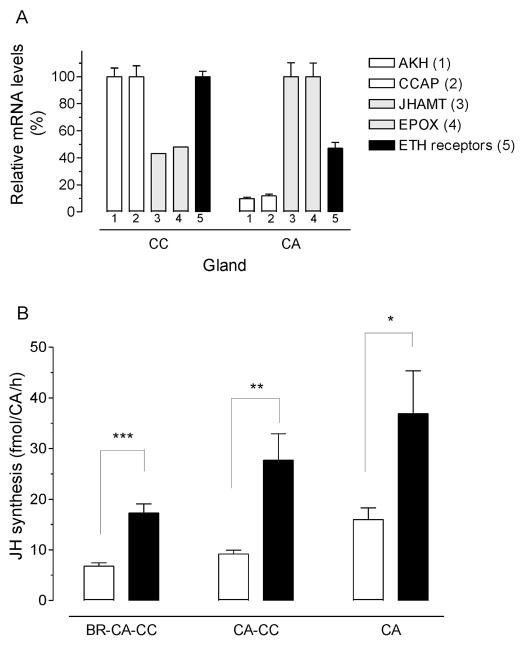
Direct effect of ETH1 on JH synthesis by isolated CA
The very low level of expression of AeaETHR mRNA in the CA made it difficult the use of in situ hybridization as the method of choice to investigate the expression of the receptor in the CA and the CC. Alternatively, to investigate the specific expression of the AeaETHR in the individual components of the CA-CC complexes, we decided to perform a surgical separation of the CA and CC, followed by analysis of expression of specific gene markers for CA and CC in the isolated glands. The minute size of the CC made it difficult to obtain glands that were not contaminated with tissue from the larger CA gland; on the other hand our CA samples had only minor contaminations with CC tissue. As a result, we expected that genes normally expressed in the CC were going to be highly enriched in the CC samples, with a very low relative expression in the CA samples. On the other hand, transcripts expressed in the CA were going to be enriched in this gland, but also present in significant amounts in the CC sample. This is exactly what it was observed (Fig. 4A). CC markers such as adipokinetic hormone (AKH) and crustacean cardioacceleratory peptide (CCAP) (Gäde and Goldsworthy, 2003) had very low expression in the CA (about 10%) when compared with transcript levels in the CC. Juvenile hormone biosynthetic enzymes, such as juvenile hormone acid methyltransferase (JHAMT) and methyl farneosate epoxidase (EPOX), that are known to be expressed in the CA (Ueda et al., 2009), were enriched in the CA, but also present as contaminants in the CC samples (about 40–45% of the CA levels). There was a high expression of AeaETHR in the CC, much higher that the levels of the CA contaminants (JHAMT and EPOX); and there was an important expression of AeaETHR in the CA, which was significantly higher that the expression of CC contaminants (AKH and CCAP), suggesting that the ETH receptor is also expressed in the CA.
Fig. 4. Tissue localization of AeaETH-receptor and effect of ETH on different CA preparations.
Corpora allata (CA) and corpora cardiaca CC were individually dissected from pharate adult female mosquitoes. A) Expression of the AeaETHRs and four additional marker genes in CC and CA. AKH (1): adipokinetic hormone; CCAP (2): crustacean cardioacceleratory peptide; JHAMT (3): juvenile hormone acid methyltransferase; EPOX (4): epoxidase; ETH receptors (5): ecdysis triggering hormone receptors. Transcripts levels are expressed as percentage of the maximum value found in the two glands. Each q-PCR data point is the mean ± S.E.M. of two independent biological replicates of 20 tissue samples. B) Different types of CA preparations were incubated with AeaETH-1 (10−9 M), and JH biosynthesis was evaluated by HPLC-FD. Each data point represents the means ± S.E.M. of 2–4 independent determinations. Asterisks denote significant differences (unpaired t-test; *P ≤ 0.05;**P ≤ 0.01; *** P ≤ 0.001).
The effect of AeaETH1 on JH biosynthesis was tested in three different types of CA preparations: BR-CA-CC, “denervated” CA-CC and isolated CA. Treatment with AeaETH1 caused significantly increases of JH synthesis in the three types of CA preparations (Fig. 4B). Separations of the CA from the CC and brain caused by itself an increase in JH biosynthesis in the untreated samples; but treatment with AeaETH1 resulted in a significant increase of JH synthesis by the isolated CA.
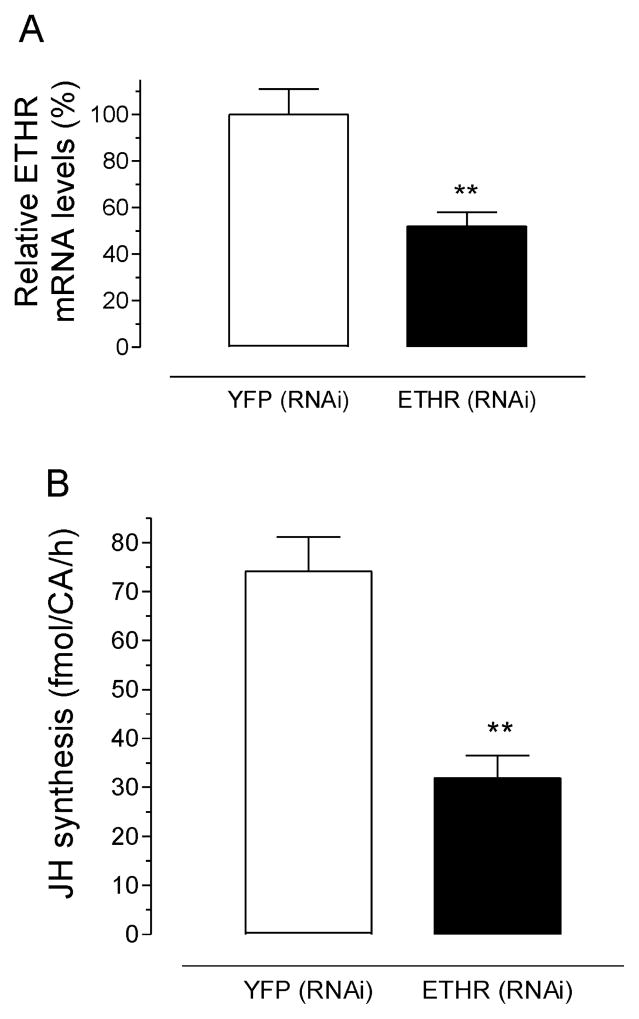
RNAi-mediated depletion of ETH receptor mRNA in pupa results in a reduction of JH synthesis by BR-CA-CC complexes of adult females
Early pupae (− 48 h) were injected with 1) dsRNA targeting the 2 isoforms of AeaETH receptors (AeaETHR-A and AeaETHR-B), or 2) a control dsRNA for YFP. To verify the efficiency of RNAi-mediated in vivo depletion of AeaETHRs, abdomens from 18 h old-adult females were used to assess AeaETHR transcript amounts. AeaETHR transcript levels were significantly decreased (52%) when compared with YFP controls (Fig. 5A). BR-CA-CC complexes dissected from 18 h old adult females treated with AeaETHR dsRNA produced significantly less JH than that from YFP controls (Fig. 5B).
Fig. 5. Effect of AeaETHRs RNAi on JH biosynthesis.
A) AeaETHRs RNA levels were evaluated in abdomens 18 h after emergence. Each data point represents the mean ± S.E.M. of 5 biological replicates. Asterisks denote significant difference (unpaired t-test; **P ≤ 0.01). B) Effect of AeaETHRs RNAi on JH biosynthesis. BR-CA-CC were dissected 18 h after emergence (~66 h post injection) and incubated for 4 h in tissue culture media. JH levels were measured using HLPC-FD. Each data point represents the mean ± S.E.M. of six independent determinations of three CA complexes. Asterisk denote significant differences (unpaired t-test; **P ≤ 0.01).
ETH stimulates JHAMT activity in CA extracts of pharate adult
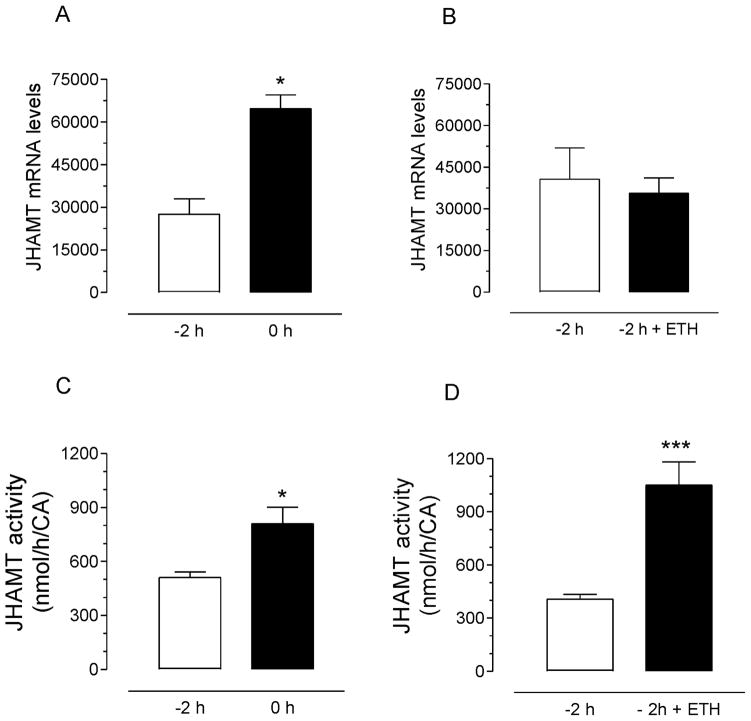
Juvenile hormone acid methyltransferase mRNA levels in CA dramatically increased during the last 4 hours in pharate adults (Supplemental Fig. 3); corresponding well with the increases in JHAMT enzymatic activities previously described (Rivera-Perez et al., 2014). Significant increases in JHAMT transcripts and enzymatic activities in the CA of pupae were detected during the last 2 hours before eclosion (Fig. 6A and C). CA were dissected from pharate adults two hours before eclosion and incubated in vitro in the presence of ETH. AaeETH1-treatment did not result in an increase in JHAMT transcript levels, but triggered a very significant increase in JHAMT activity (Fig. 6B and D).
Fig. 6. Effect of ETH on JHAMT mRNA levels and enzymatic activity.
A) Endogenous JHAMT mRNA levels in CA-CC complexes dissected at −2 h and 0 h. B) Effect of AeaETH on JHAMT mRNA levels in CA-CC complexes dissected at −2 h and 0 h. Each data point in A and B represents the mean ± S.E.M. of 2 biological replicates. Asterisk denotes significant difference (unpaired t-test; *P ≤ 0.05). C) Endogenous JHAMT enzymatic activity in CA-CC extracts dissected at −2 h and 0 h. D) Effect of AeaETH on JHAMT enzymatic activity in CA-CC extracts dissected at −2 h and 0 h. Each data point in C and D represents the mean ± S.E.M. of three independent determinations of five CA complex. Asterisk denotes significant difference (unpaired t-test; * P ≤ 0.05; *** P ≤ 0.001).
Inhibition of IP3R-operated mobilization of endoplasmic reticulum Ca2+ stores prevents the ETH-dependent increases of JH synthesis and JHAMT activity
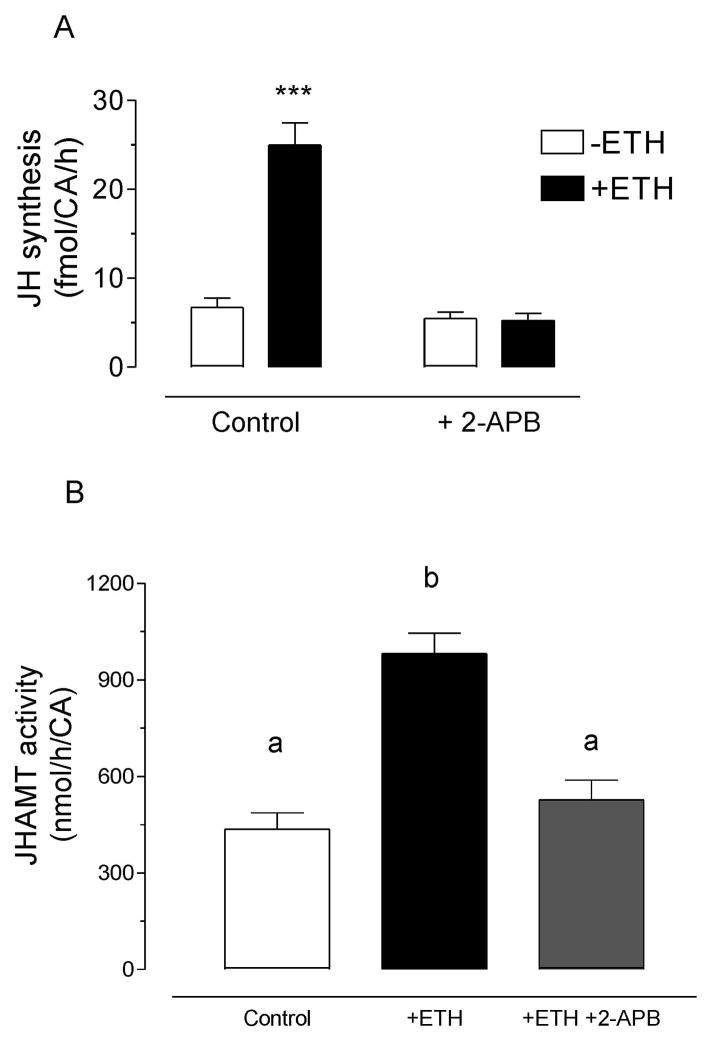
To test the hypothesis that activation of ETH receptors increases JH synthesis by mobilizing calcium from intracellular stores, we evaluated the effect of 2-APB, an antagonist of the IP3 receptor and a regulator of store-operated Ca2+ entry (SOC) channels in the endoplasmic reticulum (ER). 2-APB blocked the ETH-dependent stimulation of JH biosynthesis (Fig. 7). In addition, 2-APB effectively blocked the ETH-mediated increase in JHAMT activity.
Fig. 7. Effect of inhibition of IP3R-operated mobilization of endoplasmic reticulum Ca2+ stores on the ETH-dependent increases of JH synthesis and JHAMT activity.
A) Effect of 2-APB on JH synthesized in vitro: CA were dissected from pharate adult females and incubated for 4 h in presence of ETH and 2-APB. Each data point represents the mean ± S.E.M. of 3 independent determinations. Asterisk denotes significant difference (unpaired t-test; *** P ≤ 0.001). B) Effect of 2-APB on JHAMT activity in CA-CC extracts: Br-CA-CC were dissected from pharate adult females and incubated for 4 h in presence of ETH and 2-APB. Consequently CA-CC extracts were prepared and JHAMT activity was measured. Each data point represents the mean ± S.E.M. of 3 independent determinations of individual CA-CC complexes. Different letters above the columns indicate significant differences among treatments (ANOVA P<0.05, with Tukey’s test for multiple comparison).
DISCUSSION
Mosquito post-eclosion increase in JH biosynthesis is a developmentally and nutritionally regulated two-step process
The activation of JH synthesis in female adult mosquitoes occurs in 2 steps (Rivera-Perez et al., 2014). First there is 2-fold increase in JH synthesis that coincides with eclosion and brings JH synthetic rates to a value of 10–15 fmol/h. We would like to propose that this increase is the result of a developmentally regulated ETH-dependent process. It functions as a signal that indicates the female has eclosed to the adult stage and should activate the early-posteclosion JH-dependent gene cluster. A major increase follows around 12 h post-ecdysis, but only if teneral nutrients are above a particular threshold (Caroci et al., 2004; Rivera et al., 2014). Decapitation during these first 12 h of imaginal life prevents this nutritionally-dependent second increase of JH synthesis, suggesting that the brain plays a key role sensing the nutritional status and stimulating CA to its maximum activity (Hernandez-Martinez et al., 2007). Only when reserves are appropriate would the brain command the CA to synthesize enough JH to activate previtellogenic reproductive maturation (Caroci et al., 2004; Hagedorn et al., 1977). This second activation seems to be an “all-or-nothing” process that requires a general increase in flux efficiency and therefore concurs with increases of activities of all the JH synthetic enzymes (Rivera-Perez et al., 2014). The two-step activation guarantee that a proper rise of JH synthesis concurs with adult eclosion to induce expression of the early cluster of genes, without allocating resources to reproduction. So we can define the activation of JH biosynthesis as a process “associated” to ecdysis, and its correct timing is critical. Factors involved in the initiation and scheduling of the ecdysis sequence, such as ETH, are ideal to time CA activation with molt.
Ecdysis triggering hormone ensures proper timing of juvenile hormone biosynthesis in pharate adult mosquitoes
Previous work on Bombyx mori neuropeptide GCPRs has shown that the CA of 4th and 5th instar larva have high expression of ETH receptors, suggesting a role of ETH in the regulation of JH biosynthesis (Yamanaka et al., 2008; Hiruma and Kaneko, 2013). In the yellow fever mosquito, two distinct AeaETHR are encoded by a single gene via alternative splicing of two 3′-exons (Dai and Adams, 2009). Our studies have proved the presence of ETH receptors transcripts in the CA-CC complexes of A. aegypti pharate adult; revealing a very significant increase in mRNA levels in the last hours before eclosion. The presence of ETH receptors suggested that the CA-CC complex could be sensitive to the cognate peptide, and prompted us to test its effect using our JH biosynthesis in vitro assay (Li et al., 2003; Rivera-Perez et al., 2012).
Behavioral studies have previously shown that both synthetic AeaETHs (1 and 2) triggered ecdysis behavioral sequences in larval and pupal mosquitoes when injected prematurely (Dai and Adams, 2009). AeaETH-1 and -2 are 17-mers with a highly conserved sequence; differing at only one position in the C-terminal 12-mer, and explaining why both peptides were equipotent as activators of the AeaETHRs and in their ability to induce ecdysis behavior (Dai and Adams, 2009). Our studies revealed that AeaETH-1 also induced premature JH III biosynthesis in vitro when Br-CC-CAs were dissected from pharate adult females during a period ranging from −8 h to −2 h prior to eclosion. In contrast, ETH failed to stimulate JH synthesis by CA dissected from newly pupated females (−50 h), as well as from −24, −12 h or −10h pupae; indicating that regardless of the levels of ETH receptor transcripts, JH stimulation by ETH is not feasible before the transcriptional activation of the genes encoding JH biosynthetic enzymes (Nouzova et al., 2011).
Although we detected the expression of AeaETHR mRNA in CC and CA glands, the strong stimulation of JH biosynthesis observed when ETH was applied to “isolated CAs”, suggests a direct interaction of ETH ligand with receptors in the CA. The decrease in JH biosynthesis observed in AeaETHR-depleted adult females further supported a modulatory effect of ETH on CA activity. RNAi-dependent knock-down efficiency of ETH transcripts was verified by real-time q-PCR to be ~ 48 %. In spite of this moderate knock-down efficiency, ETHR silencing resulted in a consistent reduction in JH III synthesis.
Mechanism of action of ETH-dependent activation of JH synthesis
There is ample evidence that calcium-dependent signaling pathways are involved in the action of many insect neuropeptides; for example prothoracicotropic hormone (PTTH) stimulates ecdysteroidogenesis in M. sexta prothoracic glands (PGs) through a signal transduction cascade that involves an early influx of Ca2+ (Fellner et al., 2005). Although PTTH-stimulated IP3 generation has not been detected in Manduca PGs (Girgenrath and Smith, 1996), 2-APB blocked the Ca2+-mediated response of PTTH, suggesting that IP3 participates in the PTTH-stimulated increase in intracellular [Ca2+] (Birkenbeil, 1998).
It was previously reported that activation of AeaETHR-A and AeaETHR-B expressed in CHO-K1 cells mobilizes intracellular calcium (Dai and Adams, 2009). Similar results were described for ETHRs in Drosophila and M. sexta (Iversen et al., 2002; Kim et al., 2006a; Park et al., 2003). We established that 2-APB, an antagonist of the inositol triphosphate receptor (IP3R) and a regulator of store-operated Ca2+ entry (SOC) channels in the ER, blocked the ETH-dependent stimulation of JH biosynthesis; advocating that activation of ETH receptors mobilizes calcium from intracellular stores in the CA of mosquitoes.
How does ETH increase JH biosynthesis in the CA of the pharate adult? The biosynthetic pathway of JH-III in A. aegypti involves 13 sequential enzymatic steps and is conventionally divided into early (mevalonate pathway) and late (JH-branch) steps. The early steps follow the mevalonate pathway up to the formation of farnesyl diphosphate (FPP); in the late steps, FPP is transformed sequentially to farnesol, farnesal, farnesoic acid, methyl farnesoate (MF) and ultimately JH III (Rivera-Perez et al., 2014). JH biosynthesis is controlled by the rate of flux of isoprenoids in the pathway, which is the outcome of a complex interplay of changes in transcripts, enzyme activities and metabolites (Nouzova et al., 2011; Rivera-Perez et al., 2014). Previous studies suggested that an increase in JHAMT enzymatic activity is critical for increases in JH biosynthesis in pharate adult and newly eclosed females (Rivera-Perez et al., 2014); therefore we focused on the effect of ETH on JHAMT. ETH is not directly involved in the transcriptional activation of the JHAMT gene, as the 2.3-fold increase of JHAMT mRNA levels typically observed when −2 h and 0 h CA are compared was not prematurely induced by ETH treatment. On the other hand addition of ETH to −2 h CA resulted in a 2.6-fold increase of JHAMT enzymatic activity, with the concomitant increase of JH biosynthesis.
CONCLUSIONS
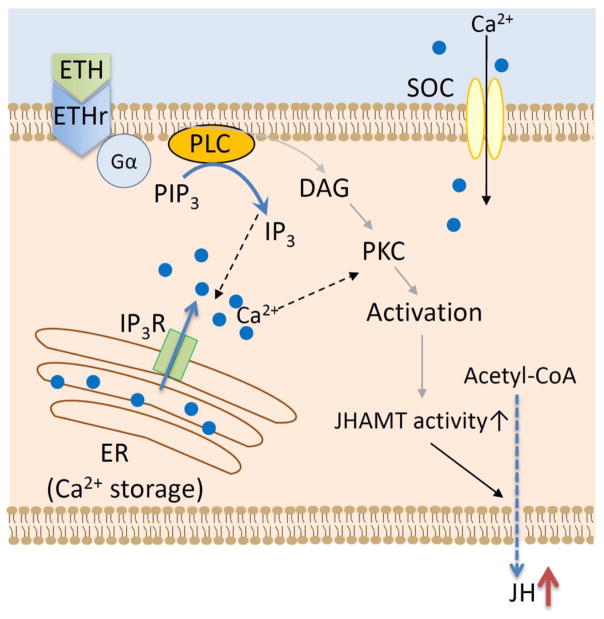
Our results support the hypothesis that ETH plays two roles in the adult pharate female mosquito: 1) orchestrates ecdysis and 2) ensures proper timing of CA activation at adult eclosion. In addition, we would like to propose that ETH modifies JH biosynthesis via a mechanism that involves IP3 activation of Ca2+ release from ER storage and stimulation of JHAMT activity (Fig. 8). This study provides valuable insight into a novel function for ETH as an allatoregulatory peptide modulating mosquito JH synthesis.
Fig. 8. Model of ETH signal transduction in CA.
Binding of ETH to the AeaETH-receptor results in increases of IP3 synthesis and IP3R-dependent release of Ca2+ from the ER. Ca2+ increases JHAMT activity and JH biosynthesis. ETH: ecdysis triggering hormone; GPCR: G-protein coupled receptor; IPI2: phosphatidylinositol 3,4 biphosphate; IP3: inositol triphosphate; IP3R: Inositol triphosphate receptor; PLC: phospholipase C; PKC: phosphokinase C; DAG: diacylglycerol; SOC: store-operated Ca2+ entry channel; ER: endoplasmic reticulum; JH: juvenile hormone.
Supplementary Material
Female pupae were collected at 30 min intervals as they molted from fourth instar larvae into the pupal stage. The duration of the pupal stage was determined by scoring at hourly intervals the number of eclosing adults. The eclosion to adult stage began 46 h after pupation (1%), with the majority of insects emerging at 50 h (40%) and the last at 53 h (1.5%). Each data point represents the mean ± S.E.M. of at least three independent determinations.
The effects of increasing concentrations of AeaETH1 on JH synthesis were tested on BR-CA-CC complexes dissected from −2 h pupa. A series of ETH1 concentrations from 10−6 to 10−12M were tested using the in vitro JH biosynthesis assay. Each data point represents the mean ± S.E.M of two independent biological replicates. Statistical analyses were performed using the GraphPad Prism Software (San Diego, CA, USA). The results are expressed as means ± S.E.M. Significant differences (p< 0.05) were determined by one-way ANOVA followed by Tukey’s test.
Juvenile hormone acid methyltransferase mRNA levels dramatically increased during the last 4 hours in pharate adults. Transcript levels were measured by Quantitative Real-Time PCR. Reactions were run in triplicate in a 20 μl volume and normalized to the house keeping gene 60S ribosomal protein L32 (rpL32) mRNA expression for each sample. Each data point represents the mean ± S.E.M. of two independent determination of 20 CA-CC complexes. Statistical analyses were performed using the GraphPad Prism Software (San Diego, CA, USA). The results are expressed as means ± S.E.M. Significant differences (p< 0.05) were determined by one-way ANOVA followed by Tukey’s test.
Accession numbers and primer sequences for the genes included in the studies.
In vitro stimulation of the pupal corpora allata with ETH resulted in an increase in JH synthesis.
Stimulation with ETH increased the activity of juvenile hormone acid methyltransferase.
Ca2+ mobilization is critical for the ETH action on JH biosynthesis and JHAMT activity.
ETH ensures proper developmental timing of JH synthesis in pharate adult mosquitoes
Acknowledgments
We thank Dr. Dusan Žitňan for providing the PETH antiserum. We thanks Dr. Michael Adams for critical reading of the manuscript. This work was supported by NIH Grant No AI 45545 to F.G.N.
Footnotes
AUTHOR CONTRIBUTIONS
MA, MN, CRP and FGN developed the concepts and approaches. MA, MN and CRP performed experiments. MA, MN, CRP and FGN did data analysis. MA, MN, CRP and FGN prepared and edited the manuscript prior to submission.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams ME, Kim YJ, Park Y, Žitňan D. Developmental Peptides: ETH, Corazonin, and PTTH. In: Kastin Abba J., editor. Handbook of Biologically Active Peptides. 2. Vol. 33. Academic Press; 2013. pp. 222–228. [Google Scholar]
- Birkenbeil H. Intracellular calcium in PTTH-stimulated prothoracic glands of Manduca sexta (Lepidoptera: Sphingidae) Eur J Entomol. 1998;96:295–298. [Google Scholar]
- Caroci A, Li Y, Noriega FG. Reduced juvenile hormone synthesis in mosquitoes with low teneral reserves prevents ovarian previtellogenic development in Aedes aegypti. J Exp Biol. 2004;207:2685–2690. doi: 10.1242/jeb.01093. [DOI] [PubMed] [Google Scholar]
- Dai L, Adams ME. Ecdysis triggering hormone signaling in the yellow fever mosquito Aedes aegypti. Gen Comp Endoc. 2009;162:43–51. doi: 10.1016/j.ygcen.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellner SK, Rybczynski R, Gilbert LI. Ca2+ signaling in prothoracicotropic hormone-stimulated prothoracic gland cells of Manduca sexta: Evidence for mobilization and entry mechanisms. Insect Biochem Mol Biol. 2005;35:263–275. doi: 10.1016/j.ibmb.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Gäde G, Goldsworthy GJ. Insect peptide hormones: a selective review of their physiology and potential application for pest control. Pest Manag Sci. 2003;59:1063–1075. doi: 10.1002/ps.755. [DOI] [PubMed] [Google Scholar]
- Girgenrath S, Smith WA. Investigation of presumptive mobilization pathways for calcium in the steroidogenic action of big prothoracicotropic hormone. Insect Biochem Mol Biol. 1996;26:455–463. doi: 10.1016/0965-1748(96)00001-x. [DOI] [PubMed] [Google Scholar]
- Goodman WG, Cusson M. The Juvenile Hormones. In: Gilbert LI, editor. Insect Endocrinology. San Diego: Academic Press; 2012. pp. 310–365. [Google Scholar]
- Gwadz RW, Spielman A. Corpus allatum control of ovarian development in Aedes aegypti. J Insect Physiol. 1973;19:1441–1448. doi: 10.1016/0022-1910(73)90174-1. [DOI] [PubMed] [Google Scholar]
- Hagedorn HH, Turner S, Hagedorn EA, Pontecorvo D, Greenbaum P, Pfeiffer D, Flanagan TR. Postemergence growth of the ovarian follicles of Aedes aegypti. J Insect Physiol. 1977;23:203–206. doi: 10.1016/0022-1910(77)90030-0. [DOI] [PubMed] [Google Scholar]
- Hernandez-Martinez S, Mayoral JG, Li Y, Noriega FG. Role of juvenile hormone and allatotropin on nutrient allocation, ovarian development and survivorship in mosquitoes. J Insect Physiol. 2007;53:230–234. doi: 10.1016/j.jinsphys.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiruma K, Kaneko Y. Hormonal Regulation of Insect Metamorphosis with Special Reference to Juvenile Hormone Biosynthesis. In: Shi YB, editor. Current Topics in Developmental Biology. Vol. 103. Academic Press; 2013. pp. 73–100. [DOI] [PubMed] [Google Scholar]
- Iversen A, Cazzamali G, Williamson M, Hauser F, Grimmelikhuijzen CJP. Molecular identification of the first insect ecdysis triggering hormone receptors. Biochem Bioph Research Commun. 2002;299:924–931. doi: 10.1016/s0006-291x(02)02798-5. [DOI] [PubMed] [Google Scholar]
- Kim YJ, Žitňan D, Galizia CG, Cho KH, Adams ME. A Command Chemical Triggers an Innate Behavior by Sequential Activation of Multiple Peptidergic Ensembles. Current Biology. 2006;16:1395–1407. doi: 10.1016/j.cub.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Kingan TG, Gray W, Zitnan D, Adams ME. Regulation of ecdysis-triggering hormone release by eclosion hormone. J Exp Biol. 1997;200:3245–3256. doi: 10.1242/jeb.200.24.3245. [DOI] [PubMed] [Google Scholar]
- Kingan TG, Adams ME. Ecdysteroids regulate secretory competence in Inka cells. J Exp Biol. 2000;203:3011–3018. doi: 10.1242/jeb.203.19.3011. [DOI] [PubMed] [Google Scholar]
- Klowden MJ. Endocrine aspects of mosquito reproduction. Arch Ins Biochem Physiol. 1997;35:491–512. [Google Scholar]
- Li Y, Hernandez-Martinez S, Unnithan GC, Feyereisen R, Noriega FG. Activity of the corpora allata of adult female Aedes aegypti: effects of mating and feeding. Insect Biochem Mol Biol. 2003;33:1307–1315. doi: 10.1016/j.ibmb.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Margam VM, Gelman DB, Palli SR. Ecdysteroid titers and developmental expression of ecdysteroid-regulated genes during metamorphosis of the yellow fever mosquito, Aedes aegypti (Diptera: Culicidae) J Ins Physiol. 2006;52:558–568. doi: 10.1016/j.jinsphys.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Noriega FG, Wells MA. A comparison of three methods for isolating RNA from mosquitoes. Ins Mol Biol. 1993;2:21–24. doi: 10.1111/j.1365-2583.1993.tb00121.x. [DOI] [PubMed] [Google Scholar]
- Noriega FG, Shaa D, Wells M. Juvenile Hormone controls early trypsin gene expression in the midgut of Aedes aegypti. Insect Mol Biol. 1997;6:63–66. doi: 10.1046/j.1365-2583.1997.00154.x. [DOI] [PubMed] [Google Scholar]
- Noriega FG. Nutritional regulation of JH synthesis: a mechanism to control reproductive maturation in mosquitoes? Ins Biochem Mol Biol. 2004;34:687–693. doi: 10.1016/j.ibmb.2004.03.021. [DOI] [PubMed] [Google Scholar]
- Nouzova M, Edwards MJ, Mayoral JG, Noriega FG. A coordinated expression of biosynthetic enzymes controls the flux of juvenile hormone precursors in the corpora allata of mosquitoes. Insect Biochem Mol Biol. 2011;41:660–669. doi: 10.1016/j.ibmb.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouzova M, Mayoral JM, Brockhoff A, Goodwin M, Meyerhof W, Noriega FG. Functional characterization of an allatotropin receptor expressed in the corpora allata of mosquitoes. Peptides. 2012;34:201–208. doi: 10.1016/j.peptides.2011.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y, Žitňan D, Gill SS, Adams ME. Molecular cloning and biological activity of ecdysis-triggering hormones in Drosophila melanogaster. FEBS Letters. 1999;463:133–138. doi: 10.1016/s0014-5793(99)01622-1. [DOI] [PubMed] [Google Scholar]
- Park Y, Kim YJ, Dupriez V, Adams ME. Two Subtypes of Ecdysis-triggering Hormone Receptor in Drosophila melanogaster. J Biol Chem. 2003;278:17710–17715. doi: 10.1074/jbc.M301119200. [DOI] [PubMed] [Google Scholar]
- Pérez-Hedo M, Rivera-Perez C, Noriega FG. The insulin/TOR signal transduction pathway is involved in the nutritional regulation of juvenile hormone synthesis in Aedes aegypti. Ins Biochem Mol Biol. 2013;43:495–500. doi: 10.1016/j.ibmb.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raikhel AS, Lea AO. Previtellogenic development and vitellogenin synthesis in the fat body of a mosquito: An ultrastructural and immunocytochemical study. Tissue Cell. 1983;15:281–299. doi: 10.1016/0040-8166(83)90023-x. [DOI] [PubMed] [Google Scholar]
- Raikhel AS, Lea AO. Hormone-mediated formation of the endocytic complex in mosquito oocytes. Gen Comp Endocrinol. 1985;57:422–433. doi: 10.1016/0016-6480(85)90224-2. [DOI] [PubMed] [Google Scholar]
- Raikhel AS, Lea AO. Juvenile hormone controls previtellogenic proliferation of ribosomal RNA in the mosquito fat body. Gen Comp Endocrinol. 1990;77:423–434. doi: 10.1016/0016-6480(90)90233-c. [DOI] [PubMed] [Google Scholar]
- Raikhel AS, Lea AO. Control of follicular epithelium development and vitelline envelope formation in the mosquito; role of juvenile hormone and 20-hydroxyecdysone. Tissue and Cell. 1991;23:577–591. doi: 10.1016/0040-8166(91)90015-l. [DOI] [PubMed] [Google Scholar]
- Riddiford LM. How does juvenile hormone control insect metamorphosis and reproduction? Gen Comp Endocrinol. 2012;179:477–484. doi: 10.1016/j.ygcen.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Riddiford LM. Microarrays reveal discrete phases in juvenile hormone regulation of mosquito reproduction. Proc Nat Acad Sci USA. 2013;110:9623–9624. doi: 10.1073/pnas.1307487110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Perez C, Nouzova M, Noriega FG. A quantitative assay for the juvenile hormones and their precursors using fluorescent tags. PLoS ONE. 2012;7(8):e43784. doi: 10.1371/journal.pone.0043784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Perez C, Nouzova M, Lamboglia I, Noriega FG. Metabolic analysis reveals changes in the mevalonate and juvenile hormone synthesis pathways linked to the mosquito reproductive physiology, Insect. Biochem Mol Biol. 2014;51:1–9. doi: 10.1016/j.ibmb.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol PA, Spielman A, Jacobs MS. Rough endoplasmic-reticulum in midgut cells of mosquitos (Diptera,Culicidae) – Aggregation stimulated by juvenile-hormone. J Med Entomol. 1982;19:719–721. [Google Scholar]
- Smykal V, Daimon T, Kayukawa T, Takaki K, Shinoda T, Jindra M. Importance of juvenile hormone signaling arises with competence of insect larvae to metamorphosis. Developmental Biology. 2014;390:221–230. doi: 10.1016/j.ydbio.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Tobe SS, Stay B. Structure and regulation of the corpus allatum. Adv Ins Phys. 1985;18:305–431. [Google Scholar]
- Ueda H, Shinoda T, Hiruma K. Spatial expression of the mevalonate enzymes involved in juvenile hormone biosynthesis in the corpora allata in Bombyx mori. J Insect Physiol. 2009;55:798–9804. doi: 10.1016/j.jinsphys.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Yamanaka N, Yamamoto S, Žitňan D, Watanabe K, Kawada T, Satake H, Kataoka H. Neuropeptide receptor transcriptome reveals unidentified neuroendocrine pathways. PLoS ONE. 2008;3(8) doi: 10.1371/journal.pone.0003048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Žitňan D, Adams ME. Neuroendocrine Regulation of Ecdysis. In: Gilbert LI, editor. Insect Endocrinology. San Diego: Academic Press; 2012. pp. 253–309. [Google Scholar]
- Žitňan D, Ross LS, Žitňanova I, Hermesman JL, Gill SS, Adams ME. Steroid induction of a peptide hormone gene leads to orchestration of a defined behavioral sequence. Neuron. 1999;23:523–535. doi: 10.1016/s0896-6273(00)80805-3. [DOI] [PubMed] [Google Scholar]
- Žitňan D, Hollar L, Spalovská I, Takáč P, Žitňanová I, Gill SS, Adams ME. Molecular cloning and function of ecdysis-triggering hormones in the silkworm Bombyx mori. J Exp Biol. 2002;205:3459–3473. doi: 10.1242/jeb.205.22.3459. [DOI] [PubMed] [Google Scholar]
- Žitňan D, Žitňanova I, Spalovská I, Takáč P, Park Y, Adams ME. Conservation of ecdysis-triggering hormone signaling in insects. J Exp Biol. 2003;206:1275–1289. doi: 10.1242/jeb.00261. [DOI] [PubMed] [Google Scholar]
- Žitňan D, Kim YJ, Žitňanová I, Roller L, Adams ME. Complex steroid–peptide–receptor cascade controls insect ecdysis. Gen Comp Endoc. 2007;153:88–96. doi: 10.1016/j.ygcen.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Žitňanová I 2001.Žitňanová I, Adams ME, Žitňan D. Dual ecdysteroid action on the epitracheal glands and central nervous system preceding ecdysis of Manduca sexta. J Exp Biol. 2001;204:3483–3495. doi: 10.1242/jeb.204.20.3483. [DOI] [PubMed] [Google Scholar]
- Zou Z, Saha TT, Roy S, Shin SW, Backman TWH, Girke T, Raikhel AS. Juvenile hormone and its receptor, methoprene-tolerant, control the dynamics of mosquito gene expression. Proc Nat Acad Sci USA. 2013;110:E2173–E2181. doi: 10.1073/pnas.1305293110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Female pupae were collected at 30 min intervals as they molted from fourth instar larvae into the pupal stage. The duration of the pupal stage was determined by scoring at hourly intervals the number of eclosing adults. The eclosion to adult stage began 46 h after pupation (1%), with the majority of insects emerging at 50 h (40%) and the last at 53 h (1.5%). Each data point represents the mean ± S.E.M. of at least three independent determinations.
The effects of increasing concentrations of AeaETH1 on JH synthesis were tested on BR-CA-CC complexes dissected from −2 h pupa. A series of ETH1 concentrations from 10−6 to 10−12M were tested using the in vitro JH biosynthesis assay. Each data point represents the mean ± S.E.M of two independent biological replicates. Statistical analyses were performed using the GraphPad Prism Software (San Diego, CA, USA). The results are expressed as means ± S.E.M. Significant differences (p< 0.05) were determined by one-way ANOVA followed by Tukey’s test.
Juvenile hormone acid methyltransferase mRNA levels dramatically increased during the last 4 hours in pharate adults. Transcript levels were measured by Quantitative Real-Time PCR. Reactions were run in triplicate in a 20 μl volume and normalized to the house keeping gene 60S ribosomal protein L32 (rpL32) mRNA expression for each sample. Each data point represents the mean ± S.E.M. of two independent determination of 20 CA-CC complexes. Statistical analyses were performed using the GraphPad Prism Software (San Diego, CA, USA). The results are expressed as means ± S.E.M. Significant differences (p< 0.05) were determined by one-way ANOVA followed by Tukey’s test.
Accession numbers and primer sequences for the genes included in the studies.