Abstract
The lung is constantly exposed to a variety of inhaled foreign antigens, many of which are harmless to the body. Therefore, the mucosal immune system must not only have the capacity to distinguish self from non-self, but also harmless versus dangerous non-self. To address this, mucosal immune cells establish an anti-inflammatory steady state in the lung that must be overcome by inflammatory signals in order to mount an effector immune response. In the case of inhaled allergens, the false detection of dangerous non-self results in inappropriate immune activation and eventual allergic asthma. Both basic and clinical studies suggest that the balance between tolerogenic and inflammatory immune responses is a key feature in the outcome of health or disease. This Review is focused on what we term ‘regulatory tone’: the immunosuppressive environment in the lung that must be overcome to induce inflammatory responses. We will summarize the current literature on this topic, with a particular focus on the role of regulatory T cells in preventing allergic disease of the lung. We propose that inter-individual differences in regulatory tone have the potential to not only establish the threshold for immune activation in the lung, but also shape the quality of resulting effector responses following tolerance breakdown.
Keywords: Asthma, Regulatory T cells, Dendritic cells, Regulatory tone, Late-onset asthma
1. Introduction
The most prominent duty of the immune system is to remain tolerant to innocuous antigens while appropriately responding to those antigens with the potential to cause tissue destruction and/or death. In order to properly carry out this duty, the first line of immune education occurs in the thymus, where positive and negative selection of developing lymphocytes exposed to self antigens efficiently sets the signaling threshold required for T cell activation. This is due to the fact that cells responding too weakly or strongly to antigenic stimulation in the thymus are deleted (1). The second level of immune education occurs in the periphery, where the context in which antigens are encountered determines how a T cell will respond. Particularly in mucosal tissues like the lung, discrimination of self from non-self by T cells is not sufficient to dictate immune responsiveness, since harmless foreign antigens are inhaled constitutively, but rarely induce an inflammatory response. Twenty years ago Matzinger proposed the Danger model (2, 3), which moved away from strict self non-self discrimination to suggest that immune responsiveness is driven primarily by signals that antigen-presenting cells (APCs) receive from neighboring cells in the tissue in conjunction with antigen uptake. When the neighboring cells are healthy and do not communicate the presence of danger, APCs will promote immune unresponsiveness or suppression to a given antigen (4). However, when tissue damage has occurred in neighboring cells, the APCs receive endogenous ‘danger signals’ that result in APC maturation and antigen specific immune activation. Danger signals derived from dead or injured cells are termed damage-associated molecular patterns, or DAMPs (5). Together with the engagement of receptors for pattern-associated molecular patterns (PAMPs) (6, 7), these signals catalyze the maturation of APCs and activation of innate cells that can result in protective and/or pathogenic inflammatory responses in the tissue.
1.1 Threshold matters
The decision between immune tolerance versus activation in response to inhaled allergens is at the foundation of determining health versus asthmatic airway inflammation. In the steady state, the immune system tends toward induction of tolerance, possibly as a mechanism of defense against the continual barrage of innocuous foreign material to which it is exposed (8-10). Therefore, an inhaled allergen that will generate an inflammatory immune response must deliver sufficient signals to overcome a level of pre-existing tolerance. Engagement of PAMPs and DAMPs, in conjunction with allergen uptake, is one way to accomplish this goal. While it is well established that strong inflammatory signals are capable of overcoming steady state tolerance to induce an effector immune response, some outstanding questions remain. For example, can the size of the ‘hill’ of steady state tolerance be different from one individual to another? Can this factor alter the strength of stimulus required for generation of an effector immune response? We term this feature of the tissue as ‘regulatory tone’, and the concept is illustrated in Figure 1. For the remainder of this Review, we will highlight the literature regarding the role of dendritic cells and T regulatory cells (Tregs) in determining regulatory tone, and how this feature could potentially impact allergic immune responses in the lung.
Figure 1. Regulatory Tone Model.
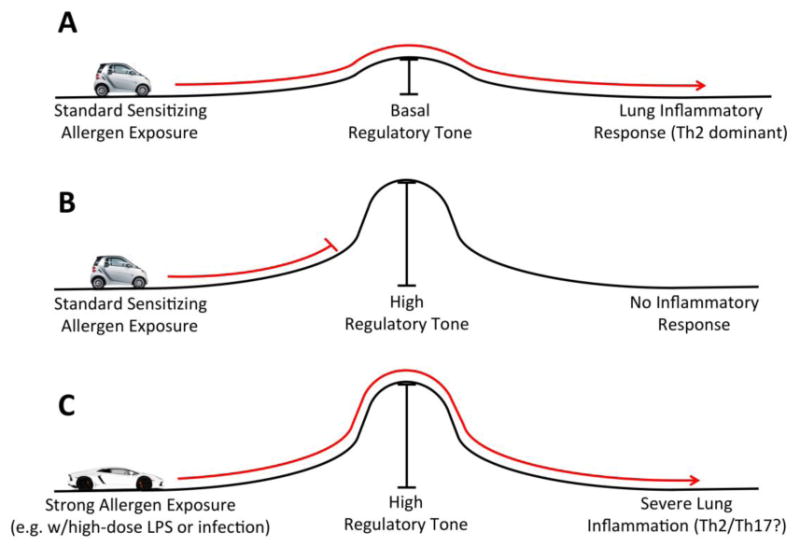
The outcome of allergen exposure depends on both properties of the allergen as well as airway regulatory tone. Under standard sensitizing conditions (e.g. low level allergen exposure), basal regulatory tone is overcome and Th2-type allergic inflammation results (A). Basal regulatory tone is dependent on IL-10 secreting tolerogenic DC and natural Treg. If previous encounter with allergen induced peripheral regulatory mechanisms (i.e. induced Tregs) that raised regulatory tone, then standard allergen exposure will not result in sensitization or allergic airway inflammation (B). However in the context of a strong inflammatory stimulus, allergen exposure will overcome even high levels of regulatory tone (C). In this case, the inflammatory milieu may promote qualitative differences in the allergic immune response compared to that seen in (A), resulting in potentially more severe allergen-specific airway immune responses. Example of strong inflammatory stimuli that could overcome high regulatory tone include those generated during airway infections, or inhalation of allergen together with strong adjuvants such as high doses of LPS or other danger signals. Independent of genetic factors, this is one possible explanation for the heterogeneity observed in asthma phenotypes.
2. Defining the hill of regulatory tone: dendritic cells and steady state surveillance
Like other mucosal tissues, the lung is under constant immune surveillance, and much study has been invested in understanding the mechanisms by which dendritic cells (DCs) take up antigen and migrate to lymph nodes to present it. With the ability to study experimental animals in specific pathogen-free conditions, it is possible to investigate the behavior of APCs and T cells during the steady state. Inhaled particles can be taken up by immature DCs in the sub-epithelial space (11). Once a DC takes up antigen, it migrates to the draining lymph node in a CCR7-dependent manner in order to engage lymph node T cells (12-15). Steinman and colleagues showed that immature DCs loaded with antigen in the absence of adjuvant or overt inflammation induce antigen-specific tolerance in vivo (16, 17). These DCs had low expression of MHC Class II and costimulatory molecules. In contrast, providing CD40 costimulation directly to these DCs caused the expansion of effector T cells (16), indicating that the presence of signal 2 (costimulation) on DCs was a critical factor controlling tolerance versus immunity. During the same time frame as Steinman's work, Umetsu and colleagues used a method of intranasal delivery of ovalbumin (OVA) in the absence of adjuvant to show that IL-10 production from antigen-loaded DCs was critical for induction of antigen-specific immune tolerance (18, 19). Interestingly, in their model the tolerance-inducing DCs expressed the same levels of CD40, CD80 and CD86 as DCs that promoted a strong Th2 response (18). This increase in costimulation compared to Steinman's approach may have been the result of choice of antigen, since the OVA used in these studies has been shown to have high endotoxin contamination (20). The addition of IL-4 or IL-12 during intranasal OVA immunization prevented the induction of tolerance (19). These results suggest, at least for the lung that the degree of DC costimulation is not the distinguishing feature dictating the type of immune response, but rather the specific cytokines and polarizing signals produced by the DC (21, 22). Further support for this hypothesis comes from studies showing CD28 expression is important for the generation and maintenance of peripheral Tregs (23-26). Therefore, expression of DC costimulatory molecules is important for basal T cell activation, but the resulting phenotype of the activated T cell is strongly influenced by the cytokines expressed and secreted by antigen-bearing DC.
DCs that express various levels of MHC Class II and costimulation, but primarily promote the activation and differentiation of Tregs rather than effector cell lineages, are termed ‘semi-mature’ or tolerogenic DC (27, 28). There are many experimental methods that have been used to generate tolerogenic DC, and they have been reviewed by Rutella, et. al. (28). However, the functional definition of a tolerogenic DC is one that causes the differentiation and expansion of antigen-specific peripherally induced Tregs (iTregs). In many cases, tolerogenic DCs promote iTreg formation through the secretion of IL-10 (22, 28-35), and IL-10 is also required to induce the tolerogenic phenotype in DCs (22, 28, 33, 36-38). In studies of TCR- transgenic RAG-deficient mice, where deficiency of natural, thymic-derived Tregs allows the direct study of iTreg induction in vivo, formation of iTreg cells was also shown to depend on TGF-β (39-41). iTregs are also critical for mucosal tolerance (40, 42), highlighting the importance of these cells in preventing mucosal sensitization to innocuous antigens. Since both human exposure to inhaled allergens and immune surveillance of the lung is continuous, the expectation from the above studies is that iTreg populations specific for various allergens would circulate in healthy, non-asthmatic subjects, while asthmatics would have circulating effector T cells specific for the same allergens. Indeed, published evidence suggests this result. Importantly, healthy non-allergic and allergic subjects both have circulating T cells that recognize the same allergen-derived peptides (43-45), suggesting that environmental allergen exposure induces an allergen-specific adaptive immune response in both healthy and asthmatic subjects. Akdis, et. al. went on to measure IL-4, IL-10 and IFN-γ in human peripheral blood CD4+ T cells specific for various immunodominant allergen epitopes. They found that healthy, non-asthmatic controls had a generally dominant IL-10 response to allergens, while asthmatic subjects had a switch to IL-4 dominance in response to the same allergens (46). They further showed that IL-10 secreting cells functionally suppressed allergen specific responses ex vivo, suggesting the iTregs in healthy subjects have the capacity to actively suppress allergen-specific effector responses (46). Taken together, these data suggest that regulatory tone is not the same for all individuals, and that proper steady state responses to allergens that result in the production of iTregs may effectively increase regulatory tone, thus further inhibiting future unwanted inflammatory responses to allergens.
3. Getting over the hill: signals that overcome regulatory tone
In recent years there has been an impressive expansion of new information regarding the innate inflammatory signals generated by PAMPs and DAMPs in tissue sites of injury or infection. Many of these innate signals converge to induce inflammasome activation and production of downstream cytokines. Current findings related to PAMPs, DAMPs and the inflammasome have been well covered in excellent recent reviews (5-7, 47). While many factors have been shown to act as adjuvants in the lung and cause allergic inflammation in naïve mice, in this Review we will focus specifically on a subset of cytokines and signals that have been shown to directly overcome or inhibit Tregs and induce allergic inflammation.
3.1 NFκB
The activation of NFκB in DCs and epithelial cells has been shown to be a key signal to induction of effector immune responses in the lung. For epithelial cells, active NFκB signaling has been shown in response to nitrogen dioxide, a major gaseous component of air pollution (48-50). In addition, Poynter and colleagues developed a mouse model of constitutive NFκB activation in airway epithelial cells (51). These mice developed a mixed Th2/Th17 type allergic response in the lung after OVA challenge, characterized by mucus metaplasia, IL-5, IL-13 and IL-17 production (51). Interestingly, these mice did not have increased expression of epithelial-derived cytokines IL-33, IL-25 or TSLP, suggesting multiple mechanisms exist by which epithelial cell activation can induce allergic inflammation in the lung (51, 52). For dendritic cells, NFκB activation is the classic signal that triggers production of IL-2, IFN-γ, IL-12 and other cytokines that induce effector T cell expansion and differentiation (53). In addition RelB, a component of the activating NFκB signaling complex, functions in both the canonical and non-canonical NFκB pathways, and is an important signal in the upregulation of the critical costimulatory receptor CD40 (54, 55). Recent work dissecting the intricate NFκB signaling module has shown that some components of this pathway are active in DCs in the steady state, and are required to actively promote DC immaturity during immune surveillance (56). For instance, in a mouse model of diabetes, NFκB1-/- DCs induce disease in the absence of adjuvant in a TNF-α dependent manner (56, 57). These data suggest NFκB1 is important in steady state maintenance of immature DC status, and that perturbation of NFκB1 signaling by activating signals is key to DC maturity.
3.2 OX40-OX40L
The TNF superfamily members OX40 (on T cells) and OX40L (on DCs) have been shown to be central costimulatory molecules driving Th2 allergic disease in the lung. OX40 expression can be driven by IL-4 and TSLP but not IFN-γ (58-60), and OX40 signaling results in decreased IL-10 production and increased Th2 priming (59, 61). Inhalation of lipopolysaccharide (LPS) in experimental animals was shown to induce OX40L expression on DCs that, in concert with TLR4 signals, promoted IL-4, IL-6 and IFN-γ production and subsequent decreased Treg development (62). Croft and colleagues studied the potency of OX40 signaling in mice that were first tolerized via intravenous injection of high dose peptide (63). Importantly, they found that injection of an OX40 agonist antibody broke peripheral tolerance (63), thus demonstrating the importance of OX40 signaling in activating Th2 responses. OX40 signaling has also been shown to activate NFκB (64, 65), which may be the signal that overcomes pre-existing immune suppression.
3.3 STAT6/IL-4
IL-4 induction via STAT6 is the classic signal inducing the differentiation of Th2 cells, the main cell type involved in the pathogenesis of asthma (66-68). However, some published data suggests an additional role for STAT6 and IL-4 signaling in directly inhibiting Tregs. In addition to the induction of OX40L mentioned above, TSLP signaling in DCs can induce STAT6 expression (34). STAT6-/- mice are reported to have an increase in Tregs (69), and STAT6 was further shown to suppress Tregs during allergic lung inflammation in vivo (70, 71). Ray and colleagues also showed an effect of IL-4 and STAT6 on Treg target cells, specifically that STAT6 caused T helper cells to be resistant to Treg-mediated suppression, thereby permitting the development of an adaptive immune response (72). Thus, while much work has highlighted the importance of IL-4 induced Th2 responses in promoting inflammation, it also appears that the same signaling pathway can have direct effects on alleviating Treg-mediated suppression.
3.4 IL-6
IL-6 is a pleiotropic cytokine first discovered as a secreted B cell factor that has been classically studied as an innate inflammatory cytokine released in the tissue in response to danger signals (73, 74). More recently, IL-6 has been shown to be an important mediator of the adaptive immune Th17 response (73, 75), a phenotype often present in severe, neutrophilic and/or steroid resistant asthma (76-80). In response to LPS exposure, TLR4 signaling blocked the suppressive effect of Tregs, and this effect was dependent on IL-6 secretion from DCs (81). This suggests IL-6 not only functions as a positive inflammatory mediator, but also as a direct inhibitor of tissue Tregs during sensitization. Later it was found that IL-6 diverts T cells to the Th17 lineage in large part by direct suppression of Foxp3+ cells (82-84). A similar effect of IL-6 on blockade of Treg expansion and IL-10 secretion was found in a model of double stranded RNA exposure (85), further supporting this model. For DCs, c-Kit expression has been shown to promote IL-6 secretion, which results in robust Th2 and Th17 type allergic airway inflammation (86). Strikingly, in a mouse model of autoimmune arthritis, a recent paper by Komatsu, et. al. reported a direct conversion of synovial Tregs into pathogenic Th17 cells (87). This conversion was dependent on IL-6. These data strongly suggest that IL-6 signaling represents an important mechanism in the direct inhibition of tolerance and promotion of inflammatory immune responses, with the potential to promote allergic lung inflammation.
4. Can increasing regulatory tone prevent allergic sensitization?
One of the unique features of many allergens that distinguish them from protein antigens is the ability to act as their own adjuvants. Allergens from dust mite, cockroach and others have protease-dependent and –independent effects when inhaled that can result in breakdown of epithelial junctions, direct engagement of toll-like receptors, and subsequent tissue inflammation (88). Therefore, it is possible that many ubiquitous allergen exposures carry with them an intrinsic ability to damage tissue and induce inflammation, thus placing a continuous demand on the suppression system of iTregs and tolerogenic DCs to inhibit unwanted sensitizing exposures in the lung. As illustrated in Figure 1A, if regulatory tone is not sufficient to inhibit allergen sensitization following exposure, then allergic airway inflammation can be the result. In contrast, if lung regulatory tone is boosted, allergen exposures that would typically result in allergic inflammation would not be predicted to generate a deleterious immune response (Figure 1B).
In a classic study, the Umetsu lab adapted a well-known immunologic method of inducing systemic tolerance with administration of high dose protein to the lung by giving chicken OVA repeatedly via the intranasal route (18, 19). When this protocol was performed on mice prior to systemic OVA/Alum administration, they were protected from allergic inflammation in an IL-10 dependent manner (18, 89). These studies established that, in the lung, increasing regulatory tone could result in prevention of allergic sensitization in response to an exposure regimen that is well documented to cause severe lung inflammation in naïve mice. In follow up studies, several groups have demonstrated that increasing regulatory tone in naïve mice by peptide or protein immunization, or even direct injection of tolerogenic DCs or Tregs, significantly suppress subsequent allergic inflammatory responses in the lung (90-96). Therefore, there is strong evidence that robust regulatory tone can prevent allergic sensitization.
One caveat in the above studies is that they all employed systemic intraperitoneal injection of model allergen and adjuvant as a means of inducing allergic sensitization. As a result, it is still unclear whether increasing regulatory tone can prevent allergic sensitization via the natural and physiologically relevant inhalation route. Much recent published work has demonstrated the unique role of airway epithelial cell-derived cytokines and lung innate lymphoid cells in promoting and shaping allergic responses (97, 98). In addition, the type and severity of lung inflammation present after allergen challenge are clearly affected by the route of llergen sensitization (99). To address these caveats, we used a mouse model of mucosal sensitization via the respiratory tract involving pre-exposure to either intranasal OVA (for the induction of iTregs) or PBS control. For mucosal sensitization we chose low-dose LPS as the adjuvant, since it has been shown to induce Th2-type allergic inflammation (100). Mucosal sensitization resulted in robust allergic airway inflammation in PBS treated mice, but mice pre-exposed to intranasal OVA were completely protected from the effects of sensitization (101). These findings directly showed that increasing lung regulatory tone could result in suppression of allergic airway inflammation from an inhaled allergen exposure, and further suggest the role of iTregs in this suppression.
5. Impact of regulatory tone on clinical outcomes in asthma
5.1 Allergen immunotherapy
While much of this Review has focused on the preventative function of iTregs, the therapeutic potential of iTregs in suppressing allergic responses is also highlighted by the benefits of allergen immunotherapy (AIT) in humans. This well-studied strategy of tolerance induction in allergic individuals through repeated administration of high-dose allergen suppresses immediate-type hypersensitivity, with compensatory increases in regulatory cells that promote disease suppression (102). This is a clear example of the important relationship between effector and regulatory cells responsive to allergen exposure. It also suggests the immune response to allergens can be shaped from an initial dominant inflammatory response that damages tissues to one characterized by ongoing immune suppression of the allergic response. In mouse studies, allergic immune responses established to OVA can be therapeutically suppressed by repeated administration of OVA in the absence of adjuvant (103-106). Repeated antigen exposure results in expansion of iTregs and localization of these cells to the airways (103-105). In depletion and re-constitution experiments, it has further been demonstrated that therapeutic suppression of the allergic effector response is dependent on Tregs (103-105, 107). Therefore, increasing regulatory tone against specific allergens may be an effective strategy to not only prevent the induction of allergic responses, but to also shift the immune balance in allergic asthma from inflammation to suppression.
5.2 Allergen exposure during infection: too much for regulatory tone to handle?
In the case of increased regulatory tone (Figure 1B), it is possible to prevent sensitization to allergen exposures co-administered with adjuvants that would otherwise promote classic Th2-type allergic inflammation. However, a clinically significant cohort of asthmatics has a more severe disease characterized by mixed eosinophil and neutrophil infiltrates and increased Th17 effector cells, resulting in a phenotype that is often refractory to standard corticosteroid therapy (78). In order to investigate the immune mechanisms behind this severe asthma phenotype, Kolls and colleagues linked Th17 responses to steroid-insensitive asthma in mice by demonstrating that polarized Th2 cells could be suppressed by dexamethasone treatment, while Th17 cells with the same specificity were resistant to dexamethasone suppression and amelioration of airway hyperreactivity in vivo (80). Interestingly, IL-6 is required for the development of Th17 responses, and also functions to directly inhibit the development of iTregs. In the model we described above (101), since Th2-promoting doses of LPS did not overcome regulatory tone, we administered high doses of LPS to simulate allergen exposure in the setting of robust lung inflammation (e.g. during bacterial infection). In these experiments, when we used high-dose LPS as adjuvant with OVA, tolerance broke down and resulted in a mixed Th2 and Th17 response with increased airway neutrophilia (101). When we measured cytokine secretion in the lung following low-dose (Th2) or high-dose (Th2/Th17) LPS exposure, IL-6 displayed the most prominent difference between the two, with an approximate 20-fold increase observed during breakdown of tolerance (101). The close relationship between Th17 and Treg cell development spurs a compelling argument for the unique ability of Th17 cells to effectively overcome regulatory tone and induce allergic inflammation. In addition to the dual role of IL-6 in suppressing iTreg formation and promoting Th17 differentiation, Tregs express the high affinity IL-2 receptor and have been shown to promote Th17 formation through the consumption of IL-2 (108, 109). Th17 cell development is boosted in the absence of IL-2 signaling (110). Furthermore, a high IL-6, Th17-promoting inflammatory milieu may even promote the direct conversion of Tregs to inflammatory Th17 cells (87), thus promoting deleterious immune responses through the employment of ‘turncoat’ Tregs. Taken together, these data suggest that while standard approaches to inducing Th2-dominant allergic asthma can be inhibited by increasing regulatory tone, allergen exposure in the context of robust pathogen-induced inflammation preferentially generates Th17 responses when regulatory tone is overcome. We speculate that these Th17 (or mixed Th2/Th17) responses may be the trigger to turning the tables on suppression for the induction of severe asthma.
Allergen exposure during infection may not only induce qualitative differences in the immune response, but could also boost the effective immune exposure to allergen through disruption of epithelial barrier. Recent published work has demonstrated that viral infection induces significant breakdown of the tight junctions between epithelial cells, which normally restrict macromolecule access to the sub-epithelial space (111). Administration of the TLR3 ligand poly i:c, or direct infection of epithelial cells with RSV results in increased epithelial permeability to macromolecules (112, 113). In addition, more severe inflammation resulting from infection with lytic viruses like influenza can break down physical barriers in the lung through epithelial cell death (114). Administration of fluorescent protein to the lung following infection with RSV in the same site resulted in increased uptake of the protein by lung DCs compared to control (unpublished observations), suggesting that virus-induced changes in airway permeability influence the ‘effective dose’ of inhaled exposure to antigen. Exactly how perturbed epithelial barrier function affects mucosal immune responses in the setting of airway infections remains to be determined, but this may be a key feature to determining both the size and quality of the immune response to an inhaled allergen.
5.3 Potential implications for severe late-onset asthma
In healthy non-asthmatic adults, ubiquitous allergen exposures can result in the formation of allergen-specific iTregs, increased regulatory tone, and therefore increased defense against future allergen exposures. In contrast, late-onset asthmatics often have features of severe asthma (115-117). The incidence rate and low remission rate of late-onset asthma indicates the importance of this understudied sub-population of asthmatics (115, 118, 119). Also, the unique features of late-onset asthma compared to childhood-onset suggest distinct immune mechanisms of sensitization likely exist (116, 120-122). It is possible that late-onset asthmatics are sensitized to new allergens not previously encountered in life, for example as in occupational asthma. However, it is also possible that asthma is induced to allergens already encountered and properly dealt with immunologically in the past, if regulatory tone is overcome by strong inflammatory stimuli. Unfortunately, to our knowledge there has not been a prospective clinical study that measures the immune response to common allergens both before and after the manifestation of asthma. If our model is correct, then a significant proportion of late-onset asthma could result from sensitizing allergen exposures in individuals that once made the appropriate suppressive response to the same allergen. In this case, we predict that a strong inflammatory stimulus (such as pulmonary infection) is necessary to provide an environment that overcomes regulatory tone and fosters allergen sensitization (Figure 1C). If Th17-type features are associated with these responses, then we predict that this would contribute to severe asthma in late-onset patients. Indeed, pre-existing tolerance has been shown to enhance allergic Th17 responses in a model of allergic asthma in vivo (101). It will be informative to perform future clinical studies that determine the unique immunologic features of late-onset asthma, thus opening the door for much-needed targeted therapies.
6. Conclusions
It is clear that steady state immune surveillance and thymic-derived natural Tregs, as well as tolerogenic responses that induce iTregs, are critical for the prevention of unwanted inflammatory responses to inhaled allergens. We collectively call this regulatory tone, and highlight the importance of regulatory tone in shaping the immune response to allergen. With this in mind, in the absence of pre-existing genetic factors or immune deficiency, the inflammatory responses with the potential to overcome prevailing immune suppression seem likely to activate a coordinate pathway of cytokine and costimulatory receptor expression that directly antagonize formation of iTregs. We propose that IL-6 and Th17 responses may be important players in this process. Pathogen infection, or co-factors such as smoking (123) and particulate matter exposure (124) that increase IL-6 production, may promote the environment required to overcome regulatory tone and induce pathologic allergic inflammation. Interestingly, while human IL-6 receptor monoclonal antibody (called tocilizumab) administration has proven efficacy in arthritis and has been tested in several other disease states (125), to our knowledge this therapy has not been tested in patients with severe neutrophilic asthma. Based on our findings and the literature reviewed here, we propose tocilizumab therapy may be a fruitful treatment for this sub-population of asthmatics. Furthermore, while AIT is a promising therapeutic strategy for treating asthma, it is interesting to also consider preventative strategies that increase regulatory tone to allergens in healthy non-asthmatics. However, the possibility exists that immune cells that promote regulatory tone could also amplify inflammatory responses if regulatory tone is overcome. Further work focused on understanding the mechanisms that establish and overcome regulatory tone in the lung will be of benefit to instructing effective immunotherapy for asthma.
Highlights.
We provide a review of the concept of “regulatory tone” in asthma.
This is comprised of tolerogenic dendritic cells and regulatory T cells.
Prior encounter with allergen can raise regulatory tone in the airway.
Regulatory tone determines the outcome of allergen inhalation.
This model may explain heterogeneity in immune responses to ubiquitous allergens.
Acknowledgments
Funding sources: NIH R01 HL071933, ES01247, and P30 ES001247 to SNG; NIH F32 HL110718-01 to TJC
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moran AE, Hogquist KA. T-cell receptor affinity in thymic development. Immunology. 2012;135:261–267. doi: 10.1111/j.1365-2567.2011.03547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matzinger P. Essay 1: The danger model in its historical context. Scandinavian journal of immunology. 2001;54:4–9. doi: 10.1046/j.1365-3083.2001.00974.x. [DOI] [PubMed] [Google Scholar]
- 3.Matzinger P. The danger model: A renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 4.Steinman RM. Decisions about dendritic cells: Past, present, and future. Annual review of immunology. 2012;30:1–22. doi: 10.1146/annurev-immunol-100311-102839. [DOI] [PubMed] [Google Scholar]
- 5.Zelenay S, Reis e Sousa C. Adaptive immunity after cell death. Trends Immunol. 2013;34:329–335. doi: 10.1016/j.it.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 6.Broz P, Monack DM. Newly described pattern recognition receptors team up against intracellular pathogens. Nature reviews Immunology. 2013;13:551–565. doi: 10.1038/nri3479. [DOI] [PubMed] [Google Scholar]
- 7.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 8.Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor dec-205 in the steady state leads to antigen presentation on major histocompatibility complex class i products and peripheral cd8+ t cell tolerance. J Exp Med. 2002;196:1627–1638. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohnmacht C, Pullner A, King SB, Drexler I, Meier S, Brocker T, Voehringer D. Constitutive ablation of dendritic cells breaks self-tolerance of cd4 t cells and results in spontaneous fatal autoimmunity. J Exp Med. 2009;206:549–559. doi: 10.1084/jem.20082394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steinman RM, Nussenzweig MC. Avoiding horror autotoxicus: The importance of dendritic cells in peripheral t cell tolerance. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:351–358. doi: 10.1073/pnas.231606698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wikstrom ME, Stumbles PA. Mouse respiratory tract dendritic cell subsets and the immunological fate of inhaled antigens. Immunol Cell Biol. 2007;85:182–188. doi: 10.1038/sj.icb.7100039. [DOI] [PubMed] [Google Scholar]
- 12.del Rio ML, Rodriguez-Barbosa JI, Kremmer E, Forster R. Cd103- and cd103+ bronchial lymph node dendritic cells are specialized in presenting and cross-presenting innocuous antigen to cd4+ and cd8+ t cells. J Immunol. 2007;178:6861–6866. doi: 10.4049/jimmunol.178.11.6861. [DOI] [PubMed] [Google Scholar]
- 13.Hammad H, Lambrecht BN, Pochard P, Gosset P, Marquillies P, Tonnel AB, Pestel J. Monocyte-derived dendritic cells induce a house dust mite-specific th2 allergic inflammation in the lung of humanized scid mice: Involvement of ccr7. J Immunol. 2002;169:1524–1534. doi: 10.4049/jimmunol.169.3.1524. [DOI] [PubMed] [Google Scholar]
- 14.Hintzen G, Ohl L, del Rio ML, Rodriguez-Barbosa JI, Pabst O, Kocks JR, Krege J, Hardtke S, Forster R. Induction of tolerance to innocuous inhaled antigen relies on a ccr7-dependent dendritic cell-mediated antigen transport to the bronchial lymph node. J Immunol. 2006;177:7346–7354. doi: 10.4049/jimmunol.177.10.7346. [DOI] [PubMed] [Google Scholar]
- 15.Ohl L, Mohaupt M, Czeloth N, Hintzen G, Kiafard Z, Zwirner J, Blankenstein T, Henning G, Forster R. Ccr7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity. 2004;21:279–288. doi: 10.1016/j.immuni.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 16.Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M, Ravetch JV, Steinman RM, Nussenzweig MC. Dendritic cells induce peripheral t cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194:769–779. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annual review of immunology. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 18.Akbari O, DeKruyff RH, Umetsu DT. Pulmonary dendritic cells producing il-10 mediate tolerance induced by respiratory exposure to antigen. Nat Immunol. 2001;2:725–731. doi: 10.1038/90667. [DOI] [PubMed] [Google Scholar]
- 19.Tsitoura DC, Blumenthal RL, Berry G, Dekruyff RH, Umetsu DT. Mechanisms preventing allergen-induced airways hyperreactivity: Role of tolerance and immune deviation. J Allergy Clin Immunol. 2000;106:239–246. doi: 10.1067/mai.2000.108429. [DOI] [PubMed] [Google Scholar]
- 20.Peters M, Dudziak K, Stiehm M, Bufe A. T-cell polarization depends on concentration of the danger signal used to activate dendritic cells. Immunol Cell Biol. 2010;88:537–544. doi: 10.1038/icb.2010.3. [DOI] [PubMed] [Google Scholar]
- 21.Reis e Sousa C. Dendritic cells in a mature age. Nature reviews Immunology. 2006;6:476–483. doi: 10.1038/nri1845. [DOI] [PubMed] [Google Scholar]
- 22.Saei A, Hadjati J. Tolerogenic dendritic cells: Key regulators of peripheral tolerance in health and disease. Int Arch Allergy Immunol. 2013;161:293–303. doi: 10.1159/000350328. [DOI] [PubMed] [Google Scholar]
- 23.Bour-Jordan H, Esensten JH, Martinez-Llordella M, Penaranda C, Stumpf M, Bluestone JA. Intrinsic and extrinsic control of peripheral t-cell tolerance by costimulatory molecules of the cd28/ b7 family. Immunological reviews. 2011;241:180–205. doi: 10.1111/j.1600-065X.2011.01011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA. B7/cd28 costimulation is essential for the homeostasis of the cd4+cd25+ immunoregulatory t cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 25.Sansom DM, Walker LS. The role of cd28 and cytotoxic t-lymphocyte antigen-4 (ctla-4) in regulatory t-cell biology. Immunological reviews. 2006;212:131–148. doi: 10.1111/j.0105-2896.2006.00419.x. [DOI] [PubMed] [Google Scholar]
- 26.Tang Q, Henriksen KJ, Boden EK, Tooley AJ, Ye J, Subudhi SK, Zheng XX, Strom TB, Bluestone JA. Cutting edge: Cd28 controls peripheral homeostasis of cd4+cd25+ regulatory t cells. J Immunol. 2003;171:3348–3352. doi: 10.4049/jimmunol.171.7.3348. [DOI] [PubMed] [Google Scholar]
- 27.Lutz MB, Schuler G. Immature, semi-mature and fully mature dendritic cells: Which signals induce tolerance or immunity? Trends Immunol. 2002;23:445–449. doi: 10.1016/s1471-4906(02)02281-0. [DOI] [PubMed] [Google Scholar]
- 28.Rutella S, Danese S, Leone G. Tolerogenic dendritic cells: Cytokine modulation comes of age. Blood. 2006;108:1435–1440. doi: 10.1182/blood-2006-03-006403. [DOI] [PubMed] [Google Scholar]
- 29.Bimczok D, Rau H, Wundrack N, Naumann M, Rothkotter HJ, McCullough K, Summerfield A. Cholera toxin promotes the generation of semi-mature porcine monocyte-derived dendritic cells that are unable to stimulate t cells. Veterinary research. 2007;38:597–612. doi: 10.1051/vetres:2007020. [DOI] [PubMed] [Google Scholar]
- 30.Dillon S, Agrawal S, Banerjee K, Letterio J, Denning TL, Oswald-Richter K, Kasprowicz DJ, Kellar K, Pare J, van Dyke T, Ziegler S, Unutmaz D, Pulendran B. Yeast zymosan, a stimulus for tlr2 and dectin-1, induces regulatory antigen-presenting cells and immunological tolerance. J Clin Invest. 2006;116:916–928. doi: 10.1172/JCI27203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGuirk P, McCann C, Mills KH. Pathogen-specific t regulatory 1 cells induced in the respiratory tract by a bacterial molecule that stimulates interleukin 10 production by dendritic cells: A novel strategy for evasion of protective t helper type 1 responses by bordetella pertussis. J Exp Med. 2002;195:221–231. doi: 10.1084/jem.20011288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Munn DH, Sharma MD, Lee JR, Jhaver KG, Johnson TS, Keskin DB, Marshall B, Chandler P, Antonia SJ, Burgess R, Slingluff CL, Jr, Mellor AL. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science. 2002;297:1867–1870. doi: 10.1126/science.1073514. [DOI] [PubMed] [Google Scholar]
- 33.Pacciani V, Gregori S, Chini L, Corrente S, Chianca M, Moschese V, Rossi P, Roncarolo MG, Angelini F. Induction of anergic allergen-specific suppressor t cells using tolerogenic dendritic cells derived from children with allergies to house dust mites. J Allergy Clin Immunol. 2010;125:727–736. doi: 10.1016/j.jaci.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 34.Pulendran B, Tang H, Manicassamy S. Programming dendritic cells to induce t(h)2 and tolerogenic responses. Nat Immunol. 2010;11:647–655. doi: 10.1038/ni.1894. [DOI] [PubMed] [Google Scholar]
- 35.Rutella S, Bonanno G, Pierelli L, Mariotti A, Capoluongo E, Contemi AM, Ameglio F, Curti A, De Ritis DG, Voso MT, Perillo A, Mancuso S, Scambia G, Lemoli RM, Leone G. Granulocyte colony-stimulating factor promotes the generation of regulatory dc through induction of il-10 and ifn-alpha. European journal of immunology. 2004;34:1291–1302. doi: 10.1002/eji.200324651. [DOI] [PubMed] [Google Scholar]
- 36.Boks MA, Kager-Groenland JR, Haasjes MS, Zwaginga JJ, van Ham SM, ten Brinke A. Il-10-generated tolerogenic dendritic cells are optimal for functional regulatory t cell induction--a comparative study of human clinical-applicable dc. Clin Immunol. 2012;142:332–342. doi: 10.1016/j.clim.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 37.Sato K, Yamashita N, Baba M, Matsuyama T. Modified myeloid dendritic cells act as regulatory dendritic cells to induce anergic and regulatory t cells. Blood. 2003;101:3581–3589. doi: 10.1182/blood-2002-09-2712. [DOI] [PubMed] [Google Scholar]
- 38.Wakkach A, Fournier N, Brun V, Breittmayer JP, Cottrez F, Groux H. Characterization of dendritic cells that induce tolerance and t regulatory 1 cell differentiation in vivo. Immunity. 2003;18:605–617. doi: 10.1016/s1074-7613(03)00113-4. [DOI] [PubMed] [Google Scholar]
- 39.Cobbold SP, Castejon R, Adams E, Zelenika D, Graca L, Humm S, Waldmann H. Induction of foxp3+ regulatory t cells in the periphery of t cell receptor transgenic mice tolerized to transplants. J Immunol. 2004;172:6003–6010. doi: 10.4049/jimmunol.172.10.6003. [DOI] [PubMed] [Google Scholar]
- 40.Curotto de Lafaille MA, Lafaille JJ, Graca L. Mechanisms of tolerance and allergic sensitization in the airways and the lungs. Curr Opin Immunol. 2010;22:616–622. doi: 10.1016/j.coi.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mucida D, Kutchukhidze N, Erazo A, Russo M, Lafaille JJ, Curotto de Lafaille MA. Oral tolerance in the absence of naturally occurring tregs. J Clin Invest. 2005;115:1923–1933. doi: 10.1172/JCI24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Curotto de Lafaille MA, Kutchukhidze N, Shen S, Ding Y, Yee H, Lafaille JJ. Adaptive foxp3+ regulatory t cell-dependent and -independent control of allergic inflammation. Immunity. 2008;29:114–126. doi: 10.1016/j.immuni.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 43.Carballido JM, Carballido-Perrig N, Terres G, Heusser CH, Blaser K. Bee venom phospholipase a2-specific t cell clones from human allergic and non-allergic individuals: Cytokine patterns change in response to the antigen concentration. European journal of immunology. 1992;22:1357–1363. doi: 10.1002/eji.1830220605. [DOI] [PubMed] [Google Scholar]
- 44.Ebner C, Schenk S, Najafian N, Siemann U, Steiner R, Fischer GW, Hoffmann K, Szepfalusi Z, Scheiner O, Kraft D. Nonallergic individuals recognize the same t cell epitopes of bet v 1, the major birch pollen allergen, as atopic patients. J Immunol. 1995;154:1932–1940. [PubMed] [Google Scholar]
- 45.Ebner C, Siemann U, Najafian N, Scheiner O, Kraft D. Characterization of allergen (bet v 1)-specific t cell lines and clones from non-allergic individuals. Int Arch Allergy Immunol. 1995;107:183–185. doi: 10.1159/000236971. [DOI] [PubMed] [Google Scholar]
- 46.Akdis M, Verhagen J, Taylor A, Karamloo F, Karagiannidis C, Crameri R, Thunberg S, Deniz G, Valenta R, Fiebig H, Kegel C, Disch R, Schmidt-Weber CB, Blaser K, Akdis CA. Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific t regulatory 1 and t helper 2 cells. J Exp Med. 2004;199:1567–1575. doi: 10.1084/jem.20032058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nature reviews Immunology. 2013;13:397–411. doi: 10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bevelander M, Mayette J, Whittaker LA, Paveglio SA, Jones CC, Robbins J, Hemenway D, Akira S, Uematsu S, Poynter ME. Nitrogen dioxide promotes allergic sensitization to inhaled antigen. J Immunol. 2007;179:3680–3688. doi: 10.4049/jimmunol.179.6.3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krishna MT, Chauhan AJ, Frew AJ, Holgate ST. Toxicological mechanisms underlying oxidant pollutant-induced airway injury. Reviews on environmental health. 1998;13:59–71. [PubMed] [Google Scholar]
- 50.Shima M, Adachi M. Effect of outdoor and indoor nitrogen dioxide on respiratory symptoms in schoolchildren. International journal of epidemiology. 2000;29:862–870. doi: 10.1093/ije/29.5.862. [DOI] [PubMed] [Google Scholar]
- 51.Ather JL, Hodgkins SR, Janssen-Heininger YM, Poynter ME. Airway epithelial nf-kappab activation promotes allergic sensitization to an innocuous inhaled antigen. Am J Respir Cell Mol Biol. 2011;44:631–638. doi: 10.1165/rcmb.2010-0106OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Poynter ME. Airway epithelial regulation of allergic sensitization in asthma. Pulm Pharmacol Ther. 2012;25:438–446. doi: 10.1016/j.pupt.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hayden MS, Ghosh S. Nf-kappab in immunobiology. Cell research. 2011;21:223–244. doi: 10.1038/cr.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martin E, O'Sullivan B, Low P, Thomas R. Antigen-specific suppression of a primed immune response by dendritic cells mediated by regulatory t cells secreting interleukin-10. Immunity. 2003;18:155–167. doi: 10.1016/s1074-7613(02)00503-4. [DOI] [PubMed] [Google Scholar]
- 55.Shih VF, Davis-Turak J, Macal M, Huang JQ, Ponomarenko J, Kearns JD, Yu T, Fagerlund R, Asagiri M, Zuniga EI, Hoffmann A. Control of relb during dendritic cell activation integrates canonical and noncanonical nf-kappab pathways. Nat Immunol. 2012;13:1162–1170. doi: 10.1038/ni.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Johnson DJ, Ohashi PS. Molecular programming of steady-state dendritic cells: Impact on autoimmunity and tumor immune surveillance. Annals of the New York Academy of Sciences. 2013;1284:46–51. doi: 10.1111/nyas.12114. [DOI] [PubMed] [Google Scholar]
- 57.Dissanayake D, Hall H, Berg-Brown N, Elford AR, Hamilton SR, Murakami K, Deluca LS, Gommerman JL, Ohashi PS. Nuclear factor-kappab1 controls the functional maturation of dendritic cells and prevents the activation of autoreactive t cells. Nat Med. 2011;17:1663–1667. doi: 10.1038/nm.2556. [DOI] [PubMed] [Google Scholar]
- 58.Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, Watanabe N, Qin FX, Yao Z, Cao W, Liu YJ. Tslp-activated dendritic cells induce an inflammatory t helper type 2 cell response through ox40 ligand. J Exp Med. 2005;202:1213–1223. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu YJ. Thymic stromal lymphopoietin and ox40 ligand pathway in the initiation of dendritic cell-mediated allergic inflammation. J Allergy Clin Immunol. 2007;120:238–244. doi: 10.1016/j.jaci.2007.06.004. quiz 245-236. [DOI] [PubMed] [Google Scholar]
- 60.Roos A, Schilder-Tol EJ, Weening JJ, Aten J. Strong expression of cd134 (ox40), a member of the tnf receptor family, in a t helper 2-type cytokine environment. Journal of leukocyte biology. 1998;64:503–510. doi: 10.1002/jlb.64.4.503. [DOI] [PubMed] [Google Scholar]
- 61.Ito T, Wang YH, Duramad O, Hanabuchi S, Perng OA, Gilliet M, Qin FX, Liu YJ. Ox40 ligand shuts down il-10-producing regulatory t cells. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:13138–13143. doi: 10.1073/pnas.0603107103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Duan W, So T, Croft M. Antagonism of airway tolerance by endotoxin/lipopolysaccharide through promoting ox40l and suppressing antigen-specific foxp3+ t regulatory cells. J Immunol. 2008;181:8650–8659. doi: 10.4049/jimmunol.181.12.8650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bansal-Pakala P, Jember AG, Croft M. Signaling through ox40 (cd134) breaks peripheral t-cell tolerance. Nat Med. 2001;7:907–912. doi: 10.1038/90942. [DOI] [PubMed] [Google Scholar]
- 64.Arch RH, Thompson CB. 4-1bb and ox40 are members of a tumor necrosis factor (tnf)-nerve growth factor receptor subfamily that bind tnf receptor-associated factors and activate nuclear factor kappab. Molecular and cellular biology. 1998;18:558–565. doi: 10.1128/mcb.18.1.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kawamata S, Hori T, Imura A, Takaori-Kondo A, Uchiyama T. Activation of ox40 signal transduction pathways leads to tumor necrosis factor receptor-associated factor (traf) 2- and traf5-mediated nf-kappab activation. The Journal of biological chemistry. 1998;273:5808–5814. doi: 10.1074/jbc.273.10.5808. [DOI] [PubMed] [Google Scholar]
- 66.Kaplan MH, Schindler U, Smiley ST, Grusby MJ. Stat6 is required for mediating responses to il-4 and for development of th2 cells. Immunity. 1996;4:313–319. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- 67.Shimoda K, van Deursen J, Sangster MY, Sarawar SR, Carson RT, Tripp RA, Chu C, Quelle FW, Nosaka T, Vignali DA, Doherty PC, Grosveld G, Paul WE, Ihle JN. Lack of il-4-induced th2 response and ige class switching in mice with disrupted stat6 gene. Nature. 1996;380:630–633. doi: 10.1038/380630a0. [DOI] [PubMed] [Google Scholar]
- 68.Takeda K, Tanaka T, Shi W, Matsumoto M, Minami M, Kashiwamura S, Nakanishi K, Yoshida N, Kishimoto T, Akira S. Essential role of stat6 in il-4 signalling. Nature. 1996;380:627–630. doi: 10.1038/380627a0. [DOI] [PubMed] [Google Scholar]
- 69.Chapoval SP, Dasgupta P, Smith EP, DeTolla LJ, Lipsky MM, Kelly-Welch AE, Keegan AD. Stat6 expression in multiple cell types mediates the cooperative development of allergic airway disease. J Immunol. 2011;186:2571–2583. doi: 10.4049/jimmunol.1002567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chapoval S, Dasgupta P, Dorsey NJ, Keegan AD. Regulation of the t helper cell type 2 (th2)/t regulatory cell (treg) balance by il-4 and stat6. Journal of leukocyte biology. 2010;87:1011–1018. doi: 10.1189/jlb.1209772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dorsey NJ, Chapoval SP, Smith EP, Skupsky J, Scott DW, Keegan AD. Stat6 controls the number of regulatory t cells in vivo, thereby regulating allergic lung inflammation. J Immunol. 2013;191:1517–1528. doi: 10.4049/jimmunol.1300486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pillemer BB, Qi Z, Melgert B, Oriss TB, Ray P, Ray A. Stat6 activation confers upon t helper cells resistance to suppression by regulatory t cells. J Immunol. 2009;183:155–163. doi: 10.4049/jimmunol.0803733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dienz O, Rincon M. The effects of il-6 on cd4 t cell responses. Clin Immunol. 2009;130:27–33. doi: 10.1016/j.clim.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hirano T, Yasukawa K, Harada H, Taga T, Watanabe Y, Matsuda T, Kashiwamura S, Nakajima K, Koyama K, Iwamatsu A, et al. Complementary DNA for a novel human interleukin (bsf-2) that induces b lymphocytes to produce immunoglobulin. Nature. 1986;324:73–76. doi: 10.1038/324073a0. [DOI] [PubMed] [Google Scholar]
- 75.Weaver CT, Hatton RD, Mangan PR, Harrington LE. Il-17 family cytokines and the expanding diversity of effector t cell lineages. Annual review of immunology. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 76.Al-Ramli W, Prefontaine D, Chouiali F, Martin JG, Olivenstein R, Lemiere C, Hamid Q. T(h)17-associated cytokines (il-17a and il-17f) in severe asthma. J Allergy Clin Immunol. 2009;123:1185–1187. doi: 10.1016/j.jaci.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 77.Chakir J, Shannon J, Molet S, Fukakusa M, Elias J, Laviolette M, Boulet LP, Hamid Q. Airway remodeling-associated mediators in moderate to severe asthma: Effect of steroids on tgf-beta, il-11, il-17, and type i and type iii collagen expression. J Allergy Clin Immunol. 2003;111:1293–1298. doi: 10.1067/mai.2003.1557. [DOI] [PubMed] [Google Scholar]
- 78.Lloyd CM, Hessel EM. Functions of t cells in asthma: More than just t(h)2 cells. Nature reviews Immunology. 2010;10:838–848. doi: 10.1038/nri2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Molet S, Hamid Q, Davoine F, Nutku E, Taha R, Page N, Olivenstein R, Elias J, Chakir J. Il-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J Allergy Clin Immunol. 2001;108:430–438. doi: 10.1067/mai.2001.117929. [DOI] [PubMed] [Google Scholar]
- 80.McKinley L, Alcorn JF, Peterson A, Dupont RB, Kapadia S, Logar A, Henry A, Irvin CG, Piganelli JD, Ray A, Kolls JK. Th17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J Immunol. 2008;181:4089–4097. doi: 10.4049/jimmunol.181.6.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pasare C, Medzhitov R. Toll pathway-dependent blockade of cd4+cd25+ t cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 82.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector th17 and regulatory t cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 83.Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the t(h)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 84.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. Tgfbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of il-17-producing t cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 85.Matsumoto K, Asai Y, Fukuyama S, Kan OK, Matsunaga Y, Noda N, Kitajima H, Tanaka K, Nakanishi Y, Inoue H. Il-6 induced by double-stranded rna augments allergic inflammation via suppression of foxp3+ t-cell/il-10 axis. Am J Respir Cell Mol Biol. 2012;46:740–747. doi: 10.1165/rcmb.2010-0479OC. [DOI] [PubMed] [Google Scholar]
- 86.Krishnamoorthy N, Oriss TB, Paglia M, Fei M, Yarlagadda M, Vanhaesebroeck B, Ray A, Ray P. Activation of c-kit in dendritic cells regulates t helper cell differentiation and allergic asthma. Nat Med. 2008;14:565–573. doi: 10.1038/nm1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Komatsu N, Okamoto K, Sawa S, Nakashima T, Oh-hora M, Kodama T, Tanaka S, Bluestone JA, Takayanagi H. Pathogenic conversion of foxp3+ t cells into th17 cells in autoimmune arthritis. Nat Med. 2014;20:62–68. doi: 10.1038/nm.3432. [DOI] [PubMed] [Google Scholar]
- 88.Georas SN, Rezaee F, Lerner L, Beck L. Dangerous allergens: Why some allergens are bad actors. Current allergy and asthma reports. 2010;10:92–98. doi: 10.1007/s11882-010-0090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Akbari O, Freeman GJ, Meyer EH, Greenfield EA, Chang TT, Sharpe AH, Berry G, DeKruyff RH, Umetsu DT. Antigen-specific regulatory t cells develop via the icos-icos-ligand pathway and inhibit allergen-induced airway hyperreactivity. Nat Med. 2002;8:1024–1032. doi: 10.1038/nm745. [DOI] [PubMed] [Google Scholar]
- 90.Fear VS, Burchell JT, Lai SP, Wikstrom ME, Blank F, von Garnier C, Turner DJ, Sly PD, Holt PG, Strickland DS, Stumbles PA. Restricted aeroallergen access to airway mucosal dendritic cells in vivo limits allergen-specific cd4+ t cell proliferation during the induction of inhalation tolerance. J Immunol. 2011;187:4561–4570. doi: 10.4049/jimmunol.1004189. [DOI] [PubMed] [Google Scholar]
- 91.Fujita S, Yamashita N, Ishii Y, Sato Y, Sato K, Eizumi K, Fukaya T, Nozawa R, Takamoto Y, Taniguchi M. Regulatory dendritic cells protect against allergic airway inflammation in a murine asthmatic model. J Allergy Clin Immunol. 2008;121:95–104. e107. doi: 10.1016/j.jaci.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 92.Hall G, Houghton CG, Rahbek JU, Lamb JR, Jarman ER. Suppression of allergen reactive th2 mediated responses and pulmonary eosinophilia by intranasal administration of an immunodominant peptide is linked to il-10 production. Vaccine. 2003;21:549–561. doi: 10.1016/s0264-410x(02)00394-8. [DOI] [PubMed] [Google Scholar]
- 93.Lee SM, Batzer G, Ng N, Lam D, Pattar SS, Patel ND, Horner AA. Regulatory t cells contribute to allergen tolerance induced by daily airway immunostimulant exposures. Am J Respir Cell Mol Biol. 2011;44:341–349. doi: 10.1165/rcmb.2010-0001OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Marazuela EG, Rodriguez R, Fernandez-Garcia H, Garcia MS, Villalba M, Batanero E. Intranasal immunization with a dominant t-cell epitope peptide of a major allergen of olive pollen prevents mice from sensitization to the whole allergen. Molecular immunology. 2008;45:438–445. doi: 10.1016/j.molimm.2007.05.030. [DOI] [PubMed] [Google Scholar]
- 95.Torres-Aguilar H, Aguilar-Ruiz SR, Gonzalez-Perez G, Munguia R, Bajana S, Meraz-Rios MA, Sanchez-Torres C. Tolerogenic dendritic cells generated with different immunosuppressive cytokines induce antigen-specific anergy and regulatory properties in memory cd4+ t cells. J Immunol. 2010;184:1765–1775. doi: 10.4049/jimmunol.0902133. [DOI] [PubMed] [Google Scholar]
- 96.Zhang-Hoover J, Finn P, Stein-Streilein J. Modulation of ovalbumin-induced airway inflammation and hyperreactivity by tolerogenic apc. J Immunol. 2005;175:7117–7124. doi: 10.4049/jimmunol.175.11.7117. [DOI] [PubMed] [Google Scholar]
- 97.Licona-Limon P, Kim LK, Palm NW, Flavell RA. Th2, allergy and group 2 innate lymphoid cells. Nat Immunol. 2013;14:536–542. doi: 10.1038/ni.2617. [DOI] [PubMed] [Google Scholar]
- 98.Swamy M, Jamora C, Havran W, Hayday A. Epithelial decision makers: In search of the ‘epimmunome’. Nat Immunol. 2010;11:656–665. doi: 10.1038/ni.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wilson RH, Whitehead GS, Nakano H, Free ME, Kolls JK, Cook DN. Allergic sensitization through the airway primes th17-dependent neutrophilia and airway hyperresponsiveness. American journal of respiratory and critical care medicine. 2009;180:720–730. doi: 10.1164/rccm.200904-0573OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent t helper cell type 2 responses to inhaled antigen. J Exp Med. 2002;196:1645–1651. doi: 10.1084/jem.20021340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chapman TJ, Emo JA, Knowlden SA, Rezaee F, Georas SN. Pre-existing tolerance shapes the outcome of mucosal allergen sensitization in a murine model of asthma. J Immunol. 2013;191:4423–4430. doi: 10.4049/jimmunol.1300042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy: Multiple suppressor factors at work in immune tolerance to allergens. J Allergy Clin Immunol. 2014;133:621–631. doi: 10.1016/j.jaci.2013.12.1088. [DOI] [PubMed] [Google Scholar]
- 103.Boudousquie C, Pellaton C, Barbier N, Spertini F. Cd4+cd25+ t cell depletion impairs tolerance induction in a murine model of asthma. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2009;39:1415–1426. doi: 10.1111/j.1365-2222.2009.03314.x. [DOI] [PubMed] [Google Scholar]
- 104.Burchell JT, Wikstrom ME, Stumbles PA, Sly PD, Turner DJ. Attenuation of allergen-induced airway hyperresponsiveness is mediated by airway regulatory t cells. American journal of physiology Lung cellular and molecular physiology. 2009;296:L307–319. doi: 10.1152/ajplung.00521.2007. [DOI] [PubMed] [Google Scholar]
- 105.Strickland DH, Stumbles PA, Zosky GR, Subrata LS, Thomas JA, Turner DJ, Sly PD, Holt PG. Reversal of airway hyperresponsiveness by induction of airway mucosal cd4+cd25+ regulatory t cells. J Exp Med. 2006;203:2649–2660. doi: 10.1084/jem.20060155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Van Hove CL, Maes T, Joos GF, Tournoy KG. Prolonged inhaled allergen exposure can induce persistent tolerance. Am J Respir Cell Mol Biol. 2007;36:573–584. doi: 10.1165/rcmb.2006-0385OC. [DOI] [PubMed] [Google Scholar]
- 107.Lewkowich IP, Herman NS, Schleifer KW, Dance MP, Chen BL, Dienger KM, Sproles AA, Shah JS, Kohl J, Belkaid Y, Wills-Karp M. Cd4+cd25+ t cells protect against experimentally induced asthma and alter pulmonary dendritic cell phenotype and function. J Exp Med. 2005;202:1549–1561. doi: 10.1084/jem.20051506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen Y, Haines CJ, Gutcher I, Hochweller K, Blumenschein WM, McClanahan T, Hammerling G, Li MO, Cua DJ, McGeachy MJ. Foxp3(+) regulatory t cells promote t helper 17 cell development in vivo through regulation of interleukin-2. Immunity. 2011;34:409–421. doi: 10.1016/j.immuni.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 109.Pandiyan P, Conti HR, Zheng L, Peterson AC, Mathern DR, Hernandez-Santos N, Edgerton M, Gaffen SL, Lenardo MJ. Cd4(+)cd25(+)foxp3(+) regulatory t cells promote th17 cells in vitro and enhance host resistance in mouse candida albicans th17 cell infection model. Immunity. 2011;34:422–434. doi: 10.1016/j.immuni.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank RB, Meylan F, Siegel R, Hennighausen L, Shevach EM, O'Shea JJ. Interleukin-2 signaling via stat5 constrains t helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 111.Rezaee F, Georas SN. Breaking barriers: New insights into airway epithelial barrier function in health and disease. Am J Respir Cell Mol Biol. 2014 doi: 10.1165/rcmb.2013-0541RT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rezaee F, DeSando SA, Ivanov AI, Chapman TJ, Knowlden SA, Beck LA, Georas SN. Sustained protein kinase d activation mediates respiratory syncytial virus-induced airway barrier disruption. Journal of virology. 2013;87:11088–11095. doi: 10.1128/JVI.01573-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rezaee F, Meednu N, Emo JA, Saatian B, Chapman TJ, Naydenov NG, De Benedetto A, Beck LA, Ivanov AI, Georas SN. Polyinosinic:Polycytidylic acid induces protein kinase d-dependent disassembly of apical junctions and barrier dysfunction in airway epithelial cells. J Allergy Clin Immunol. 2011;128:1216–1224. e1211. doi: 10.1016/j.jaci.2011.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sanders CJ, Doherty PC, Thomas PG. Respiratory epithelial cells in innate immunity to influenza virus infection. Cell and tissue research. 2011;343:13–21. doi: 10.1007/s00441-010-1043-z. [DOI] [PubMed] [Google Scholar]
- 115.Ronmark E, Lindberg A, Watson L, Lundback B. Outcome and severity of adult onset asthma--report from the obstructive lung disease in northern sweden studies (olin) Respiratory medicine. 2007;101:2370–2377. doi: 10.1016/j.rmed.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 116.Siroux V, Basagana X, Boudier A, Pin I, Garcia-Aymerich J, Vesin A, Slama R, Jarvis D, Anto JM, Kauffmann F, Sunyer J. Identifying adult asthma phenotypes using a clustering approach. Eur Respir J. 2011;38:310–317. doi: 10.1183/09031936.00120810. [DOI] [PubMed] [Google Scholar]
- 117.Tsukioka K, Toyabe S, Akazawa K. relationship between asthma severity and age at onset in japanese adults. Nihon Kokyuki Gakkai zasshi = the journal of the Japanese Respiratory Society. 2010;48:898–905. [PubMed] [Google Scholar]
- 118.Lembke B, Janson C, Norback D, Rask-Andersen A. High risk of adult-onset asthma and work-related wheeze in farmers despite low prevalence of asthma in young farmers. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2004;8:1285–1291. [PubMed] [Google Scholar]
- 119.Varraso R, Oryszczyn MP, Mathieu N, Le Moual N, Boutron-Ruault MC, Clavel-Chapelon F, Romieu I, Kauffmann F. Farming in childhood, diet in adulthood and asthma history. Eur Respir J. 2012;39:67–75. doi: 10.1183/09031936.00115010. [DOI] [PubMed] [Google Scholar]
- 120.Amelink M, de Groot JC, de Nijs SB, Lutter R, Zwinderman AH, Sterk PJ, ten Brinke A, Bel EH. Severe adult-onset asthma: A distinct phenotype. J Allergy Clin Immunol. 2013;132:336–341. doi: 10.1016/j.jaci.2013.04.052. [DOI] [PubMed] [Google Scholar]
- 121.Jamrozik E, Knuiman MW, James A, Divitini M, Musk AW. Risk factors for adult-onset asthma: A 14-year longitudinal study. Respirology. 2009;14:814–821. doi: 10.1111/j.1440-1843.2009.01562.x. [DOI] [PubMed] [Google Scholar]
- 122.Miranda C, Busacker A, Balzar S, Trudeau J, Wenzel SE. Distinguishing severe asthma phenotypes: Role of age at onset and eosinophilic inflammation. J Allergy Clin Immunol. 2004;113:101–108. doi: 10.1016/j.jaci.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 123.Spears M, McSharry C, Chaudhuri R, Weir CJ, de Wet C, Thomson NC. Smoking in asthma is associated with elevated levels of corticosteroid resistant sputum cytokines-an exploratory study. PloS one. 2013;8:e71460. doi: 10.1371/journal.pone.0071460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Porter M, Karp M, Killedar S, Bauer SM, Guo J, Williams D, Breysse P, Georas SN, Williams MA. Diesel-enriched particulate matter functionally activates human dendritic cells. Am J Respir Cell Mol Biol. 2007;37:706–719. doi: 10.1165/rcmb.2007-0199OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tanaka T, Kishimoto T. Targeting interleukin-6: All the way to treat autoimmune and inflammatory diseases. International journal of biological sciences. 2012;8:1227–1236. doi: 10.7150/ijbs.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]


