Abstract
Despite advances in neonatal care, the burden of preterm birth remains high. Preterm birth is a multifactorial problem and strategies to identify and treat medical risk factors in early pregnancy have not been effective in reducing preterm birth rates. In a sentinel clinical trial, prophylactic therapy with 17-hydoxyprogesterone caproate (17-OHPC) reduced the risk of recurrent, spontaneous preterm birth in 34% of women1. As a result, clinical practice changed and extensive research on 17-OHPC followed. The increasing body of evidence demonstrated a variable efficacy of the drug. This review will examine the plausibility, pharmacology, clinical efficacy and safety of 17-OHPC when used in the setting of preterm birth prevention. We will also discuss pharmacokinetic and pharmacodynamics data to highlight drug metabolism and mechanism of action; which will help clarify the variability in clinical outcomes and efficacy.
Introduction
Premature birth in the United States accounts for 35% of deaths in the first year of life at an estimated annual cost exceeding $26 billion2,3. Efforts to identify and prevent preterm birth have led to a modest decrease in the annual rate in the United States from a peak of 12.8% in 2006 to 11.5% in 20124. In addition to mortality, long-term morbidity is a significant risk of preterm birth and both are inversely related to gestational age at birth. The majority of preterm births occur in singleton pregnancies following the spontaneous early onset of the parturition process. However, certain factors are associated with an increased risk for preterm birth: multifetal gestation, shortened cervical length, genitourinary infection, smoking, and a prior preterm birth.
Clinical interventions to decrease the rate of preterm birth have focused on identifying women at increased risk for preterm birth and the use of prophylactic and therapeutic options in such subjects. Unfortunately, despite vigorous efforts at treatment of preterm labor with labor inhibiting drugs (tocolytics), intensive prenatal care, patient education and bed rest, rates of preterm birth have not decreased significantly over the past 40 years. One of the very few preventive measures to have shown some promise in randomized trials is the use of 17-OHPC, a progestational agent. In a sentinel article that drastically changed clinical practice, Meis et. al. demonstrated a 33% reduction in delivery before 37 weeks in women with a prior preterm birth, who received weekly injections of 17-OHPC1. Treatment with 17-OHPC has proven effective in reducing the rate of preterm birth in women with a history of spontaneous preterm delivery, but it appears to be ineffective in other high-risk categories such as women with a short cervical length and those with multi-fetal gestation. This review will focus on the pharmacologic properties of 17-OHPC, which may explain the variability in outcomes noted in clinical trials.
Plausibility of progesterone for preterm birth
Progesterone is thought to act in support of gestation and to inhibit uterine activity. The concept of a “progesterone block” was advanced and championed by Csapo in the 1950’s based on his extensive and pioneering experiments in pregnant rabbits5. This formed the basis for the study of progesterone supplementation and the role of progesterone in the onset of labor. As such, numerous animal studies support the importance of progesterone in regulating the onset of labor5–7. In sheep, goats, and many other mammalian species, a decrease in plasma progesterone (P) and an increase in estrogen (E) preceded the onset of labor. The ability of progesterone to maintain uterine quiescence during pregnancy has been shown in lower mammalian species. In these species, progesterone withdrawal is a necessary step in the events leading to parturition. The role of progesterone and changes in P/E ratio on the onset of labor in human beings and other primates is less well known. Although some investigators have described low progesterone concentrations or low P/E ratios in the plasma of women destined to deliver prematurely, no consistent evidence exists documenting decreases in plasma progesterone or P/E ratio prior to the onset of labor at or before term8. Nonetheless, some evidence exists that local changes in progesterone or the P/E ratio in the placenta, decidua, or fetal membranes may be important in the initiation of labor9. The progesterone withdrawal theory remains a leading hypothesis because no other mechanism for the onset of human parturition has been definitively established and because synthetic antiprogestins stimulate myometrial contractions.
In light of these findings, progesterone was theorized as an agent to prevent preterm birth. Early studies yielded conflicting results regarding its efficacy in preventing preterm birth. These studies were limited by small sample sizes and inclusion of heterogeneous patient populations. With increasing evidence, it appears that 17-OHPC therapy has condition-specific efficacy in decreasing the risk for preterm birth. A meta-analysis published by Keirse in 1990, included only studies that evaluated the effect of 17-OHPC on a range of outcomes including preterm birth. This meta-analysis found a pooled odds ratio of roughly 0.50 (95%CI: 0.30–0.85) for preterm birth among women treated with 17-OHPC10. Since then, various trials have focused on progesterone use for the prevention of preterm birth. In 463 women with singleton gestations and a history of spontaneous preterm birth, weekly 17-OHPC intramuscular injections, was associated with a decreased incidence of preterm birth (RR 0.66, 95% CI, 0.54–0.81) compared to placebo1. In women with twin gestation, weekly 17-OHPC injections did not reduce preterm birth or adverse neonatal outcomes compared to placebo11–13. In a multicenter randomized controlled trial, weekly 17-OHPC injections did not decrease the risk of preterm delivery in nulliparous women with a singleton gestation and a short cervical length (<30 mm)14. Furthermore, two randomized controlled trials also demonstrated a lack of benefit for 17-OHPC in triplet gestation15,16. Based on these randomized controlled trials current guidelines recommend the use of 17-OHPC only for the prevention of preterm birth in singleton pregnancies with a history of preterm birth17,18. As a tocolytic agent, 17-OHPC has proven unsuccessful19–21.
Pharmacokinetic properties of 17-OHPC
Progesterone and its naturally occurring metabolite 17-hydroxyprogesterone are produced in large amounts in human pregnancy. On the other hand 17-OHPC is not a naturally occurring substance but rather is synthesized through the acetylation of 17- hydroxyprogesterone with caproic acid in the presence of toluene sulfonic acid22. The structures of progesterone, 17-OHP and 17-OHPC are depicted in Figure 1. Despite widespread usage of 17-OHPC in the 1950s through 1970s little information about the pharmacology of this agent is available. It initially gained Federal Drug Administration (FDA) approval in 1956 (NDA 10–347) and was marketed under the trade name Delalutin as a treatment for menstrual disorders (such as dysmenorrhea, pre-menstrual tension, cyclomastopathies, adenosis, and mastodynia), threatened miscarriage and uterine cancer.
Figure 1.
Chemical structure of progesterone, 17-hydroxyprogesterone and 17-OHPC
17-OHPC is a lipophilic drug and is highly protein-bound in blood. In humans, it is predominantly metabolized in the liver by the cytochrome P450 (CYP) enzymes, primarily CYP3A4 and to a lesser extent CYP3A5. Metabolites of 17-OHPC have been identified and differ from 17-hydroxyprogesterone or progesterone. The caproic acid moiety of 17-OHPC is not removed during metabolism, rather the steroid rings are primarily hydroxylated. After intramuscular administration, elimination of metabolites occurs primarily through feces (~50%) and urine (~30%). Factors that alter CYP expression and activity can affect the clearance of 17-OHPC. CYP3A activity increases with advancing gestation and genetic polymorphisms of CYP3A5 influence clearance of CYP3A5 substrates23. Interestingly, CYP3A5 is the major CYP3A enzyme in more than half of African American adults compared to a third of Caucasians23. This finding could explain the increased clearance noted in African American pregnant women, but fails to explain the similar plasma concentrations for all races in the same study24. Not surprisingly, concomitant use of medications that are CYP3A inhibitors or inducers could lead to decrease or increase, respectively, in the clearance of 17-OHPC. At clinically relevant concentrations, 17-OHPC does not appear to have any effect on CYP1A, CYP2D6, CYP2C9 or CYP3A4, while the activity of CYP2C19 is modestly increased in primary cultures of human hepatocytes (Yang et al. AJOG In Press. Accepted March 28 2014). Dosage of CYP2C19 substrates, for example tricyclic antidepressants, proton pump inhibitors and propranolol, may have to be increased in patients on 17-OHPC. Endogenous steroids also appear to affect 17-OHPC metabolism. In human liver microsomes, a combination of steroid hormones and progesterone alone inhibited 37% and 28% of 17-OHPC metabolism, respectively25. Future clinical studies should aim to substantiate the physiologic relevance of these in vitro findings.
In women with a singleton gestation receiving the recommended dose of 17-OHPC (250 mg weekly) there is a large variation in the maternal plasma concentrations of 17-OHPC (median 15.2 ng/mL, range 3.2–64.6 ng/mL) and the drug remains detectable weeks after the last injection24. The half -life of 17-OHPC is reported to be 16 days (±6 days24. The prolonged half-life may result from a slow release of 17-OHPC from the castor oil depot and/or the maternal adipose tissue, which may also explain the detection of the drug in maternal samples 44 days after the last intramuscular injection. Across a dosing interval, maximal plasma concentration occurred a day after drug injection (Figure 2), but samples were not taken at earlier times. The area under the plasma concentration × time curve (AUC) of 17-OHPC was higher at 31–34+6 weeks compared to 20–24+6 weeks (21.0 vs. 24.3 ng/mL, p=0.007), suggesting sustained and continuous release of 17-OHPC from multiple administration sites24. In women with a singleton gestation, race did not alter drug concentrations; though the study was not powered to detect such an effect24. In women with multifetal gestation 17-OHPC trough concentrations were 40% lower compared to women with a singleton gestation (9.7 vs 14.1ng/mL24. Women with twins and triplets also exhibited a slightly shorter half–life (10 days26. These findings were attributed to an increase in apparent clearance of 17-OHPC26. Drug clearance and volume of distribution were highly variable between subjects, 24% and 49%, respectively. Fetal number was not associated with any changes in drug concentration or clearance26. In both cohorts, BMI exhibited an inverse relationship with drug concentration accounting for about 20% of the variation in drug concentrations, whereas parity did not have an effect24. The large variation in plasma 17-OHPC concentration may be explained by variability in the physiologic changes in pregnancy as well as maternal BMI, alterations in the metabolism of 17-OHPC by endogenous steroids, especially progesterone and competition for metabolism from other medications that are CYP3A substrates that the patient may be taking25. The pharmacokinetic parameters of 17-OHPC in pregnancy are summarized in Table 1.
Figure 2. 17-OHPC concentrations during one dosing interval.
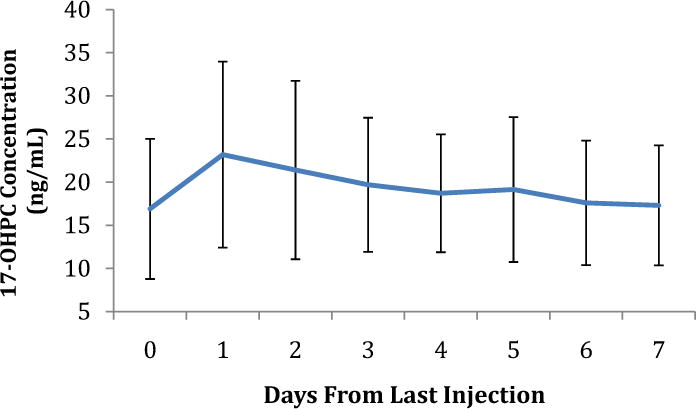
Data points represent plasma 17-OHPC concentration ± standard deviation.
Table 1. Pharmacokinetic Parameters of 17-OHPC in Pregnancy.
Absorption: Slow absorption over a prolonged time period
Distribution: Highly protein-bound in blood
High volume of distribution – extensive distribution in the body
Metabolism: Mono hydroxyl, di hydroxyl and trihydroxylated metabolites formed in liver
Excretion: Minimal unchanged drug excreted in urine and feces
| Singleton Gestation24 | Twin Gestation26 | |
|---|---|---|
| CTrough (ng/mL): | 14.1 ± 5.6 (10–18.1) | 11.2 ± 4.6 (4.8–16.3) |
| CMAX (ng/mL) | 22.6 ± 9.5 (15.8–27.4) | 17.3 ± 6.7 (12–27) |
| Apparent Half-life (d) | 16.2 (10.6–21.0) | 10 (6–16) |
| TMAX (d) | 1.0 ± 1.9 (1–3) | 1.2 ± 0.41(1–2) |
| Vd/F (*103) (L) | 56 (25.2–69.6) | 16.9 (9.1–24.5) |
| Cl/F (*103) (L) | 2.1 (1.5–2.7) | 1.2 (0.9–1.7) |
Data are mean ± standard deviation (interquartile range)
CTrough, the minimum concentration just before the next dose; CMAX, maximum concentration during the dosing interval; TMAX, time to maximum concentration; Vd/F, apparent volume of distribution/bioavailability; Cl/F, clearance/bioavailability.
Pharmacodynamic properties of 17-OHPC
Although most data support the concept that progesterone plays a role in maintaining gestation in human beings, the actual mechanisms by which exogenous progesterone therapy, including 17-OHPC, may avert preterm labor and delivery are less well determined. Most studies support a likely anti-inflammatory effect and a local increase in progesterone concentrations in gestational tissues, counteracting the functional decrease in progesterone that leads to preterm birth.
The effects of 17-OHPC on the uterine cervix in pregnant women, animals, and in the context of in vitro experiments have not been studied to the same extent as those of progesterone. One study examined the effect of 17-OHPC on cervical length in patients with a history of 1 or more preterm births who were allocated to receive 17-OHPC and were compared to an untreated control group27. No difference in cervical length measurements over time was observed in women who received 17-OHPC. In contrast, vaginal progesterone reduced the rate of cervical shortening in patients with a history of preterm birth or premature cervical shortening28. More recently, progesterone supplementation decreased the risk of preterm delivery in asymptomatic women with a short cervix29. Progesterone is also associated with a reduction in uterine contraction frequency30,31. By comparison, ex vivo exposure to 17-OHPC is associated with no change or an increase in contraction frequency, reaffirming its lack of benefit as a tocolytic agent30,31. Cellular preparations from cervix, decidua, and fetal membranes revealed an effect of progestins on collagen synthesis. Progesterone activity appeared to limit collagenolysis, altered production of cytokines, nitric oxide, and prostaglandins, and limited apoptosis32–38. In contrast, progesterone antagonism resulted in impaired decidual functioning and trophoblast proliferation, and accelerated cervical ripening by collagenolysis39–42. These differences highlight the characteristic indications and benefits for different progesterone compound and underline the need to think of each formulation distinctly.
The human progesterone receptor (PR) is a member of the steroid and thyroid reception superfamily. It is mainly located in the nucleus and exists in two isoforms (A and B43. Both receptor isoforms have been described in gestational tissues including the chorion and amnion9,43. PR isoforms likely differ in their biological roles. PR-A is thought to inhibit the transcription of progesterone receptor genes. In contrast, PR-B increases the transcription of progesterone genes and promotes myometrial quiescence44. Thus, the responsiveness of a tissue to progesterone may depend on the concentration of circulating progesterone and the ratio of PR isoforms45,46. In fact, it has been hypothesized that an increase in PR-A to PR-B ratio and an inhibition of PR-B activation by PR-A, may contribute to a qualitative decrease in progesterone activity and lead to the initiation of labor47,48. Recently, studies have described an association between different single nucleotide polymorphisms (SNPs) of the PR gene promoter and preterm birth49,50. Furthermore, Manuck et. al. described an effect of PR polymorphism on the clinical efficacy of 17-OHPC in preventing recurrent preterm birth51. In addition, a specific PR genotype was associated with an increased risk for preterm birth in Caucasian/Hispanic women who received 17-OHPC. In contrast, other genotypes were associated with a high rate of preterm birth in the placebo group and a significant reduction in recurrent preterm birth in women exposed to 17-OHPC51. Data has also emerged on the interaction between PR SNPs, BMI and race suggesting a complex genetic background of women with preterm birth49,52.
Few studies have reported on the association between progesterone concentration and possible drug targets. In 315 women with a singleton gestation receiving 17-OHPC weekly, the concentrations ranged between 3.7–56ng/mL between 25 and 28 weeks of gestation53. Women with 17-OHPC plasma concentrations in the lowest quintile had a higher risk of recurrent spontaneous preterm birth compared to women in the third to fifth quintiles (36.5% vs. 25%, Figure 3). In addition, these women delivered at an earlier gestational age than did women in the third to fifth quintiles. The authors also noted that the lowest rates of preterm birth were seen when 17-OHPC concentration was lower than 6.4ng/mL, but drug concentration and preterm birth risk were not linearly associated (Figure 3)53. Somewhat alarmingly, another report demonstrated an association between 17-OHPC plasma concentrations higher than 14ng/mL and an earlier gestational age at delivery in women carrying twins54. The study was unable to uncover the underlying mechanism for the negative association between 17-OHPC supplementation and recurrent preterm birth. The complex nature of the preterm birth phenotype, the lack of knowledge of an exact mechanism and the variation in progesterone receptor polymorphisms limit any conclusion regarding the pharmacodynamic properties of 17-OHPC and the existence of a dose response relationship. In order to characterize the relationship of 17-OHPC and preterm birth, future studies should take into consideration the current knowledge of variation in clinical efficacy and safety of 17-OHPC, identify subsets of women who may most benefit from this therapy, and define groups that may not benefit or may be harmed.
Figure 3. Relationship between median 17-OHPC concentration and preterm birth rate within each quintile.
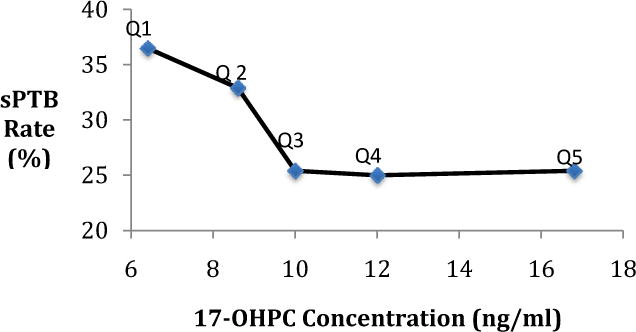
Relationship between median 17-OHPC concentration from each quintile (Q1–Q5) of 17- OHPC plasma concentrations and the rate of spontaneous preterm birth for each of these quintiles. sPTB: spontaneous preterm birth
Placental transport and fetal exposure to 17-OHPC
Placental transport of 17-OHPC has been demonstrated. At the time of delivery, 1–44 days following the last maternal dose, the range of cord blood 17-OHPC concentration was 0.4–12ng/mL24. Not surprisingly, in specimens collected at the time of delivery, the ratio of fetal to maternal concentrations was 0.2 (range 0.06–0.51) and absolute drug concentrations in the fetus were a function of time since the last dose24.
Fetal hepatocytes were found to metabolize 17-OHPC55. The active enzyme was identified as CYP3A7, the fetal form of CYP3A; and the metabolites were noted to be similar but not identical to those resulting from adult hepatocytes55. Interestingly, the placenta was also noted to metabolize 17-OHPC to its own distinct metabolites, which differed from those of fetal and adult livers56.
Although 17-OHPC is detectable in cord plasma, evidence to date is reassuring in terms of fetal/neonatal safety. Animal studies using doses much higher than those used in humans failed to exhibit any androgenic effect or a risk of congenital anomalies57–60. A number of authors have also reported the results of well-controlled clinical studies to examine the safety of 17-OHPC in human pregnancy. Varma and Morsman examined the outcome of 150 pregnancies treated with 17-OHPC because of threatened abortion, and compared them with 150 patients who experienced early pregnancy bleeding but were not treated with the drug61. No evidence was found that the drug had any adverse effect on the fetus or the outcome of the pregnancy. Michaelis, et al. in a cohort study of 13,643 pregnancies in West Germany found no increase in malformations in infants exposed in utero to 17-OHPC compared with controls62. Resseguie, et al. examined a cohort of 24,000 pregnancies delivered in Olmstead County, Minnesota from 1936 to 1974 and found that the 649 offspring exposed to 17-OHPC showed no increase in congenital anomalies or other ill effects compared with controls63. A notable feature of this study was the long period of follow-up of the children, with a mean of 11.5 years. Check, et al. performed a follow-up questionnaire study of 382 women treated with progestins during pregnancy and found no increase in anomalies compared with control offspring64. Most recently, Northen et. al. evaluated the offspring of women treated with 17-OHPC in the original Meis study65. They found no relationship between intrauterine exposure to 17-OHPC and physical findings or developmental characteristics, including gross and fine motor skills, problem solving, and personal/social interactions or gender-specific roles at a mean age of 48 months65.
Conclusion
Preterm birth is a complex phenotype with significant morbidity but few successful interventions. Weekly 17-OHPC injections have been proven to decrease rates of recurrent preterm birth. This treatment is associated with limited fetal and maternal risks. However, its efficacy can vary with maternal BMI and in relation to hepatic metabolism and progesterone receptor polymorphisms. While an exact mechanism of progesterone supplementation in preventing preterm birth remains unknown, recent data are shedding light on the variation in outcomes noted in prior clinical studies. The large variation in plasma 17-OHPC concentrations following a fixed dose, and the demonstration of improved efficacy with higher plasma concentrations suggests an opportunity to improve efficacy perhaps by tailoring the dose or by evaluating alternate routes of administration of this essential agent.
Acknowledgments
Dr Caritis and Dr Venkataramanan are supported in part by the Obstetric-Fetal Pharmacology Research Unit Network, Eunice Kennedy Shriver National Institute of Child Health and Human Development, grant no. 5U10 HD 047905.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: The authors report no conflict of interest.
References
- 1.Meis PJ, Klebanoff M, Thom E, et al. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. The New England journal of medicine. 2003;348(24):2379–2385. doi: 10.1056/NEJMoa035140. [DOI] [PubMed] [Google Scholar]
- 2.MacDorman MF, Hoyert DL, Mathews TJ. Recent declines in infant mortality in the United States, 2005–2011. NCHS data brief. 2013;(120):1–8. [PubMed] [Google Scholar]
- 3.Martin JA, Osterman MJ, Centers for Disease C, Prevention Preterm births – United States, 2006 and 2010. Morbidity and mortality weekly report. Surveillance summaries. 2013;62(Suppl 3):136–138. [PubMed] [Google Scholar]
- 4.Hamilton BE, Martin JA, Ventura SJ. Births: preliminary data for 2012. National vital statistics reports : from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2013;62(3):1–20. [PubMed] [Google Scholar]
- 5.Csapo AI. The ‘see-saw’ theory of parturition. Ciba Foundation symposium. 1977;(47):159–210. [PubMed] [Google Scholar]
- 6.Liggins GC, Fairclough RJ, Grieves SA, Forster CS, Knox BS. Parturition in the sheep. Ciba Foundation symposium. 1977;(47):5–30. doi: 10.1002/9780470720295.ch2. [DOI] [PubMed] [Google Scholar]
- 7.Liggins GC, Grieves SA, Kendall JZ, Knox BS. The physiological roles of progesterone, oestradiol-17 and prostaglandin F 2 in the control of ovine parturition. Journal of reproduction and fertility. Supplement. 1972;16(Suppl 16):85–10. [PubMed] [Google Scholar]
- 8.Mazor M, Hershkowitz R, Ghezzi F, et al. Maternal plasma and amniotic fluid 17 beta-estradiol, progesterone and cortisol concentrations in women with successfully and unsuccessfully treated preterm labor. Archives of gynecology and obstetrics. 1996;258(2):89–96. doi: 10.1007/BF00626029. [DOI] [PubMed] [Google Scholar]
- 9.Haluska GJ, Wells TR, Hirst JJ, Brenner RM, Sadowsky DW, Novy MJ. Progesterone receptor localization and isoforms in myometrium, decidua, and fetal membranes from rhesus macaques: evidence for functional progesterone withdrawal at parturition. Journal of the Society for Gynecologic Investigation. 2002;9(3):125–136. [PubMed] [Google Scholar]
- 10.Keirse MJ. Progestogen administration in pregnancy may prevent preterm delivery. British journal of obstetrics and gynaecology. 1990;97(2):149–154. doi: 10.1111/j.1471-0528.1990.tb01740.x. [DOI] [PubMed] [Google Scholar]
- 11.Combs CA, Garite T, Maurel K, Das A, Porto M, Obstetrix Collaborative Research N 17-hydroxyprogesterone caproate for twin pregnancy: a double-blind, randomized clinical trial. American journal of obstetrics and gynecology. 2011;204(3):221 e221–228. doi: 10.1016/j.ajog.2010.12.042. [DOI] [PubMed] [Google Scholar]
- 12.Lim AC, Schuit E, Bloemenkamp K, et al. 17alpha-hydroxyprogesterone caproate for the prevention of adverse neonatal outcome in multiple pregnancies: a randomized controlled trial. Obstetrics and gynecology. 2011;118(3):513–520. doi: 10.1097/AOG.0b013e31822ad6aa. [DOI] [PubMed] [Google Scholar]
- 13.Rouse DJ, Caritis SN, Peaceman AM, et al. A trial of 17 alpha-hydroxyprogesterone caproate to prevent prematurity in twins. The New England journal of medicine. 2007;357(5):454–461. doi: 10.1056/NEJMoa070641. [DOI] [PubMed] [Google Scholar]
- 14.Grobman WA, Thom EA, Spong CY, et al. 17 alpha-hydroxyprogesterone caproate to prevent prematurity in nulliparas with cervical length less than 30 mm. American journal of obstetrics and gynecology. 2012;207(5):390 e391–398. doi: 10.1016/j.ajog.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caritis SN, Rouse DJ, Peaceman AM, et al. Prevention of preterm birth in triplets using 17 alpha-hydroxyprogesterone caproate: a randomized controlled trial. Obstetrics and gynecology. 2009;113(2 Pt 1):285–292. doi: 10.1097/AOG.0b013e318193c677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Combs CA, Garite T, Maurel K, Das A, Porto M, Obstetrix Collaborative Research N Failure of 17-hydroxyprogesterone to reduce neonatal morbidity or prolong triplet pregnancy: a double-blind, randomized clinical trial. American journal of obstetrics and gynecology. 2010;203(3):248 e241–249. doi: 10.1016/j.ajog.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 17.Society for Maternal Fetal Medicine Publications C. ACOG Committee Opinion number 419 October 2008 (replaces no. 291, November 2003). Use of progesterone to reduce preterm birth. Obstetrics and gynecology. 2008;112(4):963–965. doi: 10.1097/AOG.0b013e31818b1ff6. [DOI] [PubMed] [Google Scholar]
- 18.Society for Maternal-Fetal Medicine Publications Committee waoVB. Progesterone and preterm birth prevention: translating clinical trials data into clinical practice. American journal of obstetrics and gynecology. 2012;206(5):376–386. doi: 10.1016/j.ajog.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 19.Borna S, Sahabi N. Progesterone for maintenance tocolytic therapy after threatened preterm labour: a randomised controlled trial. The Australian & New Zealand journal of obstetrics & gynaecology. 2008;48(1):58–63. doi: 10.1111/j.1479-828X.2007.00803.x. [DOI] [PubMed] [Google Scholar]
- 20.Facchinetti F, Paganelli S, Comitini G, Dante G, Volpe A. Cervical length changes during preterm cervical ripening: effects of 17-alpha-hydroxyprogesterone caproate. American journal of obstetrics and gynecology. 2007;196(5):453 e451–454. doi: 10.1016/j.ajog.2006.09.009. discussion 421. [DOI] [PubMed] [Google Scholar]
- 21.Rozenberg P, Chauveaud A, Deruelle P, et al. Prevention of preterm delivery after successful tocolysis in preterm labor by 17 alpha-hydroxyprogesterone caproate: a randomized controlled trial. American journal of obstetrics and gynecology. 2012;206(3):206 e201–209. doi: 10.1016/j.ajog.2011.12.026. [DOI] [PubMed] [Google Scholar]
- 22.Florey K. Analytical profiles of drug substances. Vol. 4. New York, NY: Academic Press; 1975. Hydroxyprogesterone caproate; pp. 208–224. [Google Scholar]
- 23.Kuehl P, Zhang J, Lin Y, et al. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nature genetics. 2001;27(4):383–391. doi: 10.1038/86882. [DOI] [PubMed] [Google Scholar]
- 24.Caritis SN, Sharma S, Venkataramanan R, et al. Pharmacology and placental transport of 17-hydroxyprogesterone caproate in singleton gestation. American journal of obstetrics and gynecology. 2012;207(5):398 e391–398. doi: 10.1016/j.ajog.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cuppett CD, Zhao Y, Caritis S, Zhang S, Zhao W, Venkataramanan R. Effect of endogenous steroid hormones on 17-alpha-hydroxyprogesterone caproate metabolism. American journal of obstetrics and gynecology. 2013;208(1):86 e81–86. doi: 10.1016/j.ajog.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 26.Caritis SN, Sharma S, Venkataramanan R, et al. Pharmacokinetics of 17- hydroxyprogesterone caproate in multifetal gestation. American journal of obstetrics and gynecology. 2011;205(1):40 e41–48. doi: 10.1016/j.ajog.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Durnwald CP, Lynch CD, Walker H, Iams JD. The effect of treatment with 17 alpha-hydroxyprogesterone caproate on changes in cervical length over time. American journal of obstetrics and gynecology. 2009;201(4):410 e411–415. doi: 10.1016/j.ajog.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Brien JM, Defranco EA, Adair CD, et al. Effect of progesterone on cervical shortening in women at risk for preterm birth: secondary analysis from a multinational, randomized, double-blind, placebo-controlled trial. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2009;34(6):653–659. doi: 10.1002/uog.7338. [DOI] [PubMed] [Google Scholar]
- 29.Hassan SS, Romero R, Vidyadhari D, et al. Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter, randomized, double-blind, placebo-controlled trial. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2011;38(1):18–31. doi: 10.1002/uog.9017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson L, Martin W, Higgins C, Nelson SM, Norman JE. The effect of progesterone on myometrial contractility, potassium channels, and tocolytic efficacy. Reproductive sciences. 2009;16(11):1052–1061. doi: 10.1177/1933719109340926. [DOI] [PubMed] [Google Scholar]
- 31.Ruddock NK, Shi SQ, Jain S, et al. Progesterone, but not 17-alpha-hydroxyprogesterone caproate, inhibits human myometrial contractions. American journal of obstetrics and gynecology. 2008;199(4):391 e391–397. doi: 10.1016/j.ajog.2008.06.085. [DOI] [PubMed] [Google Scholar]
- 32.Cakmak H, Schatz F, Huang ST, et al. Progestin suppresses thrombin- and interleukin-1beta-induced interleukin-11 production in term decidual cells: implications for preterm delivery. The Journal of clinical endocrinology and metabolism. 2005;90(9):5279–5286. doi: 10.1210/jc.2005-0210. [DOI] [PubMed] [Google Scholar]
- 33.Hardy DB, Janowski BA, Corey DR, Mendelson CR. Progesterone receptor plays a major antiinflammatory role in human myometrial cells by antagonism of nuclear factor-kappaB activation of cyclooxygenase 2 expression. Molecular endocrinology. 2006;20(11):2724–2733. doi: 10.1210/me.2006-0112. [DOI] [PubMed] [Google Scholar]
- 34.Ji H, Long V, Briody V, Chien EK. Progesterone modulates integrin {alpha}2 (ITGA2) and {alpha}11 (ITGA11) in the pregnant cervix. Reproductive sciences. 2011;18(2):156–163. doi: 10.1177/1933719110382305. [DOI] [PubMed] [Google Scholar]
- 35.Loudon JA, Elliott CL, Hills F, Bennett PR. Progesterone represses interleukin-8 and cyclo-oxygenase-2 in human lower segment fibroblast cells and amnion epithelial cells. Biology of reproduction. 2003;69(1):331–337. doi: 10.1095/biolreprod.102.013698. [DOI] [PubMed] [Google Scholar]
- 36.Marx SG, Wentz MJ, Mackay LB, et al. Effects of progesteron on iNOS, COX-2, and collagen expression in the cervix. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 2006;54(6):623–639. doi: 10.1369/jhc.5A6759.2006. [DOI] [PubMed] [Google Scholar]
- 37.Murtha AP, Feng L, Yonish B, Leppert PC, Schomberg DW. Progesterone protects fetal chorion and maternal decidua cells from calcium-induced death. American journal of obstetrics and gynecology. 2007;196(3):257 e251–255. doi: 10.1016/j.ajog.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 38.Xu H, Gonzalez JM, Ofori E, Elovitz MA. Preventing cervical ripening: the primary mechanism by which progestational agents prevent preterm birth? American journal of obstetrics and gynecology. 2008;198(3):314 e311–318. doi: 10.1016/j.ajog.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 39.Chwalisz K, Stockemann K, Fuhrmann U, Fritzemeier KH, Einspanier A, Garfield RE. Mechanism of action of antiprogestins in the pregnant uterus. Annals of the New York Academy of Sciences. 1995;761:202–223. doi: 10.1111/j.1749-6632.1995.tb31380.x. [DOI] [PubMed] [Google Scholar]
- 40.Clark K, Ji H, Feltovich H, Janowski J, Carroll C, Chien EK. Mifepristone-induced cervical ripening: structural, biomechanical, and molecular events. American journal of obstetrics and gynecology. 2006;194(5):1391–1398. doi: 10.1016/j.ajog.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 41.Leonhardt SA, Edwards DP. Mechanism of action of progesterone antagonists. Experimental biology and medicine. 2002;227(11):969–980. doi: 10.1177/153537020222701104. [DOI] [PubMed] [Google Scholar]
- 42.Lockwood CJ, Krikun G, Papp C, Aigner S, Schatz F. Biological mechanisms underlying the clinical effects of RU 486: modulation of cultured endometrial stromal cell plasminogen activator and plasminogen activator inhibitor expression. The Journal of clinical endocrinology and metabolism. 1995;80(4):1100–1105. doi: 10.1210/jcem.80.4.7714076. [DOI] [PubMed] [Google Scholar]
- 43.Mills AA, Yonish B, Feng L, Schomberg DW, Heine RP, Murtha AP. Characterization of progesterone receptor isoform expression in fetal membranes. American journal of obstetrics and gynecology. 2006;195(4):998–1003. doi: 10.1016/j.ajog.2006.06.040. [DOI] [PubMed] [Google Scholar]
- 44.Kastner P, Krust A, Turcotte B, et al. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. The EMBO journal. 1990;9(5):1603–1614. doi: 10.1002/j.1460-2075.1990.tb08280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Conneely OM, Mulac-Jericevic B, Lydon JP, De Mayo FJ. Reproductive functions of the progesterone receptor isoforms: lessons from knock-out mice. Molecular and cellular endocrinology. 2001;179(1–2):97–103. doi: 10.1016/s0303-7207(01)00465-8. [DOI] [PubMed] [Google Scholar]
- 46.Goldman S, Shalev E. Progesterone receptor isoforms profile, modulate matrix metalloproteinase 2 expression in the decidua. American journal of obstetrics and gynecology. 2007;197(6):604 e601–608. doi: 10.1016/j.ajog.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 47.Merlino AA, Welsh TN, Tan H, et al. Nuclear progesterone receptors in the human pregnancy myometrium: evidence that parturition involves functional progesterone withdrawal mediated by increased expression of progesterone receptor-A. The Journal of clinical endocrinology and metabolism. 2007;92(5):1927–1933. doi: 10.1210/jc.2007-0077. [DOI] [PubMed] [Google Scholar]
- 48.Pieber D, Allport VC, Bennett PR. Progesterone receptor isoform A inhibits isoform B-mediated transactivation in human amnion. European journal of pharmacology. 2001;427(1):7–11. doi: 10.1016/s0014-2999(01)01189-x. [DOI] [PubMed] [Google Scholar]
- 49.Guoyang L, Morgan T, Bahtiyar MO, et al. Single nucleotide polymorphisms in the human progesterone receptor gene and spontaneous preterm birth. Reproductive sciences. 2008;15(2):147–155. doi: 10.1177/1933719107310990. [DOI] [PubMed] [Google Scholar]
- 50.Manuck TA, Major HD, Varner MW, Chettier R, Nelson L, Esplin MS. Progesterone receptor genotype, family history, and spontaneous preterm birth. Obstetrics and gynecology. 2010;115(4):765–770. doi: 10.1097/AOG.0b013e3181d53b83. [DOI] [PubMed] [Google Scholar]
- 51.Manuck TA, Lai Y, Meis PJ, et al. Progesterone receptor polymorphisms and clinical response to 17-alpha-hydroxyprogesterone caproate. American journal of obstetrics and gynecology. 2011;205(2):135 e131–139. doi: 10.1016/j.ajog.2011.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ehn NL, Cooper ME, Orr K, et al. Evaluation of fetal and maternal genetic variation in the progesterone receptor gene for contributions to preterm birth. Pediatric research. 2007;62(5):630–635. doi: 10.1203/PDR.0b013e3181567bfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Caritis SN, Venkataramanan R, Thom E, et al. Relationship between 17-alpha hydroxyprogesterone caproate concentration and spontaneous preterm birth. American journal of obstetrics and gynecology. 2014;210(2):128 e121–126. doi: 10.1016/j.ajog.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Caritis SN, Simhan HN, Zhao Y, et al. Relationship between 17-hydroxyprogesterone caproate concentrations and gestational age at delivery in twin gestation. American journal of obstetrics and gynecology. 2012;207(5):396 e391–398. doi: 10.1016/j.ajog.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sharma S, Ellis EC, Dorko K, et al. Metabolism of 17alpha-hydroxyprogesterone caproate, an agent for preventing preterm birth, by fetal hepatocytes. Drug metabolism and disposition: the biological fate of chemicals. 2010;38(5):723–727. doi: 10.1124/dmd.109.029918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yan R, Nanovskaya TN, Zharikova OL, Mattison DR, Hankins GD, Ahmed MS. Metabolism of 17alpha-hydroxyprogesterone caproate by hepatic and placental microsomes of human and baboons. Biochemical pharmacology. 2008;75(9):1848–1857. doi: 10.1016/j.bcp.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carbone JP, Brent RL. Genital and nongenital teratogenesis of prenatal progestogen therapy: the effects of 17 alpha-hydroxyprogesterone caproate on embryonic and fetal development and endochondral ossification in the C57B1/6J mouse. American journal of obstetrics and gynecology. 1993;169(5):1292–1298. doi: 10.1016/0002-9378(93)90296-u. [DOI] [PubMed] [Google Scholar]
- 58.Johnstone EE, Franklin RR. Assay of Progestins for Fetal Virilizing Properties Using the Mouse. Obstetrics and gynecology. 1964;23:359–362. [PubMed] [Google Scholar]
- 59.Seegmiller RE, Nelson GW, Johnson CK. Evaluation of the teratogenic potential of delalutin (17 alpha-hydroxyprogesterone caproate) in mice. Teratology. 1983;28(2):201–208. doi: 10.1002/tera.1420280208. [DOI] [PubMed] [Google Scholar]
- 60.Suchowsky G, Junkmann K. Research on the maintenance of pregnancy by 17 alpha-hydroxyprogesterone caproate in the castrated pregnant rabbit. Acta endocrinologica. 1958;28(2):129–131. [PubMed] [Google Scholar]
- 61.Varma TR, Morsman J. Evaluation of the use of Proluton-Depot (hydroxyprogesterone hexanoate) in early pregnancy. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 1982;20(1):13–17. doi: 10.1016/0020-7292(82)90039-x. [DOI] [PubMed] [Google Scholar]
- 62.Michaelis J, Michaelis H, Gluck E, Koller S. Prospective study of suspected associations between certain drugs administered during early pregnancy and congenital malformations. Teratology. 1983;27(1):57–64. doi: 10.1002/tera.1420270109. [DOI] [PubMed] [Google Scholar]
- 63.Resseguie LJ, Hick JF, Bruen JA, Noller KL, O’Fallon WM, Kurland LT. Congenital malformations among offspring exposed in utero to progestins, Olmsted County, Minnesota, 1936–1974. Fertility and sterility. 1985;43(4):514–519. doi: 10.1016/s0015-0282(16)48490-6. [DOI] [PubMed] [Google Scholar]
- 64.Check JH, Rankin A, Teichman M. The risk of fetal anomalies as a result of progesterone therapy during pregnancy. Fertility and sterility. 1986;45(4):575–577. doi: 10.1016/s0015-0282(16)49292-7. [DOI] [PubMed] [Google Scholar]
- 65.Northen AT, Norman GS, Anderson K, et al. Follow-up of children exposed in utero to 17 alpha-hydroxyprogesterone caproate compared with placebo. Obstetrics and gynecology. 2007;110(4):865–872. doi: 10.1097/01.AOG.0000281348.51499.bc. [DOI] [PubMed] [Google Scholar]


