Abstract
Whether patients receive guideline-concordant opioid therapy (OT) is largely unknown and may vary based on provider and patient characteristics. We assessed the extent to which HIV-infected and uninfected patients initiating long-term (≥90-days) OT received care concordant with American Pain Society/American Academy of Pain Medicine and Department of Veterans Affairs/Department of Defense guidelines by measuring receipt of 17 indicators during the first 6 months of OT. Of 20,753 patients, HIV-infected patients (n= 6,604) were more likely than uninfected patients to receive a primary care provider (PCP) visit within 1-month (52.0% vs. 30.9%) and 6-months (90.7% vs. 73.7%) and urine drug tests (UDTs) within 1-month (14.8% vs. 11.5%) and 6-months (19.5% vs. 15.4%; all p < .001). HIV-infected patients were also more likely to receive OT concurrent with sedatives (24.6% vs. 19.6%) and an untreated substance use disorder (SUD; 21.6% vs. 17.2%). Among both patient groups, only modest changes in guideline-concordance were observed over time: UDTs and OT concurrent with untreated SUDs increased, while sedative co-prescriptions decreased (all p for trend < .001). Over a 10-year period, on average, patients received no more than 40% of recommended indicators. OT guideline-concordant care is rare in primary care, varies by patient/provider characteristics, and has undergone few changes over time.
Perspective
The promulgation of OT clinical guidelines has not resulted in substantive changes over time in OT management, which falls well short of the standard recommended by leading medical societies. Strategies are needed to increase the provision of OT guideline-concordant care for all patients.
Keywords: Opioid analgesics, practice guideline, quality of health care, chronic pain, HIV
Prescription opioids, medications once largely reserved for the treatment of severe acute pain and end-of-life cancer pain, are now routinely used by primary care physicians for the treatment of moderate to severe chronic non-cancer pain,6, 8, 26, 41, 50 a trend that is increasingly controversial.25, 26, 31, 45, 48 Rates of opioid-related serious adverse events, including unprecedented rates of addiction to prescription opioids as well as deaths from unintentional overdose, have risen in parallel with opioid prescribing.5, 10, 11, 15, 17, 32, 40
Partly in response to these trends, the American Pain Society (APS)/American Academy of Pain Medicine (AAPM) and the Department of Veterans Affairs (VA)/Department of Defense (DoD) have published guidelines and consensus statements over the past 17 years to assist clinicians in managing chronic pain with opioid therapy (OT).1, 12, 39, 53, 54 These documents stipulate that initiation of long-term opioid therapy should be preceded by a risk assessment, followed by frequent monitoring.
Adherence to clinical guidelines varies by medical specialty, provider expertise, and patient population.19, 24, 34, 43, 44 We hypothesize that HIV-infection, in particular, and its association with chronic pain and medical and psychiatric comorbidities, is likely to present obstacles to the receipt of guideline-concordant care for patients receiving long-term OT. Specifically, providers and patients must manage OT in the context of the competing demands of medical, psychiatric and substance use comorbidities, and accordingly, polypharmacy.7, 14, 18, 30, 38, 42, 46 Moreover, military veterans, in general, suffer from a high prevalence of chronic pain, particularly veterans returning from Afghanistan and Iraq (i.e., Operation Enduring Freedom and Operation Iraqi Freedom, respectively).46 To date, no studies have examined the provision of OT guideline-concordant care. The objective of this study is to examine the extent to which OT guideline-concordant care was provided in HIV-infected and uninfected veterans.
Materials and Methods
Study Overview
We conducted a retrospective analysis to examine the extent to which patient care was concordant with select APS/AAPM1, 12 and VA/DoD53, 54 OT recommendations among a large sample of patients receiving care in the VA healthcare system. Using electronic medical record (EMR) data, we examined receipt of OT guideline-concordant care among HIV-infected and uninfected patients initiating long-term OT as outpatients between fiscal years 1998 and 2010.
Data Source
EMR data, including administrative, clinical, laboratory, and pharmacy data, were obtained from the Veterans Aging Cohort Study-Virtual Cohort (VACS-VC), a prospective cohort of HIV-infected patients matched by age, sex, race, and VA site-of-care to uninfected controls.20 Details regarding the VACS-VC are published elsewhere.16, 21, 22
The VACS-VC is HIPAA compliant and has received approval from the Review Boards for the VA Connecticut Healthcare System and the Yale School of Medicine; the requirement for informed consent was waived.
Study Population
Patients who initiated OT as outpatients between 1998 and 2010 were eligible for inclusion. The cohort was restricted to patients who received incident long-term OT to allow us to assess OT guideline-concordant care at the beginning of treatment, starting with the first prescription written for OT, and continuing until 6 months after OT initiation (patients not reaching 6 months of OT were followed through OT stop date); a follow-up period of 6 months was chosen because it represents a time of increased risk for adverse events, particularly for opioid naïve patients.12
Patients initiating OT were identified through the VA's Pharmacy Benefits Management (PBM) database. Long-term OT was defined as greater than or equal to 90 days of prescribed opioids (allowing for a 30-day window for prescription refills).13, 15, 26 We included prescriptions for oral and transdermal opioids; methadone and buprenorphine prescribed for opioid dependence were excluded. We excluded patients who received an International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) code9 for palliative/end of life care (V66.7) prior to OT initiation (n=99), those whose follow-up period extended beyond the end of 2010 (n=1,039), and those who died prior to receiving opioids for 90 days (n=328).
Demographic and Clinical Characteristics
Demographic characteristics were derived from the VA National Patient Care Database.52 Clinical characteristics were based on ICD-9-CM codes and, when applicable, laboratory results (e.g., HIV/hepatitis C [HCV]). Variables reflecting current mental health, substance use disorder (SUD), and pain diagnoses were based on ICD-9-CM codes received between OT initiation and 6 months of follow-up (or OT stop date, when applicable); lifetime prevalence was based on ICD-9-CM codes received any time prior to OT initiation. Medication variables were identified through PBM data; treatment/procedure variables were identified through administrative codes.
Guidelines and Indicators
Operational definitions for the OT guideline indicators (Fig. 1) were based on published national documents.1, 12, 53, 54 Select indicators were chosen for study inclusion. We first identified the indicators thought to be the most important to patient safety: risk assessment, monitoring, care of high-risk patients, side-effects management, and chronic pain co-interventions. Within these indicators, we excluded those related to patient groups for which we would have insufficient power to assess guideline-concordant care (e.g., opioid use in pregnancy). We excluded indicators beyond the scope of routine care (e.g., driving and work safety) and those for which data were not contained in our databases (e.g., informal patient counseling).
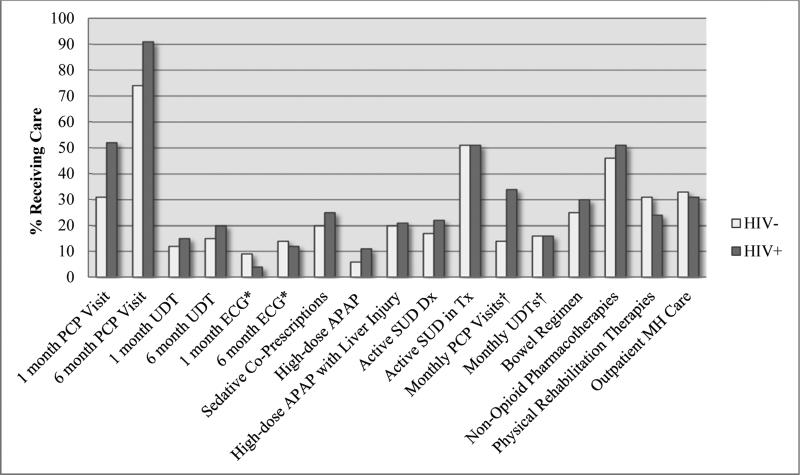
Figure 1. Receipt of OT Guideline-Concordant Care by HIV Status.
Abbreviations: APAP, acetaminophen; Dx, diagnosis; ECG, electrocardiogram; MH, mental health; PCP, primary care provider; SUD, substance use disorder; Tx, treatment; UDT, urine drug test.
*Electrocardiograms measured only among patients receiving methadone for chronic pain.
†Monthly PCP Visits and UDTs measured only among those with an active SUD.
Differences in ECG receipt (1 month or 6 months), APAP with liver injury, active SUD treatment, and monthly UDTs were not significant (P > .05); MH care was significant at P=.001; all other indicators were significant at P < .001.
Outcomes
Operational definitions (Table 1) for receipt of OT guideline indicators were operationalized a priori through consultation with the literature35 and with practicing clinicians with expertise in Addiction Medicine, Clinical Epidemiology, Health-Services Research, HIV, Pain Management, and Primary Care. We based operational definitions on specific recommendations from the guidelines regarding how often patients should be seen or monitored. When these details were missing, we used consensus definitions based on a minimum standard of care. Unless otherwise noted, the follow-up period of interest is from OT start date through 6 months. With the exception of indicators related to urine drug tests (UDTs), all indicators were assessed from 1998 to 2010; UDT data were available starting in 2000.
Table 1.
Opioid Therapy Guideline Indicators, Operational Definitions, and Sources
| Guideline Indicators | Operational Definition(s) | Source |
|---|---|---|
| Monitoring | ||
| Clinicians should conduct a follow-up visit within 2-4 weeks of OT initiation. This initial phase should be considered a therapeutic trial, for which opioid-naive patientsa are particularly at risk.b | 1. Any documented outpatient PCP visit (VA general medical or HIV-specialty clinic) between OT start date and end of 30-days of OT. | APS/AAPM, 199710 & 200911 VA/DoD 200312 & 201013 |
| As part of a comprehensive patient assessment, clinicians should obtain a UDT to assess for aberrant drug-related behaviors in all patients prior to initiating OT. | 2. Laboratory documentation of UDT (i.e., toxicology) 30-days before or after OT start date. | APS/AAPM, 2009 VA/DoD 2003 & 2010 |
| For patients receiving methadone for chronic pain, clinicians should obtain a pretreatment ECG to measure QTc interval before initiating OT. | 3. ECG results obtained 30-days before or after OT start date. | VA/DoD 2003 & 2010 |
| Clinicians should routinely reassess all patients on OT every 1 to 6 months for risks and benefits of treatment for duration of OT.b | 4. Any documented outpatient PCP visit between OT start date and end of 180-days of OT (or OT stop date for patients on OT < 6 months). | APS/AAPM, 1997 & 2009 VA/DoD 2003 & 2010 |
| Clinicians should routinely confirm adherence to OT plan of care in all patients through periodic UDTs. | 5. Laboratory documentation of UDT between OT start date and end of 180-days of OT (or OT stop date). | APS/AAPM, 2009 VA/DoD 2003 & 2010 |
| For patients receiving methadone for chronic pain, clinicians should obtain a follow-up ECG to measure QTc interval once methadone dose is stabilized. | 6. ECG results for test(s) obtained between OT start date and end of 180-days of OT (or OT stop date). | VA/DoD, 2010 |
| Co-Prescription of High-Risk Medications | ||
| Clinicians should avoid co-prescription of sedatives and OT. | 7. Pharmacy documentation that patient prescribed benzodiazepines (≥ 7-days so as to exclude prescriptions for acute indications [e.g., pre-operative sedation]), carisoprodol, or barbiturates between OT start date and end of 180-days of OT (or OT stop date). | VA/DoD, 2010 |
| When using opioid combination products, clinicians should not exceed maximum recommended daily doses of prescribed acetaminophen. | 8.Among all patients, pharmacy documentation that patient prescribed an average daily dose ≥ 4 grams/day57 between OT start date and end of 180-days of OT (or OT stop date). 9.Among patients with liver injury (hepatitis C virus, end-stage liver disease, decompensated liver disease, or Fib-4 Index > 3.25), pharmacy documentation that patient prescribed an average daily dose ≥ 2 grams/day58,59 between OT start date and end of 180-days of OT (or OT stop date). |
VA/DoD 2003 & 2010 |
| High-Risk Patients | ||
| Clinicians may consider OT for patients with a history of SUD only if they are able to implement more frequent and stringent monitoring parameters. | 10. Documentation of monthly VA PCP visits between OT start date and end of 180-days of OT (or OT stop date). 11. Documentation of monthly UDTs between OT start date and end of 180-days of OT (or OT stop date). |
APS/AAPM, 1997 & 2009 VA/DoD, 2003 & 2010 |
| Clinicians should initiate OT with caution in patients with a history of SUD and should never initiate OT in patients with an active disorder who are not in SUD treatment. | 12. Examined by SUD treatment status: Documentation of any of following between OT start and end of 180-days of OT (or OT stop date): ICD-9-CM code for an alcohol or drug use disorder SUD treatment: 1 inpatient bed day or 1 outpatient SUD-specialty clinic visit Audit-C score ≥ 4 |
VA/DoD, 2003 & 2010 |
| Side-Effects Management | ||
| Clinicians should consider prescribing a bowel regimen to all OT patients. | 13. Pharmacy documentation that patient prescribed stool softeners and/or laxatives between OT start date and end of 180-days of OT (or OT stop date). | APS/AAPM, 1997 & 2009 VA/DoD, 2003 & 2010 |
| Chronic Pain Co-Interventions | ||
| Clinicians should avoid relying exclusively on opioids for the management of chronic pain and should routinely take a multidisciplinary approach to pain management that includes the integration of non-opioid pharmacotherapies, rehabilitation or functional restoration, and psychotherapeutic interventions. | 14.Non-Opioid Pharmacotherapies Pharmacy documentation that patient prescribed tricyclic antidepressants, gabapentin, or NSAIDsc between OT start date and end of 180-days of OT (or OT stop date). 15.Physical Rehabilitation Therapies Any documented outpatient visits to a VA physical therapy, occupational therapy, or rehabilitation clinic anytime between OT start date and end of 180-days of OT (or OT stop date). 16.Psychotherapeutic Co-Interventions Any two documented outpatient visits to a VA mental health clinic between OT start date and end of 180-days of OT (or OT stop date). |
APS/AAPM, 1997 & 2009 VA/DoD, 2003 & 2010 |
Abbreviations: APS, American Pain Society; AAPM; American Academy of Pain Medicine; AUDIT-C, Alcohol Use Disorders Identification Test-Consumption; DoD, Department of Defense; ECG, electrocardiogram; ICD-9-CM, International Classification of Disease, Ninth Revision, Clinical Modification codes; NSAIDs, non-steroidal anti-inflammatory drugs; OT, opioid therapy; PCP, primary care provider; QTc, rate-corrected QT interval; SUD, substance use disorder; UDT, urine drug test; VA, Veterans Administration.
All patients in this current study are considered opioid-naive (i.e., incident OT patients).
Only the VA/DoD guidelines specify an exact time period.
Does not include acetaminophen.
Temporal Trends
Individual OT guideline-indicators
In addition to examining receipt of OT guideline indicators using pooled data (1998-2010), we examined temporal trends by categorizing yearly data into three distinct time periods: 1998-2003, 2004-2009, and 2010. These periods were chosen to allow for the implementation of new or updated guidelines.
Summary scores
To determine the proportion of patients receiving OT guideline-concordant care annually, we generated summary scores27, 33, 47 by dividing the number of OT guideline indicators received per patient by the number of indicators for which the patient was eligible; scores were then multiplied by 100 and expressed as percentages. All indicators were assigned equal weight, as the association between OT guideline indicators and outcomes is unknown. For each year of data, a mean summary score was then calculated from these patient-specific scores (Fig. 2). As UDT data, a component of the summary score, were not available until 2000, summary scores were evaluated from 2000-2010.
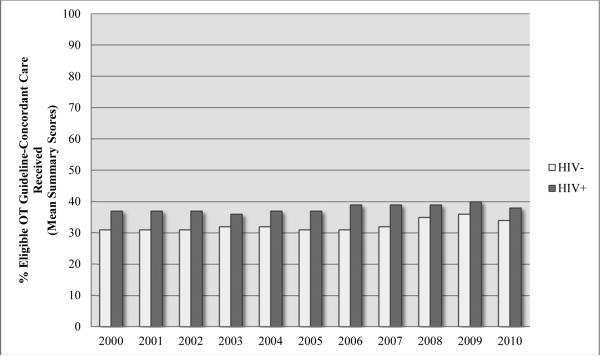
Figure 2. Temporal Trends in Receipt of OT Guideline-Concordant Care.
Abbreviations: OT, opioid therapy.
HIV+: p=.27; HIV-: p <.001
Electronic Medical Record Review of PCP and Mental Health Visits
To provide insight into the extent to which PCP visits identified in the EMR addressed OT, two reviewers independently conducted chart reviews on a random sample of 100 patients (stratified by HIV status) receiving a PCP visit during the follow-up period. From the progress notes, reviewers cataloged whether opioids were listed (e.g. present on a computer generated medication list); commented on in the narrative notes; and/or assessed with respect to safety and/or efficacy. We repeated this process among patients receiving two or more outpatient mental health visits and cataloged whether chronic pain was commented on in the narrative notes, whether visits focused on chronic pain management, and whether psychotherapeutic interventions for chronic pain were provided.
Statistical Analyses
Frequencies, means, and proportions were used to characterize the sample at baseline (i.e., date of OT initiation) and to describe receipt of OT guideline indicators. Bivariate comparisons by HIV status were assessed with χ2-tests and ANOVA, as appropriate. Nonparametric methods were used when indicated. Associations between HIV status and receipt of OT guideline indicators were quantified by odds ratios and corresponding 95% confidence intervals using unadjusted and multivariable logistic regression. Temporal trends in the receipt of OT guideline indicators were assessed using χ2-tests for trend. Cohen's kappa (k) statistics were calculated to assess inter-rater agreement for the chart review. All analyses were performed using SAS software, version 9.2 (SAS Institute, Cary, NC). As a conservative measure, we chose to apply a Bonferroni correction to adjust for multiple comparisons. Specifically, a 2-sided statistical significance level of 0.001 was applied to all analyses.
Results
We identified 20,753 patients initiating long-term OT between 1998 and 2010, among whom 6,604 (31.8%) were HIV-infected (Table 2). We report key findings by HIV status below and in Figures 1 and 2. Multivariable associations are shown in Table 3.
Table 2.
Patient Demographic and Clinical Characteristics at OT Initiation: Overall and by HIV status (n=20,753)
| Overall (n=20,753) | HIV+ (n=6,604) | HIV-(n=14,149) | P Value* | |
|---|---|---|---|---|
| Age, mean (SD), y | 49.6 (9.2) | 49.7 (8.9) | 49.5 (9.4) | .42 |
| Gender, n (%) | ||||
| Male | 20,276 (97.7) | 6,428 (97.3) | 13,848 (97.9) | .02 |
| Race/Ethnicity, n (%) | < .001 | |||
| White | 10,169 (49.0) | 3,136 (47.5) | 7,033 (49.7) | |
| Black | 8,682 (41.8) | 2,898 (43.8) | 5,784 (40.9) | |
| Hispanic | 1,333 (6.4) | 376 (5.7) | 957 (6.8) | |
| Other | 569 (2.7) | 194 (2.9) | 375 (2.7) | |
| HCV-Infected, n (%) | 6,002 (28.9) | 2,971 (45.0) | 3,031 (21.4) | < .001 |
| Diabetes, n (%) | 6,269 (30.2) | 1,566 (23.7) | 4,703 (33.2) | < .001 |
| BMI, mean (SD) | 28.4 (6.4) | 25.6 (5.2) | 29.6 (6.5) | < .001 |
| Pain Comorbidities, n (%)a | ||||
| Chronic Painb | 11,836 (57.0) | 3,129 (47.4) | 8,707 (61.5) | < .001 |
| Acute Painc | 2,700 (13.0) | 936 (14.2) | 1,764 (12.5) | < .001 |
| No Pain Diagnosis | 7,855 (37.9) | 3,025 (45.8) | 4,830 (34.1) | < .001 |
| Any Mental Illness, n (%)a | 7,126 (34.3) | 2,185 (33.1) | 4,941 (34.9) | <.01 |
| Anxiety/Depression | 4,564 (22.0) | 1,520 (23.0) | 3,044 (21.5) | .01 |
| Serious Mental Illnessd | 4,138 (19.9) | 1,114 (16.9) | 3,024 (21.4) | <.001 |
| History of Mental Illnesse | 11,164 (53.8) | 3,693 (55.9) | 7,471 (52.8) | <.001 |
| Substance Use Disorder, n (%)a | 3,439 (16.6) | 1,279 (19.4) | 2,160 (15.3) | <.001 |
| Alcohol Use Disorder | 2,177 (10.5) | 729 (11.0) | 1,448 (10.2) | .08 |
| Drug Use Disorder | 2,214 (10.7) | 943 (14.3) | 1,271 (9.0) | <.001 |
| History of Substance Use Disorder, n (%)e | 7,867 (37.9) | 2,853 (43.2) | 5,014 (35.4) | <.001 |
| VACS Index, mean (SD) | 25.0 (21.0) | 36.9 (23.7) | 18.2 (15.8) | <.001 |
| CD4 count, median (IQR), cells/μL | --- | 386.5 (165, 543) | --- | --- |
| HIV-1 RNA, Log 10 Viral Load, < 500 copies/ml, n (%) | --- | 2,409 (54) | --- | --- |
| OT Duration, median (IQR), days | 225 (139, 576) | 235 (141, 605) | 220 (138, 561) | .002 |
Abbreviations: BMI, body mass index; HCV, hepatitis C virus; IQR, interquartile range; OT, opioid therapy; PTSD, post-traumatic stress disorder; SD, standard deviation; TMJ, temporomandibular disorder; VACS, Veterans Aging Cohort Study.
P values: t-test (or non-parametric equivalent) for continuous variables. c2-test for categorical variables.
Current diagnosis: from OT index date through follow-up.
Chronic pain: headache, TMJ, neck, back, extremity, arthritis, neuropathy, other.
Acute pain: abdominal, chest fracture, or kidney stones.
Serious Mental Illness: bipolar disorder, PTSD, schizophrenia, schizoaffective disorder, and psychosis.
Lifetime prevalence.
Table 3.
Odds Ratios for Receipt of Guideline-Concordant Care for HIV-infected vs. Uninfected Patients
| Guideline Indicators | Unadjusted OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|
| 1 month PCP Visit | 2.40 (2.26, 2.55) | 2.49 (2.28, 2.70) |
| 6 month PCP Visit | 3.48 (3.18, 3.81) | 5.94 (5.13, 6.87) |
| 1 month UDT | 1.33 (1.21, 1.46) | 1.00 (0.88, 1.14) |
| 6 month UDT | 1.33 (1.22, 1.45) | 1.12 (1.00, 1.27) |
| 1 month ECGab | 0.43 (0.17, 1.09) | ----- |
| 6 month ECGab | 0.87 (0.47, 1.58) | ----- |
| Sedative Co-Prescriptions | 1.34 (1.25, 1.44) | 1.56 (1.41, 1.73) |
| Benzodiazepines Co-Prescriptions | 1.40 (1.31, 1.51) | 1.57 (1.41, 1.74) |
| APAP Exceeding Recommended Doses | 2.03 (1.83, 2.26) | 1.45 (1.25, 1.69) |
| APAP Exceeding Recommended Doses Concurrent with Liver Injury | 1.08 (0.95, 1.22) | 1.06 (0.89, 1.25) |
| Opioids Concurrent with Active SUD | 1.33 (1.23, 1.43) | 0.92 (0.82, 1.04) |
| SUD Treatmentc | 1.01 (0.88, 1.14) | 1.17 (0.96, 1.41) |
| Monthly PCP Visitsc | 3.14 (2.65, 3.70) | 3.81 (3.03, 4.81) |
| Monthly UDTsc | 1.05 (0.86, 1.29) | 0.97 (0.74, 1.28) |
| Provision of Bowel Regimen | 1.28 (1.20, 1.37) | 0.78 (0.71, 0.86) |
| Provision of Non-Opioid Pharmacotherapies | 1.22 (1.15, 1.30) | 1.71 (1.57, 1.86) |
| Provision of Physical Rehabilitative Therapies | 0.72 (0.67, 0.77) | 0.82 (0.75, 0.91) |
| Provision of Outpatient Mental Health Care | 0.90 (0.85, 0.96) | 0.84 (0.76, 0.93) |
Abbreviations: APAP, acetaminophen; ECG, electrocardiogram; OR, odds ratio, PCP, primary care provider; SUD, substance use disorder; UDT, urine drug test.
Electrocardiograms measured only among patients receiving methadone for chronic pain.
Adjusted models were not evaluated for ECGs due to the small number of outcome events relative to predictors.
SUD treatment, monthly PCP Visits, and monthly UDTs measured only among those with an active SUD.
Patient Monitoring
The median (interquartile range [IQR]) number of PCP visits over the 6 months of observation was 3.0 (2.0, 6.0) for HIV-infected patients compared to 2.0 (1.0, 3.0) for uninfected patients (p<.001). HIV-infected patients were more likely than uninfected patients to receive PCP visits within 1 month (52.0% vs. 30.9%) and 6 months (90.7% vs. 73.7%) and UDTs within 1 month (14.8% vs. 11.5%) and 6 months (19.5% vs. 15.4%) (all p<.001). Among patients prescribed methadone for chronic pain (n=397), electrocardiogram (ECG) receipt was similar for HIV-infected and uninfected patients within 1 month (3.9% vs. 8.6%; p=.07) and 6 months (12.3% vs. 14.0%; p=.64).
Co-Prescription of High-Risk Medications
HIV-infected patients were more likely to receive sedative co-prescriptions (24.6% vs. 20.0%; p <.001); ninety-two percent of these were for benzodiazepines (23.2% vs. 17.7%; p <.001). HIV-infected patients were also more likely to receive acetaminophen exceeding daily-recommended doses (11.1% vs. 5.8%; p<.001). Among patients with liver injury (n=6,305), receipt of acetaminophen exceeding daily-recommended doses was similar for HIV-infected and uninfected patients (21.0% vs. 19.8%; p=.23).
Opioid Prescribing in High-Risk Patients
HIV-infected patients were more likely to have an active SUD (21.6% vs. 17.2%; p<.001). Among active SUD patients (n=3,855), HIV-infected patients were more likely to receive monthly PCP visits (33.6% vs. 14.4%; p<.001); there was no difference between groups in the percentage engaged in SUD treatment (51.2% vs. 51.0%; p=.91) or in receipt of monthly UDTs (15.9% vs. 15.7%; p=.88).
Management of Side Effects
HIV-infected patients were more likely than uninfected patients to be prescribed a bowel regimen (29.8% vs. 24.9%; p<.001).
Provision of Chronic Pain Co-Interventions
HIV-infected patients were more likely to be prescribed concurrent non-opioid pain pharmacotherapies (51.5% vs. 46.4%; p<.001) but less likely to receive physical rehabilitation therapies (i.e., physical, occupational, or rehabilitation therapies; 24.3% vs. 30.8%; p<.001). There was no difference according to HIV status in receipt of outpatient mental health care (30.9% vs. 33.1%; p=.001) at our previously established significance level.
Temporal Trends
Individual OT guideline-indicators
Over time, among both HIV-infected and uninfected patients, there was an increase in UDTs (p for trend <.001) and OT concurrent with active SUDs (p for trend <.001), while there was decrease in high-risk co-prescribing, including for benzodiazepines (all p for trend <.001). For HIV-infected patients there was an increase in physical rehabilitative therapies (HIV-infected, p for trend <.001). Among patients with an active SUD, there was a decrease in monthly PCP visits for HIV-infected patients (p<.001) and a decrease in SUD treatment engagement for uninfected patients (p for trend=.03). ECG receipt was not evaluated due to low frequency.
Summary scores
From 2000 to 2010, receipt of guideline-concordant care varied from 36.8% to 37.5% for HIV-infected patients (p=.21) and from 31.4% to 33.4% for uninfected patients (p<.001). (Fig. 2).
Multivariable Analyses
Results from the multivariable-adjusted logistic regression models support the majority of bivariate associations. After adjustment, however, there were no longer differences in receipt of UDTs within 1 month (AOR 1.07, 95% CI 0.96-1.18) or in receipt of OT concurrent with an active SUD (AOR 1.05, 95% CI 0.96-1.16). Models were not evaluated for ECGs due to the small number of outcome events relative to predictors.
Electronic Medical Record Review of PCP and Mental Health Visits
Opioids were listed in 45% of PCP visits reviewed (k=.92) and commented on in the narrative notes in 57% of visits (k=1.00). There was mention of safety or efficacy relative to opioids for 36% of visits (k=.92). For each of these measures, results were similar for HIV-infected and uninfected patients: listed (47% vs. 44%; p=.76), commented (59% vs. 55%; p=.66), safety/efficacy (31% vs. 41%; p=.27).
Pain was commented on in 44% of mental health visits (k=.90) and the focus of 27% of visits (k=.66). Psychotherapeutic interventions for pain were provided in 3% of visits (k=1.00). HIV-infected patients were less likely than uninfected patients to have pain commented on during a mental health visit (32% vs. 56%; p=.02). For the remaining measures, results were similar for HIV-infected and uninfected patients: focus (20% vs. 34%; p=.12), intervention (2% vs. 4%; p=.56).
Discussion
From 1998 to 2010, the majority of patients initiating long-term OT did not receive OT guideline-concordant care. This was true for all patients, regardless of HIV status, and evident across all domains. While HIV-infected patients were more likely than uninfected patients to receive guideline-concordant care for the majority of measures, patient care, overall, fell short of that recommended by the guidelines. For example, we found that at most 52% of patients had a primary care visit within 1 month of starting OT. Moreover, although we found that by 6 months the majority of patients had been seen in primary care, a review of the medical records of a subset of these patients suggests that only one-third were assessed for opioid-related safety and efficacy during such visits. In addition, the vast majority of patients did not undergo a UDT within the first 6 months of care.
We also found that among the 3,855 patients (approximately 20% of the sample) receiving long-term OT concurrent with an active SUD, only 51% were engaged in SUD treatment. According to the 2003 VA/DoD guidelines, OT in the presence of an active SUD is considered a “relative” contraindication for patients not engaged in SUD treatment; with the publication of the 2010 guidelines, it was deemed an “absolute” contraindication.53, 54 Additionally, among all patients, we found that 25% of HIV-infected patients and 20% of uninfected patients were prescribed sedative medications (benzodiazepines primarily) concurrent with OT, increasing the risk for adverse outcomes, such as respiratory depression and overdose.12, 53, 54
Temporal trends in the receipt of OT guideline-concordant care suggest that clinicians are performing UDTs more frequently and prescribing benzodiazepines concurrent with OT less frequently. Less encouragingly, we found that timely PCP visits decreased over time among HIV-infected patients, while prescriptions for OT in the presence of an active SUD increased among both HIV-infected and uninfected patients. Moreover, mean summary scores indicate that over a 10-year period, patients received no more than 40% of recommended care.
Many of our findings run counter to our hypothesis that HIV-infected patients would be less likely to receive OT guideline-concordant care, possibly reflecting the complexities of caring for veterans in general. Similar to HIV-infected patients, 53% of uninfected patients had a history of mental illness and 35% a history of a SUD (Table 2). In addition, 21% of HIV-uninfected patients were HCV-infected and 33% had diabetes (Table 2). Thus, these patients presented to primary care with clinical challenges comparable to those of HIV-infected patients. Moreover, that HIV-infected patients were more likely to receive guideline-concordant care primarily for indicators related to monitoring (i.e., PCP visits/UDTs) is likely due to the increased frequency with which HIV-infected patients, in accordance with HIV guidelines, are seen in primary care.51 Additionally, many of the differences that we found, due to our large sample size, while statistically significant, may not be clinically meaningful; conversely, our findings from the chart review, where we had a much smaller sample size, may reflect the opposite (i.e., clinically relevant differences in treatment). The important message from all of these findings, we believe, is that in both groups, patients received care that did not meet the standard set by the guidelines.
Although prior research has shown that for many conditions and patient populations, clinician adherence to clinical guidelines is suboptimal,2, 4, 33, 43 this is the first study to present an extensive evaluation of longitudinal data on receipt of guideline-concordant OT. Three of the four studies that have been published in this area have focused on a subset of OT patients,36, 37, 42 and all involved regional data.28, 36, 37, 42 Consistent with our findings, these studies show deficiencies in the provision of OT across patient groups,28, 36, 37, 42 with little evidence that high-risk patients are monitored more frequently.36, 37, 49 Moreover, one study demonstrated that OT is often provided to those with untreated SUDs or with benzodiazepine co-prescriptions, and that few patients receive counseling regarding side-effects management, recommendations for nonpharmacological approaches to pain management, or mental health co-interventions.36
Our study has limitations. Specifically, we were unable to determine whether clinicians attempted to deliver OT guideline-concordant care but failed because patients did not adhere to prescribed treatments. And although we observed in a review of randomly sampled PCP and mental health visits that OT was infrequently addressed, it is possible that these issues were addressed but not documented. Additionally, for some indicators, such as adjunctive treatments, we were unable to determine whether some OT guideline indicators, such as adjunctive treatments, were provided specifically to address pain or for treatment of other comorbid conditions (e.g., rehabilitation following a stroke). Similarly, we lacked the data for this current study to determine what proportion of patients failed to receive any non-opioid interventions prior to beginning long-term OT. We also relied on ICD-9-CM data for defining co-morbidities, as a number of validation studies support the use of diagnostic codes for this purpose.3, 22, 23, 29 For ICD-9-CM codes related to chronic pain, we found that HIV-infected patients were less likely to receive a chronic pain diagnosis; we postulate that these findings may reflect differences in coding practices between Infectious Disease vs. General Medicine providers, in particular differences in their approach to pain management, with Infectious Disease providers more likely to code for infectious or medical comorbidities.13 Finally, our use of two OT guidelines, both of which were published and subsequently updated during the period of observation, would appear to present a challenge to assessing guideline-concordant care. The VA/DoD guidelines, however, were modeled on the APS/AAPM document and a review of both reveals substantial overlap.1, 12, 53, 54 Furthermore, the indicators we assessed did not vary substantively across documents and represent a minimum standard of care. In addition, by anchoring the assessment of temporal trends to one calendar year after major guideline publication dates, we were able to show that only modest changes in guideline-concordance occurred over time. The increased attention that long-term OT has received recently, however, may have resulted in more substantive changes in patient care in the time since our window of observation ended.
While our study has implications for research, policy, and clinical care, we caution against making comparisons between the observed quality of care within the VA and other medical settings. Limited information regarding OT guideline-concordant care outside of the VA exists to support such comparisons. In addition, efforts are underway in the VA to reduce harmful and ineffective care for patients receiving OT.35 Future efforts should focus on educating clinicians in all settings on the risks associated with opioid prescribing, improving awareness of guideline recommendations, and implementing tools in the clinical practice setting, such as systems-based approaches and patient-centered medical homes, to facilitate safe and effective care for patients receiving long-term OT. In addition, research is needed to understand the barriers encountered by clinicians in delivering OT guideline-concordant care.43 Importantly, research is also needed to improve the evidence-base from which the guidelines are drawn, specifically research that addresses associations between individual recommendations and patient outcomes. Only when such research is available will clinicians be able to prioritize recommended care and optimize safety and effectiveness.
Highlights.
Opioid therapy in primary care falls short of guideline recommendations
Substantive changes have not occurred in guideline-concordant care over time
Strategies are needed to increase the provision of opioid therapy guideline-concordant care
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
Research Funding: Research reported in this paper was supported by grants from the National Institute on Drug Abuse, National Institute on Alcohol Abuse and Alcoholism, National Institute of Mental Health, VA Health Services Research and Development Research Enhancement Award Program, and the Agency for Healthcare Research and Quality. These organizations had no role in the design, conduct, or reporting of this study. The content of this paper is solely the responsibility of the authors and does not necessarily reflect the official views of the National Institutes of Health, the Agency for Healthcare Research and Quality, or the Department of Veterans Affairs.
Conflicts of Interest: Dr. Fiellin received honoraria to serve on an external advisory board monitoring the diversion and abuse of buprenorphine for Pinney Associates.
References
- 1.American Pain Society/American Academy of Pain Medicine The use of opioids for treatment of chronic pain. A consensus statement from the American Academy of Pain Medicine and the American Pain Society. Clin. J. Pain. 1997;13:6–8. [PubMed] [Google Scholar]
- 2.Asch SM, Kerr EA, Keesey J, Adams JL, Setodji CM, Malik S, McGlynn EA. Who is at greatest risk for receiving poor-quality health care? N. Engl. J. Med. 2006;354:1147–1156. doi: 10.1056/NEJMsa044464. [DOI] [PubMed] [Google Scholar]
- 3.Bebu I, Tate J, Rimland D, Mesner O, Macalino GE, Ganesan A, Okulicz JF, Bavaro M, Weintrob AC, Justice AC, Agan BK. Infectious Disease Clinical Research Program HIVWG. The VACS Index Predicts Mortality in a Young, Healthy HIV Population Starting Highly Active Antiretroviral Therapy. J. Acquir. Immune Defic. Syndr. 2014;65:226–230. doi: 10.1097/QAI.0000000000000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berkenblit GV, Sosman JM, Bass M, Gebrekristos HT, Cofrancesco JJ, Sullivan LE, Cook RL, Edison M, Bashook PG, Korthuis PT. Factors affecting clinician educator encouragement of routine HIV testing among trainees. J. Gen. Intern. Med. 2012;27:839–844. doi: 10.1007/s11606-012-1985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bohnert AS, Valenstein M, Bair MJ, Ganoczy D, McCarthy JF, Ilgen MA, Blow FC. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305:1315–1321. doi: 10.1001/jama.2011.370. [DOI] [PubMed] [Google Scholar]
- 6.Braden JB, Fan MY, Edlund MJ, Martin BC, DeVries A, Sullivan MD. Trends in use of opioids by noncancer pain type 2000-2005 among Arkansas Medicaid and HealthCore enrollees: results from the TROUP study. J. Pain. 2008;9:1026–1035. doi: 10.1016/j.jpain.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breitbart W, Rosenfeld BD, Passik SD, McDonald MV, Thaler H, Portenoy RK. The undertreatment of pain in ambulatory AIDS patients. Pain. 1996;65:243–249. doi: 10.1016/0304-3959(95)00217-0. [DOI] [PubMed] [Google Scholar]
- 8.Caudill-Slosberg MA, Schwartz LM, Woloshin S. Office visits and analgesic prescriptions for musculoskeletal pain in US: 1980 vs. 2000. Pain. 2004;109:514–519. doi: 10.1016/j.pain.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention . International Classification of Diseases, Ninth Revision, Clinical Modification. National Center for Health Statistics; Hyattsville, MD: 2010. [Google Scholar]
- 10.Centers for Disease Control and Prevention Unintentional poisoning deaths--United States, 1999-2004. MMWR Morb. Mortal. Wkly. Rep. 2007;56:93–96. [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention Vital Signs: Overdoses of Prescription Opioid Pain Relievers-United States, 1999-2008. MMWR Morb. Mortal. Wkly. Rep. 2011;60:1487–1492. [PubMed] [Google Scholar]
- 12.Chou R, Fanciullo GJ, Fine PG, Adler JA, Ballantyne JC, Davies P. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J. Pain. 2:2009. doi: 10.1016/j.jpain.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edelman EJ, Gordon K, Becker WC, Goulet JL, Skanderson M, Gaither JR, Brennan Braden J, Gordon AJ, Kerns RD, Justice AC, Fiellin DA. Receipt of opioid analgesics by HIV-infected and uninfected patients. J. Gen. Intern. Med. 2013;28:82–90. doi: 10.1007/s11606-012-2189-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edelman EJ, Gordon KS, Glover J, McNicholl IR, Fiellin DA, Justice AC. The next therapeutic challenge in HIV: polypharmacy. Drugs Aging. 2013;30:613–628. doi: 10.1007/s40266-013-0093-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edlund MJ, Steffick D, Hudson T, Harris KM, Sullivan M. Risk factors for clinically recognized opioid abuse and dependence among veterans using opioids for chronic non-cancer pain. Pain. 2007;129:355–362. doi: 10.1016/j.pain.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 16.Fultz SL, Skanderson M, Mole LA, Gandhi N, Bryant K, Crystal S, Justice AC. Development and verification of a “virtual” cohort using the National VA Health Information System. Med. Care. 2006;44:S25–30. doi: 10.1097/01.mlr.0000223670.00890.74. [DOI] [PubMed] [Google Scholar]
- 17.Hall AJ, Logan JE, Toblin RL, Kaplan JA, Kraner JC, Bixler D, Crosby AE, Paulozzi LJ. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA. 2008;300:2613–2620. doi: 10.1001/jama.2008.802. [DOI] [PubMed] [Google Scholar]
- 18.Hansen L, Penko J, Guzman D, Bangsberg DR, Miaskowski C, Kushel MB. Aberrant behaviors with prescription opioids and problem drug use history in a community-based cohort of HIV-infected individuals. J. Pain Symptom Manage. 2011;42:893–902. doi: 10.1016/j.jpainsymman.2011.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jatulis DE, Meng YY, Elashoff RM, Schocket AL, Evans RM, Hasan AG, Legorreta AP. Preventive pharmacologic therapy among asthmatics: five years after publication of guidelines. Ann. Allergy. Asthma. Immunol. 1998;81:82–88. doi: 10.1016/S1081-1206(10)63113-4. [DOI] [PubMed] [Google Scholar]
- 20. [June 16, 2014];Journal of Pain Author Information. http://www.jpain.org/authorinfo - idp1328240.
- 21.Justice AC, Dombrowski E, Conigliaro J, Fultz SL, Gibson D, Madenwald T, Goulet J, Simberkoff M, Butt AA, Rimland D, Rodriguez-Barradas MC, Gibert CL, Oursler KA, Brown S, Leaf DA, Goetz MB, Bryant K. Veterans Aging Cohort Study (VACS): Overview and description. Med. Care. 2006;44:S13–24. doi: 10.1097/01.mlr.0000223741.02074.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Justice AC, Erdos J, Brandt C, Conigliaro J, Tierney W, Bryant K. The Veterans Affairs Healthcare System: A unique laboratory for observational and interventional research. Med. Care. 2006;44:S7–12. doi: 10.1097/01.mlr.0000228027.80012.c5. [DOI] [PubMed] [Google Scholar]
- 23.Justice AC, Lasky E, McGinnis KA, Skanderson M, Conigliaro J, Fultz SL, Crothers K, Rabeneck L, Rodriguez-Barradas M, Weissman SB, Bryant K, Team VP. Medical disease and alcohol use among veterans with human immunodeficiency infection: A comparison of disease measurement strategies. Med. Care. 2006;44:S52–60. doi: 10.1097/01.mlr.0000228003.08925.8c. [DOI] [PubMed] [Google Scholar]
- 24.Koethe JR, Moore RD, Wagner KR. Physician specialization and women's primary care services in an urban HIV clinic. AIDS Patient Care STDS. 2008;22:373–380. doi: 10.1089/apc.2007.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korff MV. Long-term opioid therapy reconsidered. Ann. Intern. Med. 2011;155:325–328. doi: 10.1059/0003-4819-155-5-201109060-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korff MV, Saunders K, Thomas Ray G, Boudreau D, Campbell C, Merrill J, Sullivan MD, Rutter CM, Silverberg MJ, Banta-Green C, Weisner C. De facto long-term opioid therapy for noncancer pain. Clin. J. Pain. 2008;24:521–527. doi: 10.1097/AJP.0b013e318169d03b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Korthuis PT, Fiellin DA, Fu R, Lum PJ, Altice FL, Sohler N, Tozzi MJ, Asch SM, Botsko M, Fishl M, Flanigan TP, Boverman J, McCarty D, Collaborative B. Improving adherence to HIV quality of care indicators in persons with opioid dependence: the role of buprenorphine. J. Acquir. Immune Defic. Syndr. 2011;56(Suppl 1):S83–90. doi: 10.1097/QAI.0b013e31820bc9a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krebs EE, Ramsey DC, Miloshoff JM, Bair MJ. Primary care monitoring of long-term opioid therapy among veterans with chronic pain. Pain Med. 2011;12:740–746. doi: 10.1111/j.1526-4637.2011.01099.x. [DOI] [PubMed] [Google Scholar]
- 29.Lo Re V, III, Lim JK, Goetz MB, Tate J, Bathulapalli H, Klein MB, Rimland D, Rodriguez-Barradas MC, Butt AA, Gibert CL, Brown ST, Kidwai F, Brandt C, Dorey-Stein Z, Reddy KR, Justice AC. Validity of diagnostic codes and liver-related laboratory abnormalities to identify hepatic decompensation events in the Veterans Aging Cohort Study. Pharmacoepidemiol. Drug Saf. 2011;20:689–699. doi: 10.1002/pds.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lum PJ, Little S, Botsko M, Hersh D, Thawley RE, Egan JE, Mitty J, Boverman J, Fiellin DA, Collaborative B. Opioid-prescribing practices and provider confidence recognizing opioid analgesic abuse in HIV primary care settings. J. Acquir. Immune Defic. Syndr. 2011;56(Suppl 1):S91–97. doi: 10.1097/QAI.0b013e31820a9a82. [DOI] [PubMed] [Google Scholar]
- 31.Manchikanti L, Ailinani H, Koyyalagunta D, Datta S, Singh V, Eriator I, Sehgal N, Shah R, Benyamin R, Vallejo R, Fellows B, Christo PJ. A systematic review of randomized trials of long-term opioid management for chronic non-cancer pain. Pain physician. 2011;14:91–121. [PubMed] [Google Scholar]
- 32.Manchikanti L, Singh A. Therapeutic opioids: a ten-year perspective on the complexities and complications of the escalating use, abuse, and nonmedical use of opioids. Pain physician. 2008;11:S63–88. [PubMed] [Google Scholar]
- 33.McGlynn EA, Asch SM, Adams J, Keesey J, Hicks J, DeCristofaro A, Kerr EA. The quality of health care delivered to adults in the United States. N. Engl. J. Med. 2003;348:2635–2645. doi: 10.1056/NEJMsa022615. [DOI] [PubMed] [Google Scholar]
- 34.Meng YY, Leung KM, Berkbigler D, Halbert RJ, Legorreta AP. Compliance with US asthma management guidelines and specialty care: a regional variation or national concern? J. Eval. Clin. Pract. 1999;5:213–221. doi: 10.1046/j.1365-2753.1999.00177.x. [DOI] [PubMed] [Google Scholar]
- 35.Midboe AM, Lewis ET, Paik MC, Gallagher RM, Rosenberg JM, Goodman F, Kerns RD, Becker WC, Trafton JA. Measurement of adherence to clinical practice guidelines for opioid therapy for chronic pain. Transl. Behav. Med. 2012;2:57–64. doi: 10.1007/s13142-011-0104-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morasco BJ, Cavanagh R, Gritzner S, Dobscha SK. Care management practices for chronic pain in veterans prescribed high doses of opioid medications. Fam. Pract. 2013;30:671–678. doi: 10.1093/fampra/cmt038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morasco BJ, Duckart JP, Dobscha SK. Adherence to clinical guidelines for opioid therapy for chronic pain in patients with substance use disorder. J. Gen. Intern. Med. 2011;26:965–971. doi: 10.1007/s11606-011-1734-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morrison RE, Brint JM, Smith WR, Arheart KL, Wray D, Palte SB, Ackerman TF. Appropriate and inappropriate prescribing of narcotics for ambulatory HIV-positive patients. J. Gen. Intern. Med. 1994;9:301–305. doi: 10.1007/BF02599175. [DOI] [PubMed] [Google Scholar]
- 39.Nuckols TK, Anderson L, Popescu I, Diamant AL, Doyle B, Di Capua P, Chou R. Opioid Prescribing: A Systematic Review and Critical Appraisal of Guidelines for Chronic Pain. Ann. Intern. Med. 2013 doi: 10.7326/0003-4819-160-1-201401070-00732. [published online ahead of print November 13, 2013] doi: 10.7326/0003-4819-160-1-201401070-00732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Office of National Drug Control Policy: Epidemic: Responding to America's prescription drug abuse crisis. 2011 [Google Scholar]
- 41.Olsen Y, Daumit GL, Ford DE. Opioid prescriptions by U.S. primary care physicians from 1992 to 2001. J. Pain. 2006;7:225–235. doi: 10.1016/j.jpain.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 42.Önen NF, Barrette EP, Shacham E, Taniguchi T, Donovan M, Overton ET. A review of opioid prescribing practices and associations with repeat opioid prescriptions in a contemporary outpatient HIV clinic. Pain Pract. 2012;12:440–448. doi: 10.1111/j.1533-2500.2011.00520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pronovost P. Enhancing physicians’ use of clinical guidelines. JAMA. 2013;310:2501–2502. doi: 10.1001/jama.2013.281334. [DOI] [PubMed] [Google Scholar]
- 44.Rosenblatt RA, Baldwin LM, Chan L, Fordyce MA, Hirsch IB, Palmer JP, Wright GE, Hart LG. Improving the quality of outpatient care for older patients with diabetes: lessons from a comparison of rural and urban communities. J. Fam. Pract. 2001;50:676–680. [PubMed] [Google Scholar]
- 45.Rosenblum A, Marsch LA, Joseph H, Portenoy RK. Opioids and the treatment of chronic pain: controversies, current status, and future directions. Exp. Clin. Psychopharmacol. 2008;16:405–416. doi: 10.1037/a0013628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seal KH, Shi Y, Cohen G, Cohen BE, Maguen S, Krebs EE, Neylan TC. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307:940–947. doi: 10.1001/jama.2012.234. [DOI] [PubMed] [Google Scholar]
- 47.Shrank WH, Asch SM, Adams J, Setodji C, Kerr EA, Keesey J, Malik S, McGlynn EA. The quality of pharmacologic care for adults in the United States. Med. Care. 2006;44:936–945. doi: 10.1097/01.mlr.0000223460.60033.79. [DOI] [PubMed] [Google Scholar]
- 48.Silverberg MJ. Prescription long-term opioid use in HIV-infected patients. Clin. J. Pain. 2012;28:39–46. doi: 10.1097/AJP.0b013e3182201a0f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Starrels JL, Becker WC, Weiner MG, Li X, Heo M, Turner BJ. Low use of opioid risk reduction strategies in primary care even for high risk patients with chronic pain. J. Gen. Intern. Med. 2011;26:958–964. doi: 10.1007/s11606-011-1648-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sullivan MD, Edlund MJ, Fan MY, Devries A, Brennan Braden J, Martin BC. Trends in use of opioids for non-cancer pain conditions 2000-2005 in commercial and Medicaid insurance plans: the TROUP study. Pain. 2008;138:440–449. doi: 10.1016/j.pain.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thompson MA, Aberg JA, Hoy JF, Telenti A, Benson C, Cahn P, Eron JJ, Gunthard HF, Hammer SM, Reiss P, Richman DD, Rizzardini G, Thomas DL, Jacobsen DM, Volberding PA. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society-USA panel. JAMA. 2012;308:387–402. doi: 10.1001/jama.2012.7961. [DOI] [PubMed] [Google Scholar]
- 52.US Department of Veterans Affairs National Patient Care Database (NPCD) [December 18, 2013]; http://www.virec.research.va.gov/NPCD/Overview.htm.
- 53.US Department of Veterans Affairs/Department of Defense Clinical Practice Guideline for Management of Opioid Therapy for Chronic Pain. 2003 [Google Scholar]
- 54.US Department of Veterans Affairs/Department of Defense Clinical Practice Guideline for Management of Opioid Therapy for Chronic Pain. 2010 [Google Scholar]