Abstract
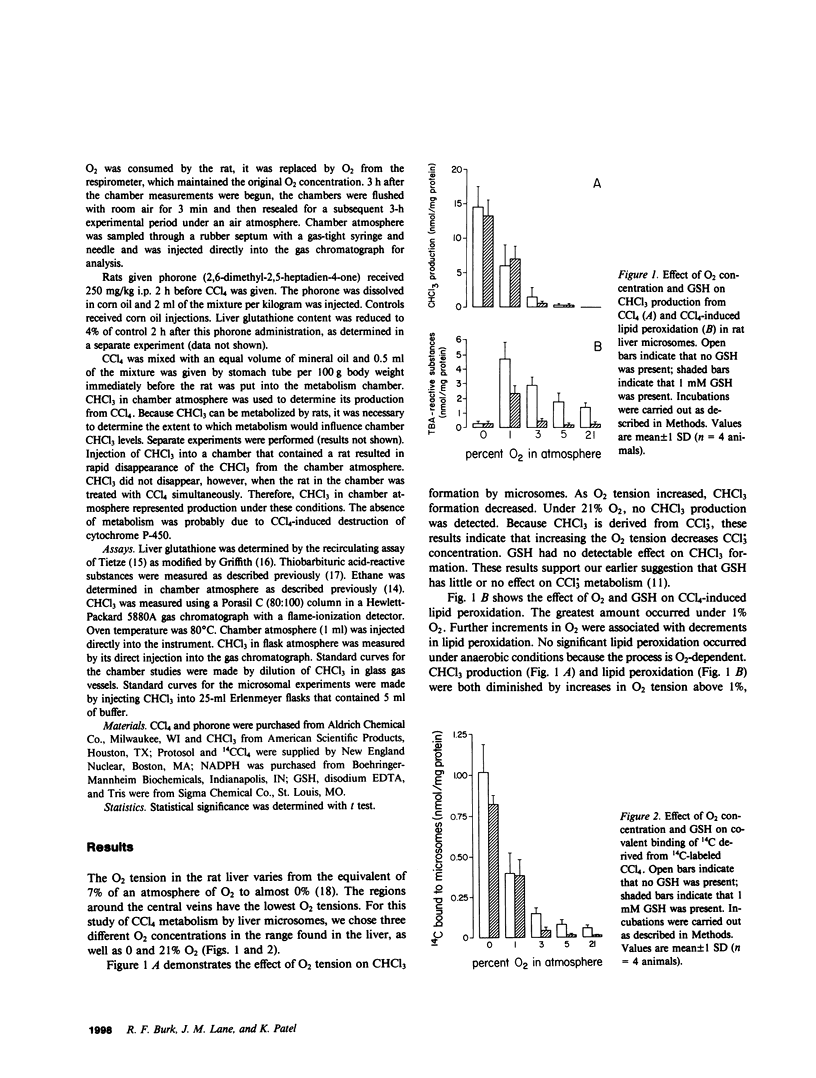
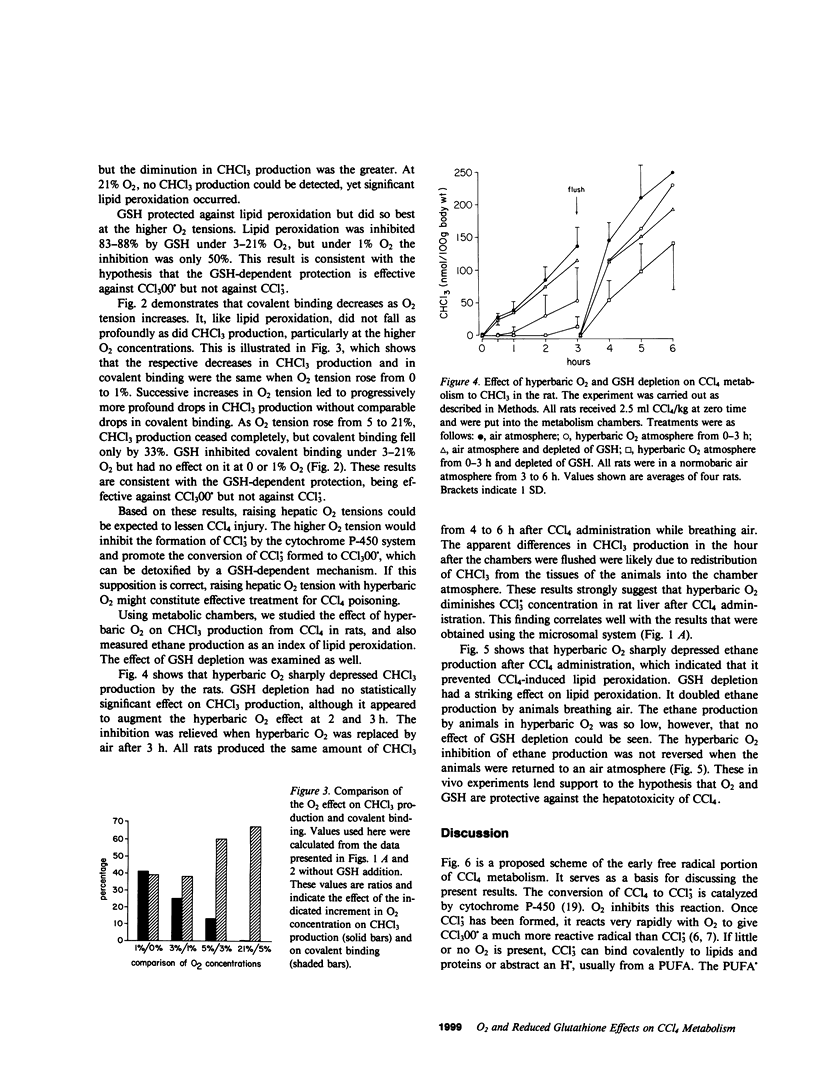
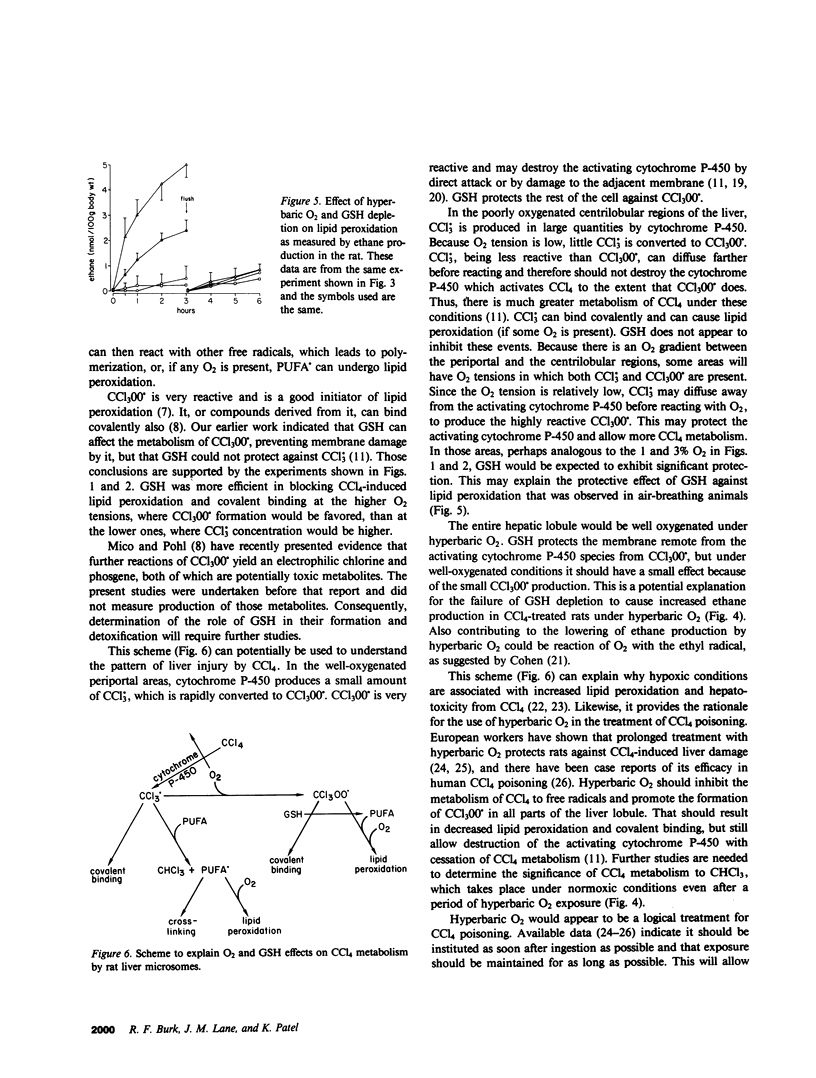
CCl4 exerts its toxicity through its metabolites, including the free radicals CCl3. and CCl(3)00.. Oxygen strongly inhibits the hepatic cytochrome P-450-mediated formation of CCl3. from CCl4 and promotes the conversion of CCl3. to CCl(3)00.. Both these free radicals injure the hepatocyte by causing lipid peroxidation and binding covalently to cell structures. A reduced glutathione (GSH)-dependent mechanism can protect the liver microsomal membrane against CCl4-induced damage under aerobic conditions but not under anaerobic conditions (Burk, R.F., K. Patel, and J.M. Lane, 1983, Biochem. J., 215:441-445). Experiments were carried out using rat liver microsomes to examine the effect of O2 tensions found in the liver and of GSH on CCl4-induced covalent binding and lipid peroxidation. An NADPH-supplemented microsomal system was used. CCl4 or 14CCl4 was added to the sealed flask that contained the system, and after 20 min CHCl3 production, thiobarbituric acid-reactive substances (an index of lipid peroxidation), and covalent binding of 14C were measured. O2 tensions of 0, 1, 3, 5, and 21% were studied. Increases in O2 tension caused a fall in CHCl3 production, which indicated that it decreased CCl3.. GSH had no significant effect on CHCl3 production at any O2 tension. Lipid peroxidation and covalent binding of 14C fell progressively as O2 tension was increased from 1 to 21%. The addition of GSH decreased both lipid peroxidation and covalent binding, but did so better at the higher O2 tensions than at the lower ones. These results indicate that low O2 tensions such as are found in the centrilobular areas of the liver favor conversion of CCl4 to free radical products which cannot be detoxified by the GSH-dependent mechanism. They suggest that hyperbaric O2 might decrease free radical formation in the liver in vivo and promote formation of CCl(3)00. from CCl3.. This should result in diminished CCl4-induced lipid peroxidation and liver damage. Rats given CCl4 (2.5 ml/kg) were studied in metabolic chambers. Production of CHCl3 and ethane, the latter an index of lipid peroxidation, were measured. Rats in two atmospheres of 100% O2 produced much less CHCl3 and ethane than rats in air. This strongly suggests that hyperbaric O2 is decreasing free radical formation from CCl4 and/or promoting the formation of CCl(3)00. from CCl3.. These results provide the rationale for the use of hyperbaric O2 in the treatment of CCl4 ingestion.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUTLER T. C. Reduction of carbon tetrachloride in vivo and reduction of carbon tetrachloride and chloroform in vitro by tissues and tissue constituents. J Pharmacol Exp Ther. 1961 Dec;134:311–319. [PubMed] [Google Scholar]
- Burk R. F., Lane J. M. Ethane production and liver necrosis in rats after administration of drugs and other chemicals. Toxicol Appl Pharmacol. 1979 Sep 30;50(3):467–478. doi: 10.1016/0041-008x(79)90400-9. [DOI] [PubMed] [Google Scholar]
- Burk R. F., Masters B. S. Some effects of selenium deficiency on the hepatic microsomal cytochrome P-450 system in the rat. Arch Biochem Biophys. 1975 Sep;170(1):124–131. doi: 10.1016/0003-9861(75)90103-4. [DOI] [PubMed] [Google Scholar]
- Burk R. F., Patel K., Lane J. M. Reduced glutathione protection against rat liver microsomal injury by carbon tetrachloride. Dependence on O2. Biochem J. 1983 Dec 1;215(3):441–445. doi: 10.1042/bj2150441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forni L. G., Packer J. E., Slater T. F., Willson R. L. Reaction of the trichloromethyl and halothane-derived peroxy radicals with unsaturated fatty acids: a pulse radiolysis study. Chem Biol Interact. 1983 Jul 15;45(2):171–177. doi: 10.1016/0009-2797(83)90066-2. [DOI] [PubMed] [Google Scholar]
- Fowler J. S. Carbon tetrachloride metabolism in the rabbit. Br J Pharmacol. 1969 Nov;37(3):733–737. doi: 10.1111/j.1476-5381.1969.tb08512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank H., Haussmann H. J., Remmer H. Metabolic activation of carbon tetrachloride: induction of cytochrome P-450 with phenobarbital or 3-methylcholanthrene and its effect on covalent binding. Chem Biol Interact. 1982 Jun;40(2):193–208. doi: 10.1016/0009-2797(82)90101-6. [DOI] [PubMed] [Google Scholar]
- Gordis E. Lipid metabolites of carbon tetrachloride. J Clin Invest. 1969 Jan;48(1):203–209. doi: 10.1172/JCI105969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith O. W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980 Jul 15;106(1):207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- Kieczka H., Kappus H. Oxygen dependence of CCl4-induced lipid peroxidation in vitro and in vivo. Toxicol Lett. 1980 Mar;5(3-4):191–196. doi: 10.1016/0378-4274(80)90058-2. [DOI] [PubMed] [Google Scholar]
- Kubic V. L., Anders M. W. Mechanism of the microsomal reduction of carbon tetrachloride and halothane. Chem Biol Interact. 1981 Mar 1;34(2):201–207. doi: 10.1016/0009-2797(81)90131-9. [DOI] [PubMed] [Google Scholar]
- Lawrence R. A., Burk R. F. Species, tissue and subcellular distribution of non Se-dependent glutathione peroxidase activity. J Nutr. 1978 Feb;108(2):211–215. doi: 10.1093/jn/108.2.211. [DOI] [PubMed] [Google Scholar]
- Mico B. A., Pohl L. R. Reductive oxygenation of carbon tetrachloride: trichloromethylperoxyl radical as a possible intermediate in the conversion of carbon tetrachloride to electrophilic chlorine. Arch Biochem Biophys. 1983 Sep;225(2):596–609. doi: 10.1016/0003-9861(83)90071-1. [DOI] [PubMed] [Google Scholar]
- Montani S., Perret C. Oxygénation hyperbare dans l'intoxication expérimentale au tétrachlorure de carbone. Rev Fr Etud Clin Biol. 1967 Mar;12(3):274–278. [PubMed] [Google Scholar]
- Nastainczyk W., Ahr H. J., Ullrich V. The reductive metabolism of halogenated alkanes by liver microsomal cytochrome P450. Biochem Pharmacol. 1982 Feb 1;31(3):391–396. doi: 10.1016/0006-2952(82)90187-3. [DOI] [PubMed] [Google Scholar]
- Noguchi T., Fong K. L., Lai E. K., Alexander S. S., King M. M., Olson L., Poyer J. L., McCay P. B. Specificity of a phenobarbital-induced cytochrome P-450 for metabolism of carbon tetrachloride to the trichloromethyl radical. Biochem Pharmacol. 1982 Mar 1;31(5):615–624. doi: 10.1016/0006-2952(82)90440-3. [DOI] [PubMed] [Google Scholar]
- Packer J. E., Slater T. F., Willson R. L. Reactions of the carbon tetrachloride-related peroxy free radical (CC13O.2) with amino acids: pulse radiolysis evidence. Life Sci. 1978 Dec 25;23(26):2617–2620. doi: 10.1016/0024-3205(78)90378-8. [DOI] [PubMed] [Google Scholar]
- Poyer J. L., McCay P. B., Lai E. K., Janzen E. G., Davis E. R. Confirmation of assignment of the trichloromethyl radical spin adduct detected by spin trapping during 13C-carbon tetrachloride metabolism in vitro and in vivo. Biochem Biophys Res Commun. 1980 Jun 30;94(4):1154–1160. doi: 10.1016/0006-291x(80)90540-9. [DOI] [PubMed] [Google Scholar]
- Rapin M., Got C., Le Gall J. R., Goulon M. Effet de l'oxygène hyperbare sur la toxicité hépatique du tétrachlorure de carbone chez le rat. Rev Fr Etud Clin Biol. 1967 Jun-Jul;12(6):594–599. [PubMed] [Google Scholar]
- Shen E. S., Garry V. F., Anders M. W. Effect of hypoxia on carbon tetrachloride hepatotoxicity. Biochem Pharmacol. 1982 Dec 1;31(23):3787–3793. doi: 10.1016/0006-2952(82)90294-5. [DOI] [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969 Mar;27(3):502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- Truss C. D., Killenberg P. G. Treatment of carbon tetrachloride poisoning with hyperbaric oxygen. Gastroenterology. 1982 Apr;82(4):767–769. [PubMed] [Google Scholar]


