Abstract
Electroencephalography (EEG) systems can enable us to study cerebral activation patterns during performance of swallowing tasks and possibly infer about the nature of abnormal neurological conditions causing swallowing difficulties. While it is well known that EEG signals are non-stationary, there are still open questions regarding the stationarity of EEG during swallowing activities and how the EEG stationarity is affected by different viscosities of the fluids that are swallowed by subjects during these swallowing activities. In the present study, we investigated the EEG signal collected during swallowing tasks by collecting data from 55 healthy adults (ages 18–65). Each task involved the deliberate swallowing of boluses of fluids of different viscosities. Using time-frequency tests with surrogates, we showed that the EEG during swallowing tasks could be considered non-stationary. Furthermore, the statistical tests and linear regression showed that the parameters of fluid viscosity, sex, and different brain regions significantly influenced the index of non-stationarity values. Therefore, these parameters should be considered in future investigations which use EEG during swallowing activities.
Keywords: Swallowing, dysphagia, brain activity, EEG, viscosity, stationarity
1. Introduction
1.1. Deglutition and dysphagia
Deglutition (i.e., swallowing) is a fundamental, but complex human function which involves groups of muscles in the head and neck and activity across several regions of the central and peripheral nervous systems, that produces the transportation of food from the oral cavity via pharynx and esophagus to the stomach (Ertekin and Aydogdu, 2003; Stevenson and Allaire, 1991). This transportation involves several swallowing phases (i.e., oral preparatory, oral transit, pharyngeal, and esophageal) which consists of decreasingly voluntary and increasingly automatized sensorimotor activity from top to bottom (Dodds, 1989). Although not reflexive by definition, the majority of involuntary activity occurring in the pharyngeal phase is mediated by a swallowing center: a central pattern generator in the dorsolateral medulla and adjacent reticular formation that initiates a patterned response when specific sensorimotor input to the center is delivered (Miller, 1986). Of particular importance to dysphagic swallowing disorders are events occurring during the pharyngeal swallowing phase including displacement and closure of the larynx to prevent aspiration (Dodds et al., 1990).
It is well known that swallowing is mediated by both cerebral cortical and brainstem activity. However, recent studies have produced evidence emphasizing the importance of the role of cerebral cortex in the involuntary and voluntary parts of the swallow (Martin et al., 2001a), which demonstrates neuronal activation of different parts of cerebral cortex, namely the sensory and motor cortices, the cingulate gyrus and the insula (Hamdy et al., 1998, 1999b).
Nearly 15 million Americans are affected by the swallowing disorder, dysphagia (Logemann, 1998; Krishnamurthy et al., 2012). As many as 40,000 people die each year in the United States from aspiration pneumonia, a medical complication strongly associated with dysphagia (Aviv et al., 1996). Neurogenic dysphagia is primarily caused by lesions in disparate cortical and subcortical regions (Smithard et al., 1996), and these lesions occur most frequently in people who are suffering from neurological conditions such as stroke (Gottlieb et al., 1996), brain injuries (Lazarus and Logemann, 1987), cerebral palsy (Rogers et al., 1994), Parkinson’s and other neurodegenerative diseases (Murray, 1999). Even though many different specific cortical lesions that produce dysphagia have been identified (Robbins and Levine, 1988; Veis and Logemann, 1985), one previous study investigating stroke patients with dysphagia concluded that the insular cortex is the most common cerebral lesion site in patients with stroke-related dysphagia (Daniels et al., 1996), and conjectured accordingly, that the insular cortex may be the most important activated region of the brain during swallowing in general.
1.2. Electroencephalography
Electroencephalography (EEG) records electrical activity in the brain (Suzuki et al., 1983). Neural activity in the brain generates electrical signals, which can be recorded by electrodes (i.e., sensors) placed on the surface of scalp (Niedermeyer and da Silva, 2005). EEG is used across a broad range of clinical applications when diagnosing an array of conditions (Montalenti et al., 2001; Connell et al., 1989; Armitage, 1995). Brain EEG waves form sinusoidal shapes where certain frequencies are more dominant depending on the brain activity during different tasks (Lykken et al., 1974; Sarnthein et al., 2006). Using sophisticated computerized analysis tools, it is possible to determine brain mapping for specific activities from the EEG time series (Nuwer, 1997). This is called quantitative EEG (Nuwer, 1997). With quantitative EEG, it is possible to determine spatial structures and localize areas with brain activity and abnormality (Nuwer, 1988; Cook et al., 1998).
Even though there are few documented studies about characterization of EEG during swallowing, EEG is considered as one of the few possible techniques that can be used for evaluating cortical brain activity during swallowing (Huckabee et al., 2003; Hiraoka, 2004). For example, Huckabee at al. (Huckabee et al., 2003) found that the supplementary motor cortex is activated right before the volitional part of the swallow, while volitional swallowing did not seem to activate the primary motor cortex. Hiraoka at all. (Hiraoka, 2004) investigated swallowing of saliva and water boluses, and found that the type of fluid greatly influences activation of the cortex associated with pharyngeal swallowing. Normal deglutition has a somewhat distinctive EEG wave representation (Stern and Engel, 2005). This representation can be detected and recorded through the electrode channels when they are placed on the scalp above active regions of the brain during swallowing. The neurological conditions that cause dysphagia also cause changes in the brain’s neuronal activity (Aydogdu et al., 2001; Kern et al., 2001). Few studies showed that abnormal changes in the brain’s neuronal activity due to neurological conditions can be recognized in the EEG signal during the swallowing task (Wilson and Oliver, 1988; Saint-Martin et al., 1999). In order to recognize these abnormalities, it is important to investigate normal deglutition and to become familiar with the origins of all EEG wavelets in different scalp regions.
1.3. Research objective
In this study, we investigate the stationarity of the EEG signal during swallowing because these findings could be important in future investigations. We hypothesized that EEG signal stationarity would be significantly affected by sex, age, different brain regions, and by the viscosity of the swallowed liquids. Stationarity describes the statistical behavior of the signal in time. If the signal has statistical characteristics which are not changing in time, the signal can be considered as stationary, otherwise it is non-stationary. Recognizing a non-stationary process in the EEG and identification of the components that are responsible for affecting the signal’s stationarity could be important for different reasons. These components may contain certain telltale patterns (e.g., different types of background activity) that elucidate the underlying nature of neurogenic dysphagia, and becoming familiar with these patterns could provide a pathway to new intervention strategies for neurogenic dysphagia caused by abnormal brain activity during swallowing. By monitoring the post-processed (i.e., in possible future investigations) EEG waveforms, it could be possible to recognize these clinically significant changes, which may lead to influencing the treatment of patients with neurogenic dysphagia, either by visual inspection or even by some other automatic method (i.e., software tools for automatic detection of changes).
2. Results
We examined the stationarity of 64209 EEG channel signals using time-frequency approach with surrogates. Figure 1 shows the percentage of non-stationarities in EEG for different stimuli. Figure 2. shows boxplots with the distribution of index of non-stationarity values for different types of fluids. According to our results, it can be seen that saliva swallowing contained more non-stationarity than other stimuli.
Figure 1.
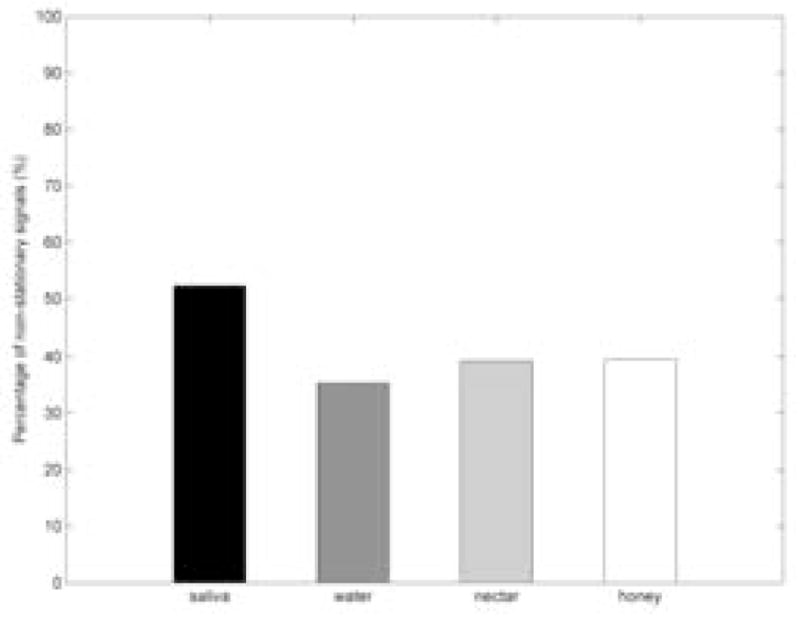
Percentage of non-stationary in EEG signals for the different fluid stimuli.
Figure 2.
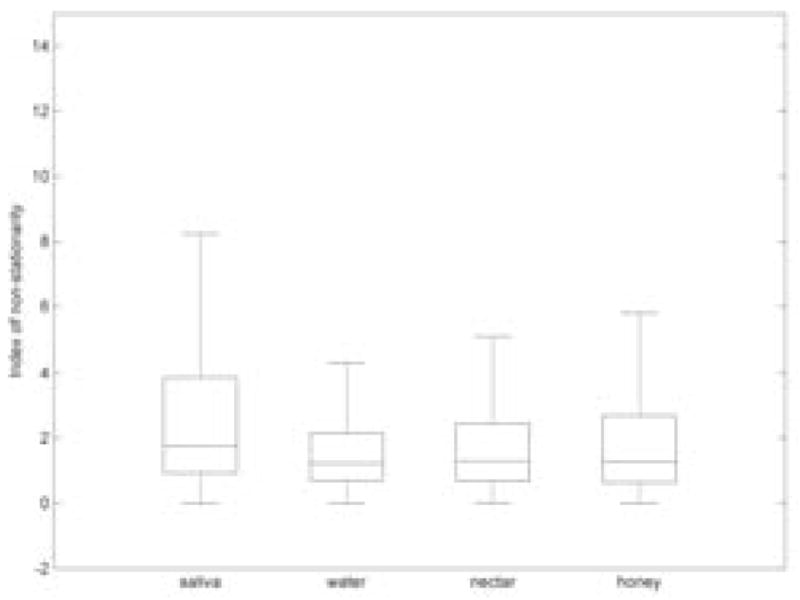
Distribution of the index of non-stationarity for different fluid stimuli.
Statistical tests failed to show differences for the index of non-stationarity only between nectar-thick and honey-thick swallows (p = 0.63). All other pairwise comparisons between different stimuli (i.e., saliva and water; saliva and nectar-thick; saliva and honey-thick; water and nectar-thick; water and honey-thick) showed significant differences (p ≪ 0.01). Sex differences were absent only for water swallows (p = 0.44), while male participants exhibited a higher non-stationarity for all other swallows (p ≪ 0.01). Comparisons between the left and right frontal sides and comparisons between left and right back sides (Figure 3.) did not show statistical difference (p > 0.05) for each respective comparison pair. However, both the left and right frontal sides have a higher index of non-stationarity than both the left and right back sides (p ≪ 0.01). According to the linear regression, age did not affect the percent of stationarity test statistic values (p = 0.65).
Figure 3.
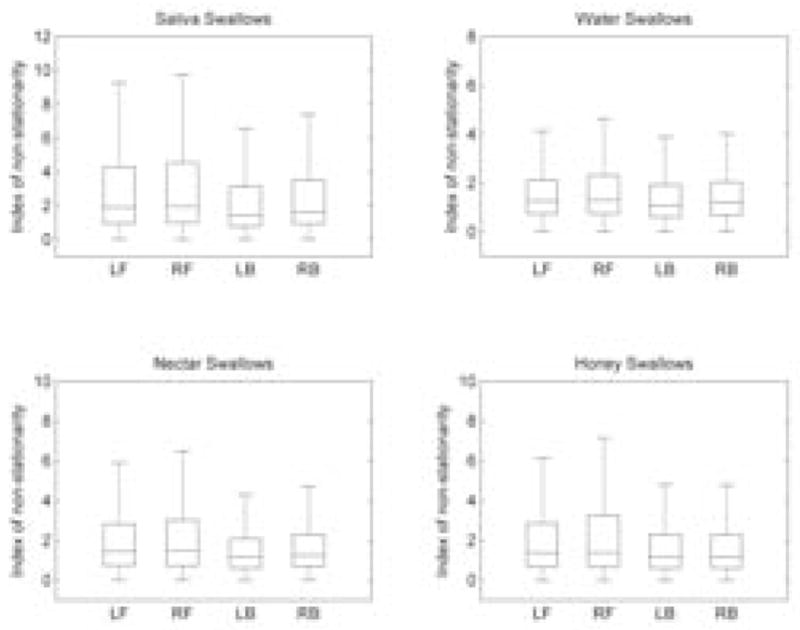
Distribution of the index of non-stationarity for each brain region for different fluid stimuli.
According to Figure 4. most of the non-stationarity can be attributed to a change in the mean over time (50–60%). Less than 10% of EEG demonstrated time-dependent variance alone. Changes in both mean and variance significantly contributed to the non-stationarity (i.e., 15–25%), as well as unknown causes (15–21%). However, the time-varying mean was a major cause of non-stationarity.
Figure 4.
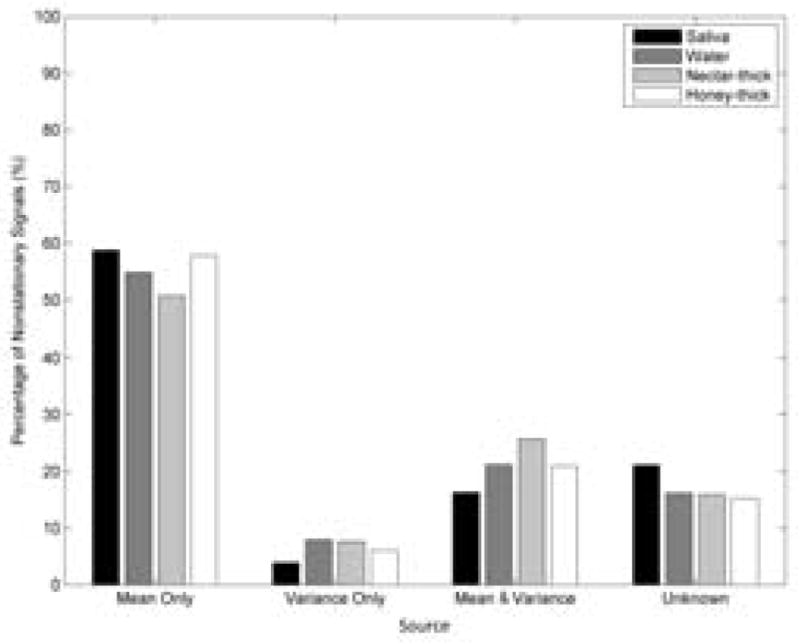
Percent of source contribution to non-stationarity for different fluid stimuli.
3. Discussion
Our hypotheses, that sex, liquid viscosity, and brain region would significantly affect the EEG signals stationarity, were supported in most comparisons, while our hypothesis that age affects EEG signals stationarity was not supported. The fact that swallowing is one of the most complicated tasks performed by the central nervous system (Bieger, 1993; Martin et al., 2001a), led us to intuitively expect non-stationarities in its EEG representation. EEG signals tend to be more non-stationary with an increasing fluid viscosity. This means that the collected EEG signals during swallowing tasks using thicker liquids contain more changes in mean and variance along the signal sample duration (Papoulis, 1991). Studies that have investigated brain activity related to eating have discovered neurons that are capable of responding to either the viscosity or the taste of food and to both the viscosity and the taste (De Araujo and Rolls, 2004). The neuronal responses to increasing fluid viscosity during swallowing tasks manifests as modulation of the response from the neurons (i.e., increase or decrease) or even as activation thresholding where neurons respond only for a range of fluid viscosities (Rolls et al., 2003). The cumulative effect of neuronal activity in a given area of the cortex will proportionally change the behavior of the EEG signal and subsequently affect the number of stationary test statistic values (Niedermeyer and da Silva, 2005).
Additionally, our results reported that sex affects the stationary test statistic values. Even though there exists little documented information about sex differences in brain activity for food stimuli (Smeets et al., 2006), there is evidence of gender differences regarding the ability to inhibit brain activation elicited by food stimulation (Wang et al., 2009). In women, it was shown that the brain regions responsible for processing visual and taste sensations showed greater activity to food stimuli than the same brain regions for men (Uher et al., 2006). Food stimuli are associated with greater activity in the orbitofrontal cortex and prefrontal dorsolateral cortex regions of the brain; these regions are responsible mostly for emotions/rewards with regard to decision making and memory, respectively. It is known that the frontal cortex is associated with the presence of sex hormones levels during the menstrual cycle during a memory task in women (Schöning et al., 2007; Del Parigi et al., 2002). This leads to the conclusion that modulation of brain activity (i.e., when recording with EEG equipment) during a swallowing task (i.e., food) in these regions is correlated with gender and explains our result. This means that one of our future investigations could focus on the characterization of swallowing EEG signals with females at known points in the menstrual cycle and testing for differences. Also, this difference in stationarity between gender, could be attributed to bolus size. It was shown that comfortable blouse sizes are different between men and women (Adnerhill et al., 1989). Men have significantly larger bolus sizes than women. This difference may produce different brain activation patterns during swallowing activity. There is also evidence of possible gender differences in aspects of peripheral swallowing kinematics and physiology including the duration of laryngeal closure during the swallow in healthy adults and those with dysphagia (Hiss et al., 2001; Kent-Braun et al., 2002; Kurosu and Logemann, 2010, 2011). However it remains uncertain whether these are actual gender differences caused by anatomic and physiologic differences between adult females and males, or artifacts of experimental design that did not correct for proportional differences in average adult female and male aerodigestive tract caliber and size.
One of the challenges in the investigation of swallowing’s neural origins is determining the brain regions involved in swallowing activities. Advanced imaging techniques, such as functional magnetic resonance imaging (fMRI), positron emission tomography (PET), and magnetoencephalography (MEG) provided significant contributions to the swallowing field and they were used to identify many different origins of swallowing within the brain (Hamdy et al., 1999a,b; Martin et al., 2001b; Kern et al., 2001; Mosier and Bereznaya, 2001; Zald and Pardo, 1999). Our findings showed differences in stationarity between the frontal and the back part of the brain. Luan et al. (2013), in their fMRI studies related to swallowing, summarize 23 different brain regions which are activated during swallowing activities. According to their report, it can be seen that the brain regions involved in swallowing are mostly concentrated in the frontal portion. This explains the statistical difference for stationarity in our data.
Stationarity describes if a signals’ statistical behavior from the future has the same statistical behavior from the past. Our investigation of stationarity determined that EEG signals during swallowing can be considered as non-stationary, and changes in mean of the signal are mostly responsible for producing the non-stationarities. Studies which investigated artifacts in EEG signals showed that swallowing activities produce a burst of brain activity with higher frequency content (i.e., compared with the baseline) and a differing amplitude variation (i.e., again, compared to the baseline) (Yong et al., 2009). This variation of the amplitude explains origins of non-stationarities related to changes in the mean. This provides us an answer which allows us to appropriately select which technique and which signal duration length we should use in future analyses of EEG (i.e., the wavelet-based approach (Simonsen et al., 1998), time-frequency based phase synchrony measure (Aviyente et al., 2011), which are suitable for analyzing of non-stationary signals). Clinically, appropriately choosing the technique for analysis is very important, because the proper choice can significantly reduce the number of false positive and false negative detection of swallowing abnormalities. The major future goal for this research and all future investigations is to obtain a faster and more efficient method to determine abnormalities in patients with dysphagia. It is our aim to reduce the clinical burden associated with dysphagia screening and diagnosis, and increase its accuracy and efficiency. To expound on the direction of our aims, further investigation would determine and recognize exact components which cause non-stationarity and their origins. Furthermore, these investigations would aim to determine if those components are repeating and to what extent (i.e., does the signal have a noticeable pattern or are the patterns random), and also determining if these components are important or necessitate removal from our EEG signals.
The clinical implications of this line of research may lead to as to yet unexplored avenues of intervention for people with swallowing disorders. Currently, revived interest in the afferent/sensory portion of the sensorimotor systems subserving swallowing function. Early research investigating peripheral stimulation of sensory receptors produced equivocal evidence of a transient increase in motor output in dysphagic patients after high doses of stimulation (Hamdy et al., 2003; Power et al., 2006; Rosenbek et al., 1991, 1996). However only recently are methods of measuring the central effects of peripheral stimulation increasingly available. Electroencephalography, a relatively portable and noninvasive procedure, may offer significant value in the treatment of neurogenic dysphagia if researchers could elucidate whether central plasticity can be shown to occur as a result of sensorimotor treatments, and if manipulation of the process of reorganization might improve clinical outcomes.
A shortcoming of our study is that the volume of swallowed boluses was not controlled for, so the possibility that bolus volume affected signal stationarity cannot be excluded. Also, in this study, swallowing tasks are administered in a specific order (saliva, water, nectar-thick, honey-thick) which leaves an open question about the influence of order to our results. In order to eliminate these limitations, a future study could include investigations with specific bolus size as well as randomizing the order of administered stimuli.
4. Conclusion
In this study we investigated the stationarity of EEG signals during swallowing activities. Swallowing EEG signals were collected from 55 healthy adults who performed four different types of swallowing tasks, each with a unique viscosity fluid. We demonstrated that the EEG during swallowing tasks, can be considered as non-stationary. Additionally, we found that the viscosity of the fluids, sex, and different brain regions, affects the index of non-stationarity values. And lastly, we found that the origins of non-stationarities were mainly due to variations in the mean.
5. Methods and materials
5.1. Data acquisition from participants
55 healthy adults (28 males and 27 females), aged 18 to 65, participated in the data acquisition process. Each participant signed consent forms, and they provided information about age, gender, height, and weight. The Institutional Review Board at the University of Pittsburgh approved this study.
EEG data was collected with a 64 channels system, arranged according to the 10–20 international electrode system (Jasper, 1958), using actiCAP active electrodes and an actiCHamp amplifier (BrainProducts, Germany). The impedance of each electrode was below 15 kΩ, and the electrode marked P1 was chosen as the reference electrode. All data was recorded with the PyCorder acquisition software, which provided a 10 kHz sampling rate on each channel. In order to determine the swallowing segments (i.e., the starting and ending points of the swallow), the EEG signal was recorded during swallowing simultaneously while dual axis accelerometer sensor attached to the participants’ anterior necks overlying the larynx, recorded vibratory signals associated with laryngeal movement that occurs during swallowing. The methods describing the implementation of the accelerometer, pre-processing steps, and swallowing segmentations are described in a previous study (Jestrović et al., 2013).
After appropriately affixing the accelerometer and EEG cap along with the rest of the system (Figure 5), participants were asked to perform five swallows with fluids of four different viscosities. Fluids were served chilled (i.e., between 3° – 5 °C) in individual cups filled to approximately 30 ml. Participants were instructed, after the instructor signaled for the participant to begin the swallowing task, and then to pause 2–3 seconds between each swallow. The order of presentation of the four conditions was not randomized. Participants swallowed five saliva boluses, followed by five water boluses (viscosity of water is 1 cP), followed by five mildly thick (nectar-thick, Nestlé Health Care Inc., Florham Park, N.J.) liquid boluses with a viscosity of 150cP, and followed finally by five moderately thick (honey-thick, Nestlé Health Care Inc., Florham Park, N.J.) liquid boluses (400cP). Bolus volume was not controlled for or measured, and participants were asked to consume a comfortable bolus volume as there are sex based differences in a comfortable bolus size (Adnerhill et al., 1989).
Figure 5.
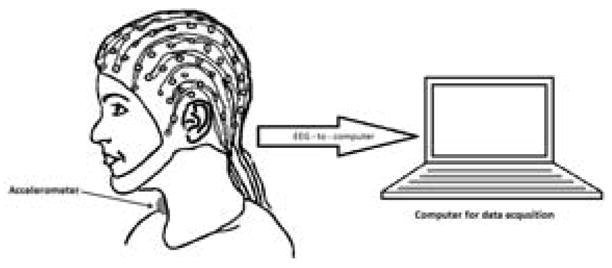
The experimental setups used in the study.
5.2. Pre-processing steps
Pre-processing of the EEG signals were performed using the EEGlab (Delorme and Makeig, 2004) toolbox running on MATLAB Version 2013a. The first step in pre-processing of the EEG signals began with downsampling the signal to 256Hz. Next, the signal was filtered using a short band-pass elliptical IIR filter with a cut-off frequency between 0.1Hz to 100Hz. To remove power supply noise, the EEG signal was filtered with a short notch elliptical IIR filter with a bandwidth between 58Hz to 62Hz. Next, the signal was segmented into separate swallows using segmentation points based on the accelerometer signal. Lastly, artifacts were removed. Previous studies have shown that independent Component Analysis (ICA) (Hyvärinen and Oja, 2000) implemented in EEGlab is a convenient method for removing EEG artifacts (Olbrich et al., 2011; Hoffmann and Falkenstein, 2008; Srivastava et al., 2005); therefore, this same method was used to remove artifacts in the EEG signals collected in this study. Only the channels, which were contaminated with artifacts, were considered for the ICA algorithm. ICA components corresponding to artifacts were identified by visual inspection and then removed. Also, channels that contained unreasonable values due to artifacts which couldn’t be removed, were excluded from study (i.e. less then 5% were excluded).
5.3. Stationarity and time-frequency approach test
Assume that each EEG channel is represented as separate discrete time series of the length n, X(t) = {x1, x2, …, xn}. In this case X(t) is a family of real-valued random variables and t is an integer (t ∈
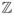 ). If the statistical properties of the given data series are shift-invariant,
). If the statistical properties of the given data series are shift-invariant,
| (1) |
where h ∈
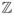 , then we would say that this time series is strongly or strict-sense stationary (Papoulis, 1991). However, if only first two moments of the series are time-invariant,
, then we would say that this time series is strongly or strict-sense stationary (Papoulis, 1991). However, if only first two moments of the series are time-invariant,
| (2) |
| (3) |
then the time series would have a weakly or wide-sense stationarity.
Testing stationarity with surrogates is a time-frequency approach proposed by Borgnat et al. (2010) for testing wide-sense stationarity. The essence of this approach is to compare the local spectra statistics of the signal with the global spectrum of the signal. Depending on the result of this comparison, the stationarity of the time scale can be determined. The local spectra of the signal is calculated using the multitaper spectrogram, which is defined as:
| (4) |
where is the spectrogram computed with the k-th Hermite function, and it is defined as:
| (5) |
where hk(t) is k-th Hermite function of the length Th, defined by:
| (6) |
where g(t) = exp{−t2/2}.
A surrogate data set stationary version of the signal of interest. A surrogate data set is formed by multiplying the amplitude of the Fourier transform of the original signal by independent identically distributed phase sequence. After this multiplication, the inverse Fourier transform is applied to the result. Since the computed result is just a realization of the random process, in order to improve the test, there is a need for greater realization. Therefore, a total of J of realizations will be obtained. After obtaining J different surrogate realizations, the distance between the local spectra and the global spectrum (GS) was calculated. Mathematically, GS can be expressed as:
| (7) |
The distance between the local spectra and the global spectrum is formed combining the Kullback-Leibler divergence and the log-spectral deviation. These two parameters are defined as (Basseville, 1989):
| (8) |
| (9) |
respectively, where L(f) is the local spectrum and G(f) is the global spectrum, and f is the frequency variable over the spaca Ω. Next, distance of our interest is computed as:
| (10) |
where L̃ and G̃ are the normalized parameters for L and G, respectively. For the original signal and each surrogate signal, N distances between N local spectra and GS were calculated. Furthermore, from this set of N distances, the variance was calculated. The variance for the distances of the original signal is indexed as Θ1, while the variance for the distances of each surrogate is indexed as vector Θ0. Since elements of the vector Θ0 has Gamma distribution (Borgnat et al., 2010), it enables determination of the value for threshold γ. This means that for all values above γ the null hypothesis is rejected.
In the case when null hypothesis is rejected, we can calculate the index of non-stationarity (INS), which is defined as:
| (11) |
where E[γ] the average value of the random variable. This value is approximate as the average value from the vector Θ0. In the case of stationary signals, INS is close to one, while non-stationary signals has higher INS value. As signal is more non stationary, INS is higher.
5.4. Data analysis
In order to determine statistical difference between stationarity of different condition, statistical test was applied on index of non-stationarity. The Kruskal-Wallis test (Kruskal and Wallis, 1952) was used for testing the statistical differences between each swallowing task types (i.e., saliva, water, nectar-thick, and honey-thick). Next, the Wilcoxon rank-sum test (Wilcoxon et al., 1963) was used for determining the statistical differences of index of non-stationarity between different fluids, between genders, and between different brain regions (left frontal, right frontal, left back, and right back). A standard linear regression was used to examine the age effects on percent of stationarity test statistic values (Montgomery et al., 2012).
Acknowledgments
Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute Of Child Health & Human Development of the National Institutes of Health under Award Number R01HD074819.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
I. Jestrović, Email: ivj2@pitt.edu.
J. L. Coyle, Email: jcoyle@pitt.edu.
E. Sejdić, Email: esejdic@ieee.org.
References
- Adnerhill I, Ekberg O, Groher ME. Determining normal bolus size for thin liquids. Dysphagia. 1989;4 (1):1–3. doi: 10.1007/BF02407395. [DOI] [PubMed] [Google Scholar]
- Armitage R. Microarchitectural findings in sleep EEG in depression: diagnostic implications. Biological Psychiatry. 1995;37 (2):72–84. doi: 10.1016/0006-3223(94)00082-E. [DOI] [PubMed] [Google Scholar]
- Aviv JE, Martin J, Sacco R, Zagar D, Diamond B, Keen M, Blitzer A. Supraglottic and pharyngeal sensory abnormalities in stroke patients with dysphagia. The Annals of Otology, Rhinology, and Laryngology. 1996;105 (2):92–97. doi: 10.1177/000348949610500202. [DOI] [PubMed] [Google Scholar]
- Aviyente S, Bernat EM, Evans WS, Sponheim SR. A phase synchrony measure for quantifying dynamic functional integration in the brain. Human Brain Mapping. 2011;32 (1):80–93. doi: 10.1002/hbm.21000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydogdu I, Ertekin C, Tarlaci S, Turman B, Kiylioglu N, Secil Y. Dysphagia in lateral medullary infarction (Wallenbergs Syndrome): An acute disconnection syndrome in premotor neurons related to swallowing activity. Stroke. 2001;32 (9):2081–2087. doi: 10.1161/hs0901.094278. [DOI] [PubMed] [Google Scholar]
- Basseville M. Distance measures for signal processing and pattern recognition. Signal Processing. 1989;18 (4):349–369. [Google Scholar]
- Bieger D. Central nervous system control mechanisms of swallowing: a neuropharmacological perspective. Dysphagia. 1993;8 (4):308–310. doi: 10.1007/BF01321768. [DOI] [PubMed] [Google Scholar]
- Borgnat P, Flandrin P, Honeine P, Richard C, Xiao J. Testing stationarity with surrogates: A time-frequency approach. IEEE Transactions on Signal Processing. 2010;58 (7):3459–3470. [Google Scholar]
- Connell J, Oozeer R, De Vries L, Dubowitz L, Dubowitz V. Continuous EEG monitoring of neonatal seizures: diagnostic and prognostic considerations. Archives of Disease in Childhood. 1989;64 (4):452–458. doi: 10.1136/adc.64.4_spec_no.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook IA, O’Hara R, Uijtdehaage SH, Mandelkern M, Leuchter AF. Assessing the accuracy of topographic EEG mapping for determining local brain function. Electroencephalography and Clinical Neurophysiology. 1998;107 (6):408–414. doi: 10.1016/s0013-4694(98)00092-3. [DOI] [PubMed] [Google Scholar]
- Daniels SK, Foundas AL, Iglesia GC, Sullivan MA. Lesion site in unilateral stroke patients with dysphagia. Journal of Stroke and Cerebrovascular Diseases. 1996;6 (1):30–34. doi: 10.1016/s1052-3057(96)80023-1. [DOI] [PubMed] [Google Scholar]
- De Araujo IE, Rolls ET. Representation in the human brain of food texture and oral fat. The Journal of Neuroscience. 2004;24 (12):3086–3093. doi: 10.1523/JNEUROSCI.0130-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Parigi A, Chen K, Gautier JF, Salbe AD, Pratley RE, Ravussin E, Reiman EM, Tataranni PA. Sex differences in the human brain’s response to hunger and satiation. The American Journal of Clinical Nutrition. 2002;75 (6):1017–1022. doi: 10.1093/ajcn/75.6.1017. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods. 2004;134 (1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Dodds WJ. The physiology of swallowing. Dysphagia. 1989;3 (4):171–178. doi: 10.1007/BF02407219. [DOI] [PubMed] [Google Scholar]
- Dodds WJ, Stewart ET, Logemann JA. Physiology and radiology of the normal oral and pharyngeal phases of swallowing. American Journal of Roentgenology. 1990;154 (5):953–963. doi: 10.2214/ajr.154.5.2108569. [DOI] [PubMed] [Google Scholar]
- Ertekin C, Aydogdu I. Neurophysiology of swallowing. Clinical Neurophysiology. 2003;114 (12):2226–2244. doi: 10.1016/s1388-2457(03)00237-2. [DOI] [PubMed] [Google Scholar]
- Gottlieb D, Kipnis M, Sister E, Vardi Y, Brill S. Validation of the 50 ml3 drinking test for evaluation of post-stroke dysphagia. Disability and Rehabilitation. 1996;18 (10):529–532. doi: 10.3109/09638289609166040. [DOI] [PubMed] [Google Scholar]
- Hamdy S, Aziz Q, Rothwell JC, Power M, Singh KD, Nicholson DA, Tallis RC, Thompson DG. Recovery of swallowing after dysphagic stroke relates to functional reorganization in the intact motor cortex. Gastroenterology. 1998;115 (5):1104–1112. doi: 10.1016/s0016-5085(98)70081-2. [DOI] [PubMed] [Google Scholar]
- Hamdy S, Jilani S, Price V, Parker C, Hall N, Power M. Modulation of human swallowing behaviour by thermal and chemical stimulation in health and after brain injury. Neurogastroenterology & Motility. 2003;15 (1):69–77. doi: 10.1046/j.1365-2982.2003.00390.x. [DOI] [PubMed] [Google Scholar]
- Hamdy S, Mikulis DJ, Crawley A, Xue S, Lau H, Henry S, Diamant NE. Cortical activation during human volitional swallowing: an event-related fMRI study. American Journal of Physiology-Gastrointestinal and Liver Physiology. 1999a;277 (1):219–225. doi: 10.1152/ajpgi.1999.277.1.G219. [DOI] [PubMed] [Google Scholar]
- Hamdy S, Rothwell JC, Brooks DJ, Bailey D, Aziz Q, Thompson DG. Identification of the cerebral loci processing human swallowing with H2 150 PET activation. Journal of Neurophysiology. 1999b;81 (4):1917–1926. doi: 10.1152/jn.1999.81.4.1917. [DOI] [PubMed] [Google Scholar]
- Hiraoka K. Movement-related cortical potentials associated with saliva and water bolus swallowing. Dysphagia. 2004;19 (3):155–159. doi: 10.1007/s00455-004-0002-9. [DOI] [PubMed] [Google Scholar]
- Hiss SG, Treole K, Stuart A. Effects of age, gender, bolus volume, and trial on swallowing apnea duration and swallow/respiratory phase relationships of normal adults. Dysphagia. 2001;16 (2):128–135. doi: 10.1007/s004550011001. [DOI] [PubMed] [Google Scholar]
- Hoffmann S, Falkenstein M. The correction of eye blink artefacts in the EEG: a comparison of two prominent methods. PLoS ONE. 2008;3 (8):3004–3015. doi: 10.1371/journal.pone.0003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckabee ML, Deecke L, Cannito MP, Gould HJ, Mayr W. Cortical control mechanisms in volitional swallowing: the Bereitschaftspotential. Brain Topography. 2003;16 (1):3–17. doi: 10.1023/a:1025671914949. [DOI] [PubMed] [Google Scholar]
- Hyvärinen A, Oja E. Independent component analysis: algorithms and applications. Neural Networks. 2000;13 (4):411–430. doi: 10.1016/s0893-6080(00)00026-5. [DOI] [PubMed] [Google Scholar]
- Jasper HH. The ten twenty electrode system of the international federation. Electroencephalography and Clinical Neurophysiology. 1958;10:371–375. [PubMed] [Google Scholar]
- Jestrović I, Dudik JM, Luan B, Coyle JL, Sejdić E. The effects of increased fluid viscosity on swallowing sounds in healthy adults. Biomedical Engineering Online. 2013;12 (1):90–107. doi: 10.1186/1475-925X-12-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent-Braun JA, Ng AV, Doyle JW, Towse TF. Human skeletal muscle responses vary with age and gender during fatigue due to incremental isometric exercise. Journal of Applied Physiology. 2002;93 (5):1813–1823. doi: 10.1152/japplphysiol.00091.2002. [DOI] [PubMed] [Google Scholar]
- Kern M, Birn R, Jaradeh S, Jesmanowicz A, Cox R, Hyde J, Shaker R. Swallow-related cerebral cortical activity maps are not specific to deglutition. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2001;280 (4):531–538. doi: 10.1152/ajpgi.2001.280.4.G531. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy C, Hilden K, Peterson KA, Mattek N, Adler DG, Fang JC. Endoscopic findings in patients presenting with dysphagia: analysis of a national endoscopy database. Dysphagia. 2012;27 (1):101–105. doi: 10.1007/s00455-011-9346-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruskal WH, Wallis WA. Use of ranks in one-criterion variance analysis. Journal of the American Statistical Association. 1952;47 (260):583–621. [Google Scholar]
- Kurosu A, Logemann JA. Gender effects on airway closure in normal subjects. Dysphagia. 2010;25 (4):284–290. doi: 10.1007/s00455-009-9257-5. [DOI] [PubMed] [Google Scholar]
- Kurosu A, Logemann JA. Gender effects on airway closure in head and neck cancer patients. Dysphagia. 2011;26 (1):18–26. doi: 10.1007/s00455-009-9262-8. [DOI] [PubMed] [Google Scholar]
- Lazarus C, Logemann J. Swallowing disorders in closed head trauma patients. Archives of Physical Medicine and Rehabilitation. 1987;68 (2):79–84. [PubMed] [Google Scholar]
- Logemann JA. Evaluation and treatment of swallowing disorders. PRO-ED, Incorporated; Austin, TX: 1998. [Google Scholar]
- Luan B, Sörös P, Sejdić E. A study of brain networks associated with swallowing using graph-theoretical approaches. PloS one. 2013;8 (8):e73577. doi: 10.1371/journal.pone.0073577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykken D, Tellegen A, Thorkelson K. Genetic determination of EEG frequency spectra. Biological Psychology. 1974;1 (4):245–259. doi: 10.1016/0301-0511(74)90001-5. [DOI] [PubMed] [Google Scholar]
- Martin RE, Goodyear BG, Gati JS, Menon RS. Cerebral cortical representation of automatic and volitional swallowing in humans. Journal of Neurophysiology. 2001a;85 (2):938–950. doi: 10.1152/jn.2001.85.2.938. [DOI] [PubMed] [Google Scholar]
- Martin RE, Goodyear BG, Gati JS, Menon RS. Cerebral cortical representation of automatic and volitional swallowing in humans. Journal of Neurophysiology. 2001b;85 (2):938–950. doi: 10.1152/jn.2001.85.2.938. [DOI] [PubMed] [Google Scholar]
- Miller AJ. Neurophysiological basis of swallowing. Dysphagia. 1986;1 (2):91–100. [Google Scholar]
- Montalenti E, Imperiale D, Rovera A, Bergamasco B, Benna P. Clinical features, EEG findings and diagnostic pitfalls in juvenile myoclonic epilepsy: a series of 63 patients. Journal of the Neurological Sciences. 2001;184 (1):65–70. doi: 10.1016/s0022-510x(00)00496-2. [DOI] [PubMed] [Google Scholar]
- Montgomery DC, Peck EA, Vining GG. Introduction to linear regression analysis. Wiley; New York City, NY: 2012. [Google Scholar]
- Mosier K, Bereznaya I. Parallel cortical networks for volitional control of swallowing in humans. Experimental Brain Research. 2001;140 (3):280–289. doi: 10.1007/s002210100813. [DOI] [PubMed] [Google Scholar]
- Murray J. Manual of dysphagia assessment in adults. Singular; San Diego, CA: 1999. [Google Scholar]
- Niedermeyer E, da Silva FHL. Electroencephalography: basic principles, clinical applications, and related fields. Wolters Kluwer Health; Alphen aan den Rijn, Netherlands: 2005. [Google Scholar]
- Nuwer M. Assessment of digital EEG, quantitative EEG, and EEG brain mapping: Report of the American Academy of Neurology and the American Clinical Neurophysiology Society. Neurology. 1997;49 (1):277–292. doi: 10.1212/wnl.49.1.277. [DOI] [PubMed] [Google Scholar]
- Nuwer MR. Quantitative EEG: I. Techniques and problems of frequency analysis and topographic mapping. Journal of Clinical Neurophysiology. 1988;5 (1):1–44. [PubMed] [Google Scholar]
- Olbrich S, Jödicke J, Sander C, Himmerich H, Hegerl U. ICA-based muscle artefact correction of EEG data: What is muscle and what is brain?: Comment on McMenamin et al. Neuroimage. 2011;54 (1):1–3. doi: 10.1016/j.neuroimage.2010.04.256. [DOI] [PubMed] [Google Scholar]
- Papoulis A. Probability, random variables, and stochastic processes. 3. McGraw-Hill; New York City, NY: 1991. [Google Scholar]
- Power ML, Fraser CH, Hobson A, Singh S, Tyrrell P, Nicholson DA, Turnbull I, Thompson DG, Hamdy S. Evaluating oral stimulation as a treatment for dysphagia after stroke. Dysphagia. 2006;21 (1):49–55. doi: 10.1007/s00455-005-9009-0. [DOI] [PubMed] [Google Scholar]
- Robbins J, Levine RL. Swallowing after unilateral stroke of the cerebral cortex: preliminary experience. Dysphagia. 1988;3 (1):11–17. doi: 10.1007/BF02406275. [DOI] [PubMed] [Google Scholar]
- Rogers B, Arvedson J, Buck G, Smart P, Msall M. Characteristics of dysphagia in children with cerebral palsy. Dysphagia. 1994;9 (1):69–73. doi: 10.1007/BF00262762. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Verhagen JV, Kadohisa M. Representations of the texture of food in the primate orbitofrontal cortex: neurons responding to viscosity, grittiness, and capsaicin. Journal of Neurophysiology. 2003;90 (6):3711–3724. doi: 10.1152/jn.00515.2003. [DOI] [PubMed] [Google Scholar]
- Rosenbek JC, Robbins J, Fishback B, Levine RL. Effects of thermal application on dysphagia after stroke. Journal of Speech, Language, and Hearing Research. 1991;34 (6):1257–1268. doi: 10.1044/jshr.3406.1257. [DOI] [PubMed] [Google Scholar]
- Rosenbek JC, Roecker EB, Wood JL, Robbins J. Thermal application reduces the duration of stage transition in dysphagia after stroke. Dysphagia. 1996;11 (4):225–233. doi: 10.1007/BF00265206. [DOI] [PubMed] [Google Scholar]
- Saint-Martin A, Petiau C, Massa R, Maquet P, Marescaux C, Hirsch E, Metz-Lutz MN. Idiopathic rolandic epilepsy with interictal facial myoclonia and oromotor deficit: a longitudinal EEG and PET study. Epilepsia. 1999;40 (5):614–620. doi: 10.1111/j.1528-1157.1999.tb05564.x. [DOI] [PubMed] [Google Scholar]
- Sarnthein J, Stern J, Aufenberg C, Rousson V, Jeanmonod D. Increased EEG power and slowed dominant frequency in patients with neurogenic pain. Brain. 2006;129 (1):55–64. doi: 10.1093/brain/awh631. [DOI] [PubMed] [Google Scholar]
- Schöning S, Engelien A, Kugel H, Schäfer S, Schiffbauer H, Zwitserlood P, Pletziger E, Beizai P, Kersting A, Ohrmann P, Greb R, Lehmann W, Heindel W, Arolt V, Konrad C. Functional anatomy of visuo-spatial working memory during mental rotation is influenced by sex, menstrual cycle, and sex steroid hormones. Neuropsychologia. 2007;45 (14):3203–3214. doi: 10.1016/j.neuropsychologia.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Simonsen I, Hansen A, Nes OM. Determination of the hurst exponent by use of wavelet transforms. Physical Review E. 1998;58 (3):2779–2787. [Google Scholar]
- Smeets PA, de Graaf C, Stafleu A, van Osch MJ, Nievelstein RA, van der Grond J. Effect of satiety on brain activation during chocolate tasting in men and women. The American Journal of Clinical Nutrition. 2006;83 (6):1297–1305. doi: 10.1093/ajcn/83.6.1297. [DOI] [PubMed] [Google Scholar]
- Smithard D, O’neill P, Park C, Morris J, Wyatt R, England R, Martin D. Complications and outcome after acute stroke does dysphagia matter. Stroke. 1996;27 (7):1200–1204. doi: 10.1161/01.str.27.7.1200. [DOI] [PubMed] [Google Scholar]
- Srivastava G, Crottaz-Herbette S, Lau K, Glover G, Menon V. ICA-based procedures for removing ballistocardiogram artifacts from EEG data acquired in the MRI scanner. NeuroImage. 2005;24 (1):50–60. doi: 10.1016/j.neuroimage.2004.09.041. [DOI] [PubMed] [Google Scholar]
- Stern JM, Engel J. Atlas of EEG patterns. Wolters Kluwer Health; Alphen aan den Rijn, Netherlands: 2005. [Google Scholar]
- Stevenson RD, Allaire JH. The development of normal feeding and swallowing. Pediatric Clinics of North America. 1991;38 (6):1439–1453. doi: 10.1016/s0031-3955(16)38229-3. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Yamaguchi T, Li CL, Klatzo I. The effects of 5-minute ischemia in Mongolian gerbils: II. Changes of spontaneous neuronal activity in cerebral cortex and CA1 sector of hippocampus. Acta Neuropathologica. 1983;60 (3–4):217–222. doi: 10.1007/BF00691869. [DOI] [PubMed] [Google Scholar]
- Uher R, Treasure J, Heining M, Brammer MJ, Campbell IC. Cerebral processing of food-related stimuli: effects of fasting and gender. Behavioural Brain Research. 2006;169 (1):111–119. doi: 10.1016/j.bbr.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Veis S, Logemann J. Swallowing disorders in persons with cerebrovascular accident. Archives of Physical Medicine and Rehabilitatione. 1985;66 (6):372–375. [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Telang F, Jayne M, Ma Y, Pradhan K, Zhu W, Wong CT, Thanos PK, Geliebter A, Biegon A, Fowler JS. Evidence of gender differences in the ability to inhibit brain activation elicited by food stimulation. Proceedings of the National Academy of Sciences. 2009;106 (4):1249–1254. doi: 10.1073/pnas.0807423106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcoxon F, Katti S, Wilcox RA. Critical values and probability levels for the Wilcoxon rank sum test and the Wilcoxon signed rank test. American Cyanamid Company; New York City, NY: 1963. [Google Scholar]
- Wilson GN, Oliver W. Further delineation of the G syndrome: a manageable genetic cause of infantile dysphagia. Journal of Medical Genetics. 1988;25 (3):157–163. doi: 10.1136/jmg.25.3.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong X, Ward RK, Birch GE. Artifact removal in EEG using morphological component analysis. IEEE International Conference on Acoustics, Speech and Signal Processing; IEEE; 2009. pp. 345–348. [Google Scholar]
- Zald DH, Pardo JV. The functional neuroanatomy of voluntary swallowing. Annals of Neurology. 1999;46 (3):281–286. [PubMed] [Google Scholar]


