Abstract
Apolipoprotein (apo) E is a multifunctional protein with central roles in lipid metabolism, neurobiology, and neurodegenerative diseases. It has three major isoforms (apoE2, apoE3, and apoE4) with different effects on lipid and neuronal homeostasis. A major function of apoE is to mediate the binding of lipoproteins or lipid complexes in the plasma or interstitial fluids to specific cell-surface receptors. These receptors internalize apoE-containing lipoprotein particles; thus, apoE participates in the distribution/redistribution of lipids among various tissues and cells of the body. In addition, intracellular apoE may modulate various cellular processes physiologically or pathophysiologically, including cytoskeletal assembly and stability, mitochondrial integrity and function, and dendritic morphology and function. Elucidation of the functional domains within this protein and of the three-dimensional structure of the major isoforms of apoE has contributed significantly to our understanding of its physiological and pathophysiological roles at a molecular level. It is likely that apoE, with its multiple cellular origins and multiple structural and biophysical properties, is involved widely in processes of lipid metabolism and neurobiology, possibly encompassing a variety of disorders of neuronal repair, remodeling, and degeneration by interacting with different factors through various pathways.
Introduction
ApoE functions as a component of plasma lipoproteins in the transport of lipids among cells of different organs and within specific tissues (Mahley, 1988; Mahley and Huang, 1999; Mahley et al., 1999; Mahley and Ji, 1999; Mahley and Rall, 2001; Weisgraber, 1994). Discovered in the early 1970s, it is one of several apolipoproteins associated with very low density lipoproteins (VLDL), intermediate density lipoproteins, chylomicron remnants, and certain subclasses of high-density lipoproteins (HDL). ApoE plays a key role in regulating the clearance of these lipoproteins from the plasma by serving as the ligand for binding to specific cell-surface receptors, including the LDL receptor family members and heparan sulfate proteoglycans (HSPGs) (Mahley, 1988; Mahley and Huang, 1999; Mahley et al., 1999; Mahley and Ji, 1999; Mahley and Rall, 2001; Weisgraber, 1994).
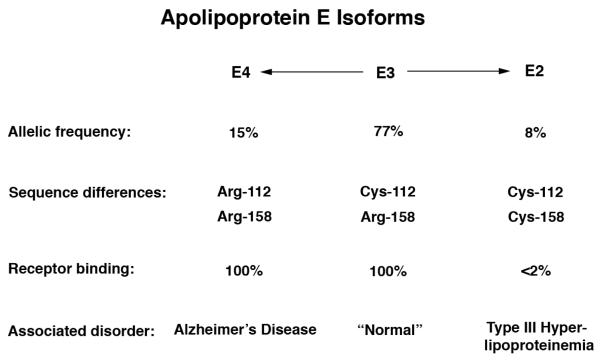
ApoE3, the most common of the three isoforms, is considered to be the normal form. ApoE2 and apoE4 differ from apoE3 by single amino acid substitutions at position 112 or 158 (Fig. 1). Early studies established the amino acid and structural differences among the various apoE isoforms and advanced our understanding of the roles of apoE in various metabolic pathways. Understanding of the role of apoE in lipid metabolism was further advanced by the discovery that apoE2 is defective in lipoprotein receptor binding and is associated with the genetic disorder type III hyperlipoproteinemia (Mahley, 1988; Mahley et al., 1999; Mahley and Rall, 2001). The genetic linkage of apoE4 to the pathogenesis of AD has refocused attention on the importance of this apolipoprotein in neurobiology and neurodegenerative diseases (Fig. 1) (Bu, 2009; Herz and Beffert, 2000; Huang, 2010; Huang and Mucke, 2012; Huang et al., 2004; Kim et al., 2009; Mahley and Huang, 2012a; Mahley et al., 2006; Roses, 1996).
Figure 1. ApoE isoforms and their properties.
E2, apoE2; E3, apoE3; E4, apoE4.
Synthesis of ApoE in Different Tissues and Cells
ApoE is synthesized and secreted from a variety of tissues and several types of cells and is abundant in the interstitial fluid and lymph, as well as in the plasma (Huang, 2010; Huang and Mucke, 2012; Huang et al., 2004; Mahley, 1988; Mahley and Huang, 1999, 2012a; Mahley et al., 2006). ApoE may be secreted by cells in a lipid-poor form; however, because of its avidity for lipids (especially phospholipids), apoE almost certainly always exists in association with lipids and most likely acquires them from the cell surface or from secretory vesicles as it is secreted. In lymph, plasma, and cerebral spinal fluid (CSF), it always appears to be associated with lipids and occurs on lipoprotein particles or phospholipid discs.
Studies in rats, marmosets, and humans have shown that hepatocytes are major sites of apoE synthesis. ApoE production is also readily detected in the brain (second to the liver in quantity), adrenal gland, testis, skin, kidney, spleen, and adipose tissue and in macrophages in a variety of tissues. In both human and rat brains, apoE mRNA is abundant in the cerebral cortex, hippocampus, cerebellum, and medulla, as well as other regions that have been examined.
In the central nervous system (CNS), astrocytes are primarily responsible for the production of apoE; however, specialized astrocytic cell types also synthesize apoE (e.g., Bergmann glia of the cerebellum, tanycytes of the third ventricle, pituicytes of the neurohypophysis, Muller cells of the retina). Neuronal expression of apoE has also been suggested (Huang, 2010; Huang and Mucke, 2012; Huang et al., 2004; Mahley and Huang, 2012a; Mahley et al., 2006). Using knock-in mice in which enhanced green fluorescent protein cDNA was inserted into the mouse apoE locus immediately after the translation initiation site (EGFPapoE reporter mice), we demonstrated conclusively that hippocampal and cortical neurons express apoE in response to injury (Xu et al., 2006). We hypothesize that apoE generated in different types of cells in the CNS plays distinct roles in both physiological and pathophysiological pathways (Huang, 2010; Huang and Mucke, 2012; Huang et al., 2004; Mahley and Huang, 2012a; Mahley et al., 2006).
In the peripheral nervous system, apoE is present in glia surrounding sensory and motor neurons. It is also present in nonmyelinating Schwann cells but not in myelinating Schwann cells. Macrophages are responsible for apoE synthesis and secretion in injured peripheral nerves. Resident macrophages and monocyte-derived macrophages recruited to the site of injury produce large quantities of apoE that accumulate in the extracellular matrix of the degenerating stump and the regenerating nerve (Huang et al., 2004; Mahley, 1988; Mahley et al., 2006).
Structure and Function of ApoE Isoforms in Lipid Metabolism
A major function of apoE is to transport lipids among various cells and tissues of the body (Herz and Bock, 2002; Mahley, 1988; Mahley and Huang, 1999; Mahley et al., 1999; Mahley and Ji, 1999; Mahley and Rall, 2001; Weisgraber, 1994). ApoE is a key regulator of plasma lipid levels and participates in the homeostatic control of plasma and tissue lipid content. This is accomplished in part because apoE binds with high affinity to cell-surface lipoprotein receptors. ApoE mediates the interaction of apoE-containing lipoproteins and lipid complexes to the LDL receptor, the LDL receptor-related protein (LRP), the VLDL receptor, the apoE receptor-2, and gp330. The apoE isoforms differ in their ability to interact with these receptors. In addition, apoE binds to cell surface HSPGs, again with isoform-specific differences in binding affinity. Interaction with HSPGs appears to attract and sequester apoE-containing lipoproteins at cell surfaces and to facilitate their interaction with the LRP and possibly other receptors. HSPGs alone can also mediate the internalization of the apoE-containing lipoprotein particles directly (Mahley and Huang, 2007; Mahley and Ji, 1999). Furthermore, the distribution of apoE among the various lipoproteins is isoform specific and reflects the lipid binding activities and structural differences of apoE2, apoE3, and apoE4 (Mahley and Rall, 2001; Weisgraber, 1994).
Receptor and Heparan Sulfate Proteoglycan Binding Activity
ApoE possesses two structural domains that are connected by 20 to 30 amino acids that may serve as a hinge between the two domains. The N-terminal two thirds of apoE contains the receptor binding region. Six to eight critical arginine and lysine residues and a histidine residue in the region of amino acids 136–150 mediate the interaction of apoE with the ligand binding domain of the LDL receptor (Mahley, 1988; Mahley and Huang, 1999; Mahley et al., 1999; Mahley and Ji, 1999; Mahley and Rall, 2001; Weisgraber, 1994). Arginine 158 appears to be involved in modulating the conformation of the 136–150 region and is involved only indirectly in receptor binding activity. ApoE3 and apoE4, which display normal receptor binding activity, have arginine at residue 158, whereas apoE2, which is defective in receptor binding activity, has cysteine.
Several naturally occurring mutants of apoE have helped to define the receptor binding region (Mahley, 1988; Mahley and Huang, 1999; Mahley et al., 1999; Mahley and Ji, 1999; Mahley and Rall, 2001; Weisgraber, 1994). Amino acid substitutions at residues 136, 142, 145, and 146 result in defective receptor binding and are associated with the development of dominant type III hyperlipoproteinemia. The mutations at residues 136, 142, and 145 involve the substitution of a neutral amino acid for arginine. The mutations at residue 146 involve the substitution of neutral (glutamine) or acidic (glutamate) amino acids for lysine. The substitution of cysteine at residue 158 for the normally occurring arginine results in the common apoE2 variant, which is defective in LDL receptor binding and is associated with the recessive form of type III hyperlipoproteinemia (Mahley, 1988; Mahley and Huang, 1999; Mahley et al., 1999; Mahley and Ji, 1999; Mahley and Rall, 2001; Weisgraber, 1994).
The three-dimensional structure of the receptor binding region of apoE has shed light on the mechanism whereby these specific residues are involved in receptor binding. As shown by X-ray crystallography, the N-terminal two thirds of the apoE molecule (residues 1–191) is a four-helix bundle (Mahley and Huang, 1999; Mahley et al., 1999; Mahley and Rall, 2001; Weisgraber, 1994). Helix 4 (residues 130–164) contains the receptor binding region. The basic amino acids in the 136–150 region are largely solvent-exposed, extend away from the backbone of the molecule, and form a 20-Angstroms basic field of charge that could be available to interact directly with the receptor. Residue 158 (arginine in apoE3 and apoE4) lies outside this highly basic region and is involved in receptor binding indirectly, as confirmed by comparison of the crystal structures of apoE2 and apoE3 (Mahley and Huang, 1999; Mahley et al., 1999; Mahley and Rall, 2001; Weisgraber, 1994).
Cell-surface HSPGs also play an important role in the binding and uptake of apoE-containing lipoproteins either by transferring them to the LDL receptor related protein (LRP) and other lipoprotein receptors or directly by HSPGs (Mahley and Huang, 2007; Mahley and Ji, 1999; Mahley and Rall, 2001). It appears that interaction of apoE with HSPGs is a necessary first step for the LRP-mediated uptake of chylomicron remnants by hepatocytes. This is referred to as the HSPG-LRP pathway. In the absence of cell-surface HSPGs, the apoE-containing remnant lipoproteins do not bind and are not internalized by LRP in in vitro studies. The HSPG-LRP pathway is not restricted to hepatocyte uptake of lipoproteins; it is also operative in other cells (including neurons) and mediates the uptake of lipoproteins enriched in apoE. It is thought that apoE is secreted from the cells, enriching the environment with extracellular apoE and facilitating high-affinity binding of lipoproteins to HSPGs. This process has been termed the secretion-capture role for apoE. ApoE-enriched lipoproteins are captured by binding to HSPGs, brought into the environment of the LRP, and then transferred to the LRP for internalization by the cells. Alternatively, HSPG-LRP may form a complex that is internalized. HSPGs can also serve as receptors for direct binding and internalizing the apoE-containing lipoproteins (Mahley and Huang, 2007; Mahley and Ji, 1999; Mahley and Rall, 2001).
Lipid and Lipoprotein Binding Activity
The isoforms of apoE display preferences for specific classes of lipoproteins (Mahley, 1988; Mahley and Huang, 1999; Mahley et al., 1999; Mahley and Ji, 1999; Mahley and Rall, 2001; Weisgraber, 1994). Examination of the distribution of apoE among the various plasma lipoproteins has shown that apoE4 has a preference for large, triglyceride-rich VLDL particles, whereas apoE3 and apoE2 associate preferentially with the small, phospholipid-rich HDL.
The C-terminal one third of the apoE molecule is critical for lipid binding (Mahley, 1988; Mahley and Huang, 1999; Mahley et al., 1999; Mahley and Ji, 1999; Mahley and Rall, 2001; Weisgraber, 1994). In fact, residues in the 244–272 region of apoE form amphipathic α-helices that mediate the binding of apoE to lipoproteins. A truncated segment of apoE encompassing residues 1–244 possesses markedly reduced ability to bind to lipoproteins, whereas a segment encompassing residues 1–272 possesses full lipid-lipoprotein binding activity (full-length apoE molecule has 299 amino acids). AD brains contain truncated, neurotoxic forms of apoE4, in which the lipid binding domain is responsible for the neurotoxicity (see below) (Chang et al., 2005).
Intramolecular Domain Interaction May Explain ApoE Isoform-Specific Activities
The residues that distinguish the apoE isoforms are in the N-terminus (apoE4, arginine 112; apoE3 and apoE2, cysteine 112). However, the lipid-binding region is in the C-terminus (residues 244–272). This suggests that the N- and C-terminal domains interact to determine the preference of apoE4 for VLDL and of apoE3 and apoE2 for HDL.
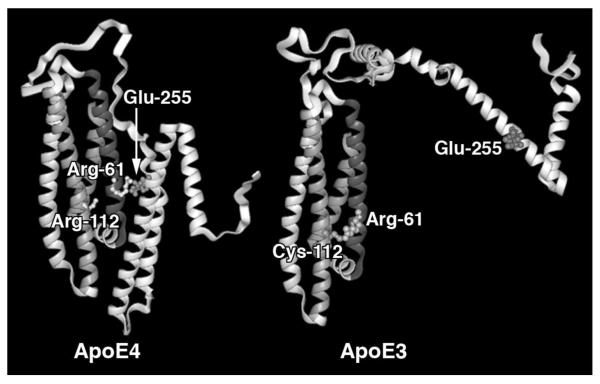
Comparison of the three-dimensional structures of the N-terminal domains of apoE3 and apoE4 and site-directed mutagenesis have provided insights into the functional differences among the isoforms and have defined how domain interaction in apoE4 might occur (Huang, 2010; Huang and Mucke, 2012; Mahley et al., 2006). The only major differences in the crystallographic structures of apoE3 and apoE4 are in the local environment of residue 112. In apoE3, cysteine 112 is in close proximity to glutamic acid 109 in helix 3 and arginine 61 in helix 2. However, in apoE4, with arginine at residue 112, there is a markedly different orientation for glutamic acid 109 and arginine 61. A salt bridge forms between arginine 112 and glutamic acid 109, and the side chain of arginine 61 is reoriented away from the helix and presumably more available for interaction with other residues, including those in the C-terminal domain. In fact, the exposed side chain of arginine 61 in apoE4, but not in the other isoforms, interacts through a salt bridge with the side chain of glutamic acid 255 within the critical lipid binding region of the C-terminal domain. Domain interaction profoundly alters the protein conformation and somehow directs the preference of apoE4 for binding to VLDL, as mutation of either arginine 61 or glutamic acid 255 changes the lipoprotein preference of apoE4 from VLDL to HDL (Mahley and Huang, 1999; Mahley et al., 1999; Mahley and Ji, 1999; Mahley and Rall, 2001; Weisgraber, 1994). Domain interaction occurs predomaintly in apoE4 and is probably responsible for several apoE4-specific roles (Fig. 2).
Figure 2. ApoE4 domain interaction.
In apoE4 (left), arginine 112 orients the side chain of arginine 61 into the aqueous environment, where it can interact with glutamic acid 255, resulting in interaction between the N-terminal and C-terminal domains. In apoE3 (right), arginine 61 is not available to interact with residues in the C-terminal domain, resulting in a very different overall conformation.
Arginine 61 is one feature of human apoE that distinguishes it from apoE in lower species, where residue 61 is a threonine (Weisgraber, 1994). Despite having arginine 112, apoE in lower species associates preferentially with HDL. This further suggests that arginine 61 (in the presence of arginine 112) preferentially directs apoE to the large VLDL particles. The importance of domain interaction extends beyond the isoform-specific roles of apoE in lipid binding and eventually may help us to understand the isoform-specific roles of apoE in AD. In fact, it has been demonstrated that apoE4 domain interaction occurs in living neuronal cells, which might contribute directly to the detrimental effects of apoE4 in AD pathogenesis (Xu et al., 2004).
Structure and Function of ApoE in Neurobiology and Alzheimer’s Disease
Several lines of evidence have linked apoE to neurobiology and Alzheimer’s disease. By the mid-1980s, clues had begun to surface that apoE plays an important role in neurological diseases. ApoE is produced in abundance in the brain and serves as the principal lipid transport vehicle in CSF. It is induced at high concentration in peripheral nerve injury and appears to play a key role in repair by redistributing lipids to regenerating axons and to Schwann cells during remyelination. It modulates neurite outgrowth in cultured rabbit dorsal root ganglion cells and neuroblastoma (Neuro-2a) cells (Huang, 2010; Huang and Mucke, 2012; Huang et al., 2004; Mahley and Huang, 2012a; Mahley et al., 2006). Later, Roses and associates discovered that apoE is a major susceptibility gene associated with sporadic and familial AD, increasing the occurrence and lowering the age of onset of the disease (Roses, 1996).
Aβ-Dependent Effects of ApoE4 on AD Pathogenesis
Aβ overproduction and depositions may play a central role in AD pathogenesis (Bu, 2009; Kim et al., 2009; Selkoe, 2001). Clearly, apoE has isoform-specific effects on Aβ metabolism and catabolism, as it exacerbates Aβ-caused neuropathology and cognitive decline. In vivo, apoE is associated with neuritic amyloid plaques (Namba et al., 1991; Strittmatter et al., 1993a; Wisniewski and Frangione, 1992). In vitro, lipid-free apoE3 and apoE4 can form stable complexes with Aβ peptides; these complexes are resistant to degradation by sodium dodecyl sulfate and guanidine hydrochloride–stable complex and form more rapidly and effectively with apoE4 (Cho et al., 2001; Strittmatter et al., 1993b). In addition, apoE4 enhances zinc- and copper-induced Aβ aggregation (Moir et al., 1999). Thus, apoE seems to display isoform-specific differences in binding to the Aβ peptide, with apoE4 binding more rapidly and effectively under certain conditions. In addition, as compared to apoE3, apoE4 seems also to decrease Aβ clearance in mice (Castellano et al., 2011; Deane et al., 2008). Decreased Aβ clearance and increased amyloid fibril formation associated with apoE4 probably triggers or exacerbates neurodegeneration and the development of AD. Studies in apoE-deficient mice expressing amyloid protein precursor (APP)-V717F demonstrated that apoE is actually required for amyloid plaque formation, at least in mice (Bales et al., 1999). In line with this observation, recent studies showed that increasing expression levels of apoE3 or apoE4 in mutant hAPP or hAPP/PS1 transgenic mice actually increased amyloid deposition in their brains, suggesting that reducing, rather than increasing, apoE expression could be a promising approach to lowering brain Aβ levels and decreasing plaque loads (Bien-Ly et al., 2012; Kim et al., 2011).
However, when incubated with Aβ peptide, apoE3 or apoE4 isolated from stably transfected HEK cells expressing apoE yielded different results (LaDu et al., 1994). ApoE3 bound with 20-fold greater affinity than apoE4 to the Aβ peptide, suggesting that apoE derived from HEK cells and purified recombinant apoE differ in their ability to interact with Aβ peptides in vitro (LaDu et al., 1994). It has been suggested that the avid binding of apoE3 to the Aβ peptide may enhance clearance of the complex, preventing the conversion of Aβ into a neurotoxic species (LaDu et al., 1994). However, the significance of this observation needs to be further evaluated since the apoE3 and apoE4 secreted from HEK cells are multimeric but not lipidated, which is clearly non-physiological and different from apoE in CSF or secreted from astrocytes and neurons (Fagan et al., 1999). Furthermore, a recent study demonstrates that apoE influences soluble Aβ metabolism not through direct binding to Aβ in solution but through its actions with other interacting receptors or transporters and cell surfaces (Verghese et al., 2013).
Studies of transgenic mice expressing human apoE3 or apoE4 have also provided insights into the role of apoE in Aβ metabolism. When hAPP-V717F mice were crossed onto the apoE-null background, Aβ deposition in the brain decreased dramatically, suggesting that mouse apoE enhances Aβ deposition (Bales et al., 1999). However, mice expressing human apoE3 or apoE4 in the absence of mouse apoE had less Aβ deposition than mice expressing mouse apoE, suggesting that human apoE stimulates Aβ clearance (Holtzman et al., 2000a). Interestingly, apoE2 and apoE3 cleared more Aβ than apoE4 in transgenic mice (Dodart et al., 2005; Holtzman et al., 2000a), which was confirmed via gene transfer of different apoE isoforms (Hudry et al., 2013). It has been suggested that apoE isoforms differentially promote astrocyte colocalization and degradation of deposited Aβ peptides (Koistinaho et al., 2004). A recent study suggests that induction of apoE expression by RXR agonist bexarotene led to a short-term reduction in soluble Aβ levels as well as plaque loads and behavioral improvement (Cramer et al., 2012), which was partially confirmed in some (Fitz et al., 2013; Boehm-Cagan et al., 2014) but not in other studies (Veeraraghavalu et al., 2013; Tesseur et al., 2013; Price et al., 2013; LaClair et al., 2013). Interestingly, the cognitive impairment in human APP transgenic mice depends on apoE and on amyloid formation catalyzed by α1-antichymotrypsin (Nilsson et al., 2004). Furthermore, in one study, neuronal apoE4, but not glial apoE4, stimulated Aβ deposition and plaque formation in the hippocampus and cortex in hAPP-V717I transgenic mice (Van Dooren et al., 2006). In another study, however, overexpression of apoE4 in astroglia and neurons did not alter Aβ deposition in transgenic mice (Lesuisse et al., 2001; Van Dooren et al., 2006).
On the other hand, although some studies have suggested that the main effect of apoE isoforms on AD pathogenesis is through plaque formation (Bales et al., 1999; Holtzman et al., 2000a; Holtzman et al., 2000b), others have provided evidence for plaque-independent mechanisms (Buttini et al., 2002; Raber et al., 2000). A study demonstrates that modulation of AD-like synaptic and cholinergic deficits in transgenic mice by human apoE depends on isoform, aging, and overproduction of Aβ peptides but not on plaque formation (Buttini et al., 2002). Moreover, the differential effects of apoE isoforms on hAPP/Aβ-induced cognitive impairment in 6-month-old hAPP/apoE bigenic mice were independent of plaque formation and, surprisingly, Aβ levels in the brain (Raber et al., 2000). Based on our studies (Brecht et al., 2004; Harris et al., 2003; Huang et al., 2001), we hypothesize that the Aβ- and plaque-independent effects of apoE4 on neuronal and behavioral deficits are caused by neurotoxic effects of apoE fragments (see below) (Huang, 2010; Huang and Mucke, 2012; Huang et al., 2004; Mahley and Huang, 2012a; Mahley et al., 2006).
Aβ-Independent Effects of ApoE4 on AD Pathogenesis
Both in vivo and in vitro studies also suggest Aβ-independent roles of apoE4 in AD pathogenesis. The Aβ-independent detrimental effects may act in parallel with Aβ-dependent effects of apoE4, leading to neuropathology and cognitive decline.
ApoE4 causes neuronal and behavioral deficits in the absence of Aβ accumulation in transgenic mice
Several transgenic mouse lines expressing apoE3 or apoE4 have been established. The neuron-specific enolase (NSE) promoter has been used to express human apoE3 or apoE4 at similar levels in neurons of transgenic mice lacking endogenous mouse apoE (Buttini et al., 1999; Raber et al., 1998). NSE-apoE4 mice showed impairments in a water maze test and in vertical exploratory behavior not observed in NSE-apoE3 mice or wild-type controls. These impairments increased with age and were observed primarily in female apoE4 transgenic mice, suggesting that human apoE isoforms differ in their effects on brain function in vivo and that the susceptibility to apoE4-induced deficits is critically influenced by age and gender (Buttini et al., 1999; Raber et al., 1998). Morphological studies of these transgenic mouse lines demonstrated that human apoE3 prevents the age-dependent neurodegeneration seen in apoE-null mice and prevents kainic acid–induced neurodegeneration; human apoE4 is not protective (Buttini et al., 1999). ApoE4 knock-in mice also show age- and sex-dependent impairment of spatial learning and memory (Andrews-Zwilling et al., 2010; Leung et al., 2012). Transgenic mice expressing apoE4 in astrocytes had impairment of working memory, although no significant neuropathological changes were found in the brains of these mice (Hartman et al., 2001). Since Aβ does not accumulate in any of these apoE isoform transgenic mouse models, these data strongly suggest an Aβ-independent role of apoE4 in causing neuronal and behavioral deficits in vivo.
ApoE4 proteolysis and neurotoxicity
ApoE4 is more susceptible to proteolytic cleavage than apoE3, as determined in vitro in transfected neuronal cells and in vivo in transgenic mice expressing apoE3 or apoE4 in CNS neurons (Brecht et al., 2004; Harris et al., 2003; Huang et al., 2001). Brain levels of these apoE fragments were present at much higher levels in AD patients than in age- and sex-matched nondemented controls, with an apoE4 gene dose-dependent effect (Harris et al., 2003; Huang et al., 2001; Jones et al., 2011). Carboxyl-terminal-truncated fragments of apoE also appeared to accumulate in neurofibrillary tangles and amyloid plaques in AD brains (Huang et al., 2001; Jones et al., 2011).
To determine if expression of carboxyl-terminal-truncated apoE4 in transgenic mice induces AD-like neuropathological and behavioral changes, transgenic mouse lines expressing various levels of apoE4(Δ272–299) in CNS neurons were established (Harris et al., 2003). Hippocampal or cortical neurons in these transgenic mice had numerous inclusion bodies containing phosphorylated tau that were positive for Gallyas silver staining, suggesting neurodegeneration. Staining with hematoxylin/eosin revealed degeneration of neurons expressing truncated apoE4. Morris water maze testing revealed learning and memory deficits of the truncated apoE4 transgenic mice. Thus, the carboxyl-terminal-truncated apoE4 is neurotoxic in vivo in transgenic mice and leads to AD-like neurodegeneration and behavioral deficits (Harris et al., 2003). We hypothesize that, in response to brain injury, neuronal apoE expression is induced or enhanced for purposes of repair or remodeling. However, in the context of apoE4, these events trigger proteolytic processing and fragment generation, which are detrimental to repair and remodeling and lead to neurodegeneration (Fig. 3) (Huang, 2010; Huang and Mucke, 2012; Huang et al., 2004; Mahley and Huang, 2012a; Mahley et al., 2006).
Figure 3. ApoE proteolysis and AD.
In response to stressors and injurious agents, neurons turn on or increase their expression of apoE to repair or remodel the damaged neurons. However, neuronal apoE undergoes proteolytic processing, which generates C-terminal truncated fragments of apoE; apoE4 is more susceptible than apoE3 to the cleavage. These apoE fragments cause cytoskeletal changes, such as tau phosphorylation, neurofibrillary tangle formation, and mitochondrial dysfunction, finally leading to neurodegeneration.
ApoE4 fragments disrupt cytoskeletal structure and impair mitochondrial function
It has also been demonstrated that the carboxyl-terminal-truncated fragments of apoE4 enter the cytosol and cause neurotoxicity (Brecht et al., 2004; Harris et al., 2003; Huang et al., 2001). Cytoskeletal components, such as tau and neurofilaments, are a target of these fragments (Fig. 3) (Huang et al., 2001). In vitro, the carboxyl-terminal-truncated fragments of apoE are toxic when expressed in neuronal cells or added to the cultures, leading to the formation of cytoplasmic neurofibrillary tangle-like inclusions in some cells (Huang et al., 2001; Ljungberg et al., 2002). Thus, neurotoxicity induced by the apoE4 fragments might be related to cytoskeletal disruption.
ApoE4 fragments also target the mitochondria of neurons, leading to mitochondrial dysfunction and neurotoxicity (Fig. 3) (Chang et al., 2005). Importantly, the receptor binding region of apoE is required for escape from the secretory pathway, and the lipid binding region is required for mitochondrial interaction (Chang et al., 2005). It appears that positively charged amino acids in the receptor-binding region, a feature shared among the protein translocation domains of many viral proteins (Frankel and Pabo, 1988; Green and Loewenstein, 1988), enable apoE4 fragments to translocate across membrane compartments of the secretory pathway and enter the cytosol, whereas the lipid binding region interacts directly with the mitochondria (Chang et al., 2005). Biophysical studies suggest that the lipid binding domain within the C-terminal-truncated apoE4 has a less organized structure and greater exposure of the hydrophobic residues than full-length apoE4 (Chou et al., 2006; Tanaka et al., 2006), which might increase the interaction with mitochondrial membranes. Time-lapse recordings of cultured neuronal cells demonstrate that apoE decreases mitochondrial mobility in an isoform-specific manner (apoE4 fragment > apoE4 > apoE3) (Brodbeck et al., 2011). Likewise, apoE4 impairs axonal transport of mitochondria in transgenic mice with neuron-specific expression of apoE4 (Tesseur et al., 2000a).
Mitochondrial dysfunction in AD is modulated by apoE genotype (Ghosh et al., 1999; Hirai et al., 2001; Kamino et al., 2000; Trimmer and Borland, 2005), and the effects are greater in apoE4 than in apoE3 carriers (Gibson et al., 2000). In both AD patients and age-matched nondemented subjects, apoE4 is associated with decreased cerebral glucose metabolism (Drzezga et al., 2005; Hirono et al., 2002; Mosconi et al., 2005; Mosconi et al., 2004a; Mosconi et al., 2004b; Mosconi et al., 2004c; Reiman et al., 2004, 2005; Small, 2001; Small et al., 2004), an effect that occurs decades before cognitive impairment becomes apparent (Reiman et al., 2004, 2005; Scarmeas et al., 2005) and probably before significant Aβ deposition occurs. Thus, apoE4 may cause mitochondrial dysfunction at very early stages of pathogenesis in vivo.
ApoE4 stimulates tau phosphorylation
In vitro, apoE3 forms a stable complex with tau in a 1:1 ratio, whereas apoE4 does not interact significantly (Strittmatter et al., 1994b). Phosphorylation of tau by a crude brain extract inhibited the interaction of apoE3 with tau (Strittmatter et al., 1994b), suggesting that apoE3 binds to nonphosphorylated tau. Furthermore, the amino-terminal domain of apoE3 is responsible for binding to tau (Strittmatter et al., 1994b). In fact, apoE3 irreversibly binds to the microtubule-binding repeat regions of tau (Strittmatter et al., 1994a). We and others have shown increased phosphorylation of tau in transgenic mice expressing human apoE4 in neurons but not in mice expressing apoE4 in astrocytes (Brecht et al., 2004; Tesseur et al., 2000a; Tesseur et al., 2000b), indicating a neuron-specific effect of apoE4 on tau phosphorylation. Increased tau phosphorylation in apoE4 transgenic mice appears to be associated with activation of Erk that can be modified by zinc concentration (Harris et al., 2004). Thus, carboxyl-terminal-truncated apoE4 stimulates tau phosphorylation and the formation of intracellular neurofibrillary tangle-like inclusions in transgenic mice (Brecht et al., 2004; Harris et al., 2003). Importantly, removing tau protects mice from apoE4 fragments-induced neurotoxicity (Andrews-Zwilling et al., 2010). It has been reported in a human study that apoE4 has Aβ-independent effect on increasing phosphorylated tau in CSF (Cruchaga et al., 2013).
ApoE4 inhibits neurite outgrowth and impairs neuronal plasticity
In the presence of a source of lipids, apoE3 and apoE4 have markedly different effects on neurite extension (DeMattos et al., 1998; DeMattos et al., 2001; Fagan et al., 1996; Holtzman et al., 1995; Nathan et al., 1994; Nathan et al., 1995; Sun et al., 1998). In both cultured dorsal root ganglion neurons and Neuro-2a cells, apoE3 plus β-VLDL significantly stimulates neurite extension, whereas apoE4 plus β-VLDL markedly inhibits neurite branching and extension and disrupts the cytoskeleton (Holtzman et al., 1995; Nathan et al., 1994; Nathan et al., 1995). In neuronal cells in culture, apoE4 disrupts microtubule formation and decreases β-tubulin polymorization (Tesseur et al., 2000b). In addition, astrocyte-derived apoE3, but not apoE4, stimulates neurite outgrowth of rat hippocampal neurons (Sun et al., 1998). Furthermore, apoE3-transfected Neuro-2a cells grown in medium containing β-VLDL or HDL from CSF show greater neurite extension than apoE4-transfected Neuro-2a cells (Bellosta et al., 1995). ApoE4-associated inhibition of neurite extension is probably due to its effect on microtubule stability (Nathan et al., 1995) and is mediated by cell-surface lipoprotein receptors, specifically the HSPG/LRP pathway (Bellosta et al., 1995; Holtzman et al., 1995; Nathan et al., 1994). Notably, the apoE receptors mediate neurite outgrowth through activation of the Erk pathway in primary neuronal cultures (Qiu et al., 2004).
ApoE4 impairs synaptogenesis in vivo in apoE transgenic and gene-targeted mice and in vitro in primary neuronal cultures. As compared to apoE3, apoE4 decreases dendritic spine density in transgenic and gene-targeted mice (Dumanis et al., 2009; Jain et al., 2013; Ji et al., 2003). In rat primary cortical neuronal cultures, apoE4 and its fragment decrease the density of dendritic spines (Brodbeck et al., 2008). Interestingly, rosiglitazone, an insulin sensitizer and mitochondrial activator, rescues this loss of dendritic spines (Brodbeck et al., 2008), suggesting the involvement of apoE4-caused mitochondrial impairment in the detrimental effect of apoE4 and its fragment on synaptogenesis. Furthermore, apoE is involved in maintaining and regulating synaptic activity and strength. ApoE4, but not apoE3, reduces neuronal cell-surface expression of the apoE receptor-2, as well as NMDA and AMPA receptors, by sequestering them in an intracellular compartment (Chen et al., 2010). It is postulated that the apoE isoform-specific effect on apoE receptor-2 and NMDA/AMPA receptor trafficking contributes to AD pathogenesis by impairing synaptic activity.
ApoE4 also impairs adult hippocampal neurogenesis (Levi and Michaelson, 2007; Li et al., 2009). Neural stem cells express high levels of apoE (Li et al., 2009). ApoE knockout mice have significantly less hippocampal neurogenesis, but significantly more astrogenesis, than wildtype mice due to decreased Noggin expression in neural stem cells (Li et al., 2009). In contrast, neuronal maturation in apoE4 knock-in mice is impaired due to reduced survival and function of GABAergic interneurons in the hilus of the hippocampus, and a GABAA receptor potentiator rescues the apoE4-associated decrease in hippocampal neurogenesis (Li et al., 2009). Thus, apoE contributes to adult hippocampal neurogenesis, and apoE4 impairs GABAergic input to newborn neurons, leading to decreased neurogenesis (Li et al., 2009). Interestingly, exercise, which stimulates hippocampal neurogenesis, improves cognition and hippocampal plasticity in apoE4 transgenic mice (Nichol et al., 2009).
ApoE4 impairs blood-brain barrier (BBB) integrity
ApoE exhibits isoform-specific effects on BBB integrity at least in mouse models (Bell et al., 2012). In both apoE knock-in and glial fibrillary acidic protein promoter transgenic mice, expression of apoE4 increases the BBB’s susceptibility to injury by activating the proinflammatory cytokine cyclophilin A in pericytes and triggering the NF-kB/matrix metalloproteinase 9 pathway. Interestingly, BBB breakdown is independent of Aβ. It has been reported that pericytes express apoE (Xu et al., 2006), which might contribute to BBB impairment in the context of apoE4.
ApoE4 impairs GABAergic interneurons
ApoE4 knock-in mice show an age-dependent decrease in hilar GABAergic interneurons, which correlates with the extent of apoE4-induced impairments of adult hippocampal neurogenesis and with learning and memory deficits (Andrews-Zwilling et al., 2010; Leung et al., 2012; Li et al., 2009). In transgenic mice expressing neurotoxic apoE4 fragments, the loss of hilar interneurons is more pronounced and also correlates with learning and memory deficits (Andrews-Zwilling et al., 2010). These adverse effects were prevented by tau removal, but not when GABA signaling was blocked with picrotoxin (Andrews-Zwilling et al., 2010). Mice treated with the GABAA receptor potentiator pentobarbital had normal neurogenesis and learning and memory (Andrews-Zwilling et al., 2010; Li et al., 2009). These findings strongly suggest that apoE4 causes age- and tau-dependent impairment of hilar GABAergic interneurons, leading to decreased neurogenesis in the hippocampus and to learning and memory deficits.
Dysfunction of the GABAergic system may also contribute to cognitive impairment in humans. AD patients have decreased GABA and somatostatin levels in the brain and CSF (Bareggi et al., 1982; Davies et al., 1980; Hardy et al., 1987; Seidl et al., 2001; Zimmer et al., 1984) and these alterations were more severe in apoE4 carriers (Grouselle et al., 1998). ApoE4 is associated with increased brain activity at rest and in response to memory tasks (Dennis et al., 2009; Filippini et al., 2009), possibly reflecting impaired GABAergic inhibitory control. A single nucleotide polymorphism in the somatostatin gene increases the risk for AD in carriers of apoE4 but not of apoE3 (Vepsalainen et al., 2007; Xue et al., 2009). Furthermore, GABA levels in human CSF decrease with age (Bareggi et al., 1982)—the strongest risk factor for AD. We hypothesize that apoE4 contributes to AD pathogenesis, at least partially, by causing age-dependent impairment of GABAergic interneurons, leading to learning and memory deficits.
ApoE4 and Other Neurodegenerative Disorders
Although the data are not as strong as with AD, apoE4 has also been associated with progression or poor clinical outcomes in other neurological or neurodegenerative diseases, including traumatic brain injury (TBI) (Chamelian et al., 2004; Crawford et al., 2002; Friedman et al., 1999; Gandy and DeKosky, 2012; Mayeux et al., 1995; Nicoll et al., 1996; Teasdale et al., 1997), multiple sclerosis (Chapman et al., 2001; Fazekas et al., 2001), stroke (Alberts et al., 1995; McCarron et al., 1999; Slooter et al., 1997), frontotemporal dementia (Agosta et al., 2009), and Parkinson’s disease (Harhangi et al., 2000; Li et al., 2004; Martinez et al., 2005; Parsian et al., 2002). More studies are needed to confirm these observations and to dissect the underlyig mechanisms.
Other Lipid Metabolism–Related Genes and AD
AD appears to be linked to cholesterol metabolism–related genes other than apoE (Shobab et al., 2005; Wolozin, 2004). It has been reported that AD is associated with a polymorphism in ABCA1 (ATP-binding cassette, subfamily A, member 1), a cellular cholesterol transporter (Katzov et al., 2004); however, that association was not found in another study (Li et al., 2004). In mice, ABCA1 is required for maintaining normal CNS apoE levels and for lipidation of astrocyte-secreted apoE (Hirsch-Reinshagen et al., 2004; Wahrle et al., 2004), and deficiency of ABCA1 increases Aβ deposition in human APP transgenic mice (Hirsch-Reinshagen et al., 2005; Koldamova et al., 2005; Wahrle et al., 2005). Interestingly, the effect of ABCA1 deficiency on AD-like phenotype only occurs in apoE4 expressing mice, but not in apoE3 expressing mice (Fitz et al., 2012). Two studies identified an association between AD and two single nucleotide polymorphisms in Cyp46, an enzyme that converts cholesterol to 24-S-hydroxycholeaterol for excretion through the brain-blood barrier (Kölsch et al., 2002; Papassotiropoulos et al., 2003). One of the polymorphisms was also associated with increased Aβ levels in CSF (Papassotiropoulos et al., 2003). However, two other studies failed to show such an association (Desai et al., 2002; Johansson et al., 2004); one suggested that an intronic marker of CYP46 interacts with age and apoE genotype (Johansson et al., 2004). Therefore, although links between AD and cholesterol metabolism-related genes other than apoE4 seem to support the importance of abnormal cholesterol metabolism in AD pathogenesis, the association between those genes and AD, except for the case of apoE4, remains weak. The significance of those genes in the pathogenesis of AD merits further investigation both in vitro and in vivo.
Conclusion and Perspective
Biochemical, cell biological, and transgenic animal studies have suggested several mechanisms to explain the contribution of apoE4 to AD pathogenesis (Bu, 2009; Huang, 2010; Huang and Mucke, 2012; Huang et al., 2004; Kim et al., 2009; Mahley and Huang, 2012a; Mahley et al., 2006). However, the mechanisms of these apoE4-mediated effects are still poorly understood. Likewise, it is not known which of these pathophysiological effects of apoE4 is the primary effect and which are subsequent or downstream effects or the extent to which they contribute to the pathogenesis of the dementia that characterizes AD clinically. Based on both in vitro and in vivo studies reviewed above, it is very likely that apoE4 affects AD pathogenesis by interacting with different factors through various pathways (Bu, 2009; Huang, 2010; Huang and Mucke, 2012; Huang et al., 2004; Kim et al., 2009; Mahley and Huang, 2012a; Mahley et al., 2006). Thus, multiple molecular and cellular mechanisms should be considered when anti-AD drugs are developed based on apoE studies (Huang and Mucke, 2012; Mahley and Huang, 2012a).
The diverse cellular pattern of expression implies multiple functions of apoE. ApoE derived from different cellular sources probably has distinct roles in both physiological and pathophysiological pathways (Huang, 2010; Huang and Mucke, 2012; Huang et al., 2004; Mahley and Huang, 2012a; Mahley et al., 2006). Thus, determining how apoE expression is regulated in different types of cells in the brain during development and in response to various insults should provide fundamental insights into the varied effects of apoE in neurobiology and neurodegenerative disorders, including AD. Drugs that inhibit neuronal expression of apoE4 might eliminate its downstream detrimental effects.
The Aβ-dependent effects of apoE4 on AD pathogenesis could be attenuated by drugs that inhibit apoE4-stimulated Aβ deposition (Bu, 2009; Kim et al., 2009; Sadowski et al., 2006). Drugs could also be designed, based on Aβ-independent effects of apoE4, to inhibit the apoE cleaving enzyme that mediates apoE4 fragmentation or to block the interaction of apoE4 fragments with cytoskeletal elements and mitochondria, thereby protecting against fragment-induced neurotoxicity. In addition, drugs capable of increasing the activity and/or numbers of mitochondria could also be beneficial for treating AD. Finally, another potential drug target is apoE4 domain interaction, which is responsible for many, if not all, of apoE4’s detrimental effects (Huang, 2010; Huang and Mucke, 2012; Huang et al., 2004; Mahley and Huang, 2012a; Mahley et al., 2006). Small molecules have been designed to disrupt domain interaction by making apoE4 structurally and functionally more like apoE3 (Brodbeck et al., 2011; Chen et al., 2012; Mahley and Huang, 2012b).
Clearly, hope for effective therapeutics relies upon the ability of scientists to explore multiple lines of inquiry. It is certainly conceivable that there will be combination therapies, with both symptomatic drugs and those that might fundamentally alter the rate of onset and progression. The time is right to expand our therapeutic attack against this devastating disease. Clearly, the structure and pathophysiological functions of apoE4 represent such a target.
Acknowledgement
This work was supported in part by National Institutes of Heath grants P50AG023501, 1RF1AG047655, 2P50AG023501, the S.D. Bechtel, Jr. Foundation, and the Hellman Foundation. We thank Linda Turney for manuscript preparation, Gary Howard for editorial assistance, and John C.W. Carroll for graphics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Agosta F, Vossel KA, Miller BL, Migliaccio R, Bonasera SJ, Filippi M, Boxer AL, Karydas A, Possin KL, Gorno-Tempini ML. Apolipoprotein E ε4 is associated with disease-specific effects on brain atrophy in Alzheimer’s disease and frontotemporal dementia. Proc. Natl. Acad. Sci. USA. 2009;106:2018–2022. doi: 10.1073/pnas.0812697106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts MJ, Graffagnino C, McClenny C, DeLong D, Strittmatter W, Saunders AM, Roses AD. ApoE genotype and survival from intracerebral haemorrhage. Lancet. 1995;346:575. doi: 10.1016/s0140-6736(95)91411-0. [DOI] [PubMed] [Google Scholar]
- Andrews-Zwilling Y, Bien-Ly N, Xu Q, Li G, Bernardo A, Yoon SY, Zwilling D, Yan TX, Chen L, Huang Y. Apolipoprotein E4 causes age- and Tau-dependent impairment of GABAergic interneurons, leading to learning and memory deficits in mice. J Neurosci. 2010;30:13707–13717. doi: 10.1523/JNEUROSCI.4040-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales KR, Verina T, Cummins DJ, Du Y, Dodel RC, Saura J, Fishman CE, DeLong CA, Piccardo P, Petegnief V, et al. Apolipoprotein E is essential for amyloid deposition in the APPV717F transgenic mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA. 1999;96:15233–15238. doi: 10.1073/pnas.96.26.15233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bareggi SR, Franceschi M, Bonini L, Zecca L, Smirne S. Decreased CSF concentrations of homovanillic acid and γ-aminobutyric acid in Alzheimer’s disease. Age- or disease-related modifications? Arch Neurol. 1982;39:709–712. doi: 10.1001/archneur.1982.00510230035010. [DOI] [PubMed] [Google Scholar]
- Bell RD, Winkler EA, Singh Il, Sagare AP, Deane R, Wu Z, Holtzman DM, Betsholtz C, Amulik A, Sallstrom J, Berk BC, Zlokovic BV. Apolipoprotein E controls cerebrovascular integrity via yclophilin A. Nature. 2012;485:512–516. doi: 10.1038/nature11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellosta S, Nathan BP, Orth M, Dong L-M, Mahley RW, Pitas RE. Stable expression and secretion of apolipoproteins E3 and E4 in mouse neuroblastoma cells produces differential effects on neurite outgrowth. J Biol Chem. 1995;270:27063–27071. doi: 10.1074/jbc.270.45.27063. [DOI] [PubMed] [Google Scholar]
- Bien-Ly N, Gillespie AK, Walker D, Yoon SY, Huang Y. Reducing human apolipoprotein E levels attenuates age-dependent A β accumulation in mutant human amyloid precursor protein transgenic mice. J Neurosci. 2012;32:4803–4811. doi: 10.1523/JNEUROSCI.0033-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm-Cagan A, Michaelson DM. Reversal of apoE4-driven brain pathology and behavioral deficits by bexarotene. J. Neurosci. 2014;34:7293–7301. doi: 10.1523/JNEUROSCI.5198-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecht WJ, Harris FM, Chang S, Tesseur I, Yu G-Q, Xu Q, Fish JD, Wyss-Coray T, Buttini M, Mucke L, et al. Neuron-specific apolipoprotein E4 proteolysis is associated with increased tau phosphorylation in brains of transgenic mice. J Neurosci. 2004;24:2527–2534. doi: 10.1523/JNEUROSCI.4315-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodbeck J, Balestra ME, Saunders AM, Roses AD, Mahley RW, Huang Y. Rosiglitazone increases dendritic spine density and rescues spine loss caused by apolipoprotein E4 in primary cortical neurons. PNAS. 2008;105:1343–1346. doi: 10.1073/pnas.0709906104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodbeck J, McGuire J, Liu Z, Meyer-Franke A, Balestra ME, Jeong DE, Pleiss M, McComas C, Hess F, Witter D, et al. Structure-dependent impairment of intracellular apolipoprotein E4 trafficking and its detrimental effects are rescued by small-molecule structure correctors. J Biol Chem. 2011;286 doi: 10.1074/jbc.M110.217380. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu G. Apolipoprotein E and its receptors in Alzheimer’s disease: pathways, pathogenesis and therapy. Nat Rev Neurosci. 2009;10:333–344. doi: 10.1038/nrn2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttini M, Orth M, Bellosta S, Akeefe H, Pitas RE, Wyss-Coray T, Mucke L, Mahley RW. Expression of human apolipoprotein E3 or E4 in the brains of Apoe–/– mice: Isoform-specific effects on neurodegeneration. J Neurosci. 1999;19:4867–4880. doi: 10.1523/JNEUROSCI.19-12-04867.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttini M, Yu G-Q, Shockley K, Huang Y, Jones B, Masliah E, Mallory M, Yeo T, Longo FM, Mucke L. Modulation of Alzheimer-like synaptic and cholinergic deficits in transgenic mice by human apolipoprotein E depends on isoform, aging, and overexpression of amyloid β peptides but not on plaque formation. J Neurosci. 2002;22:10539–10548. doi: 10.1523/JNEUROSCI.22-24-10539.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano JM, Kim J, Stewart FR, Jiang H, DeMattos RB, Patterson BW, Fagan AM, Morris JC, Mawuenyega KG, Cruchaga C, et al. Human apoE isoforms differentially regulate brain amyloid-β peptide clearance. Sci Transl Med. 2011;3:89ra57. doi: 10.1126/scitranslmed.3002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamelian L, Reis M, Feinstein A. Six-month recovery from mild to moderate traumatic brain injury: the role of APOE-ε4 allele. Brain. 2004;127:2621–2628. doi: 10.1093/brain/awh296. [DOI] [PubMed] [Google Scholar]
- Chang S, Ma TR, Miranda RD, Balestra ME, Mahley RW, Huang Y. Lipid- and receptor-binding regions of apolipoprotein E4 fragments act in concert to cause mitochondrial dysfunction and neurotoxicity. Proc Natl Acad Sci USA. 2005;102:18694–18699. doi: 10.1073/pnas.0508254102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman J, Vinokurov S, Achiron A, Karussis DM, Mitosek-Szewczyk K, Birnbaum M, Michaelson DM, Korczyn AD. APOE genotype is a major predictor of long-term progression of disability in MS. Neurology. 2001;56:312–316. doi: 10.1212/wnl.56.3.312. [DOI] [PubMed] [Google Scholar]
- Chen HK, Liu Z, Meyer-Franke A, Brodbeck J, Miranda RD, McGuire JG, Pleiss MA, Ji ZS, Balestra ME, Walker DW, et al. Samll-molecule structure correctors abolish detrimental effects of apolipoprotein E4 in cultured neurons. J Biol Chem. 2012;287:5253–5266. doi: 10.1074/jbc.M111.276162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Durakoglugil MS, Xian X, Herz J. ApoE4 reduces glutamate receptor function and synaptic plasticity by selectively imparing apoE receptor recycling. Proc. Natl. Acad. Sci. USA. 2010;107:12011–12016. doi: 10.1073/pnas.0914984107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HS, Hyman BT, Greenberg SM, Rebeck GW. Quantitation of apoE domains in Alzheimer disease brain suggests a role for apoE in Aβ aggregation. J Neuropathol Exp Neurol. 2001;60:342–349. doi: 10.1093/jnen/60.4.342. [DOI] [PubMed] [Google Scholar]
- Chou C-Y, Jen W-P, Hsieh Y-H, Shiao M-S, Chang G-G. Structural and functional variations in human apolipoprotein E3 and E4. J Biol Chem. 2006;281:13333–13344. doi: 10.1074/jbc.M511077200. [DOI] [PubMed] [Google Scholar]
- Cramer PE, Cirrito JR, Wesson DW, Lee CY, Karlo JC, Zinn AE, Casali BT, Restivo JL, Goebel WD, James MJ, Brunden KR, Wilson DA, Landreth GE. ApoE-directed therapeutics rapidly clear β-amyloid and reverse deficits in AD mouse models. Science. 2012;335:1503–1506. doi: 10.1126/science.1217697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford FC, Vanderploeg RD, Freeman MJ, Singh S, Waisman M, Michaels L, Abdullah L, Warden D, Lipsky R, Salazar A, Mullan MJ. APOE genotype influences acquisition and recall following traumatic brain injury. Neurology. 2002;58:1115–1118. doi: 10.1212/wnl.58.7.1115. [DOI] [PubMed] [Google Scholar]
- Cruchaga C, Kauwe JSK, Harari O, Jin SC, Cai Y, Karch CM, Benitez BA, Jeng AT. GWAS of cerebrospinal fluid tau levels identifies ris variants for Alzheimer’s disease. Neuron. 2013;78:256–268. doi: 10.1016/j.neuron.2013.02.026. al, e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P, Katzman R, Terry RD. Reduced somatostatin-like immunoreactivity in cerebral cortex from cases of Alzheimer disease and Alzheimer senile dementia. Nature. 1980;288:279–280. doi: 10.1038/288279a0. [DOI] [PubMed] [Google Scholar]
- Deane R, Sagare A, Hamm K, Parisi M, Lane S, Finn MB, Holtzman DM, Zlokovic BV. ApoE isoform-specific disruption of amyloid beta peptide clearance from mouse brain. J Clin Invest. 2008;118:4002–4013. doi: 10.1172/JCI36663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMattos RB, Curtiss LK, Williams DL. A minimally lipidated form of cell-derived apolipoprotein E exhibits isoform-specific stimulation of neurite outgrowth in the absence of exogenous lipids or lipoproteins. J Biol Chem. 1998;273:4206–4212. doi: 10.1074/jbc.273.7.4206. [DOI] [PubMed] [Google Scholar]
- DeMattos RB, Rudel LL, Williams DL. Biochemical analysis of cell-derived apoE3 particles active in stimulating neurite outgrowth. J Lipid Res. 2001;42:976–987. [PubMed] [Google Scholar]
- Dennis NA, Browndyke JN, Stokes J, Need A, Burke JR, Welsh-Bohmer KA, Cabeza R. Temporal lobe functional activity and connectivity in young adult APOE e4 carriers. Alzheimer’s and Dementia. 2009;5:1–9. doi: 10.1016/j.jalz.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai P, DeKosky ST, Kamboh MI. Genetic variation in the cholesterol 24- hydroxylase (CYP46) gene and the risk of Alzheimer’s disease. Neurosci Lett. 2002;328:9–12. doi: 10.1016/s0304-3940(02)00443-3. [DOI] [PubMed] [Google Scholar]
- Dodart J-C, Marr RA, Koistinaho M, Gregersen BM, Malkani S, Verma IM, Paul SM. Gene delivery of human apolipoprotein E alters brain Aβ burden in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA. 2005;102:1211–1216. doi: 10.1073/pnas.0409072102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drzezga A, Riemenschneider M, Strassner B, Grimmer T, Peller M, Knoll A, Wagenpfeil S, Minoshima S, Schwaiger M, Kurz A. Cerebral glucose metabolism in patients with AD and different APOE genotypes. Neurology. 2005;64:102–107. doi: 10.1212/01.WNL.0000148478.39691.D3. [DOI] [PubMed] [Google Scholar]
- Dumanis SB, Tesoriero JA, Babus LW, Nguyen MT, Trotter JH, Ladu MJ, Weeber EJ, Turner RS, Xu B, Rebeck GW, et al. ApoE4 decreases spine density and dendritic complexity in cortical neurons in vivo. J Neurosci. 2009;29:15317–15322. doi: 10.1523/JNEUROSCI.4026-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan AM, Bu G, Sun Y, Daugherty A, Holtzman DM. Apolipoprotein E-containing high density lipoprotein promotes neurite outgrowth and is a ligand for the low density lipoprotein receptor-related protein. J Biol Chem. 1996;271:30121–30125. doi: 10.1074/jbc.271.47.30121. [DOI] [PubMed] [Google Scholar]
- Fagan AM, Holtzman DM, Munson G, Mathur T, Schneider D, Chang LK, Getz GS, Reardon CA, Lukens J, Shah JA, et al. Unique lipoproteins secreted by primary astrocytes from wild type, apoE (–/–), and human apoE transgenic mice. J Biol Chem. 1999;274:30001–30007. doi: 10.1074/jbc.274.42.30001. [DOI] [PubMed] [Google Scholar]
- Fazekas F, Strasser-Fuchs S, Kollegger H, Berger T, Kristoferitsch W, Schmidt H, Enzinger C, Schiefermeier M, Schwarz C, Kornek B, et al. Apolipoprotein E ε4 is associated with rapid progression of multiple sclerosis. Neurology. 2001;57:853–857. doi: 10.1212/wnl.57.5.853. [DOI] [PubMed] [Google Scholar]
- Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, Matthews PM, Beckmann CF, Mackay CE. Distinct patterns of brain activity in young carriers of the APOE-ε4 allele. Proc Natl Acad Sci USA. 2009;106:7209–7214. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitz NF, Cronican AA, Lefterov I, Koldamova R. Comment on “ApoE-directed therapeutics rapidly clear b-amyloid and reverse deficits in AD mouse models. Science. 2013;340:924–c. doi: 10.1126/science.1235809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitz NF, Cronican AA, Saleem M, Fauq AH, Chapman R, Lefterov I, Koldamova R. Abac1 deficiency affects Alzheimer’s disease-like phenotype in human apoE4 but not in apoE3-targeted replacement mice. J Neurosci. 2012;32:13125–13136. doi: 10.1523/JNEUROSCI.1937-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman G, Froom P, Sazbon L, Grinblatt I, Shochina M, Tsenter J, Babaey S, Yehuda B, Groswasser Z. Apolipoprotein E-ε4 genotype predicts a poor outcome in survivors of traumatic brain injury. Neurology. 1999;52:244–248. doi: 10.1212/wnl.52.2.244. [DOI] [PubMed] [Google Scholar]
- Frankel AD, Pabo CO. Cellular uptake of the tat protein from human immunodeficiency virus. Cell. 1988;55:1189–1193. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- Gandy S, DeKosky ST. APOE ε4 status and traumatic brain injury on the gridiron or the battlefield. Sci. Transl. Med. 2012;4:134. doi: 10.1126/scitranslmed.3004274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh SS, Swerdlow RH, Miller SW, Sheeman B, Parker WD, Jr., Davis RE. Use of cytoplasmic hybrid cell lines for elucidating the role of mitochondrial dysfunction in Alzheimer’s disease and Parkinson’s disease. Ann NY Acad Sci. 1999;893:176–191. doi: 10.1111/j.1749-6632.1999.tb07825.x. [DOI] [PubMed] [Google Scholar]
- Gibson GE, Haroutunian V, Zhang H, Park LCH, Shi Q, Lesser M, Mohs RC, Sheu RK-F, Blass JP. Mitochondrial damage in Alzheimer’s disease varies with apolipoprotein E genotype. Ann Neurol. 2000;48:297–303. [PubMed] [Google Scholar]
- Green M, Loewenstein PM. Autonomous functional domains of chemically synthesized human immunodeficiency virus tat trans-activator protein. Cell. 1988;55:1179–1188. doi: 10.1016/0092-8674(88)90262-0. [DOI] [PubMed] [Google Scholar]
- Grouselle D, Winsky-Sommerer R, David JP, Delacourte A, Dournaud P, Epelbaum J. Loss of somatostatin-like immunoreactivity in the frontal cortex of Alzheimer patients carrying the apolipoprotein epsilon 4 allele. Neuroscience Letters. 1998;255:21–24. doi: 10.1016/s0304-3940(98)00698-3. [DOI] [PubMed] [Google Scholar]
- Hardy J, Cowburn R, Barton A, Reynolds G, Dodd P, Wester P, O’Carroll AM, Lofdahl E, Winblad B. A disorder of cortical GABAergic innervation in Alzheimer’s disease. Neurosi Lett. 1987;73:192–196. doi: 10.1016/0304-3940(87)90016-4. [DOI] [PubMed] [Google Scholar]
- Harhangi BS, de Rijk MC, van Duijn CM, Van Broeckhoven C, Hofman A, Breteler MMB. APOE and the risk of PD with or without dementia in a population-based study. Neurology. 2000;54:1272–1276. doi: 10.1212/wnl.54.6.1272. [DOI] [PubMed] [Google Scholar]
- Harris FM, Brecht WJ, Xu Q, Mahley RW, Huang Y. Increased tau phosphorylation in apolipoprotein E4 transgenic mice is associated with activation of extracellular signal-regulated kinase: Modulation by zinc. J Biol Chem. 2004;279:44795–44801. doi: 10.1074/jbc.M408127200. [DOI] [PubMed] [Google Scholar]
- Harris FM, Brecht WJ, Xu Q, Tesseur I, Kekonius L, Wyss-Coray T, Fish JD, Masliah E, Hopkins PC, Scearce-Levie K, et al. Carboxyl-terminal-truncated apolipoprotein E4 causes Alzheimer’s disease-like neurodegeneration and behavioral deficits in transgenic mice. Proc Natl Acad Sci USA. 2003;100:10966–10971. doi: 10.1073/pnas.1434398100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman RE, Wozniak DF, Nardi A, Olney JW, Sartorius L, Holtzman DM. Behavioral phenotyping of GFAP-apoE3 and -apoE4 transgenic mice: ApoE4 mice show profound working memory impairments in the absence of Alzheimer’s-like neuropathology. Exp Neurol. 2001;170:326–344. doi: 10.1006/exnr.2001.7715. [DOI] [PubMed] [Google Scholar]
- Herz J, Berffert U. Apolipoprotein E receptors: linking brain development and Alzheimer’s disease. Nat. Rev. Neurosci. 2000;1:51–58. doi: 10.1038/35036221. [DOI] [PubMed] [Google Scholar]
- Herz J, Bock HH. Lipoprotein receptors in the nervous system. Annu Rev Biochem. 2002;71:405–434. doi: 10.1146/annurev.biochem.71.110601.135342. [DOI] [PubMed] [Google Scholar]
- Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, Johnson AB, Kress Y, Vinters HV, Tabaton M, et al. Mitochondrial abnormalities in Alzheimer’s disease. J Neurosci. 2001;21:3017–3023. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirono N, Hashimoto M, Yasuda M, Ishii K, Sakamoto S, Kazui H, Mori E. The effect of APOE ε4 allele on cerebral glucose metabolism in AD is a function of age at onset. Neurology. 2002;58:743–750. doi: 10.1212/wnl.58.5.743. [DOI] [PubMed] [Google Scholar]
- Hirsch-Reinshagen V, Maia LF, Burgess BL, Blain J-F, Naus KE, McIsaac SA, Parkinson PF, Chan JY, Tansley GH, Hayden MR, et al. The absence of ABCA1 decreases soluble apoE levels but does not diminish amyloid deposition in two murine models of Alzheimer disease. J Biol Chem. 2005;280:43243–43256. doi: 10.1074/jbc.M508781200. [DOI] [PubMed] [Google Scholar]
- Hirsch-Reinshagen V, Zhou S, Burgess BL, Bernier L, McIsaac SA, Chan JY, Tansley GH, Cohn JS, Hayden MR, Wellington CL. Deficiency of ABCA1 impairs apolipoprotein E metabolism in brain. J Biol Chem. 2004;279:41197–41207. doi: 10.1074/jbc.M407962200. [DOI] [PubMed] [Google Scholar]
- Holtzman DM, Bales KR, Tenkova T, Fagan AM, Parsadanian M, Sartorius LJ, Mackey B, Olney J, McKeel D, Wozniak D, et al. Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA. 2000a;97:2892–2897. doi: 10.1073/pnas.050004797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman DM, Fagan AM, Mackey B, Tenkova T, Sartorius L, Paul SM, Bales K, Ashe KH, Irizarry MC, Hyman BT. Apolipoprotein E facilitates neuritic and cerebrovascular plaque formation in an Alzheimer’s disease model. Ann Neurol. 2000b;47:739–747. [PubMed] [Google Scholar]
- Holtzman DM, Pitas RE, Kilbridge J, Nathan B, Mahley RW, Bu G, Schwartz AL. Low density lipoprotein receptor-related protein mediates apolipoprotein E-dependent neurite outgrowth in a central nervous system-derived neuronal cell line. Proc Natl Acad Sci USA. 1995;92:9480–9484. doi: 10.1073/pnas.92.21.9480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y. Aβ-independent roles of apolipoprotein E4 in the pathogenesis of Alzheimer’s disease. Trends Mol Med. 2010;16:287–294. doi: 10.1016/j.molmed.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Huang Y, Liu XQ, Wyss-Coray T, Brecht WJ, Sanan DA, Mahley RW. Apolipoprotein E fragments present in Alzheimer’s disease brains induce neurofibrillary tangle-like intracellular inclusions in neurons. Proc Natl Acad Sci USA. 2001;98:8838–8843. doi: 10.1073/pnas.151254698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Mucke L. Alzheimer mechanisms and therapeutic strategies. Cell. 2012;148:1204–1222. doi: 10.1016/j.cell.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Weisgraber KH, Mucke L, Mahley RW. Apolipoprotein E. Diversity of cellular origins, structural and biophysical properties, and effects in Alzheimer’s disease. J Mol Neurosci. 2004;23:189–204. doi: 10.1385/JMN:23:3:189. [DOI] [PubMed] [Google Scholar]
- Hudry E, Dashkoff J, Roe AD, Takeda S, Koffie RM, Hashimoto T, Scheel M, Spires-Jones T, Arbel-Ornath M, Betensky BL, Hyman BT. Gene transfer of human apoE isoforms results in differential modulation of amyloid deposition and neurotoxicity in mouse brain. Sci. Transl. Med. 2013;5:212ra162. doi: 10.1126/scitranslmed.3007000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S, Yoon SY, Leung L, Knoferle J, Huang Y. Cellular source-specific effects of apolipoprotein (apo) E4 on dendrite arborization and dendritic spine development. PLoS ONE. 2013;8:e59478. doi: 10.1371/journal.pone.0059478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y, Gong Y, Gan W, Beach T, Holtzman DM, Wisniewski T. Apolipoprotein E isoform-specific regulation of dendritic spine morphology in apolipoprotein E transgenic mice and Alzheimer’s disease patients. Neuroscience. 2003;122:305–315. doi: 10.1016/j.neuroscience.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Johansson A, Katzov H, Zetterberg H, Feuk L, Johansson B, Bogdanovic N, Andreasen N, Lenhard B, Brookes AJ, Pedersen NL, et al. Variants of CYP46A1 may interact with age and APOE to influence CSF Aβ42 levels in Alzheimer’s disease. Hum Genet. 2004;114:581–587. doi: 10.1007/s00439-004-1107-9. [DOI] [PubMed] [Google Scholar]
- Jones PB, Adams KW, Rozkalne A, Spires-Jones TL, Hshieh TT, Hashimoto T, Armin C.A.F.v., Mielke M, Bacskai BJ, Hyman BT. Apolipoprotein E: isoform specific differences in tertiary structure and interaction with amyloid-β in human Alzheimer brain. PLoS One. 2011;6:e14586. doi: 10.1371/journal.pone.0014586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamino K, Nagasaka K, Imagawa M, Yamamoto H, Yoneda H, Ueki A, Kitamura S, Namekata K, Miki T, Ohta S. Deficiency in mitochondrial aldehyde dehydrogenase increases the risk for late-onset Alzheimer’s disease in the Japanese population. Biochem Biophys Res Commun. 2000;273:192–196. doi: 10.1006/bbrc.2000.2923. [DOI] [PubMed] [Google Scholar]
- Katzov H, Chalmers K, Palmgren J, Andreasen N, Johansson B, Cairns NJ, Gatz M, Wilcock GK, Love S, Pedersen NL, et al. Genetic variants of ABCA1 modify Alzheimer disease risk and quantitative traits related to β-amyloid metabolism. Hum Mutat. 2004;23:358–367. doi: 10.1002/humu.20012. [DOI] [PubMed] [Google Scholar]
- Kim J, Basak JM, Holtzman DM. The role of apolipoprotein E in Alzheimer’s disease. Neuron. 2009;63:287–303. doi: 10.1016/j.neuron.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Jiang H, Park S, Eltorai AEM, Stewart FR, Yoon H, Basak JM, Finn MB, Holtzman DM. Haploinsufficiency of human APOE reduces amyloid deposition in a mouse model of amyloid-β amyloidosis. J Neurosci. 2011;31:18007–18012. doi: 10.1523/JNEUROSCI.3773-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koistinaho M, Lin S, Wu X, Esterman M, Koger D, Hanson J, Higgs R, Liu F, Malkani S, Bales KR, et al. Apolipoprotein E promotes astrocyte colocalization and degradation of deposited amyloid-β peptides. Nat Med. 2004;10:719–726. doi: 10.1038/nm1058. [DOI] [PubMed] [Google Scholar]
- Koldamova R, Staufenbiel M, Lefterov I. Lack of ABCA1 considerably decreases brain apoE level and increases amyloid deposition in APP23 mice. J Biol Chem. 2005;280:43224–43235. doi: 10.1074/jbc.M504513200. [DOI] [PubMed] [Google Scholar]
- Kölsch H, Lütjohann D, Ludwig M, Schulte A, Ptok U, Jessen F, von Bergmann K, Rao ML, Maier W, Heun R. Polymorphism in the cholesterol 24S-hydroxylase gene is associated with Alzheimer’s disease. Mol Psychiatry. 2002;7:899–902. doi: 10.1038/sj.mp.4001109. [DOI] [PubMed] [Google Scholar]
- LaClair KD, Manaye KF, Lee DL, Allard JS, Savonenko AV, Troncoso JC, Wong PC. Treatment with bexarotene, a compound that increases apolipoprotein E, provides no cognitive benefit in mutant APP/PS1 mice. Mol. Neurodegener. 2013;8 doi: 10.1186/1750-1326-8-18. DOI:10.1186/1750-1326-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaDu MJ, Falduto MT, Manelli AM, Reardon CA, Getz GS, Frail DE. Isoform-specific binding of apolipoprotein E to β-amyloid. J Biol Chem. 1994;269:23403–23406. [PubMed] [Google Scholar]
- Lesuisse C, Xu G, Anderson J, Wong M, Jankowsky J, Holtz G, Gonzalez V, Wong PCY, Price DL, Tang F, et al. Hyper-expression of human apolipoprotein E4 in astroglia and neurons does not enhance amyloid deposition in transgenic mice. Hum Mol Genet. 2001;10:2525–2537. doi: 10.1093/hmg/10.22.2525. [DOI] [PubMed] [Google Scholar]
- Leung L, Andrews-Zwilling Y, Yoon SY, Jain S, Ring K, Dai J, Wang MM, Tong L, Walker D, Huang Y. Apolipoprotein E4 causes age- and sex-dependent impairments of hilar GABAergic interneurons and learning and memory deficits in mice. PLoS ONE. 2012;7:e53569. doi: 10.1371/journal.pone.0053569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi O, Michaelson DM. Environmental enrichment stimulates neurogenesis in apolipoprotein E3 and neuronal apoptosis in apolipoprotein E4 transgenic mice. J Neurosci. 2007;100:202–210. doi: 10.1111/j.1471-4159.2006.04189.x. [DOI] [PubMed] [Google Scholar]
- Li G, Bien-Ly N, Andrews-Zwilling Y, Xu Q, Bernardo A, Ring K, Halabisky B, Deng C, Mahley RW, Huang Y. GABAergic interneuron dysfunction impairs hippocampal neurogenesis in adult apolipoprotein E4 knockin mice. Cell Stem Cell. 2009;5:634–645. doi: 10.1016/j.stem.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Tacey K, Doil L, van Luchene R, Garcia V, Rowland C, Schrodi S, Leong D, Lau K, Catanese J, et al. Association of ABCA1 with the late-onset Alzheimer’s disease is not observed in a case-control study. Neurosci Lett. 2004;366:268–271. doi: 10.1016/j.neulet.2004.05.047. [DOI] [PubMed] [Google Scholar]
- Li YJ, Hauser MA, Scott WK, Martin ER, Booze MW, Qin XJ, Walter JW, Nance MA, Hubble JP, Koller WC, et al. Apolipoprotein E controls the risk and age at onset of Parkinson disease. Neurology. 2004;62:2005–2009. doi: 10.1212/01.wnl.0000128089.53030.ac. [DOI] [PubMed] [Google Scholar]
- Ljungberg MC, Dayanandan R, Asuni A, Rupniak TH, Anderton BH, Lovestone S. Truncated apoE forms tangle-like structures in a neuronal cell line. Neuroreport. 2002;13:867–870. doi: 10.1097/00001756-200205070-00026. [DOI] [PubMed] [Google Scholar]
- Mahley RW. Apolipoprotein E: Cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- Mahley RW, Huang Y. Apolipoprotein E: From atherosclerosis to Alzheimer’s disease and beyond. Curr Opin Lipidol. 1999;10:207–217. doi: 10.1097/00041433-199906000-00003. [DOI] [PubMed] [Google Scholar]
- Mahley RW, Huang Y. Atherogenic remnant lipoproteins: Role for proteoglycans in trapping, transferring, and internalizing. J Clin Invest. 2007;117:94–98. doi: 10.1172/JCI30889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley RW, Huang Y. Apolipoprotein E sets the stage: response to injury triggers neuropathology. Neuron. 2012a;76:871–885. doi: 10.1016/j.neuron.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley RW, Huang Y. Small-molecule structure correctors target abnormal protein structure and function: Structure corrector rescue of apolipoprotein E4-associated neuropathology. J Med Chem. 2012b;55:8997–9008. doi: 10.1021/jm3008618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley RW, Huang Y, Rall SC., Jr. Pathogenesis of type III hyperlipoproteinemia (dysbetalipoproteinemia): Questions, quandaries, and paradoxes. J Lipid Res. 1999;40:1933–1949. [PubMed] [Google Scholar]
- Mahley RW, Ji Z-S. Remnant lipoprotein metabolism: Key pathways involving cell-surface heparan sulfate proteoglycans and apolipoprotein E. J Lipid Res. 1999;40:1–16. [PubMed] [Google Scholar]
- Mahley RW, Rall SC., Jr. Type III hyperlipoproteinemia (dysbetalipoproteinemia): The role of apolipoprotein E in normal and abnormal lipoprotein metabolism. In: Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Kinzler KW, Vogelstein B, editors. The Metabolic and Molecular Bases of Inherited Disease. McGraw-Hill; New York: 2001. pp. 2835–2862. [Google Scholar]
- Mahley RW, Weisgraber KH, Huang Y. Apolipoprotein E4: A causative factor and therapeutic target in neuropathology, including Alzheimer’s disease. Proc Natl Acad Sci USA. 2006;103:5644–5651. doi: 10.1073/pnas.0600549103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez M, Brice A, Vaughan JR, Zimprich A, Breteler MMB, Meco G, Filla A, Farrer MJ, Be’ tard C, Singleton A, et al. Apolipoprotein E4 is probably responsible for the chromosome 19 linkage peak for Parkinson’s disease. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2005;136B:72–74. doi: 10.1002/ajmg.b.30196. [DOI] [PubMed] [Google Scholar]
- Mayeux R, Ottman R, Maestre G, Ngai C, Tang M-X, Ginsberg H, Chun M, Tycko B, Shelanski M. Synergistic effects of traumatic head injury and apolipoprotein-ε4 in patients with Alzheimer’s disease. Neurology. 1995;45:555–557. doi: 10.1212/wnl.45.3.555. [DOI] [PubMed] [Google Scholar]
- McCarron MO, Delong D, Alberts MJ. APOE genotype as a risk factor for ischemic cerebrovascular disease: a meta-analysis. Neurology. 1999;53:1308–1311. doi: 10.1212/wnl.53.6.1308. [DOI] [PubMed] [Google Scholar]
- Moir RD, Atwood CS, Romano DM, Laurans MH, Huang X, Bush AI, Smith JD, Tanzi RE. Differential effects of apolipoprotein E isoforms on metal-induced aggregation of Aβ using physiological concentrations. Biochemistry. 1999;38:4595–4603. doi: 10.1021/bi982437d. [DOI] [PubMed] [Google Scholar]
- Mosconi L, Herholz K, Prohovnik I, Nacmias B, De Cristofaro MTR, Fayyaz M, Bracco L, Sorbi S, Pupi A. Metabolic interaction between apoE genotype and onset age in Alzheimer’s disease: Implications for brain reserve. J Neurol Neurosurg Psychiatry. 2005;76:15–23. doi: 10.1136/jnnp.2003.030882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, Nacmias B, Sorbi S, De Cristofaro MTR, Fayazz M, Tedde A, Bracco L, Herholz K, Pupi A. Brain metabolic decreases related to the dose of the apoE e4 allele in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2004a;75:370–376. doi: 10.1136/jnnp.2003.014993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, Perani D, Sorbi S, Herholz K, Nacmias B, Holthoff V, Salmon E, Baron J-C, De Cristofaro MTR, Padovani A, et al. MCI conversion to dementia and the APOE genotype. A prediction study with FDG-PET. Neurology. 2004b;63:2332–2340. doi: 10.1212/01.wnl.0000147469.18313.3b. [DOI] [PubMed] [Google Scholar]
- Mosconi L, Sorbi S, Nacmias B, De Cristofaro MTR, Fayyaz M, Bracco L, Herholz K, Pupi A. Age and apoE genotype interaction in Alzheimer’s disease: An FDG-PET study. Psychiatry Res. 2004c;130:141–151. doi: 10.1016/j.pscychresns.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Namba Y, Tomonaga M, Kawasaki H, Otomo E, Ikeda K. Apolipoprotein E immunoreactivity in cerebral amyloid deposits and neurofibrillary tangles in Alzheimer’s disease and kuru plaque amyloid in Creutzfeldt–Jakob disease. Brain Res. 1991;541:163–166. doi: 10.1016/0006-8993(91)91092-f. [DOI] [PubMed] [Google Scholar]
- Nathan BP, Bellosta S, Sanan DA, Weisgraber KH, Mahley RW, Pitas RE. Differential effects of apolipoproteins E3 and E4 on neuronal growth in vitro. Science. 1994;264:850–852. doi: 10.1126/science.8171342. [DOI] [PubMed] [Google Scholar]
- Nathan BP, Chang K-C, Bellosta S, Brisch E, Ge N, Mahley RW, Pitas RE. The inhibitory effect of apolipoprotein E4 on neurite outgrowth is associated with microtubule depolymerization. J Biol Chem. 1995;270:19791–19799. doi: 10.1074/jbc.270.34.19791. [DOI] [PubMed] [Google Scholar]
- Nichol K, Deeny S, Seif J, Camaclang K, Cotman C. Exercise improve cognition and hippocampal plasticity in APOE epsilon4 mice. Alzheimers Dement. 2009;5:287–294. doi: 10.1016/j.jalz.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll JAR, Roberts GW, Graham DI. Amyloid β-protein, APOE genotype and head injury. Ann. N Y Acad. Sci. 1996;777:271–275. doi: 10.1111/j.1749-6632.1996.tb34431.x. [DOI] [PubMed] [Google Scholar]
- Nilsson LNG, Arendash GW, Leighty RE, Costa DA, Low MA, Garcia MF, Cracciolo JR, Rojiani A, Wu X, Bales KR, et al. Cognitive impairment in PDAPP mice depends on apoE and ACT-catalyzed amyloid formation. Neurobiol Aging. 2004;25:1153–1167. doi: 10.1016/j.neurobiolaging.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Papassotiropoulos A, Streffer JR, Tsolaki M, Schmid S, Thal D, Nicosia F, Iakovidou V, Maddalena A, Lütjohann D, Ghebremedhin E, et al. Increased brain β-amyloid load, phosphorylated tau, and risk of Alzheimer disease associated with an intronic CYP46 polymorphism. Arch Neurol. 2003;60:29–35. doi: 10.1001/archneur.60.1.29. [DOI] [PubMed] [Google Scholar]
- Parsian A, Racette B, Goldsmith LJ, Perlmutter JS. Parkinson’s disease and apolipoprotein E: possible association with dementia but not age at onset. Genomics. 2002;79:458–461. doi: 10.1006/geno.2002.6707. [DOI] [PubMed] [Google Scholar]
- Price AR, Xu G, Siemienski ZB, Smithson LA, Borchelt DR, Golde TE, Felsenstein KM. Comment on “ApoE-directed therapeutics rapidly clear b-amyloid and reverse deficits in AD mouse models. Science. 2013;340:924–d. doi: 10.1126/science.1234089. [DOI] [PubMed] [Google Scholar]
- Qiu Z, Hyman BT, Rebeck GW. Apolipoprotein E receptors mediate neurite outgrowth through activation of p44/42 mitogen-activated protein kinase in primary neurons. J Biol Chem. 2004;279:34948–34956. doi: 10.1074/jbc.M401055200. [DOI] [PubMed] [Google Scholar]
- Raber J, Wong D, Buttini M, Orth M, Bellosta S, Pitas RE, Mahley RW, Mucke L. Isoform-specific effects of human apolipoprotein E on brain function revealed in ApoE knockout mice: Increased susceptibility of females. Proc Natl Acad Sci USA. 1998;95:10914–10919. doi: 10.1073/pnas.95.18.10914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raber J, Wong D, Yu G-Q, Buttini M, Mahley RW, Pitas RE, Mucke L. Apolipoprotein E and cognitive performance. Nature. 2000;404:352–354. doi: 10.1038/35006165. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, Saunders AM, Hardy J. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. Proc Natl Acad Sci USA. 2004;101:284–289. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, Saunders AM, Hardy J. Correlations between apolipoprotein E ε4 gene dose and brain-imaging measurements of regional hypometabolism. Proc Natl Acad Sci USA. 2005;102:8299–8302. doi: 10.1073/pnas.0500579102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roses AD. Apolipoprotein E alleles as risk factors in Alzheimer’s disease. Annu Rev Med. 1996;47:387–400. doi: 10.1146/annurev.med.47.1.387. [DOI] [PubMed] [Google Scholar]
- Sadowski MJ, Pankiewicz J, Scholtzova H, Mehta PD, Prelli F, Quartermain D, Wisniewski T. Blocking the apolipoprotein E/amyloid-β interaction as a potential therapeutic approach for Alzheimer’s disease. Proc Natl Acad Sci USA. 2006;103:18787–18792. doi: 10.1073/pnas.0604011103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarmeas N, Habeck CG, Hilton J, Anderson KE, Flynn J, Park A, Stern Y. APOE related alterations in cerebral activation even at college age. J Neurol Neurosurg Psychiatry. 2005;76:1440–1444. doi: 10.1136/jnnp.2004.053645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidl R, Cairns N, Singewald N, Kaehler ST, Lubec G. Differences between GABA levels in Alzheimer’s disease and Down syndrome with Alzheimer-like neuropathology. Naunyn Schmiedeberg Arch Pharmacol. 2001;363:139–145. doi: 10.1007/s002100000346. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Clearing the brain’s amyloid cobwebs. Neuron. 2001;32:177–180. doi: 10.1016/s0896-6273(01)00475-5. [DOI] [PubMed] [Google Scholar]
- Shobab LA, Hsiung G-YR, Feldman HH. Cholesterol in Alzheimer’s disease. Lancet Neurol. 2005;4:841–852. doi: 10.1016/S1474-4422(05)70248-9. [DOI] [PubMed] [Google Scholar]
- Slooter AJC, Tang M-X, van Duijn CM, Stern Y, Ott A, Bell K, Breteler MMB, Van Broeckhoven C, Tatemichi TK, Tycko B, et al. Apolipoprotein E ε4 and the risk of dementia with stroke. A population-based investigation. JAMA. 1997;277:818–821. doi: 10.1001/jama.277.10.818. [DOI] [PubMed] [Google Scholar]
- Small SA. Age-related memory decline. Current concepts and future directions. Arch Neurol. 2001;58:360–364. doi: 10.1001/archneur.58.3.360. [DOI] [PubMed] [Google Scholar]
- Small SA, Chawla MK, Buonocore M, Rapp PR, Barnes CA. Imaging correlates of brain function in monkeys and rats isolates a hippocampal subregion differentially vulnerable to aging. Proc Natl Acad Sci USA. 2004;101:7181–7186. doi: 10.1073/pnas.0400285101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter WJ, Saunders AM, Goedert M, Weisgraber KH, Dong L-M, Jakes R, Huang DY, Pericak-Vance M, Schmechel D, Roses AD. Isoform-specific interactions of apolipoprotein E with microtubule-associated protein tau: Implications for Alzheimer disease. Proc Natl Acad Sci USA. 1994a;91:11183–11186. doi: 10.1073/pnas.91.23.11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, Roses AD. Apolipoprotein E: High-avidity binding to β-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci USA. 1993a;90:1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter WJ, Weisgraber KH, Goedert M, Saunders AM, Huang D, Corder EH, Dong L-M, Jakes R, Alberts MJ, Gilbert JR, et al. Hypothesis: Microtubule instability and paired helical filament formation in the Alzheimer disease brain are related to apolipoprotein E genotype. Exp Neurol. 1994b;125:163–171. doi: 10.1006/exnr.1994.1019. [DOI] [PubMed] [Google Scholar]
- Strittmatter WJ, Weisgraber KH, Huang DY, Dong L-M, Salvesen GS, Pericak-Vance M, Schmechel D, Saunders AM, Goldgaber D, Roses AD. Binding of human apolipoprotein E to synthetic amyloid β peptide: Isoform-specific effects and implications for late-onset Alzheimer disease. Proc Natl Acad Sci USA. 1993b;90:8098–8102. doi: 10.1073/pnas.90.17.8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Wu S, Bu G, Onifade MK, Patel SN, LaDu MJ, Fagan AM, Holtzman DM. Glial fibrillary acidic protein–apolipoprotein E (apoE) transgenic mice: Astrocyte-specific expression and differing biological effects of astrocyte-secreted apoE3 and apoE4 lipoproteins. J Neurosci. 1998;18:3261–3272. doi: 10.1523/JNEUROSCI.18-09-03261.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Vedhachalam C, Sakamoto T, Dhanasekaran P, Phillips MC, Lund-Katz S, Saito H. Effect of carboxyl-terminal truncation on structure and lipid interaction of human apolipoprotein E4. Biochemistry. 2006;45:4240–4247. doi: 10.1021/bi060023b. [DOI] [PubMed] [Google Scholar]
- Teasdale GM, Nicoll JAR, Murray G, Fiddes M. Association of apolipoprotein E polymorphism with outcome after head injury. Lancet. 1997;350:1069–1071. doi: 10.1016/S0140-6736(97)04318-3. [DOI] [PubMed] [Google Scholar]
- Tesseur I, Lo AC, Roberfroid A, Dietvorst S, Van Broeck B, Borgers M, Gijsen H, Moechars D, Mercken M, Kemp J, D’Hooge R, De Strooper B. Comment on “ApoE-directed therapeutics rapidly clear b-amyloid and reverse deficits in AD mouse models. Science. 2013;340:924–e. doi: 10.1126/science.1233937. [DOI] [PubMed] [Google Scholar]
- Tesseur I, Van Dorpe J, Bruynseels K, Bronfman F, Sciot R, Van Lommel A, Van Leuven F. Prominent axonopathy and disruption of axonal transport in transgenic mice expressing human apolipoprotein E4 in neurons of brain and spinal cord. Am J Pathol. 2000a;157:1495–1510. doi: 10.1016/S0002-9440(10)64788-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesseur I, Van Dorpe J, Spittaels K, Van den Haute C, Moechars D, Van Leuven F. Expression of human apolipoprotein E4 in neurons causes hyperphosphorylation of protein tau in the brains of transgenic mice. Am J Pathol. 2000b;156:951–964. doi: 10.1016/S0002-9440(10)64963-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimmer PA, Borland MK. Differentiated Alzheimer’s disease transmitochondrial cybrid cell lines exhibit reduced organelle movement. Antioxid Redox Signal. 2005;7:1101–1109. doi: 10.1089/ars.2005.7.1101. [DOI] [PubMed] [Google Scholar]
- Van Dooren T, Muyllaert D, Borghgraef P, Cresens A, Devijver H, Van der Auwera I, Wera S, Dewachter I, Van Leuven F. Neuronal or glial expression of human apolipoprotein E4 affects parenchymal and vascular amyloid pathology differentially in different brain regions of double- and triple-transgenic mice. Am J Pathol. 2006;168:245–260. doi: 10.2353/ajpath.2006.050752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comment on “ApoE-directed therapeutics rapidly clear b-amyloid and reverse deficits in AD mouse models. Science. 340:924–f. doi: 10.1126/science.1233937. [DOI] [PubMed] [Google Scholar]
- Vepsalainen S, Helisalmi S, Koivisto AM, Tapaninen T, Hiltunen M, Soininen H. Somatostatin genetic variants modify the risk for Alzheimer’s disease among Finnish patients. J Neurol. 2007;254:1504–1508. doi: 10.1007/s00415-007-0539-2. [DOI] [PubMed] [Google Scholar]
- Verghese PB, Castellano JM, Garai K, Wang Y, Jiang H, Shah A, Bu G, Frieden C, Holtzman DM. ApoE influences amyloid-β clearance despite minimal apoE/Aβ association in physiological conditions. Proc Natl Acad Sci USA. 2013;110:E1807–E1816. doi: 10.1073/pnas.1220484110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahrle SE, Jiang H, Parsadanian M, Hartman RE, Bales KR, Paul SM, Holtzman DM. Deletion of Abca1 increases Aβ deposition in the PDAPP transgenic mouse model of Alzheimer disease. J Biol Chem. 2005;280:43236–43242. doi: 10.1074/jbc.M508780200. [DOI] [PubMed] [Google Scholar]
- Wahrle SE, Jiang H, Parsadanian M, Legleiter J, Han X, Fryer JD, Kowalewski T, Holtzman DM. ABCA1 is required for normal central nervous system apoE levels and for lipidation of astrocyte-secreted apoE. J Biol Chem. 2004;279:40987–40993. doi: 10.1074/jbc.M407963200. [DOI] [PubMed] [Google Scholar]
- Weisgraber KH. Apolipoprotein E: Structure–function relationships. Adv Protein Chem. 1994;45:249–302. doi: 10.1016/s0065-3233(08)60642-7. [DOI] [PubMed] [Google Scholar]
- Wisniewski T, Frangione B. Apolipoprotein E: A pathological chaperone protein in patients with cerebral and systemic amyloid. Neurosci Lett. 1992;135:235–238. doi: 10.1016/0304-3940(92)90444-c. [DOI] [PubMed] [Google Scholar]
- Wolozin B. Cholesterol and the biology of Alzheimer’s disease. Neuron. 2004;41:7–10. doi: 10.1016/s0896-6273(03)00840-7. [DOI] [PubMed] [Google Scholar]
- Xu Q, Bernardo A, Walker D, Kanegawa T, Mahley RW, Huang Y. Profile and regulation of apolipoprotein E (apoE) expression in the CNS in mice with targeting of green fluorescent protein gene to the apoE locus. J Neurosci. 2006;26:4985–4994. doi: 10.1523/JNEUROSCI.5476-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Brecht WJ, Weisgraber KH, Mahley RW, Huang Y. Apolipoprotein E4 domain interaction occurs in living neuronal cells as determined by fluorescence resonance energy transfer. J Biol Chem. 2004;279:25511–25516. doi: 10.1074/jbc.M311256200. [DOI] [PubMed] [Google Scholar]
- Xue S, Jia L, Jia J. Association between somatostatin gene polymorphisms and sporadic Alzheimer’s disease in Chinese population. Neurosci Lett. 2009;465:181–183. doi: 10.1016/j.neulet.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Zimmer R, Teelken AW, Trieling WB, Weber W, Weihmayr T, Lauter H. γ-Aminobutyric acid and homovanillic acid concentration in the CSF of patients with senile dementia of Alzheimer’s type. Arch Neurol. 1984;41:602–604. doi: 10.1001/archneur.1984.04210080010005. [DOI] [PubMed] [Google Scholar]