Abstract
Background
Previous studies have found interrelationships between the serotonin system and alcohol self-administration. The goal of this work was to directly observe in vivo effects of chronic ethanol self-administration on serotonin 5-HT1A receptor binding with [18F]mefway PET neuroimaging in rhesus monkeys. Subjects were first imaged alcohol-naïve and again during chronic ethanol self-administration to quantify changes in 5-HT1A receptor binding.
Methods
Fourteen rhesus monkey subjects (10.7-12.8 years) underwent baseline [18F]mefway PET scans prior to alcohol exposure. Subjects then drank gradually increasing ethanol doses over four months as an induction period, immediately followed by at least nine months ad libidum ethanol access. A post [18F]mefway PET scan was acquired during the final three months of ad libidum ethanol self-administration. 5-HT1A receptor binding was assayed with binding potential (BPND) using the cerebellum as a reference region. Changes in 5-HT1A binding during chronic ethanol self-administration were examined. Relationships of binding metrics with daily ethanol self-administration were also assessed.
Results
Widespread increases in 5-HT1A binding were observed during chronic ethanol self-administration, independent of the amount of ethanol consumed. A positive correlation between 5-HT1A binding in the raphe nuclei and average daily ethanol self-administration was also observed, indicating that baseline 5-HT1A binding in this region predicted drinking levels.
Conclusions
The increase in 5-HT1A binding levels during chronic ethanol self-administration demonstrates an important modulation of the serotonin system due to chronic alcohol exposure. Furthermore, the correlation between 5-HT1A binding in the raphe nuclei and daily ethanol self-administration indicates a relationship between the serotonin system and alcohol self-administration.
Keywords: Serotonin, 5-HT1A, PET Imaging, Chronic Alcohol Self-Administration, Rhesus Monkey
1. INTRODUCTION
The 5-hydroxytryptamine (5-HT), or serotonin, system is a crucial neurotransmitter system that regulates learning, mood, and anxiety. Dysfunction of this system has been implicated in many neuropsychiatric illnesses including depression, schizophrenia, and other disorders relating to mood and anxiety (Jans et al., 2007). There is also extensive evidence linking alcohol-related disorders with the serotonin system (see LeMarquand et al., 1994; Sari et al., 2011 for reviews). In particular, Type II alcoholism, which is characterized by impaired impulse control, has been heavily connected with deficits in serotonergic functioning (Cloninger, 1987). Low levels of 5-hydroxindoleacetic acid (5-HIAA), the primary metabolite of 5-HT, have been observed in the CSF of Type II alcoholics (Virkkunen and Linnoila, 1993; Linnoila et al., 1994). Additionally, studies examining the serotonin transporter (5-HTT) found reduced 5-HTT density in post-mortem alcoholics (Mantere et al., 2002).
Serotonin 5-HT1A receptors occur both as autoreceptors in the raphe nuclei that regulate 5-HT tone (Blier et al., 2003), and postsynaptically on inhibitory neurons throughout the rest of the brain (Raymond et al., 1999). The critical role these 5-HT1A receptors play in regulating 5-HT transmission makes them an important target for understanding interactions of ethanol with the serotonin system (Sari et al., 2011). Moreover, 5-HT1A receptors are heavily implicated in anxiety and depression, making them particularly vital targets for studies investigating behavioral traits associated with alcoholism.
Neuroimaging studies in humans have provided important in vivo data concerning alcohol”s effects on the serotonin system, though the findings are mixed. One study explicitly examined 5-HT1A receptors in addition to 5-HTT binding, and found no difference in binding levels between alcoholic patients and healthy controls for both serotonergic targets (Martinez et al., 2009). Further experiments examining the 5-HTT yielded varying results, with one study finding no difference in 5-HTT availability in alcoholic patients relative to control subjects (Brown et al., 2007), while earlier investigations found lower 5-HTT binding in alcoholics compared to healthy controls (Heinz et al., 1998; Szabo et al., 2004). Striatal 5-HT1B levels were also significantly increased in alcoholics (Hu et al., 2010).
The previously referenced neuroimaging experiments examined correlational relationships between the serotonin system and ethanol exposure, and are demonstrative of the majority of studies designed to link 5-HT dysfunction with alcohol exposure. However, there is a lack of experiments directly examining causal effects of ethanol on serotonin function, particularly in primate species. In rats, microdialysis and in vitro studies have shown synaptic serotonin to decrease during a chronic presence of ethanol (Kirby et al., 2011).
The use of positron emission tomography (PET) imaging offers a unique opportunity for causal experiments directly assaying receptor binding during chronic ethanol exposure in primate species. Studies of nonhuman primates combine the social and behavioral similarities between nonhuman primates and humans with advantages of greater control over experimental variables in a laboratory setting, including precise knowledge of a subject”s ethanol exposure history, data from adult subjects in an ethanol-naive state, and control over abstinence during scanning procedures (Barr and Goldman, 2006).
The goal of the present work was to use PET imaging with the 5-HT1A specific radioligand [18F]mefway to assay 5-HT1A binding before and during chronic ethanol self-administration in rhesus monkeys, allowing for direct measures of changes in 5-HT1A binding due to an extended presence of ethanol. Secondary analyses were conducted to assess the relationship between 5-HT1A availability and ethanol consumed ad libidum. These studies yield crucial insight into the modifications chronic ethanol exposure impose on serotonin 5-HT1A receptors.
2. METHODS
2.1. Subjects
The experimental cohort consisted of fourteen rhesus monkeys (Macaca mulatta; 7 males, 7 females), drawn from an experimental protocol designed to examine the effects of gestational timing of prenatal ethanol exposure on development (for details, see Schneider et al., 2001). At the start of these experiments, no post-natal alcohol exposure was experienced by any subjects. All housing and experimental guidelines were approved by the Institutional Animal Care and Use Committee. These procedures follow the guidelines addressing the ethical care and use of laboratory animals as set forth by the USDA “Federal Register” standards and the “Guide for the Care and Use of Laboratory Animals” set forth by the NIH (Bethesda, MD, USA).
2.2. Experimental Procedures
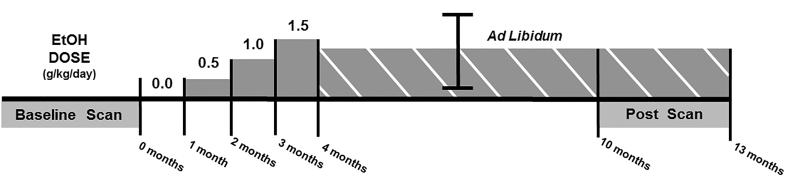
To summarize the experimental protocol, shown in Figure 1, subjects underwent a “baseline” PET scan quantifying 5-HT1A binding prior to chronic ethanol drinking. Age at the time of the first PET scan was 10.7-12.8 years. Subjects then self-administered (drank) ethanol over a 390 day (13 month) period, adapting procedures developed previously (Vivian et al., 2001). The first 120 days (4 months) consisted of a controlled ethanol dose induction period, while the final 270 days (9 months) allowed subjects to consume ethanol ad-libidum. During the final 90 days of ethanol exposure, a “post” PET scan was acquired to examine 5-HT1A binding levels during chronic alcohol self-administration.
Figure 1.
Alcohol administration protocol during these experiments. Methods are based on procedures developed by Vivian et al., 2001. The x-axis indicates time. Above the y-axis, the ethanol dose is scaled in g/kg/day, while below, periods of PET scanning are indicated.
2.2.1. Ethanol Drinking Protocol
An operant drinking panel, described in detail by Vivian and colleagues (2001), controlled and monitored subject access to food pellets, water, and ethanol solution. For every phase of the study, alcohol was available as 4% v/v ethanol water solution.
The first 120 days (4 months) of ethanol access consisted of an induction period, where subjects self-administered (drank) controlled, gradually increasing ethanol doses using scheduled-induced polydipsia. During this induction period, food pellets were regularly delivered every 180-300 seconds during a 16-hour session every day. The fixed time interval was designed to encourage animals to drink a targeted alcohol dose. To avoid conditioned taste aversion to ethanol, the induced ethanol self-administration dose was escalated in a step-wise fashion. Thus, subjects consumed only water for the first 30 days, followed by 0.5 g/kg/day ethanol for the second 30 days, 1.0 g/kg/day ethanol for the third 30 days, and 1.5 g/kg/day ethanol for the final 30 days of induction.
Immediately following ethanol induction, subjects were allowed to drink ethanol ad libidum for 270 days (9 months). During the ad libidum period, ethanol/water solution (4% v/v) and water were continuously available over a 22-hour session every day. Food was given as 3 meals available every 2 hours over the 22-hour session. Ethanol intake was quantified as average ethanol consumed per day (g/kg/day). Following the ad libidum period, ethanol access was terminated, and subjects were monitored for symptoms of withdrawal including nausea, vomiting, and dehydration. Animal weight was also monitored.
The average daily ethanol self-administration for each subject was quantified over the first 6 months of the ad-libitum period. Across all subjects, the average daily ethanol consumed was 0.93 ± 0.45 g/kg/day. No withdrawal symptoms were observed following the termination of ethanol access.
2.2.2. PET Scanning Sessions
Measures of 5-HT1A receptor binding were assayed with [18F]mefway PET imaging sessions. The PET radioligand [18F]mefway is highly specific to 5-HT1A receptors with well-validated kinetic properties in the rhesus monkey (Wooten et al., 2011). Radiotracer was produced following previously reported methods (Saigal et al., 2006), yielding high specific activity [18F]mefway (>70 GBq/μmol at injection). For post [18F]mefway scans, ethanol access was terminated approximately 90 minutes prior to scan initiation to minimize acute abstinence effects from influencing measured 5-HT1A binding levels. Since alcohol was self-administered, the resulting time between last drink and scan start ranged from two to 14 hours.
On the day of scans, subjects were anesthetized one hour prior to imaging with 10 mg/kg IM ketamine. Anesthesia was induced at 1.5% isoflurane with 2 L of oxygen and then maintained at 1.0-1.5% isoflurane with 2 L of oxygen throughout the imaging session. All vital signs, including body temperature, respiration rate, heart rate, SpO2 levels, and capnography were monitored throughout the duration of scans. A microPET P4 scanner was used for image data acquisition (Tai et al., 2001). A 518 s 57Co transmission scan was first acquired to correct for the scatter and attenuation of radiation in the tissue. A 30 s bolus injection of 59-130 MBq high specific activity [18F]mefway then initiated PET data acquisition, which lasted for 90 minutes. Following study completion, anesthesia was terminated and subjects were returned to their cages and monitored until fully alert.
2.3. PET data processing
Dynamic PET data were histogrammed into time bins up to 5 minutes long and reconstructed with filtered back projection (0.5 cm−1 ramp filter). Corrections for scanner normalization, dead time, signal attenuation, radioactive decay, and scatter were included. Final reconstructed PET images had a matrix size of 128×128×63 with spatial dimensions of 1.90×1.90×1.21 mm3.
[18F]Mefway binding was quantified by binding potential relative to nondisplaceable uptake (BPND). BPND is proportional to 5-HT1A receptor sites available for radioligand binding (Bavail) and [18F]mefway”s apparent affinity for these sites (1/KD) (Innis et al., 2007). The mulitilinear reference tissue model (MRTM2; Ichise et al., 2003) was used with the cerebellum as a reference region (Wooten et al., 2011) to produce voxel-wise BPND parametric maps. Our previous experience using [18F]mefway has yielded a BPND test-retest variability of 8-11% (unpublished data).
Since individual subject MRIs were not acquired, a template based approach was used for region of interest (ROI) definition. Parametric maps were transformed to a standardized PET space using an in-house [18F]mefway template coregistered to a rhesus monkey MRI atlas (McLaren et al., 2009). The optimal transformation matrix was determined with FSL-FLIRT (Jenkinson et al., 2002), allowing for 7 degrees of freedom. The spatially normalized images were then smoothed with a 4×4×4 mm3 Gaussian filter to reduce anatomical differences between subjects in the images.
ROIs were selected for analysis based on brain areas of [18F]mefway specific binding with similar kinetic properties and physiological significance implemented previously by our lab (Christian et al., 2013). Regions extracted with a digital rhesus monkey template included anterior cingulate gyrus (aCg), posterior cingulate gyrus (pCg), hippocampus (Hp), amygdala (Am), dorsolateral prefrontal cortex (pFC), parietal cortex (PC), occipital cortex (OC), and lateral temporal cortex (lTC). Since the raphe nuclei region (RN), which consisted of a small volume containing high 5-HT1A density, exhibited variability in location despite spatial normalization, the RN was manually defined on the normalized images with a 3 voxel diameter circle drawn on 3 consecutive transaxial planes.
2.4. Statistical Analysis
Analysis was carried out to test for differences in baseline BPND and post BPND. Paired t-tests were used to test the hypothesis of no difference between the means of baseline BPND compared to post BPND for each region. The resulting p-values from these hypotheses indicating differences between baseline BPND and post BPND were corrected for multiple comparisons due to the examination of multiple regions. The False Discovery Rate correction with α<0.05 was implemented for the multiple comparison correction (Benjamini et al., 1995). The corresponding adjusted confidence intervals are also reported (Benjamini and Yekutieli, 2005). Effect sizes for differences between baseline BPND and post BPND were computed with Cohen”s d effect sizes (Cohen, 1988).
Univariate analysis was used to test for correlations between binding measures and ethanol self-administration. Pearson”s correlation coefficients between average daily ethanol consumed (g/kg/day) over the ad libidum drinking period and baseline BPND, post BPND, and change in BPND were calculated. The 95% confidence interval was computed for the Pearson”s correlation using Fisher”s z transformation. The correlation coefficient”s p-values and their confidence interval levels were not corrected for multiple hypothesis tests. Analysis was performed using SAS (version 9.3, SAS Ipearnstitute, Cary NC) and R (version 3.0) statistical software.
3. RESULTS
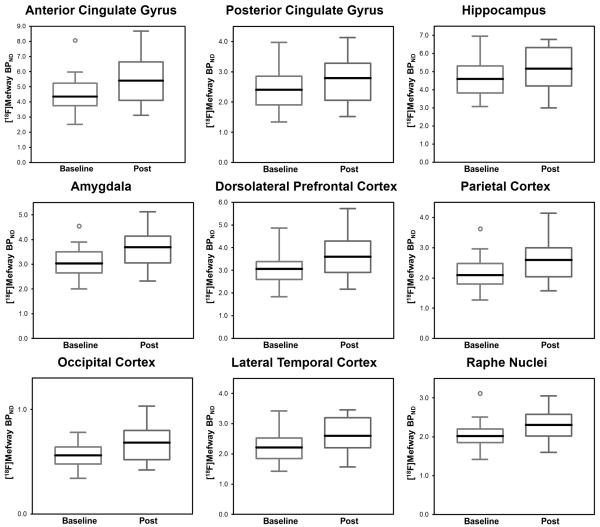
Statistically significant (α<0.05) increases in mean 5-HT1A binding ([18F]mefway BPND) following chronic ethanol self-administration were observed in all regions except for the RN, shown in Figure 2. The p-value in the RN approached but did not reach statistical significance (p=0.059). The average change in BPND for all regions was 0.34 (16%) with a standard deviation of 0.13. The average effect size for all the regions was 0.59, which is regarded as a medium-high effect size (Cohen, 1988). These data are presented for each region in Table 1, featuring observed differences between baseline and post BPND with adjusted 95% confidence intervals, p-values (both uncorrected and corrected), and Cohen”s d effect sizes.
Figure 2.
Boxplots of 5-HT1A BPND in the regions of interest. Left, in blue, shows baseline BPND, while right, in red, shows post-drinking BPND. ○ indicates outliers. Statistically significant differences were observed in all regions except the raphe nuclei, where a trend (P=0.059) was observed. Note the different scales for each panel. Solid center lines indicate the medians. Each boxplot was composed of data from 14 subjects.
Table 1.
5-HT1A binding (BPND) in all regions for baseline and post-alcohol.
| Region | Baseline BPND |
Post BPND | Estimate of BPND Difference (Adjusted 95% C.I.) |
Test statistic |
p-value (corrected) |
Effect Size (Cohen’s d) |
|---|---|---|---|---|---|---|
|
Anterior
Cingulate Gyrus |
3.25±0.95 | 3.87±1.14 | 0.61 (0.03,1.19) | t(13)=2.41 | .031 (.035) | 0.58 |
| Posterior Cingulate Gyrus |
1.77±0.52 | 2.09±0.60 | 0.32 (0.02, 0.61) | t(13)=2.44 | .030 (.035) | 0.57 |
| Hippocampus | 2.51±0.65 | 2.89±0.69 | 0.38 (0.04,0.73) | t(13)=2.55 | .024 (.035) | 0.57 |
| Amygdala | 2.51±0.56 | 2.93±0.61 | 0.42 (0.09,0.74) | t(13)=2.92 | .011 (.035) | 0.71 |
|
Dorsolateral
Prefrontal Cortex |
2.50±0.67 | 2.95±1.02 | 0.44 (0.05,0.84) | t(13)=2.58 | .023 (.035) | 0.51 |
| Parietal Cortex | 1.72±0.53 | 2.05±0.61 | 0.32 (0.05,0.60) | t(13)=2.69 | .018 (.035) | 0.57 |
| Occipital Cortex | 0.60±0.16 | 0.73±0.21 | 0.13 (0.03,0.22) | t(13)=3.04 | .009 (.035) | 0.68 |
|
Lateral Temporal
Cortex |
1.84±0.46 | 2.16±0.52 | 0.32 (0.06,0.59) | t(13)=2.78 | .015 (.035) | 0.66 |
| Raphe Nuclei | 2.10±0.34 | 2.31±0.45 | 0.21 (−0.02,0.44) | t(13)=2.07 | .059 (.059) | 0.49 |
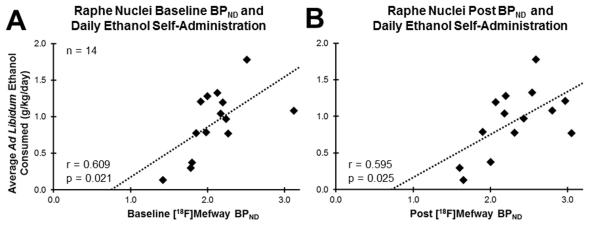
A second major finding was a positive association between baseline BPND and average daily ethanol self-administration in the RN (r=0.609; p=0.021; 95% CI (0.12, 0.86)), shown in Figure 3A. Similarly, a significant positive association was found between post BPND and average daily ethanol self-administration in RN (r=0.595; p=0.024; 95% CI (0.09, 0.86)), shown in Figure 3B. No other regions exhibited significant relationships between baseline or post BPND and daily ethanol self-administration. No significant relationships between change in BPND and daily ethanol self-administration were observed. Table 2 summarizes these results, including the Pearson”s r and p-values calculated in each region for each BPND measure (baseline, post, and change in BPND) correlated with daily average ethanol self-administration.
Figure 3.
Relationship between 5-HT1A BPND in raphe nuclei and ethanol-self administration (g/kg/day). Panel A shows baseline BPND while panel B shows post BPND. Pearson”s correlation coefficients (r) and correlation significance (p) are shown in each panel, bottom left, along with the line fit (dashed line).
Table 2.
Correlations between average daily ethanol self-administered and binding measurements.
| Average Daily Ethanol (g/kg/day) vs. Baseline BPND |
Average Daily Ethanol (g/kg/day) vs. Post BPND |
Average Daily Ethanol (g/kg/day) vs. Change in BPND |
||||
|---|---|---|---|---|---|---|
| Region | Pearson’s r |
p- value | Pearson’s r |
p- value | Pearson’s r |
p- value |
| Anterior Cingulate Gyrus |
0.40 | 0.155 | 0.32 | 0.276 | 0.02 | 0.933 |
| Posterior Cingulate Gyrus |
0.40 | 0.157 | 0.32 | 0.264 | 0.03 | 0.911 |
| Hippocampus | 0.44 | 0.118 | 0.42 | 0.131 | −0.02 | 0.957 |
| Amygdala | 0.38 | 0.183 | 0.39 | 0.178 | −0.04 | 0.890 |
| Dorsolateral Prefrontal Cortex |
0.31 | 0.276 | 0.28 | 0.335 | −0.02 | 0.939 |
| Parietal Cortex |
0.32 | 0.268 | 0.25 | 0.393 | 0.04 | 0.904 |
| Occipital Cortex |
0.13 | 0.648 | 0.11 | 0.71 | 0.00 | 0.994 |
| Lateral Temporal Cortex |
0.36 | 0.205 | 0.39 | 0.178 | −0.07 | 0.805 |
|
Raphe
Nuclei |
0.61 | 0.021 | 0.60 | 0.023 | −0.07 | 0.807 |
The column on far left indicates the region examined. The major columns indicate correlation analyses examining for relationships between daily alcohol self-administration and baseline BPND (left), post BPND (middle), and change in BPND (right). Within each main column, sub-columns containing Pearson’s correlation coefficients (r) are given on the left, with the corresponding p-value (uncorrected for multiple comparisons) on the right.
4. DISCUSSION
This study presents two important findings relating in vivo 5-HT1A binding levels with chronic ethanol self-administration. First, 5-HT1A binding levels increased during chronic ethanol self-administration in the rhesus monkey. This finding is consistent with either increased 5-HT1A receptor density, decreased synaptic 5-HT concentrations, or a combination of the two effects. Second, 5-HT1A binding in the RN both before and during ethanol self-administration positively correlated with average daily ethanol self-administration. This provides evidence for altered 5-HT function influencing ethanol self-administration. The present experiments fill a crucial gap directly examining causal modifications to 5-HT1A receptor binding in vivo following chronic ethanol self-administration in primate species.
4.1. Interpretation of Results
Precise interpretation of the primary finding, increases to 5-HT1A BPND during chronic ethanol self-administration, is complicated by the multiple influences on radioligand binding at receptor sites. Decreased extracellular 5-HT, as observed after prolonged ethanol exposure in microdialysis studies (Bare et al., 1998; Thielen et al., 2004), would reduce synaptic 5-HT competition at the 5-HT1A site, increasing [18F]mefway BPND measured with PET procedures. Alternatively, increases in receptor density (Bmax) would also increase BPND. Recent post mortem studies demonstrated increased hippocampal 5-HT1A Bmax for monkeys self-administering alcohol relative to healthy controls in cynomolgus macaques (Burnett et al., 2014). Notably, those subjects underwent a similar ethanol drinking paradigm to the present studies. The increase in 5-HT1A binding during chronic ethanol self-administration could speculatively be a combination of the two effects, where lowered synaptic 5-HT induced by chronic ethanol exposure upregulates 5-HT1A receptors as a compensatory mechanism. The likely increase in 5-HT1A receptor density represents, to our knowledge, a first reported direct in vivo observation of modulations to 5-HT1A receptors induced by chronic alcohol exposure in primate species.
A secondary result of the present work was a positive relationship between average daily ad libidum ethanol self-administration and 5-HT1A binding in the RN both at baseline and post ethanol drinking. The RN is a crucial component of the serotonin system because these 5-HT1A receptors function as autoreceptors to regulate synaptic 5-HT tone (Raymond et al., 1999; Blier et al., 2003). Furthermore, the RN is the source of dense serotonergic projections to cortico-midbrain regions (Johnson, 2004). Therefore, we interpret these findings to suggest that fundamental differences in 5-HT function may underlie intrasubject variation in ethanol self-administration.
Interestingly, for all regions no relationship between the change in 5-HT1A BPND (between baseline and post-drinking) and average daily ethanol self-administered was observed. Considering the observed global increases in binding during chronic ethanol self-administration, we speculate that a prolonged presence of ethanol induces a uniform response from the 5-HT system throughout the brain. The large inter-subject range for 5-HT1A RN BPND binding (approximately 100%) observed between the lowest and highest ethanol consumers makes it unlikely that our measures lacked the sensitivity to identify subtle changes in 5-HT1A binding. These data therefore provide crucial cohesion between the primary findings of this work by suggesting that the 5-HT involvement (via the RN) in predicted average daily ethanol self-administration is independent from the mechanistic global increases in 5-HT1A binding during chronic ethanol consumption.
4.2. Further Statistical Considerations and Limitations
The primary analysis of differences in BPND baseline and during drinking yielded a non-significant yet strong trend (p=0.059) of increased binding in RN. Considering the increased variability in PET measurements due to the small volume of this region (>400% smaller than any other region), we therefore speculate that the RN is consistent with the other regions in increased 5-HT1A binding during chronic ethanol self-administration.
Our group has previously reported significant relationships between [18F]mefway BPND and both 5-HTTLPR genotype (Christian et al., 2013) and subject sex (Wooten et al., 2013). These factors did not influence the observed increases in 5-HT1A binding following chronic ethanol exposure since each subject serves as its own control. For the correlation analyses, two-sample t-tests found no statistically significant differences in BPND between 5-HTTLPR genotypes or subject gender due to the small sample size, justifying the use of a univariate statistical model.
The present subjects were part of a cohort of animals whose mothers were exposed to alcohol while subjects were in utero (Schneider et al., 2001), with subjects drawn from all treatment groups. There was no evidence for any trends between treatment groups and 5-HT1A binding levels. Consequently, prenatal treatment group was not included as a covariate in the analysis, particularly since the small group sizes yielded poor statistical power. The potential confound of pre-natal alcohol exposure should be considered in interpretation of correlations between 5-HT1A binding in RN and average daily alcohol self-administration, as this may have conceivably influenced the serotonin system at baseline or alcohol self-administration (or both).
Sensitivity analysis suggested that the potential presence of ethanol during post PET scans also did not influence the two main findings. Four subjects consumed ethanol within four hours of their post PET scan, and thus potentially had ethanol in their system during PET procedures, while the other ten did not consume alcohol for at least eight hours prior to PET scans. For the sensitivity analysis, the data were re-analyzed excluding the four subjects who consumed ethanol within four hours of their post PET scan. Statistical significance, including the false discovery rate correction, was maintained for the primary finding of increased 5-HT1A binding across most brain regions, and a similar correlation coefficient was observed between RN post BPND and average daily ethanol self-administration (r=0.545). Previous microdialysis studies found that prior exposure to ethanol dampened the release of synaptic 5-HT following subsequent ethanol exposure (Bare et al., 1998), suggesting that the presence of ethanol during PET scans minimally influences radioligand binding levels in a chronic ethanol exposure paradigm.
4.3. Implications of Results
The positive relationship between RN 5-HT1A binding and ethanol self-administration exhibits striking similarities to studies examining CSF 5-HIAA concentrations in connection with ethanol exposure. In humans, 5-HIAA levels are lower in Type II alcoholics (Linnoila et al., 1994), and these findings have been extensively replicated in rhesus monkeys (Higley et al., 1996). Notably, in rhesus monkeys a negative correlation between 5-HIAA levels and ethanol self-administration was observed. Furthermore, inter-individual 5-HIAA levels were shown to be stable over time (Higley et al., 1992), providing evidence that low 5-HIAA levels may be an enduring trait of 5-HT deficit connected to increased ethanol self-administration. In our findings, the positive correlation between 5-HT1A binding in the RN and daily ethanol self-administration is notably present both in an alcohol-naive state and following ethanol exposure, suggesting that increased RN 5-HT1A binding may be an alternative manifestation of reduced 5-HIAA and 5-HT deficit present in increased ethanol self-administration in rhesus monkeys. In support of this interpretation, a linear model correlating RN post BPND with daily alcohol self-administration while controlling for RN pre BPND yielded a weak relationship (r=0.35). This provides additional evidence that 5-HT1A binding in the RN is trait-like, echoing findings examining 5-HIAA levels in nonhuman primates exposed to alcohol (Higley et al., 1996). These findings may potentially be extrapolated to human alcohol patients (e.g., type II alcoholism), but require further human studies to validate this conjecture.
The present results demonstrate that the use of PET imaging of 5-HT1A receptors can be an alternative tool to assess 5-HT deficits associated with increased ethanol self-administration. Sophisticated experimental techniques offer the opportunity to extend these findings by teasing apart differences in receptor upregulation from changes to synaptic serotonin concentrations (Wooten et al., 2012). Additionally, 5-HT1A autoreceptors in the RN may be a target of localized treatment for potential therapeutics in alcohol-related disorders.
Earlier experiments with a PET radioligand analog of [18F]mefway, [11C]WAY-100635, found no difference in 5-HT1A receptor binding levels between alcoholic patients and healthy controls (Martinez et al., 2009). That study, however, tested subjects following 14 days of detoxification. The experiment here minimally withheld ethanol access prior to imaging, thus the duration of ethanol abstinence may speculatively be a contributing factor to the different findings. In vitro microdialysis studies demonstrated an effect of ethanol abstinence duration on the serotonin system, as 35% lower extracellular 5-HT concentrations were observed in rats continually exposed to ethanol compared to controls, while this effect was not observed following 2 weeks ethanol deprivation (Thielen et al., 2004). The opportunity to minimize ethanol abstinence in rhesus monkeys thus offered a major advantage over clinical protocols in that the present experiments directly examined causal effects of chronic ethanol self-administration on serotonin function. No follow-up scans were performed on the present cohort after extended alcohol abstinence. The extent to which 5-HT1A binding levels may recover after alcohol abstinence remains an important question for future experiments.
Previous neuroimaging studies of the serotonin transporter (5-HTT) found decreased 5-HTT binding levels in the midbrain (interpreted as the raphe nuclei) of alcoholics compared to healthy controls (Heinz et al., 1998; Szabo et al., 2004). Later studies using [11C]DASB, a radioligand more specific to 5-HTT, found no difference in binding between alcoholic patients and healthy controls (Brown et al., 2007; Martinez et al., 2009). Notably, [11C]DASB exhibits improved kinetic properties over the radioligands used previously (Szabo et al., 2002). An earlier neuroimaging study examining 5-HT receptors with [11 1B C]P943 revealed increased binding in the 5-HT1B dense region of ventral striatum in alcoholic subjects relative to healthy controls (Hu et al., 2010), demonstrating a difference in the 5-HT system between alcoholics and healthy controls. Future longitudinal studies are needed to examine the causal effects of chronic ethanol exposure on other components of the 5-HT system.
The nonhuman primate model of ethanol self-administration used herein is a well validated tool for examining ethanol self-administration with many parallels to human alcohol use (Vivian et al., 2001; Grant et al., 2008). In this particular cohort, neither excessive amounts of ethanol self-administration nor withdrawal symptoms were observed, indicating that this study most parallels moderate alcohol drinking in humans. These experiments are crucial complements to studies of alcoholic patients in human subjects to examine causal effects of moderate alcohol drinking on neurotransmitter systems with PET imaging. Similar studies in a different cohort have demonstrated an increase in α4β2 nicotinic acetylcholine receptor binding in the cortex following chronic alcohol self-administration (Hillmer et al., 2014). Future studies are underway to exploit these advantages to further study the serotonergic system and the dopaminergic system during moderate ethanol exposure, and include behavior correlates into analyses of these data.
4.4 Conclusion
To conclude, increases in 5-HT1A-specific binding were observed following chronic ethanol self-administration in rhesus monkeys. A positive relationship between 5-HT1A binding (both pre- and post- chronic ethanol self-administration) and daily ad libidum ethanol consumed was also observed in the raphe nuclei. These findings provide crucial evidence for the modification of serotonin system by the chronic presence of ethanol, and demonstrate the utility of PET imaging in assessing abnormal 5-HT1A function in alcohol-related disorders.
Highlights.
Rhesus monkeys chronically self-administered (drank) alcohol over 12 months
PET 5-HT1A binding was measured before and after 9-12 months alcohol drinking
Global increases in 5-HT1A binding were observed during chronic alcohol drinking
Raphe Nuclei 5-HT1A binding positively correlated with average alcohol drinking
Acknowledgements
We thank R. Jerry Nickles, Hector Valdovinos, and Stephen Graves for assistance with radioisotope production. Patrick Lao, Tobey Betthauser, and Andrew Higgins provided invaluable help in image processing and data analysis. Jeff Moirano contributed help in implementing the [18F]mefway rhesus monkey atlas. The staff at Harlow Center for Biological Psychology (R000167) provided indispensable aid in animal care, handling, and data acquisition.
Role of Funding Source Support for this work was provided by NIH grants R01AA10079, R01AA12277, R03AA017706, P30HD003352, and T32CA009206.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors TEB operated the cyclotron for radioisotope production. DWW, ATH, and TEB performed the [18F]mefway radiochemistry. LMR and JAL supervised animal care, anesthesia, measurement of ethanol consumed by subjects, and processing of the ethanol consumption data. EOA conducted image data acquisition, assisted by LMR and JAL. DWW and ATH reconstructed and processed the imaging data. DWW and ATH performed image registration and creation of BPND maps. DLT performed statistical analysis. Literature search and manuscript preparation was performed by ATH, DWW, DLT, AKC, CFM, MLS, and BTC. The subjects in this study were part of a grant awarded to MLS including AKC, CFM, and BTC, all of whom oversaw research procedures. All authors have contributed to and approved the final manuscript.
Conflict of Interest The authors have no potential conflicts of interest to declare.
REFERENCES
- Bare DJ, McKinzie JH, McBride WL. Development of rapid tolerance to ethnaol-stimulated serotonin release in the ventral hippocampus. Alcohol. Clin. Exp. Res. 1998;22:1272–1276. [PubMed] [Google Scholar]
- Barr CS, Goldman D. Non-human primate models of inheritance vulnerability to alcohol use disorders. Addict. Biol. 2006;11:374–385. doi: 10.1111/j.1369-1600.2005.00034.x. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. 1995;57:289–300. [Google Scholar]
- Benjamini Y, Yekutieli D. False discovery rate-adjusted multiple confidence intervals for selected parameters. J. Am. Stat. Assoc. 2005;100:71–81K. [Google Scholar]
- Blier P, Ward NM. Is there a role for 5-HT1A agonists in the treatment of depression? Biol. Psychiatry. 2003;53:193–203. doi: 10.1016/s0006-3223(02)01643-8. [DOI] [PubMed] [Google Scholar]
- Borg S, Kvande H, Liljeberg P, Mossberg D, Valverius P. 5-hydroxyindoleacetic acid in cerebrospinal fluid in alcoholic patients under different clinical conditions. Alcohol. 1985;2:415–418. doi: 10.1016/0741-8329(85)90106-5. [DOI] [PubMed] [Google Scholar]
- Brown AK, George DT, Fujita M, Liow JS, Ichise M, Hibbeln J, Ghose S, Sangare J, Hommer D, Innis RB. PET [11C]DASB imaging of serotonin transporters in patients with alcoholism. Alcohol. Clin. Exp. Res. 2007;31:28–32. doi: 10.1111/j.1530-0277.2006.00261.x. [DOI] [PubMed] [Google Scholar]
- Burnett EJ, Grant KA, Davenport AT, Hemby SE. The effects of chronic ethanol self-administration on hippocampal 5-HT1A receptors in monkeys. Drug Alcohol Depend. 2014;136:135–142. doi: 10.1016/j.drugalcdep.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian BT, Wooten DW, Hillmer AT, Tudorascu DL, Converse AK, Moore CF, Ahlers EO, Barnhart TE, Kalin NH, Barr CS, Schneider ML. Serotonin transporter genotype affects serotonin 5-HT1A binding in primates. J. Neurosci. 2013;33:2512–2516. doi: 10.1523/JNEUROSCI.4182-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloninger CR. Neurogenetic adaptive mechanisms in alcoholism. Science. 1987;236:410–416. doi: 10.1126/science.2882604. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis For The Behavioral Sciences. 2nd Lawrence Earlbaum Associates; Hillsdale, NJ: 1988. [Google Scholar]
- Grant KA, Leng X, Green HL, Szeliga KT, Rogers LS, Gonzales SW. Drinking typography established by scheduled induction predicts chronic heavy drinking in a monkey model of ethanol self-administration. Alcohol. Clin. Exp. Res. 2008;32:1824–1838. doi: 10.1111/j.1530-0277.2008.00765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A, Ragan P, Jones DW, Hommer D, Williams W, Knable MB, Gorey JG, Doty L, Geyer C, Lee KS, Coppola R, Weinnberger DR, Linnoila M. Reduced central serotonin transporters in alcoholism. Am. J. Psychiatry. 1998;155:1544–1549. doi: 10.1176/ajp.155.11.1544. [DOI] [PubMed] [Google Scholar]
- Heinz A, Jones DW, Mazzanti C, Goldman D, Ragan P, Hommer D, Linnoila M, Weinberger M. A relationship between serotonin transporter genotype and in vivo protein expression and alcohol neurotoxicity. Biol. Psychiatry. 2000;47:643–649. doi: 10.1016/s0006-3223(99)00171-7. [DOI] [PubMed] [Google Scholar]
- Higley JD, Suomi SJ, Linnoila M. A longitudinal assessment of CSF monoamine metabolite and plasma cortisol concentrations in young rhesus monkeys. Biol. Psychiatry. 1992;32:127–145. doi: 10.1016/0006-3223(92)90016-s. [DOI] [PubMed] [Google Scholar]
- Higley JD, Suomi SJ, Linnoila M. A nonhuman primate model of type II excessive alcohol consumption? Part 1. low cerebrospinal fluid 5-hydroxyindoleacetic acid concentrations and diminished social competence correlate with excessive alcohol consumption. Alcohol. Clin. Exp. Res. 1996;20:629–642. doi: 10.1111/j.1530-0277.1996.tb01665.x. [DOI] [PubMed] [Google Scholar]
- Hillmer AT, Tudorascu DL, Wooten DW, Lao PJ, Ahlers EO, Resch L, Converse AK, Moore CF, Schneider ML, Christian BT. Changes in the α4β2* nicotinic acetylcholine system during chronic controlled alcohol exposure in nonhuman primates. Drug Alcohol Depend. 2014;138:216–219. doi: 10.1016/j.drugalcdep.2014.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Henry S, Gallezot J-D, Ropchan J, Neumaier JF, Potenza MN, Sinha R, Krystal JH, Huang Y, Ding YS, Carson RE, Neumeister A. Serotonin 1B receptor imaging in alcohol dependence. Biol. Psychiatry. 2010;67:800–803. doi: 10.1016/j.biopsych.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichise M, Liow JS, Lu JQ, Takano A, Model K, Toyama H, Suhara T, Suzuki K, Innis RB, Carson RE. Linearized reference tissue parametric imaging methods: application to [C-11]DASB positron emission tomography studies of the serotonin transporter in human brain. J. Cereb. Blood Flow Metab. 2003;23:1096–1112. doi: 10.1097/01.WCB.0000085441.37552.CA. [DOI] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang SC, Ichise M, Iida H, Ito H, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J. Cereb. Blood Flow Metab. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Jans LAW, Riedel WJ, Markus CR, Blokland A. Serotonergic vulnerability and depression: assumptions, experimental evidence and implications. Mol. Psychiatr. 2007;12:522–543. doi: 10.1038/sj.mp.4001920. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister PR, Brady JM, Smith SM. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Johnson BA. Role of the serotonergic system in the neurobiology of alcoholism. CNS Drugs. 2004;18:1105–1118. doi: 10.2165/00023210-200418150-00005. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Zeeb FD, Winstanley CA. Contributions of serotonin in addiction vulnerability. Neuropharmacology. 2011;61:421–432. doi: 10.1016/j.neuropharm.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMarquand D, Pihl RO, Benkelfat C. Serotonin and alcohol intake, abuse, and dependence: findings of animal studies. Biol. Psychiatry. 1994;36:395–421. doi: 10.1016/0006-3223(94)91215-7. [DOI] [PubMed] [Google Scholar]
- Linnoila M, Virkkunen M, George T, Eckardt M, Higley JD, Nielsen D, Goldman D. Serotonin, violent behavior, and alcohol. Experientia. 1994;71:155–163. doi: 10.1007/978-3-0348-7330-7_16. [DOI] [PubMed] [Google Scholar]
- Mantere T, Tupala E, Hall H, Sarkioja T, Rasanen P, Bergstrom K, Callway J, Tiihonen J. Serotonin transporter distribution and density in the cerebral cortex of alcoholic and nonalcoholic comparison subjects: a whole-hemisphere autoradiography study. Am. J. Psychiatry. 2002;159:599–606. doi: 10.1176/appi.ajp.159.4.599. [DOI] [PubMed] [Google Scholar]
- Martinez D, Slifstein M, Gil R, Hwang D-R, Huang Y, Perez A, Frankle WG, Laruelle M, Krystal J, Abi-DArgham A. Positron emission tomography imaging of the serotonin transporter and 5-HT1A receptor in alcohol dependence. Biol. Psychiatry. 2009;65:175–180. doi: 10.1016/j.biopsych.2008.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride WJ, Guan XM, Chernet E, Lumeng L, Li TK. Regional serotonin1A receptors in the CNS of alcohol-preferring and –nonpreferring rats. Pharmacol. Biochem. Behav. 1994;49:7–12. doi: 10.1016/0091-3057(94)90449-9. [DOI] [PubMed] [Google Scholar]
- McLaren DG, Kosmatka KJ, Oakes TR, Kroenke CD, Kohama SG, Matochik JA, Ingram DK, Johnson SC. A population-average MRI-based atlas collection of the rhesus macaque. NeuroImage. 2009;45:52–59. doi: 10.1016/j.neuroimage.2008.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabiner EA, Messa C, Sargent PA, Husted Kjaer K, Montgomery A, Lawrence AD, Bench CJ, Gunn RN, Cowen P, Grasby PM. A database of [11C]WAY-100635 binding to 5-HT1A receptors in normal male volunteers: normative data and relationship to methodological, demographic, physiological, and behavioral variables. NeuroImage. 2002;15:620–632. doi: 10.1006/nimg.2001.0984. [DOI] [PubMed] [Google Scholar]
- Raymond JR, Muklin YV, Gettys TW, Garnovskaya MN. The recombinant 5-HT1A receptor: G protein coupling and signaling pathways. Br. J. Pharmacol. 1999;127:1751–1764. doi: 10.1038/sj.bjp.0702723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saigal N, Pichika R, Easwaramoothy B, Collins D, Christian BT, Shi B, Narayanan TK, Potkin SG, Mukherjee J. Synthesis and biologic evaluation of a novel serotonin 5-HT1A receptor ardioligand, F-18-labeled mefway, in rodents and imaging by PET in a nonhuman primate. J. Nucl. Med. 2006;47:1697–1706. [PubMed] [Google Scholar]
- Sari Y, Johnson VR, Weedman JM. Role of the serotonergic system in alcohol dependence: from animal models to clinics. Prog. Mol. Biol. Sransl. Sci. 2011;98:401–443. doi: 10.1016/B978-0-12-385506-0.00010-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider ML, Moore CF, Becker EF. Timing of moderate alcohol exposure during pregnancy and neonatal outcome in rhesus monkeys (Macaca mulatta) Alcohol. Clin. Exp. Res. 2001;25:1238–1245. [PubMed] [Google Scholar]
- Szabo Z, McCann UD, Wilson AA, Scheffel U, Owonikoko T, Matthews WB, Ravert HT, Hilton J, Dannals RF, Ricaurte GA. Comparison of (+)-11C McN5652 and 11C-DASB as serotonin transporter radioligands under various experimental conditions. J. Nucl. Med. 2002;43:678–692. [PMC free article] [PubMed] [Google Scholar]
- Szabo Z, Owonikoko T, Peyrot M, Varga J, Matthews WB, Ravert HT, Dannals RF, Wand G. Positron emission tomography imaging of the serotonin transporter in subjects with a history of alcoholism. Biol. Psychiatry. 2004;55:766–771. doi: 10.1016/j.biopsych.2003.11.023. [DOI] [PubMed] [Google Scholar]
- Tai C, Chatziioannou A, Siegel S, Young J, Newport D, Goble RN, Nutt RE, Cherry SR. Performance evaluation of the microPET P4: a PET system dedicated to animal imaging. Phys. Med. Biol. 2001;46:1845–1862. doi: 10.1088/0031-9155/46/7/308. [DOI] [PubMed] [Google Scholar]
- Thielen RH, Engleman EA, Rodd ZA, Murphy JM, Lumeng L, Li T-K, McBride WJ. Ethanol drinking and deprivation alter dopaminergic and serotonergic function in the nucleus accumbens of alcohol-preferring rats. J. Pharm. Exp. Ther. 2004;309:216–225. doi: 10.1124/jpet.103.059790. [DOI] [PubMed] [Google Scholar]
- Virkkunen M, Linnoila M. Brain serotonin, type II alcoholism and impulsive violence. J. Studies Alcohol Suppl. 1993;11:163–169. doi: 10.15288/jsas.1993.s11.163. [DOI] [PubMed] [Google Scholar]
- Vivian JA, Green HL, Young JE, Majersky LS, Thomas BW, Shively CA, Tobin JR, Nader MA, Grant KA. Induction and maintenance of ethanol self-administration in cynomolgus monkeys (Macaca fascicularis): long-term characterization of sex and individual differences. Alcohol. Clin. Exp. Res. 2001;25:1087–1097. [PubMed] [Google Scholar]
- Wooten DW, Moirano JM, Hillmer AT, Engle JW, DeJesus OJ, Murali D, Barnhart TE, Nickles RJ, Davidson RJ, Schneider ML, Mukherjee J, Christian BT. In vivo kinetics of [F-18]mefway: a comparison with [C-11]WAY100635 and [F-18]MPPF in the nonhuman primate. Synapse. 2011;65:592–600. doi: 10.1002/syn.20878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooten DW, Hillmer AT, Moirano JM, Ahlers EO, Slesarev M, Barnhart TE, Mukherjee J, Schneider ML, Christian BT. Measurement of 5-HT1A receptor density and in vivo binding parameters of [18F]Mefway in the nonhuman primate. J. Cereb. Blood Flow Metab. 2012;32:1546–1558. doi: 10.1038/jcbfm.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooten DW, Hillmer AT, Moirano JM, Tudorascu DL, Ahlers EO, Slesarev MS, Barnhart TE, Mukherjee J, Schneider ML, Christian BT. 5-HT1A sex based differences in Bmax, in vivo KD, and BPND in the nonhuman primate. NeuroImage. 2013;33:2512–2516. doi: 10.1016/j.neuroimage.2013.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]