Abstract
Patients with EGFR mutant lung cancer derive significant therapeutic benefit from treatment with EGFR tyrosine kinase inhibitors (TKIs). Unfortunately, acquired resistance is an inevitable consequence of this treatment strategy, with a broad variety of resistance mechanisms including acquired EGFR mutations (e.g., T790M) and activation of bypass signaling pathways, such as MET and HER2. Several therapeutic strategies hypothesized to delay or overcome resistance have been tested in clinical trials, including ‘next-generation’ EGFR TKIs and rational combinations of targeted agents. However, to date, there are no FDA approved therapies for patients with acquired resistance to first-line EGFR TKI therapy. There remains a critical need for more effective and better tailored treatments in this setting in order to match treatments to the individual patient and specific resistance mechanism at hand. In this review, we will discuss known mechanisms of resistance to first-line EGFR TKI therapy and describe previous and ongoing strategies to overcome resistance.
Introduction
Acquired resistance to EGFR TKI therapy
For patients with advanced EGFR mutant lung cancer, treatment with an EGFR tyrosine kinase inhibitor (TKI), such as erlotinib, gefitiniborafatinib (Table 1), is associated with superior radiographic response and prolonged progression-free survival (PFS) compared to standard cytotoxic chemotherapy (1-3). EGFR TKIsare now standard first-line therapy for patients with advanced EGFRmutant lung cancer.Most patients will develop clinical evidence ofacquired resistance (AR) after a median of 12 months (1,4). Study of tumor samples from patients at the time of resistance has identified several potential mechanisms whereby tumors evade EGFR inhibition (5, 6). These resistance mechanisms can be broadly classified into four categories: (1) second site mutations within the EGFR kinase domain (7,8);(2) acquired mutations in other oncogenes such BRAF and PIK3CA (6, 9); (3) up-regulation of parallel signaling pathways, includingMET, HER2, FGFR and AXL (10-16), to bypass the inhibited EGFR protein; and (4) histological transformation, specifically epithelial to mesenchymal transition and small cell transformation (6).
Table 1.
Lineage of EGFR tyrosine kinase inhibitors in clinical development. This table provides an overview of the various EGFR inhibitors in clinical development.
| Drug | Target | Reversible/irreversible | Company | Approval status | |
|---|---|---|---|---|---|
| 1st Generation | Erlotinib | EGFR | Reversible | Astellas | FDA approved1, EMA approved2, Japan ministry of health approved, South Korea and CFDA approved |
| Gefitinib | EGFR | Reversible | AstraZeneca | EMA approved3, Japan ministry of health approved, South Korea and CFDA approved | |
| Icotinib | EGFR | Reversible | Beta Pharma | CFDA approved | |
| 2nd Generation | Afatinib | EGFR/HER2 | Irreversible | BoehringerIn gelheim | FDA approved4, EMA approved5, Japan ministry of health approved3 |
| Dacomitinib | Pan-ErbB | Irreversible | Pfizer | No approvals to date | |
| Neratinib | EGFR/HER2 | Irreversible | Puma | No approvals to date | |
| 3rd generation (mutant specific) | AP26113 | EGFR/ALK | Reversible | Ariad | No approvals to date |
| CO-1686 | Mutant EGFR | Irreversible | Clovis | No approvals to date | |
| AZD9291 | Mutant EGFR | Irreversible | AstraZeneca | No approvals to date | |
| EGF816 | Mutant EGFR | Irreversible | Novartis | No approvals to date | |
| ASP8273 | Mutant EGFR | Irreversible | Astellas | No approvals to date |
Erlotinib is FDA approved for (a) first-line treatment of patients with metastatic non-small cell lung cancer (NSCLC) whose tumors have EGFR exon 19 deletions or exon 21 (L858R) substitution mutations; (b) maintenance treatment of patients with locally advanced or metastatic NSCLC whose disease has not progressed after four cycles of first line platinumbased chemotherapy; (c) treatment of patients with locally advanced or metastatic non-small cell lung cancer after failure of at least one prior chemotherapy regimen.
Erlotinib is EMA approved for (a) first-line treatment of patients with metastatic non-small cell lung cancer (NSCLC) whose tumors have activating EGFR mutations; (b) maintenance treatment of patients with locally advanced or metastatic NSCLC whose disease has not progressed after four cycles of first line platinum-based chemotherapy; (c) treatment of patients with locally advanced or metastatic non-small cell lung cancer after failure of at least one prior chemotherapy regimen.
Gefitinib is EMA approved for the treatment of patients with metastatic lung cancer whose tumors have activating EGFR mutations.
Afatinib is FDA approved for the first-line treatment of patients with metastatic non-small cell lung cancer (NSCLC) whose tumors have EGFR exon 19 deletions or exon 21 (L858R) substitution mutations.
Afatinib is EMA approved for the treatment of patients with metastatic lung cancer whose tumors have activating EGFR mutations. FDA: Food and Drug Administration. EMA: European Medicines Agency. CFDA: China Food and Drug Administration.
Overview of therapeutic strategies
Though acquired resistanceappears to be an inevitable consequence of EGFR TKI treatment, successful treatmentstrategies to overcome acquired resistancehavebeen elusive. More than 75 studies have looked at over 50investigational drugs and drug combinations in the setting of AR to first generation EGFR TKI therapy, with the majority of studies reporting little tono efficacy in this population (Table 2). In this review, we will discuss completed and ongoing efforts to overcome resistance.
Table 2. Published or presented studies with results in patients with EGFR mutant lung cancer and acquired resistance to EGFR TKI.
| Target | Agents | Study | Phase | Eligibility | # pts / (% EGFRm+) | RR (%) | PFS (months) |
|---|---|---|---|---|---|---|---|
| 1st/2nd generationEGFR TKI | Neratinib | Ref. 24 | 2 | PR or SD × 3 months on TKI and EGFRm | 91 (100) | 3 | 3.6 |
| XL647 | Ref. 26 | 2 | PR or SD × 3 months on TKI or EGFR T790M | 33 (53) | 3 | 3.5 | |
| Afatinib vs. Placebo | Ref. 23 | 2B/3 | 1-2 lines previous chemo + PD after 3 months on TKI | 585 (16) | 7 (A) <1 (P) |
3.3 1.1 |
|
| Afatinib | Ref. 70 | 2 | PD after 3 months on TKI | 62 (73) | 8 | 4.4 | |
| Dacomitinib | Ref. 25 | 2 | PD after chemo and TKI | 50 (48) | 9 | 4.5 | |
| MM-121 + Erlotinib | Ref. 50 | 1 | Advanced NSCLC | 32 (35) | 3* | 2* | |
| AP26113 | Ref. 33 | 1 | Solid tumor, subset with EGFR T790M+ | 12 T790M+ | 0 | NR | |
| Mutant-specific TKI | CO-1686 | Ref. 35 | 1 | EGFRm and prior TKI | 40 T790M+ (100) | T790M+ 58 | NR |
| AZD9291 | Ref. 37 | 1 | EGFRm + PD on TKI | 107 T790M+ (100) | T790M+ 64 | NR | |
| HM61713 | Ref. 38 | 1 | EGFRm + PD on TKI | 48 T790M+ | T790M+ 29 | 4.4 | |
| EGFR antibodies | Cetuximab + Erlotinib | Ref. 28 | 2 | PR or SD × 3 months on TKI orEGFRm | 19 (84) | 0 | 3 |
| Cetuximab + Afatinib | Ref. 30 | 1B | Jackman criteria | 126 (98) | 29 | 4.7 | |
| Chemotherapy | Carboplatin/Pac litaxel | Ref. 71 | 3 | PD after first-line gefitinib | 52 (100) | 28.8 | NR |
| Chemo/Erlotinib vs. Chemo | Ref. 21 | Retro | Jackman criteria | 78 (100) | 41 (C+E) 18 (C) | 4.4 4.2 |
|
| Pemetrexed + Gefitinib or Erlotinib | Ref. 72 | 2 | EGFRm and PD on TKI | 27 (100) | 25.9 | 7.0 | |
| Chemo/Erlotinib vs. Chemo | Ref. 73 | 2 | PR or SD × 3 months on TKI | 46 (67) | NR | E = 4.6 No E = 5.4 | |
| mTOR | Everolimus + Gefitinib or Erlotinib | Ref. 64 | 2 | PR on TKI or 6 months SD + EGFRm | 13 (62) | 0 | 3 |
| Everolimus | Ref. 74 | 2 | Prior TKI × 1 month | 43 (0) | 2 | 2.7 | |
| HSP90 | IPI-504 | Ref. 75 | 2 | PD on TKI | 28 (100) | 4 | 2.8 |
| AUY-922 + Erlotinib | Ref. 66 | 1/2 | Jackman Criteria | 22 (100) | 16 | NR | |
| AUY-922 | Ref. 65 | 2 | EGFRm and prior EGFR TKI | 35 (100) | 18 | NR | |
| HDAC | Erlotinib + Vorinostat | Ref. 76 | 1/2 | EGFRm after PD on at least 3month TKI | 33 (100) | 0 | 2 |
| MET | INC280 + Gefitinib | Ref. 47 | 1 | EGFRm, MET positive and PD on TKI | 46 (100) | 17 | NR |
| Other | Cabozantinib | Ref. 46 | 2 | EGFRm and PD on TKI | 11 | ||
| Dasatinib + Erlotinib | Ref. 77 | 2 | PR or SD × 6 months on TKI or EGFRm | 12 (100) | 0 (D+E) | 0.9 | |
| Hydroxy-chloroquine +/- Erlotinib | Ref. 78 | 1 | PR or SD × 3 months on TKI | 27 (74) | 0 (HCQ)5 (HCQ+E) | 1.82 |
A = Afatinib; G = gefitinib; E= erlotinib; P = placebo; NR = Not Reported; EGFRm = EGFR mutant; PR = partial response; SD = stable disease; PD = progressive disease.
Response rate and PFS with MM-121 + E is in unselected patients.
Therapeutic Strategies Targeting EGFR
Standard treatment with addition of continued EGFR inhibition beyond progression
Management of patients with progression on first-line EGFR TKI therapy is largely dependent on clinical factors such as symptoms and disease burden. Some patients may have RECIST progression based on tumor measurements, but with continued clinical benefit from therapy. In this setting, many asymptomatic patients can delay a change in therapy for several months to over a year (17). This approach is being studied prospectively in the ASPIRATION trial (18). If there is limited progression, local therapy and continued EGFR inhibition canbe considered (19,20). For patients with oligometastatic disease, a prolonged PFS after local therapy of 6-10 months was achieved in several small series (19,20).
There arelimitedprospective data to support continued EGFR TKI in addition to chemotherapy in the AR setting. Erlotinib has been combined with platinum doublet chemotherapy with toxicity that is similar to chemotherapy alone (4). In aretrospective series of 78 patients with EGFRmutant lung cancer andAR, the odds ratio for response rate was 0.20 favoring chemotherapy with concurrent erlotinib compared to chemotherapy alone (21). In a trial comparing afatinib and paclitaxel to chemotherapy alone in patients with disease progression on single agent EGFR TKI, there was an increase in PFS with continuation of EGFR TKI (5.6 vs 2.8 months, HR 0.60)(22). There are several ongoing prospective studies that may result in a definitive recommendation regarding the combination of chemotherapy anderlotinib (NCT01928160, NCT02064491, NCT02098954) or gefitinib (NCT01544179) in the AR setting.
Second-generation EGFR TKIs
Second-generation EGFR TKIs bind to the EGFR tyrosine kinase domain irreversibly and have broader ERBB inhibition (Table 1). Afatinib is a dual EGFR/HER2inhibitorthatis now FDAapproved for the first-line treatment of lung cancers with EGFR L858R mutations or exon 19 deletions. This approval is based on a phase 3 trialthatdemonstrated a superiorPFS for afatinib compared to cisplatin and pemetrexed (13.6 vs 6.9 months) in patients with EGFRmutant lung cancer (3).
While afatinib has proven useful in the first-line setting, its role in AR is less clear. The LUX-Lung 1 trial randomized molecularly unselected patients, who had previously been treated with erlotinib or gefitinib for at least three months, to receive afatinib or placebo (23). The response rate (RR)(7% vs <1%) and PFS (3.3 vs 1.1 months) were superior with afatinib compared to placebo, but there was no improvement in overall survival (the primary endpoint) in all study participants or in the subset of patients with knownEGFR mutant lung cancer.
Neratinib has activity against both EGFR and HER2 and was evaluated in a phase II study in patients with non-small cell lung cancer (NSCLC)(24). The RR was 2% among those patients with EGFRmutations, but there wereno responses in patients with EGFR T790M. Dacomitinib, a pan-HER inhibitor, was administered to patients with NSCLC whose disease had progressed on chemotherapy and erlotinib (25). In the subset of patients with EGFRmutant lung cancer, the RR was 8%;no responses were seen in patients with EGFR T790M. The dual EGFR/VEGFR inhibitors, XL647 and Vandetanib, have also been tested in the acquired resistance setting with limited efficacy observed (26, 27).
EGFR antibodies
Cetuximab, an EGFR monoclonal antibody, binds to the extracellular domain of EGFR and prevents ligand-dependent receptor activation. Cetuximab has predominantly been used in combination therapy with EGFR TKIs in the resistance setting. In a phase 1/2 study of erlotinibpluscetuximab in patients with AR to erlotinib, no responses (0/19 evaluable patients) were seen (28). However, the combination of cetuximab and afatinib has shown more promise (29). In aclinical trial of afatinibandcetuximabin 100 patients with AR to erlotinib or gefitinib, treatment with the combinationled to a30% overall RR and a median PFS of 4.7 months (30). Responses were seen in patients with both EGFR T790M positive and T790M negativetumors. The most common toxicities were rash, diarrhea and fatigue. Randomized trials of afatinibpluscetuximab are planned in both the first-lineand AR settings.
Mutant specific EGFR inhibitors
A relatively new class of drugs irreversibly inhibits mutant EGFR, in particular EGFR T790M, with much lessactivity against wild-type EGFR (Table 1). The firstdrug in this class, WZ4002, is 30-100 fold more potent against EGFR T790M and up to 100 fold less potent against wild type EGFR in preclinical models (31). This discovery led to the subsequent development of other compounds in this same class. AP26113 is a reversible dual ALK and EGFR inhibitor that has selectivity for the mutant forms of EGFR, both activating and resistance mutations, in pre-clinical models (32). The phase 1/2 of AP26113 demonstrated no objective responses in 12 patients with EGFR T790M containing tumors (33).
CO-1686 targets mutant EGFR, including T790M, and is active in EGFRmutant xenografts and murine models of erlotinib resistance (34). The phase 1 study of single-agent CO-1686 is complete with a small range of doses being testedin the ongoing phase 2 study (35). CO-1686 has the notable adverse event of hyperglycemia as a frequent adverse event (38%, any grade). The reported overall RRis 58% in 40 patients with centrally confirmed EGFR T790M tumors. Median PFS has not been reached, but is estimated at >12 months (35).
AZD9291 is another mutant specific EGFR currently being tested clinically (36). A phase 1 study of AZD9291 in patients with advanced NSCLC who had progression on first- or secondgeneration EGFR TKI therapy is nearing completion (37). As of April2014, 232patients hadreceived AZD9291 with no dose limiting toxicities identified. Diarrhea (14%, any grade) and rash (24%, any grade) were the most frequent toxicities. The RR in 107 patients withEGFR T790M tumors to date was 64%.
Several other EGFR-mutant specific TKIs are being tested in phase 1 studies currently, including HM61713 (NCT01588145), EGF816 (NCT02108964), and ASP8273 (NCT02113813). Preliminary results with HM61713 were recently presented (38). 118patients have received the drug, and a RR of 22% was noted in the 83 evaluable patients. The RR was 29% in the 48 patients with EGFR T790M containing tumors. EGF816 similarly has selective activity against mutant EGFR in cellular assays and xenograft models (39).
Therapeutic Strategies Targeting Alternate Pathways
MET inhibition
MET is a receptor tyrosine kinase which, when activated by its ligand, hepatocyte growth factor (HGF), can mediate cell proliferation, evasion of apoptosis, and metastasis (40). MET amplification is seen in untreated patients with NSCLC at a rate of approximately 4%(41, 42), and is detected in approximately 5% of tumors with acquired resistance to EGFR TKI (5, 6). De novo MET amplification has been associated with primary resistance to EGFR TKIs (43). Therapeutic targeting of MET may be an effective strategy in MET amplified tumors (44,45). Results from a phase 2 study of cabozantinib (an oral MET/VEGFR2/RET inhibitor) in patients with EGFR mutant lung cancer and progression on EGFR TKI resulted in 3 partial responses out of 35 patients treated (46). There are several ongoing clinical trials of MET TKIs or MET MAbs in combination with EGFR TKIs with the majority of studies selecting for patients that are MET positive by various assays (Table 3). An ongoing phase 1 study of INC280 and gefitinib in EGFR mutant patients with AR who have either MET amplification or MET overexpressionhas modest activity, with a 15% unconfirmed RR (6/41 patients)(47). In addition to MET amplification, MET activation through increased production of HGF is a potential mechanism of resistance to EGFR TKIs (48). Lung cancer cell lines made resistant to EGFR TKIs by HGF overexpression were sensitive to dual EGFR and MET blockade (49). Prospective clinical validation of anti-HGF directed monoclonal antibodies is pending.
Table 3.
Ongoing studies without results in patients with EGFR mutant lung cancers.
| Target | Agents | Clinical trials | Phase | Status |
|---|---|---|---|---|
| 1st/2nd gen EGFR TKI | Erlotinib/Gefitinib | Pulsatile Erlotinib or Gefitinib (NCT01965275) | 2 | Ongoing |
| Erlotinib | High/low dose Erlotinib (first line) (NCT01967095) | 1 | Ongoing | |
| Afatinib | Pulsatile Afatinib (NCT01647711) | 1 | Ongoing | |
| Afatinib + Nimotuzumab | (NCT01861223) | 1B | Ongoing | |
| Mutant-specificTKI | ASP8273 | (NCT02113813) | 1 | Ongoing |
| EGF816 | (NCT02108964) | 1 | Ongoing | |
| Chemotherapy | Bevacizumab | Erlotinib +/- Bev (first-line) (NCT01532089) | 2 | Ongoing |
| Platinum/Gem | Erlotinib +/- chemo (first-line) (NCT02001896) Erlotinib + chemo (NCT02098954) |
3 2 |
Not yet open Not yet open |
|
| Platinum/Pemetrexed | Chemo +/- Erlotinib (NCT01928160) | 2 | Not yet open | |
| Platinum doublet | Chemo +/- Erlotinib (NCT02064491) | 2 | Ongoing | |
| MET | Crizotinib | Crizotinib + Dacomitinib (NCT01121575) | 1 | Completed |
| Tivatinib (ARQ197) | Erlotinib +/- ARQ197 (NCT00777309) | 2 | Completed | |
| Cabozantinib (XL184) | XL184 +/- Erlotinib (NCT00596648) XL184 + Erlotinib (NCT01866410) |
1/2 2 |
Completed Ongoing | |
| INC280 | INC280 + Gefitinib (NCT01610336) INC280 + Erlotinib (NCT01911507) |
2 1 |
Ongoing Ongoing |
|
| Onartuzumab (METMab) | Erlotinib +/-METMab (first line) (NCT01887886) | 3 | Ongoing | |
| LY2875358 | LY2875358 +/- Erlotinib (NCT01900652) | 2 | Ongoing | |
| MSC2156119J | MSC2156119J + Gefitinib in p1 (NCT01982955) | 1B/2 | Ongoing | |
| HGF | Rilotumumab (AMG102) | Rilotumumab + Erlotinib (NCT01233687) | 1/2 | Ongoing |
| HER2 | Trastuzumab | Trastuzumab (NCT00004883) | 2 | Completed |
| IGF-1R | OSI-906 | Erlotinib +/- OSI-906 (first line) (NCT01221077) | 2 | On hold |
| AXL | Foretinib | Foretinib + Erlotinib (NCT02034097) | 2 | Not yet open |
| MEK | MEK162 | MEK162 + Erlotinib (NCT01859026) | 1 | Ongoing |
| AZD6244 | AZD +/- Erlotinib (NCT01229150) | 2 | Ongoing | |
| Selumetinib | Selumetinib + Gefitinib (NCT02025114) | 1B/2 | Not yet open | |
| AKT | MK-2206 | MK-2206 + Gefitinib (NCT01147211) | 1 | Completed |
| PI3K | BKM120 | BKM120 + Erlotinib (NCT01487265) | 2 | Ongoing |
| XL147 | XL147 + Erlotinib (NCT00692640) | 1 | Completed | |
| mTOR | XL765 (also PI3K-I) | XL765 + Erlotinib (NCT00777699) | 1 | Completed |
| Sirolimus | Sirolimus + Afatinib (NCT00993499) | 1 | Ongoing | |
| Everolimus | (NCT00124280) | 2 | Completed | |
| HSP90 | SNX-5422 | SNX-5422 + Erlotinib (NCT01851096) | 1 | Completed |
| DS-2248 | DS-2248 (NCT01288430) | 1 | Ongoing | |
| Immunotherapy | MPDL3280A (PD-L1) | MPDL3280A + Erlotinib (first-line) (NCT02013219) | 1B | Ongoing |
| MK-3475 (PD-1) | MK-3475 + Erlotinib, MK-3475 + Gefitinib (NCT02039674) | 1/2 | Ongoing | |
| Other | Metformin | Gefitinib +/- Metformin (first-line) (NCT01864681) | 2 | Ongoing |
| Hydroxychloroquine | Hydroxychloroquine + Gefitinib (NCT00809237) | 1/2 | Ongoing |
A=afatinib, E=erlotinib, G=gefitinib
HER2 and HER3 inhibition
The ErbB family of receptor tyrosine kinases is composed of 4 members – EGFR (ErbB1), HER2 (ErbB2), HER3 (ErbB3), and HER4 (ErbB4). HER2 amplification has been detected in 12% of tumors with AR to EGFR TKI therapy (13), resulting in sustained downstream signaling, even in the continued presence of the EGFR TKI. Combination strategies involving dual EGFR/HER2 blockade are being explored. The combination of the dual HER2/EGFR TKI, afatinib plus cetuximab, has been studied in patients with AR to erlotinib (30), as described above. In a phase 1/2 clinical trial of erlotinib plus the HER3 monoclonal antibody, MM-121 (NCT00994123), one EGFR TKI naïve patient had a partial response and 5/8 patients with EGFR mutant/TKI resistant disease had stable disease (50).
IGF-1R inhibition
The insulin like growth factor-1 receptor (IGF-1R) is a receptor tyrosine kinase that is activated by the IGF-1 or IGF-2 ligands (51). Up-regulation of IGF-1R signaling has previously been described to mediate acquired resistance to first generation EGFR TKIs (52) as well as to the irreversible EGFR inhibitors PF299804 and WZ4002 (53). Though IGF-1R was implicated in the pathogenesis of EGFR mutant lung cancer, clinical testing of combined EGFR/IGF-1R blockade has not proven to be effective. A randomized phase 2 study of erlotinib alone or in combination with the IGF-1R/Insulin receptor TKI, OSI-906, in patients with advanced EGFR mutant NSCLC (NCT01221077) closed earlyafter an interim analysisin March 2013 showed there would be no benefit to the combination.
AXL inhibition
Activation of the receptor tyrosine kinase AXL has been reported as a mechanism of resistance to erlotinib. Recent studies have demonstrated increased AXL activation, without EGFR T790M mutation, in both in vitro and in vivo models of EGFR mutant/TKI resistant lung cancer (10). Combined inhibition of AXL and EGFR in these models restored therapeutic efficacy. More recently, increased AXL expression was observed in 5/26 patients (19%) of patients with AR to gefitinib (54). AXL inhibitors are in early clinical development (NCT00697632, ISRCTN00759419 and (55)).
FGFR inhibition
More recently, in vitro studies have demonstrated increased expression of fibroblast growth factor receptor 2 (FGFR2) and its ligand, fibroblast growth factor 1 (FGF1), in EGFR mutant cell lines derived to be resistant to gefitinib or afatinib (14,15). Combined inhibition of EGFR and FGFR in these models improved therapeutic responses in the EGFR TKI resistant cell lines. Validation of these findings in clinical models has yet to be demonstrated.
MAPK pathway inhibition
Activation of the MAPK signaling pathway (Fig. 1) can drive EGFR TKI resistance in both preclinical models as well as in patient tumor samples. In a large series of tumor biopsy samples from patients with EGFR mutant/TKI resistant lung cancer, 2/195 (1%) were found to have mutations in BRAF (9). Concomitant inhibition of EGFR and MEK was an effective therapeutic strategy in models of EGFR TKI resistance driven by MAPK pathway activation (9). Erlotinib resistance has also been associated with reduced expression of neurofibromin, a RAS GTPaseactivating protein (GAP) encoded by the NF1 gene which functions as a negative regulator of RAS (56). In preclinical models of EGFR TKI resistance, erlotinib failed to fully inhibit MAPK signaling in the context of low neurofibromin levels, however, these tumors responded to the combination of an EGFR TKI with a MEK inhibitor. Downregulation of NF1 expression was also seen in clinical samples from patients with EGFR TKI resistance. There are several ongoing clinical trials of MEK inhibitors plus EGFR TKIs in EGFR mutant lung cancer (Table 3). In addition, one of the few described mechanisms of resistance to the mutant-specific EGFR TKIs is aberrant activation of ERK signaling (57). The MAPK pathway may become increasingly relevant as we develop combination strategies to prevent or overcome resistance to EGFR mutant-specific TKIs.
Figure 1.
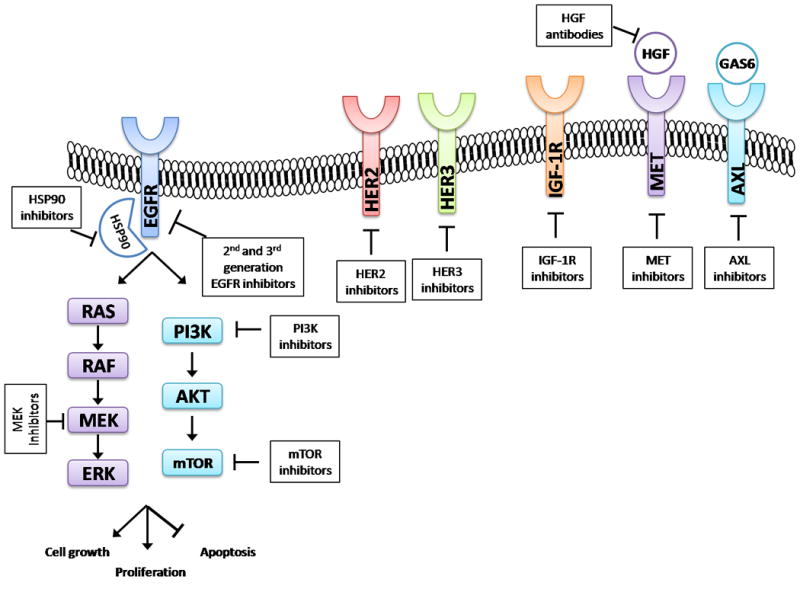
EGFR signaling pathways and receptor cross-talk. Activation of EGFR propagates downstream pro-growth signals through the MAPK pathway (RAS-RAF-MEK-ERK) and PI3K-AKT-mTOR pathway. Targets for potential combinatorial therapeutic strategies to overcome resistance are noted.
PI3K-AKT-mTOR pathway inhibition
Analogous to the MAPK pathway, activation of the PI3K-AKT-mTOR pathway (Fig. 1) also drives EGFR TKI resistance. PIK3CA mutations can co-occur with EGFR mutations and may portend a poorer response to EGFR TKIs (58-60). In addition, acquired PIK3CA mutations have been detected in a small percentage (∼5%) of EGFR mutant lung cancers with AR to EGFR TKIs (6). Preclinical data have shown that introduction of activating PIK3CA mutations into EGFR mutant cell lines confers resistance to EGFR TKIs (61). A phase 2 clinical trial combining erlotinib with the PI3K inhibitor, BKM120, is ongoing in patients with advanced NSCLC previously sensitive to erlotinib or whose tumors harbor an EGFR mutation (NCT01487265), although no PIK3CA mutation or aberration is required. A phase 2 study combining erlotinib with the AKT inhibitor, MK-2206, in patients with EGFR mutant lung cancers and progression on erlotinib demonstrated a 9% RR (4/46 patients)(62). Pre-clinical data also supports the use of mTOR inhibitors in the context of EGFR TKI resistance (63), althougha small study of EGFR TKI and everolimus in patients who previously responded to EGFR TKI demonstrated no responses (64). Concomitant inhibition of EGFR and mTOR in patients with AR is currently being evaluated further in a phase 1 trial of afatinib plus sirolimus (NCT00993499).
HSP90 inhibitors
Heat shock protein 90 (HSP90), a molecular chaperone for several oncogenic kinases, is required for protein folding and stabilization of mutant EGFR. Single agent activity of the HSP90 inhibitor, AUY922, has been documented in patients with EGFR mutant lung cancer, with a RRof 20% (7/35 patients)(65). In a phase 2 study combining AUY922 with erlotinib, in patients with EGFR mutant/TKI resistant lung cancer, the overall RRwas 2/16 (13%)(66). Several HSP90 inhibitors are in clinical development for use in both EGFR TKI sensitive and EGFR TKI resistant patient populations (Table 3).
Immune Therapy
Another potential strategy which has garnered much attention recently is the use of immune checkpoint inhibitors, either as single agents or in combination with EGFR TKIs. An intriguing hypothesis is that tumor cell death triggered by targeted therapies results in antigen release and immunomodulation that can prime tumors and potentiate anti-tumor immune responses (67). Pre-clinical data in EGFRmutant lung cancer cell lines and mouse models has demonstrated that mutant EGFR signaling drives expression of programmed death- ligand 1 (PD-L1), and that blockade of the PD-1 receptor improved survival of mice with EGFRmutant tumors (68). A phase 1 clinical trial of erlotinib plus the anti-PD-L1 antibody, MPDL3280A, in the first line setting (NCT02013219), a phase 1/2 study of pembrolizumab in combination with erlotinib or gefitinib (NCT02039674), and a study of erlotinib and nivolumab (NCT01454102) are allcurrently ongoing. Preliminary results indicate activity of the nivolumab and erlotinib combination; an overallRRof 19% (4/21 patients) was reported, with 3 of 4 responders having previously progressed on erlotinib monotherapy (69).
Future Directions
Despite the tremendous success of first generation EGFR TKIs in patients with EGFR mutant lung cancer, the emergence of resistance remains a significant barrier for the successful management of patients with this disease. Several strategies are beginningto show promise. In particular, continued clinical development of the new mutant specific EGFR TKIs has the potential to dramatically change our treatment paradigm for patients with EGFR mutant lung cancers. However, acquired resistance that does not involve EGFR T790M is a heterogeneous clinical problem with more limited success.
Moving forward, there are several critical issues that remain to be addressed: (1) How clinically relevant is heterogeneity in resistance mechanisms to first generation EGFR TKIS? Published reports have already documented co-occurrence of more than one resistance mechanism (e.g., EGFR T790M and MET amplification) within a given tumor sample, and amongdifferent sites of disease. (2) As patients are treated with multiple lines of EGFR-targeted therapies, what are the mechanisms to mutant specific EGFR inhibitors as sequential treatment? How does sequential treatment with multiple EGFR inhibitors affect tumor evolution and resistance? Will mutant specific EGFR inhibitorsmake EGFR T790Mrare? (3) What are the optimal sequences of anti-EGFR directed therapies and combination therapies? Will combination therapies and mutant-specific EGFR TKI be successful in the first line setting? Will combination strategies - including combinations of small molecules inhibiting various targets or combinations of EGFR inhibitors with chemotherapy or immune therapy – overcome resistance? (4) What are the best treatments for patient with lung cancers harboring non-canonical EGFR mutations, such as exon 20 insertions?(5) Are immune therapies useful in molecularly defined cohorts? As repeat biopsies increasingly become the standard of care, matching treatments targeting a specific resistance mechanism to patients whose tumors harbor that mechanismwill allow us to more accurately assess therapeutic efficacy. Additional study of EGFR TKI resistance may provide clinicians with a greater understanding of therapeutic resistance across the numerous other cancers in which targeted therapies are being employed.
Acknowledgments
Grant Support: G.J. Riely was supported by the NIH under award number P30CA008748. C.M. Lovly was supported by the NIH under award numbers R01CA121210 and P01CA129243.
Footnotes
Disclosure of Potential Conflicts of Interest: H.A. Yuis a consultant/advisory board member for Clovis Oncology. G.J. Riely reports receiving commercial research support from Novartis and Roche, and is a consultant/advisory board member for Novartis. C.M. Lovly reports receiving a commercial research grant from AstraZeneca;speakers bureau honoraria from Abbott Molecular, Harrison and Star, and Qiagen; and is a consultant/advisory board member for Pfizer.
References
- 1.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–57. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 2.Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–46. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 3.Sequist LV, Yang JC, Yamamoto N, O'Byrne K, Hirsh V, Mok T, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31:3327–34. doi: 10.1200/JCO.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 4.Jänne PA, Wang X, Socinski MA, Crawford J, Stinchcombe TE, Gu L, et al. Randomized phase II trial of erlotinib alone or with carboplatin and paclitaxel in patients who were never or light former smokers with advanced lung adenocarcinoma: CALGB 30406 trial. J Clin Oncol. 2012;30:2063–9. doi: 10.1200/JCO.2011.40.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu H, Arcila ME, Rekhtman N, Sima CS, Zakowski MF, Pao W, et al. Analysis of mechanisms of acquired resistance to EGFR TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013;19:2240–7. doi: 10.1158/1078-0432.CCR-12-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. SciTransl Med. 2011;3:75ra26. doi: 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balak MN, Gong Y, Riely GJ, Somwar R, Li AR, Zakowski MF, et al. Novel D761Y and common secondary T790M mutations in epidermal growth factor receptor-mutant lung adenocarcinomas with acquired resistance to kinase inhibitors. Clin Cancer Res. 2006;12:6494–501. doi: 10.1158/1078-0432.CCR-06-1570. [DOI] [PubMed] [Google Scholar]
- 8.Bean J, Riely GJ, Balak M, Marks JL, Ladanyi M, Miller VA, et al. Acquired resistance to epidermal growth factor receptor kinase inhibitors associated with a novel T854A mutation in a patient with EGFR-mutant lung adenocarcinoma. Clin Cancer Res. 2008;14:7519–25. doi: 10.1158/1078-0432.CCR-08-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohashi K, Sequist LV, Arcila ME, Moran T, Chmielecki J, Lin YL, et al. Lung cancers with acquired resistance to EGFR inhibitors occasionally harbor BRAF mutations but lack mutations in KRAS, NRAS, or MEK1. ProcNatlAcadSci USA. 2012;109:E2127–33. doi: 10.1073/pnas.1203530109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Z, Lee JC, Lin L, Olivas V, Au V, LaFramboise T, et al. Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nat Genet. 2012;44:852–60. doi: 10.1038/ng.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–43. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 12.Bean J, Brennan C, Shih JY, Riely G, Viale A, Wang L, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. ProcNatlAcadSci USA. 2007;104:20932–7. doi: 10.1073/pnas.0710370104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takezawa K, Pirazzoli V, Arcila ME, Nebhan CA, Song X, de Stanchina E, et al. HER2 amplification: a potential mechanism of acquired resistance to EGFR inhibition in EGFR mutant lung cancers that lack the second-site EGFR T790M mutation. Cancer Discov. 2012;2:922–33. doi: 10.1158/2159-8290.CD-12-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ware KE, Hinz TK, Kleczko E, Singleton KR, Marek LA, Helfrich BA, et al. A mechanism of resistance to gefitinib mediated by cellular reprogramming and the acquisition of an FGF2-FGFR1 autocrine growth loop. Oncogenesis. 2013;2:e39. doi: 10.1038/oncsis.2013.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Terai H, Soejima K, Yasuda H, Nakayama S, Hamamoto J, Arai D, et al. Activation of the FGF2-FGFR1 autocrine pathway: a novel mechanism of acquired resistance to gefitinib in NSCLC. Mol Cancer Res. 2013;11:759–67. doi: 10.1158/1541-7786.MCR-12-0652. [DOI] [PubMed] [Google Scholar]
- 16.Ware KE, Marshall ME, Heasley LR, Marek L, Hinz TK, Hercule P, et al. Rapidly acquired resistance to EGFR tyrosine kinase inhibitors in NSCLC cell lines through de-repression of FGFR2 and FGFR3 expression. PLoS One. 2010;5:e14117. doi: 10.1371/journal.pone.0014117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oxnard GR, Lo P, Jackman DM, Butaney M, Heon S, Johnson BE, et al. Delay of chemotherapy through use of post-progression erlotinib in patients with EGFR-mutant lung cancer. J Clin Oncol. 2012;30(suppl) abstr 7547. [Google Scholar]
- 18.Park K, Tsai CM, Ahn Mj, Yu CJ, Kim SW, Sriuranpon V, et al. ASPIRATION: Phase II study of continued erlotinib beyond RECIST progression in Asian patients (pts) with epidermal growth factor receptor (EGFR) mutation-positive non-small cell lung cancer (NSCLC) J Clin Oncol. 2012;30(suppl) abstr TPS7614. [Google Scholar]
- 19.Yu HA, Sima CS, Huang J, Solomon SB, Rimner A, Paik P, et al. Local therapy with continued EGFR tyrosine kinase inhibitor therapy as a treatment strategy in EGFR-mutant advanced lung cancers that have developed acquired resistance to EGFR tyrosine kinase inhibitors. J Thorac Oncol. 2013;8:346–51. doi: 10.1097/JTO.0b013e31827e1f83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weickhardt AJ, Scheier B, Burke JM, Gan G, Lu X, Bunn PA, Jr, et al. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer. JThoracOncol. 2012;7:1807–14. doi: 10.1097/JTO.0b013e3182745948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldberg SB, Oxnard GR, Digumarthy S, Muzikansky A, Jackman DM, Lennes IT, et al. Chemotherapy with Erlotinib or chemotherapy alone in advanced non-small cell lung cancer with acquired resistance to EGFR tyrosine kinase inhibitors. Oncologist. 2013;18:1214–20. doi: 10.1634/theoncologist.2013-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schuler MH, Yang CH, Park K, Bennouna J, Chen YM, Chouaid C, et al. Continuation of afatinib beyond progression: results of a randomized, open-label, phase III trial of afatanib plus paclitaxel (P) versus investigator's choice chemotherapy (CT) in patients (pts) with metastatic non-small cell lung cancer (NSCLC) progressed on erlotinib/gefitinib (E/G) and afatanib—LUXLung 5 (LL5) J Clin Oncol. 2014;32(suppl):5s. abstr 8019Λ. [Google Scholar]
- 23.Miller VA, Hirsh V, Cadranel J, Chen YM, Park K, Kim SW, et al. Afatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): a phase 2b/3 randomised trial. Lancet Oncol. 2012;13:528–38. doi: 10.1016/S1470-2045(12)70087-6. [DOI] [PubMed] [Google Scholar]
- 24.Sequist LV, Besse B, Lynch TJ, Miller VA, Wong KK, Gitlitz B, et al. Neratinib, an irreversible pan-ErbB receptor tyrosine kinase inhibitor: results of a phase II trial in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:3076–83. doi: 10.1200/JCO.2009.27.9414. [DOI] [PubMed] [Google Scholar]
- 25.Reckamp KL, Giaccone G, Camidge DR, Gadgeel SM, Khuri FR, Engelman JA, et al. A phase 2 trial of dacomitinib (PF-00299804), an oral, irreversible pan-HER (human epidermal growth factor receptor) inhibitor, in patients with advanced non-small cell lung cancer after failure of prior chemotherapy and erlotinib. Cancer. 2014;120:1145–54. doi: 10.1002/cncr.28561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pietanza MC, Lynch TJ, Jr, Lara PN, Jr, Cho J, Yanagihara RH, Vrindavanam N, et al. XL647--a multitargeted tyrosine kinase inhibitor: results of a phase II study in subjects with nonsmall cell lung cancer who have progressed after responding to treatment with either gefitinib or erlotinib. J Thorac Oncol. 2012;7:219–26. doi: 10.1097/JTO.0b013e31822eebf9. [DOI] [PubMed] [Google Scholar]
- 27.Lee JS, Hirsh V, Park K, Qin S, Blajman CR, Perng RP, et al. Vandetanib Versus placebo in patients with advanced non-small-cell lung cancer after prior therapy with an epidermal growth factor receptor tyrosine kinase inhibitor: a randomized, double-blind phase III trial (ZEPHYR) J Clin Oncol. 2012;30:1114–21. doi: 10.1200/JCO.2011.36.1709. [DOI] [PubMed] [Google Scholar]
- 28.Janjigian YY, Azzoli CG, Krug LM, Pereira LK, Rizvi NA, Pietanza MC, et al. Phase I/II trial of cetuximab and erlotinib in patients with lung adenocarcinoma and acquired resistance to erlotinib. Clin Cancer Res. 2011;17:2521–7. doi: 10.1158/1078-0432.CCR-10-2662. [DOI] [PubMed] [Google Scholar]
- 29.Regales L, Gong Y, Shen R, de Stanchina E, Vivanco I, Goel A, et al. Dual targeting of EGFR can overcome a major drug resistance mutation in mouse models of EGFR mutant lung cancer. J Clin Invest. 2009;119:3000–10. doi: 10.1172/JCI38746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Janjigian YY, Smit EF, Groen H, Horn L, Gettinger S, Camidge DR, et al. Dual inhibition of EGFR with Afatinib and Cetuximab in Kinase Inhibitor-Resistance EGFR-Mutant Lung cancer with and without T790M Mutations. Cancer Discov. 2014;4:1036–45. doi: 10.1158/2159-8290.CD-14-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou W, Ercan D, Chen L, Yun CH, Li D, Capelletti M, et al. Novel mutant-selective EGFR kinase inhibitors against EGFR T790M. Nature. 2009;462:1070–4. doi: 10.1038/nature08622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rivera VM, Wang F, Anjum R, Zhang S, Squillace R, Keats J, et al. AP26113 is a dual ALK/EGFR inhibitor: Characterization against EGFR T790M in cell and mouse models of NSCLC. Proceedings of the 103rd Annual Meeting of the American Association for Cancer Research; 2012 March 31-April 4; Chicago, IL. Philadelphia (PA). AACR; 2012. Abstract nr 1794. [Google Scholar]
- 33.Camidge DR, Bazhenova L, Salgia R, Weiss GJ, Langer CJ, Shaw AT. Updated results of a first-in-human dose-finding study of the ALK/EGFR inhibitor AP26113 in patients with advanced malignancies. Proceedings of European Cancer Congress 2013 (ECCO-ESMOESTRO); 2013 September 27-October 1; Amsterdam, Netherlands. Brussels, Belgium: ECCO; Abstract nr 3401. [Google Scholar]
- 34.Walter AO, Sjin RT, Haringsma HJ, Ohashi K, Sun J, Lee K, et al. Discovery of a mutant-selective covalent inhibitor of EGFR that overcomes T790M-mediated resistance in NSCLC. Cancer Discov. 2013;3:1404–15. doi: 10.1158/2159-8290.CD-13-0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sequist LV, Soria JC, Gadgeel SM, Wakelee HA, Camidge DR, Varga A, et al. First-in-human evaluation of CO-1686, an irreversible, highly selective tyrosine kinase inhibitor of mutations of EGFR (activating and T790M) J Clin Oncol. 2014;32(suppl):5s. abstr 8010Λ. [Google Scholar]
- 36.Cross DA, Ashton SE, Ghiorghiu S, Eberlein C, Nebhan CA, Spitzler PJ, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 2014;4:1046–61. doi: 10.1158/2159-8290.CD-14-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jänne PA, Ramalingam SS, Yang JCH, Ahn MJ, Kim DW, Kim SW, et al. Clinical activity of the mutant-selective EGFR inhibitor AZD9291 in patients (pts) with EGFR inhibitor–resistant non-small cell lung cancer (NSCLC) J Clin Oncol. 2014;32(suppl):5s. abstr 8009Λ. [Google Scholar]
- 38.Kim DW, Lee DH, Kang JH, Park K, Han JY, Lee JS, et al. Clinical activity and safety of HM61713, an EGFR-mutant selective inhibitor, in advanced non-small cell lung cancer (NSCLC) patients (pts) with EGFR mutations who had received EGFR tyrosine kinase inhibitors (TKIs) ClinOncol. 2014;32(suppl):5s. abstr 8011. [Google Scholar]
- 39.Kasibhatla S, Li J, Tompkins C, Vaillancourt MT, Anderson J, Cullazzo A, et al. EGF816, a novel covalent inhibitor of mutant-selective epidermal growth factor receptor, overcomes T790M-mediated resistance in NSCLC. Proceedings of the 105th Annual Meeting of the American Association for Cancer Research; 2014 April 5-9; San Diego, CA. Philadelphia (PA): AACR; 2014. Abstract nr 1733. [Google Scholar]
- 40.Sadiq AA, Salgia R. MET as a possible target for non-small-cell lung cancer. J Clin Oncol. 2013;31:1089–96. doi: 10.1200/JCO.2012.43.9422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Onozato R, Kosaka T, Kuwano H, Sekido Y, Yatabe Y, Mitsudomi T. Activation of MET by gene amplification or by splice mutations deleting the juxtamembrane domain in primary resected lung cancers. J Thorac Oncol. 2009;4:5–11. doi: 10.1097/JTO.0b013e3181913e0e. [DOI] [PubMed] [Google Scholar]
- 42.Cappuzzo F, Marchetti A, Skokan M, Rossi E, Gajapathy S, Felicioni L, et al. Increased MET gene copy number negatively affects survival of surgically resected non-small-cell lung cancer patients. J Clin Oncol. 2009;27:1667–74. doi: 10.1200/JCO.2008.19.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cappuzzo F, Jänne PA, Skokan M, Finocchiaro G, Rossi E, Ligorio C, et al. MET increased gene copy number and primary resistance to gefitinib therapy in non-small-cell lung cancer patients. Ann Oncol. 2009;20:298–304. doi: 10.1093/annonc/mdn635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ou SH, Kwak EL, Siwak-Tapp C, Dy J, Bergethon K, Clark JW, et al. Activity of crizotinib (PF02341066), a dual mesenchymal-epithelial transition (MET) and anaplastic lymphoma kinase (ALK) inhibitor, in a non-small cell lung cancer patient with de novo MET amplification. J Thorac Oncol. 2011;6:942–6. doi: 10.1097/JTO.0b013e31821528d3. [DOI] [PubMed] [Google Scholar]
- 45.Camidge DR, Ou SHI, Shapiro G, Otterson GA, Villaruz LC, Villalona-Calero MA, et al. Efficacy and safety of crizotinib in patients with advanced c-MET-amplified non-small cell lung cancer (NSCLC) J Clin Oncol. 2014;32(suppl):5s. abstr 8001. [Google Scholar]
- 46.Reckamp KL, Frankel PH, Mack PC, Gitliz BJ, Ruel N, Lara P, et al. Phase II trial of XL184 (cabozantinib) plus erlotinib in patients (pts) with advanced EGFR-mutant non-small cell lung cancer (NSCLC) with progressive disease (PD) on epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) therapy: a California Cancer Consortium phase II trial (NCI 9303) J Clin Oncol. 2014;32(suppl):5s. doi: 10.3389/fonc.2019.00132. abstr 8014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu YL, Yang JCH, Kim DW, Su WC, Ahn MJ, Lee DH, et al. Safety and efficacy of INC280 in combination with gefitinib (gef) in patients with EGFR-mutated (mut), MET-positive NSCLC: A single-arm phase lb/ll study. J Clin Oncol. 2014;32(suppl):5s. abstr 8017. [Google Scholar]
- 48.Yano S, Wang W, Li Q, Matsumoto K, Sakurama H, Nakamura T, et al. Hepatocyte growth factor induces gefitinib resistance of lung adenocarcinoma with epidermal growth factor receptor-activating mutations. Cancer Res. 2008;68:9479–87. doi: 10.1158/0008-5472.CAN-08-1643. [DOI] [PubMed] [Google Scholar]
- 49.Nanjo S, Yamada T, Nishihara H, Takeuchi S, Sano T, Nakagawa T, et al. Ability of the Met kinase inhibitor crizotinib and new generation EGFR inhibitors to overcome resistance to EGFR inhibitors. PLoS One. 2013;8:e84700. doi: 10.1371/journal.pone.0084700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sequist LV, Modiano MR, Rixe O, Jackman DM, Andreas K, Pearlberg J, et al. Targeting EGFR and ERBB3 in lung cancer patients: clinical outcomes in a phase I trial of MM-121 in combination with erlotinib. J Clin Oncol. 2012;30(suppl) abstr 7556. [Google Scholar]
- 51.Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8:915–28. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- 52.Guix M, Faber AC, Wang SE, Olivares MG, Song Y, Qu S, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in cancer cells is mediated by loss of IGF-binding proteins. J Clin Invest. 2008;118:2609–19. doi: 10.1172/JCI34588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cortot AB, Repellin CE, Shimamura T, Capelletti M, Zejnullahu K, Ercan D, et al. Resistance to irreversible EGFR tyrosine kinase inhibitors through a multistep mechanism involving the IGF1R pathway. Cancer Res. 2013;73:834–43. doi: 10.1158/0008-5472.CAN-12-2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ji W, Choi CM, Rho JK, Jang SJ, Park YS, Chun SM, et al. Mechanisms of acquired resistance to EGFR-tyrosine kinase inhibitor in Korean patients with lung cancer. BMC Cancer. 2013;13:606. doi: 10.1186/1471-2407-13-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wnuk-Lipinska K, Tiron C, Gausdal G, Sandal T, Frink R, Hinz R, et al. BGB324, a selective small molecule Axl kinase inhibitor to overcome EMT-associated drug resistance in carcinomas: Therapeutic rationale and early clinical studies. Proceedings of the 105th Annual Meeting of the American Association for Cancer Research; 2014 April 5-9; San Diego, CA. Philadelphia (PA): AACR; 2014. Abstract nr 1747. [Google Scholar]
- 56.de Bruin EC, Cowell C, Warne PH, Jiang M, Saunders RE, Melnick MA, et al. Reduced NF1 expression confers resistance to EGFR inhibition in lung cancer. Cancer Discov. 2014;4:606–19. doi: 10.1158/2159-8290.CD-13-0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ercan D, Xu C, Yanagita M, Monast CS, Pratilas CA, Montero J, et al. Reactivation of ERK signaling causes resistance to EGFR kinase inhibitors. Cancer Discov. 2012;2:934–47. doi: 10.1158/2159-8290.CD-12-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chaft JE, Arcila ME, Paik PK, Lau C, Riely GJ, Pietanza MC, et al. Coexistence of PIK3CA and other oncogene mutations in lung adenocarcinoma-rationale for comprehensive mutation profiling. Mol Cancer Ther. 2012;11:485–91. doi: 10.1158/1535-7163.MCT-11-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kawano O, Sasaki H, Endo K, Suzuki E, Haneda H, Yukiue H, et al. PIK3CA mutation status in Japanese lung cancer patients. Lung Cancer. 2006;54:209–15. doi: 10.1016/j.lungcan.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 60.Carcereny E, Molima MA, Sanchez JJ, Bertran-Alamillo, Mayo C, Aldeguer E, et al. Mutations of the catalytic subunit a of PI3K (PIK3CA) in erlotinib-treated non-small cell lung cancer (NSCLC) patients (p) with epidermal growth factor receptor (EGFR) mutations. J Clin Oncol. 2011;29(suppl) abstr 7588. [Google Scholar]
- 61.Engelman JA, Mukohara T, Zejnullahu K, Lifshits E, Borras AM, Gale CM, et al. Allelic dilution obscures detection of a biologically significant resistance mutation in EGFR-amplified lung cancer. J Clinical Invest. 2006;116:2695–706. doi: 10.1172/JCI28656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lara P, Longmate J, Mack PC, Kelly K, Socinski MA, Salgia R, et al. Phase II study of the AKT inhibitor MK-2206 plus erlotinib (E) in patients (pts) with advanced non-small cell lung cancer (NSCLC) who progressed on prior erlotinib: A California Cancer Consortium phase II trial (NCI 8698) J Clin Oncol. 2014;32(suppl):5s. abstr 8015. [Google Scholar]
- 63.La Monica S, Galetti M, Alfieri RR, Cavazzoni A, Ardizzoni A, Tiseo M, et al. Everolimus restores gefitinib sensitivity in resistant non-small cell lung cancer cell lines. BiochemPharmacol. 2009;78:460–8. doi: 10.1016/j.bcp.2009.04.033. [DOI] [PubMed] [Google Scholar]
- 64.Riely GJ, Kris MG, Zhao B, Akhurst T, Milton DT, Moore E, et al. Prospective assessment of discontinuation and reinitiation of erlotinib or gefitinib in patients with acquired resistance to erlotinib or gefitinib followed by the addition of everolimus. Clin Cancer Res. 2007;13:5150–5. doi: 10.1158/1078-0432.CCR-07-0560. [DOI] [PubMed] [Google Scholar]
- 65.Garon EB, Moran T, Barlesi F, Gandhi L, Sequist LV, Kim SW, et al. Phase II study of the HSP90 inhibitor AUY922 in patients with previously treated, advanced non-small cell lung cancer (NSCLC) J Clin Oncol. 2012;30(suppl) abstr 7543. [Google Scholar]
- 66.Johnson ML, Hart EM, Rademaker A, Weitner BB, Urman A, Simm HD, et al. A phase II study of HSP90 inhibitor AUY922 and erlotinib (E) for patients (pts) with EGFR-mutant lung cancer and acquired resistance (AR) to EGFR tyrosine kinase inhibitors (EGFR TKIs) J Clin Oncol. 2013;31(suppl) doi: 10.1200/JCO.2014.59.7328. abstr 8036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer. 2012;12:237–51. doi: 10.1038/nrc3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Akbay EA, Koyama S, Carretero J, Altabef A, Tchaicha JH, Christensen CL, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov. 2013;3:1355–63. doi: 10.1158/2159-8290.CD-13-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rizvi NA, Chow LQM, Borghaei H, Shen Y, Harbison C, Alaparthy S, et al. Safety and response with nivolumab (anti-PD-1; BMS-936558, ONO-4538) plus erlotinib in patients (pts) with epidermal growth factor receptor mutant (EGFR MT) advanced NSCLC. J Clin Oncol. 2014;32(suppl):5s. abstr 8022. [Google Scholar]
- 70.Katakami N, Atagi S, Goto K, Hida T, Horai T, Inoue A, et al. LUX-Lung 4: a phase II trial of afatinib in patients with advanced non-small-cell lung cancer who progressed during prior treatment with erlotinib, gefitinib, or both. J Clin Oncol. 2013;31:3335–41. doi: 10.1200/JCO.2012.45.0981. [DOI] [PubMed] [Google Scholar]
- 71.Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–8. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 72.Yoshimura N, Okishio K, Mitsuoka S, Kimura T, Kawaguchi T, Kobayashi M, et al. Prospective assessment of continuation of erlotinib or gefitinib in patients with acquired resistance to erlotinib or gefitinib followed by the addition of pemetrexed. J Thorac Oncol. 2013;8:96–101. doi: 10.1097/JTO.0b013e3182762bfb. [DOI] [PubMed] [Google Scholar]
- 73.Halmos B, Pannell NA, Otterson GA, Gadgeel SM, Mekhail T, Snell MR, et al. Erlotinib beyond progression study: Randomized phase II study comparing chemotherapy plus erlotinibwith chemotherapy alone in EGFR tyrosine kinase inhibitor (TKI)-responsive, non-small cell lung cancer (NSCLC) that subsequently progresses. J Clin Oncol. 2013;31(suppl) abstr 8114. [Google Scholar]
- 74.Soria JC, Shepherd FA, Douillard JY, Wolf J, Giaccone G, Crino L, et al. Efficacy of everolimus (RAD001) in patients with advanced NSCLC previously treated with chemotherapy alone or with chemotherapy and EGFR inhibitors. Ann Oncol. 2009;20:1674–81. doi: 10.1093/annonc/mdp060. [DOI] [PubMed] [Google Scholar]
- 75.Sequist LV, Gettinger S, Senzer NN, Martins RG, Jänne PA, Lilenbaum R, et al. Activity of IPI-504, a novel heat-shock protein 90 inhibitor, in patients with molecularly defined nonsmall-cell lung cancer. J Clin Oncol. 2010;28:4953–60. doi: 10.1200/JCO.2010.30.8338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reguart N, Rosell R, Cardenal F, Cardona AF, Isla D, Palmero R, et al. Phase I/II trial of vorinostat (SAHA) and erlotinib for non-small cell lung cancer (NSCLC) patients with epidermal growth factor receptor (EGFR) mutations after erlotinib progression. Lung Cancer. 2014;84:161–7. doi: 10.1016/j.lungcan.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 77.Johnson ML, Riely GJ, Rizvi NA, Azzoli CG, Kris MG, Sima CS, et al. Phase II trial of dasatinib for patients with acquired resistance to treatment with the epidermal growth factor receptor tyrosine kinase inhibitors erlotinib or gefitinib. J Thorac Oncol. 2011;6:1128–31. doi: 10.1097/JTO.0b013e3182161508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Goldberg SB, Supko JG, Neal JW, Muzikansky A, Digumarthy S, Fidias P, et al. A phase I study of erlotinib and hydroxychloroquine in advanced non-small-cell lung cancer. J Thorac Oncol. 2012;7:1602–8. doi: 10.1097/JTO.0b013e318262de4a. [DOI] [PMC free article] [PubMed] [Google Scholar]


