Abstract
There is a need for additional safe and effective human vaccine adjuvants. Advax is a novel adjuvant produced from semi-crystalline particles of delta inulin. In animal studies Advax enhanced humoral and cellular immunity to hepatitis B surface antigen (HBsAg) without inducing local or systemic reactogenicity. This first-in-man Phase 1 clinical trial tested the safety and tolerability of three intramuscular doses of HBsAg formulated with Advax in a group of healthy adult subjects. Advax was well tolerated with injection site pain scores not significantly different to subjects receiving HBsAg alone and no adverse events were reported in subjects that received Advax. Seroprotection and HBsAb geometric mean titers (GMT) after three immunizations were higher in the Advax 5mg (seroprotection 5/6, 83.3%, GMT 40.7, 95% CI 11.9–139.1) and 10mg (seroprotection 4/5, 80%, GMT 51.6, 95% CI 10.0–266.2) groups versus HBsAg alone (seroprotection 1/5, 20%, GMT 4.1, 95% CI 1.3–12.8). Similarly the proportion of subjects with positive CD4 T-cell responses to HBsAg was higher in the Advax 5mg (4/6, 67%) and Advax 10mg (4/5, 80%) groups versus HBsAg alone (1/5, 20%). These results confirm the safety, tolerability and immunogenicity of Advax adjuvant observed in preclinical studies. Advax may represent a suitable replacement for alum adjuvants in prophylactic human vaccines subject to confirmation of current results in larger studies. Australia and New Zealand Clinical Trial Registry: ACTRN12607000598482
Introduction
Hepatitis B virus (HBV) is the commonest cause of chronic liver infection and can lead to cirrhosis and liver cancer. Chronic infection most commonly affects infants after maternal transmission. In Africa and Asia, up to 10% of the population have chronic HBV infections with an estimated 240 million individuals globally being chronic carriers and >780,000 dying from liver failure each year[1].
Prophylactic immunization with plasma-purified inactivated HBV or yeast-expressed recombinant hepatitis B surface antigen (HBsAg) largely prevents clinical HBV infection [2]. HBsAg by itself is poorly immunogenic and therefore requires an adjuvant to be effective[3, 4]. Traditionally, aluminium hydroxide or aluminium phosphate (collectively known as alum) have been used to adjuvant HBV vaccines. Immunization of children with alum-based prophylactic HBV vaccines is 90–95% effective in preventing chronic HBV infection [3]. However, individuals over 40 years of age or with immunodeficiency, diabetes mellitus or renal impairment have suboptimal responses to alum-based vaccines[5]. There is also a need for therapeutic vaccines for those with chronic HBV infection, with alum-based HBV vaccines being ineffective in this context[6]. Attempts to improve HBV vaccine potency for poor responder populations have included use of double-dose HBsAg vaccines[7], use of preS antigens[8] and use of alternative adjuvants such as AS04, a combination of alum and monophosphoryl lipid A (Fendrix®, GlaxoSmithKline, UK) approved in Europe for renal hemodialysis patients [9, 10] and an immunostimulatory oligonucleotide adjuvant (Heplisav®, Dynavax, USA) in clinical development in the US [11, 12].
Advax™ is a novel adjuvant produced from microparticles of delta inulin (β-D-(2->1)-polyfructofuranosyl-D-glucose)[13]. Advax arose from research showing that the normally water soluble plant-derived polysaccharide inulin, is capable of crystallizing into a series of polymorphic forms distinguished by progressively higher temperatures of solubility[14] due to repeated addition of a crystal unit cell[15]. Delta inulin is distinguished from earlier described inulin isoforms (alpha, beta and gamma) by being relatively insoluble at mammalian body temperature bestowing it with unique immunological properties including potent adjuvant properties, not exhibited by more soluble inulin forms. In animal studies, Advax enhanced adaptive immune responses to a wide variety of viral and bacterial antigens including influenza [16, 17], Japanese encephalitis[18, 19], West Nile virus[20], HIV [21]and anthrax vaccines[22]. Mice immunized with HBsAg with Advax exhibited enhanced HBsAb titers along with antigen dose-sparing and enhanced cellular immune responses including CD4 and CD8 T-cell proliferation and Th1, Th2 and Th17 cytokine production [23]. The favorable effects of Advax on HBsAg immune responses were similarly evident in guinea pigs [23]. A notable finding in all animal studies conducted of Advax to date has been the lack of local or systemic reactogenicity.
This Phase 1 study was conducted to confirm safety and tolerability of Advax adjuvant in healthy adult subjects, as a prerequisite for subsequent trials to test its efficacy in poor responder populations. A secondary objective was to assess the ability of Advax to enhance the human immune response against HBsAg. As shown below, Advax was well tolerated and no safety issues were identified. When compared to HBsAg alone, formulation with Advax enhanced HBsAb titers and T-cell responses, consistent with it being an effective human adjuvant.
Methods
Study Design
The study was a single centre, randomized, observer and participant blinded, controlled Phase 1 trial conducted at Flinders Medical Centre in Adelaide, Australia. The study was conducted under the Clinical Trial Notification (CTN) provisions of the Australian Therapeutic Goods Administration, after approval by the Southern Adelaide Human Research Ethics Committee. Under the CTN process the TGA delegates responsibility for approval of a clinical trial to the Institutional Ethics Committee. After providing informed consent and being assessed for eligibility, subjects were examined and blood taken for safety analysis and measurement of baseline HBsAb titers. Eligible subjects were randomized using an on-line tool (www.randomiser.com) to receive an accelerated immunization course of three intramuscular doses a month apart of HBsAg (Butantan Institute, Brazil) alone or with Advax, 5mg or 10mg. This vaccine regimen was chosen for convenience and differed slightly from the usual administration course in Brazil of the alum-adjuvanted Butantan vaccine which is administered at 0, 1, and 3 months[24]. Randomization codes were loaded into numbered sealed envelopes sequentially opened by clinical staff to allocate subjects to study groups. On post-immunization days 1 and 7, subjects returned to the clinic for inspection of the vaccination site by a blinded observer and to have their diary reviewed. A month after each immunization subjects returned to have blood taken for HBsAb titers. The efficacy endpoint was frequency of seroprotection (HBsAb >10 mIU/mL) 1 month after the final vaccine dose. Injection site pain was assessed by visual analogue pain scores (VAPS) and safety was assessed by the frequency of local and systemic solicited and unsolicited adverse events. Blood samples were obtained at each visit for additional safety assessments.
Enrolment criteria
The study population comprised healthy adults aged 18 to 40 years without evidence of prior HBV infection or HBV immunization. Exclusion criteria included, immunodeficiency, diabetes mellitus, significant liver disease, significant kidney disease, any serious systemic illness in the last 6 months, history of vaccine allergy, women of childbearing potential unless using a reliable and appropriate contraceptive method, pregnant or lactating women, concurrent immunosuppressive therapy, including corticosteroids (with the exception of topically applied/inhaled steroids), known infection of human immunodeficiency virus (HIV), history of intravenous drug abuse or alcohol abuse or clinically-significant abnormal baseline blood count.
Study Vaccines
Current Good Manufacturing Practices (cGMP) recombinant HBsAg produced in Hansenula polymorpha was a gift of Butantan Institute, Sao Paulo, Brazil. It is a component of a licensed Brazilian vaccine formulated with alum adjuvant [24]. Each subject received 7μg HBsAg alone or combined with Advax 5mg or 10mg. Alum-adjuvanted HBsAg vaccines typically contain 10–20μg HBsAg [24], and a 7μg dose was chosen for this study to set a stringent benchmark and test whether the adjuvant could compensate for this lower antigen dose. Advax adjuvant (Vaxine Pty Ltd, Adelaide, Australia) manufactured under Good Laboratory Practices (GLP) was supplied as a sterile suspension of delta inulin microparticles in a bicarbonate buffer. Each vaccine was injected in a final volume of 0.5 mL with Advax adjuvant formulated with HBsAg immediately prior to immunization by intramuscular injection into the deltoid muscle of the non-dominant arm. We have previously shown that unlike alum adjuvant, Advax does not absorb HBsAg. In animal studies injection of Advax adjuvant 24 hours before HBsAg still potentiated the antibody response[23]. Hence antigen absorption and depot formation does not appear necessary for Advax’s action.
HBsAb assay
HBsAb titres were measured in serum separated from venous blood collected into Z Serum Separator Clot Activator Vacuette tubes (Greiner Bio-one) using a commercial AxSYM AUSAB assay (Abbott Diagnostics), following the manufacturer’s protocol.
CD4+ T-cell proliferation assay
Peripheral blood mononuclear cells (PBMC) were isolated from venous blood using BD Vacutainer CPT cell preparation tubes. Proliferation was measured using a carboxyfluorescein succinimidyl ester (CFSE) cell division assay, as previously described [25]. The stimulation index (SI) was calculated by dividing the percentage of CFSElo cells in HBsAg-stimulated wells by the percentage in non-stimulated wells. Values below 1% CFSElo cells in non-stimulated wells were set to 1, to exclude unrealistic high values in SI due to division by values <1. The CD4+ T-cell response was considered positive if the SI was >2 and percent CFSElo cells in response to HBsAg was at least 5% above the background in non-stimulated wells.
Statistics
Statistical analysis was performed using Prism 6 for MacOSX. Geometric mean titer antibody (GMT) levels were compared between groups by Mann-Whitney. The proportion of each vaccine group achieving seroprotection (>10mIU/mL) titer post-immunization was compared by Fisher’s exact test. Trends across time and study groups for each safety blood parameter were analyzed by 2-way ANOVA.
RESULTS
Vaccine and adjuvant safety
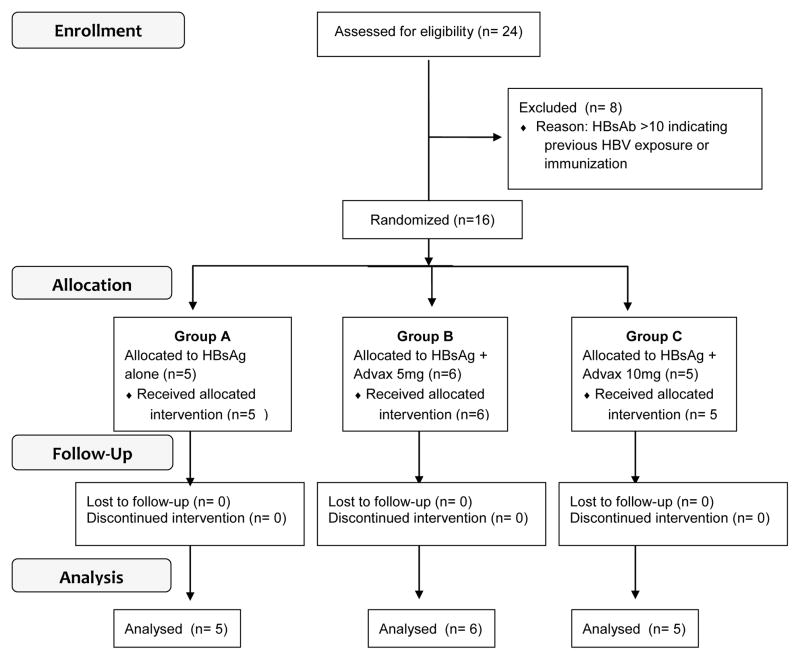
The study outline in accordance with CONSORT requirements [26] is depicted in Fig. 1. The safety analysis was performed on 16 eligible healthy male and female subjects with a mean age of 27 years (range 20–38) (Table 1). Vaccine safety was assessed by safety blood assessments at each visit and frequency of solicited and unsolicited adverse events (AE).
Figure 1.
CONSORT Flow diagram depicting the HBV001 study.
TABLE 1.
Subject demographics
| Study Group | Subject ID | Age | Sex | Race |
|---|---|---|---|---|
| HBsAg alone | #101 | 38 | F | Caucasian |
| #102 | 31 | M | Caucasian | |
| #103 | 26 | F | Caucasian | |
| #104 | 27 | F | Caucasian | |
| #105 | 26 | F | Asian | |
| HBsAg + Advax 5mg | #206 | 20 | M | Caucasian |
| #207 | 34 | F | Caucasian | |
| #208 | 22 | F | Caucasian | |
| #209 | 22 | M | Caucasian | |
| #210 | 23 | M | Asian | |
| #211 | 31 | M | Caucasian | |
| HBsAg + Advax 10mg | #312 | 20 | M | Caucasian |
| #313 | 26 | M | Asian | |
| #314 | 25 | F | Caucasian | |
| #315 | 36 | M | Caucasian | |
| #316 | 22 | F | Caucasian |
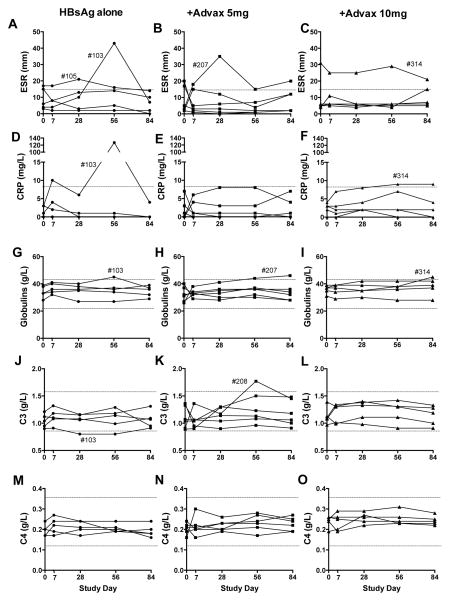
As adjuvants may induce inflammatory responses we specifically examined for changes in inflammatory markers including erythrocyte sedimentation rate (ESR), C- reactive protein (CRP) and plasma globulins that might indicate an inflammatory effect (Fig. 2). Analysis by 2-way ANOVA revealed no significant trend in the ESR over time or between groups. Only one moderately elevated ESR (43) was recorded during the study and this was at a single visit in a subject receiving HBsAg alone (Fig. 2A). Mild ESR elevations was seen occasionally in all groups most likely reflecting transient subclinical infections (Fig. 2A–C).
Figure 2. Safety assessment of inflammatory markers.
Venous blood was obtained from all subjects pre-immunization, days 7 and 28 post-1st immunization and 28 days post- each subsequent immunization and assayed for relevant inflammatory markers to identify any potential adverse effects. Depicted separated by subject group are each subject’s ESR over time (A–C), CRP (D–F), plasma globulins (G–I), complement factors C3 (J–L), and C4 (M–O). Normal laboratory ranges are indicated by dotted lines.
CRP is secreted by the liver within six hours of an acute inflammatory stimulus and is a useful marker of inflammation, with the median normal CRP concentration being 0.8 mg/L and 99% of healthy individuals having a value <12 mg/L [27]. Analysis by 2-way ANOVA revealed no significant trend in CRP over time or between groups. The only subject who developed a markedly elevated CRP (127mg/L) was subject #103 in HBsAg alone group who had an elevated ESR at the same visit (Fig. 2D). Both the CRP and ESR in subject #103 returned to normal at their next visit suggesting a transient non-study related infection as the cause. No significant trends in plasma globulin levels over time or between groups was observed (Fig. 2G–I).
As inulin particles are known to activate the alternative complement pathway [28], we examined for any changes in complement C3, or C4 (classical pathway) post- immunization (Fig. 2J–O). Analysis by 2-way ANOVA revealed no significant trend in C3 or C4 over time or between groups, suggesting Advax adjuvant was not significantly disturbing the complement pathway.
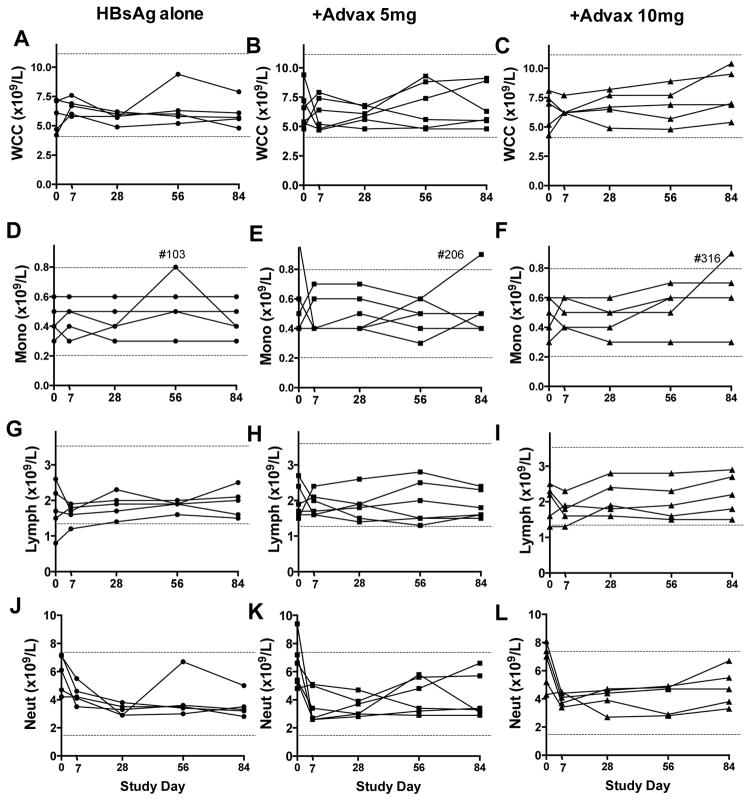
Similarly, no significant trends over time or between groups were observed in total white cell (Fig. 3A–C), monocyte (Fig. 3D–F), lymphocyte (Fig. 3G–I), or neutrophil (Fig. 3J–L) counts. Eosinophil and basophil counts remained low or undetectable in all subjects throughout the study and no clinically significant abnormalities were observed in electrolytes, creatinine, liver enzymes, bilirubin, urate, total protein, albumin, random blood glucose, cholesterol, triglyceride or thyroid stimulating hormone (data not shown).
Figure 3. Safety assessment of white cell counts.
Venous blood was obtained from all subjects pre-immunization, 7 and 28 days post-1st immunization and 28 days post subsequent immunizations for measurement of white blood cell counts. Depicted separated by subject group are total WCC (A–C), monocyte count (D–F), lymphocyte count (G–I), and neutrophil counts C3 (J–L). Normal laboratory ranges are indicated by dotted lines.
Another potential safety concern for adjuvants is their ability to induce or flare autoimmune disease, which may correlate with inflammatory activity [29–32]. We monitored for de novo development or increases in specific autoantibodies including rheumatoid factor (RF), antinuclear antibodies (ANA), and thyroid peroxidase antibodies (TPO) post-immunization. All subjects remained negative throughout the study for RF and TPO antibodies (data not shown). Five subjects had borderline titers of ANA (titer 1:40) at study commencement and no changes in ANA titer during the study were considered clinically significant. As a final part of the safety assessment, subjects were assessed for local and systemic AEs by physical examination and by use of a subject diary. No subjects reported any problems with swelling, redness or pain at the injection site in the days post-immunization and no abnormalities were detected at any vaccine injection site upon inspection at each study visit. Only two systemic AEs were reported during the study, both in subjects from the HBsAg alone group; one reported an episode of viral infection and the second diarrhea and muscle ache. Both AE’s resolved spontaneously. Overall, no safety issues were identified for the HBsAg vaccine alone or combined with Advax.
Vaccine tolerability
Many adjuvants including MF59[33], AS04[10], QS21[34] and Montanide[35] have been shown to increase vaccine reactogenicity. Hence vaccine tolerability was assessed using a visual analogue pain score (VAPS) where subjects recorded the pain at the injection site on a scale of 0 (no pain) through to 10 (most severe pain imaginable). There was no significant difference between the groups in VAPS post-immunization (Fig. 4A–C). There were three instances of VAPS ≥5 in immediate injection site pain which were spread across the HBsAg alone (2 cases) and Advax 5mg (1 case) with no cases in Advax 10mg group, suggesting that Advax does not increase moderate or severe injection pain. Interestingly, there was a significant progressive reduction in each group’s mean injection site pain post-injection at each subsequent immunization (Fig. 4). Overall the HBsAg alone or with Advax adjuvant was well tolerated with minimal injection site reactions.
Figure 4. Assessment of vaccine tolerability by visual analogue pain score (VAPS).
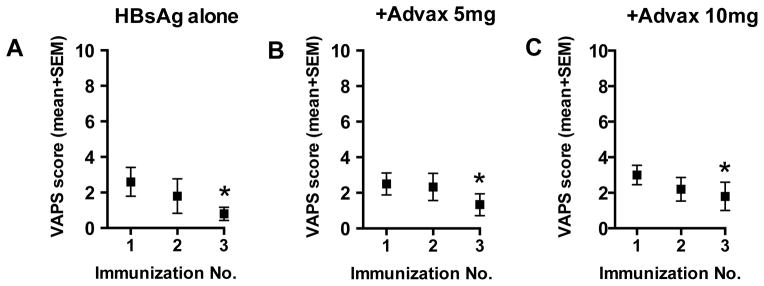
Immediately post immunization each subject scored their injection site pain in the range from from 0 (no pain) to 10 (worst imaginable pain). Shown are the injection site VAPS scores (mean + SEM) for each vaccine group immediately following each immunization. *p<0.05
Vaccine Immunogenicity
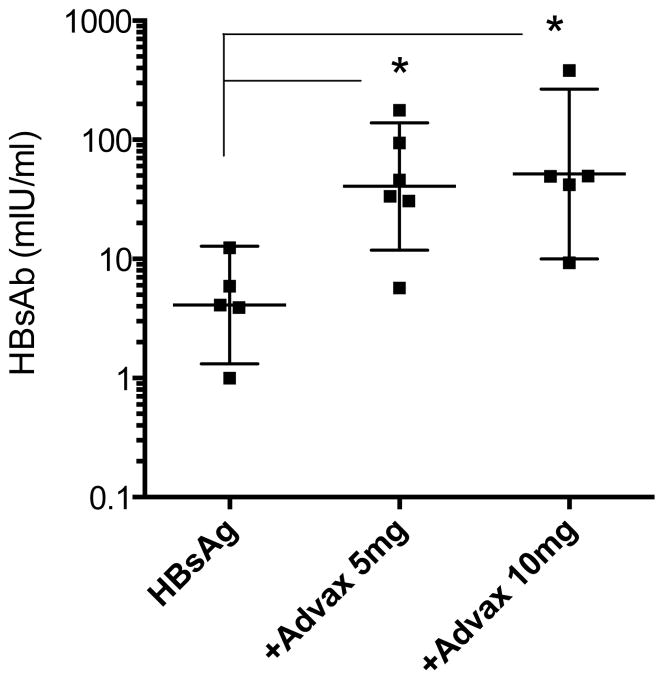
A preliminary assessment was also undertaken of the effect of Advax on vaccine immunogenicity. Only 1/5 (20%) of subjects in the HBsAg alone group achieved seroproprotection after three immunizations, and this was in Subject #105 who had a relatively high HbSAb level pre-immunisation, possibly suggesting past HBV exposure (Table 2). High levels of seroprotection were achieved in both Advax groups; Advax 5mg 5/6 (83.3%) and Advax 10mg 4/5 (80%) (Table 2). When both Advax dose groups were combined, the frequency of seroprotection was significantly higher in Advax versus HBsAg alone groups; 9/11 (82%) vs 1/5 (20%), p=0.04. HBsAb titers were significantly higher in the Advax 5mg (GMT 40.7, 95% confidence interval 11.9 – 139, p=0.02) and Advax 10mg (GMT 51.6, 95% confidence interval 10.0 – 266.2, p=0.02) groups versus the HBsAg alone group (GMT 4.1, 95% confidence interval 1.3 – 12.8) (Fig. 5). There was no significant GMT difference between the two Advax groups.
TABLE 2.
Antibody and T-cell responses
| Study Group | Subject ID | HBsAb titer (mIU/mL) | T-cell index Post 3rd dose | |||
|---|---|---|---|---|---|---|
| Baseline | Post 1st dose | Post 2nd dose | Post 3rd dose | |||
| HBsAg alone | #101 | 0 | 0 | 0 | 4.1 | 5.1 |
| #102 | 0 | 0 | 0.1 | 1.0 | 1.1 | |
| #103 | 0.1 | 0 | 0 | 3.9 | 1.2 | |
| #104 | 0 | 0 | 0 | 5.9 | 1.1 | |
| #105 | 5.8 | 7.9 | 9.3 | 12.4 | 1.2 | |
| HBsAg + Advax 5mg | #206 | 0 | 0 | 4.6 | 33.7 | 2.5 |
| #207 | 0 | 0.2 | 23.9 | 177 | 11.9 | |
| #208 | 0.7 | 0.6 | 3.8 | 30.7 | 1.7 | |
| #209 | 0 | 0 | 3.5 | 46.0 | 1.3 | |
| #210 | 0 | 0 | 5.8 | 94.3 | 3.1 | |
| #211 | 0 | 0 | 1 | 5.7 | 2.3 | |
| HBsAg + Advax 10mg | #312 | 0 | 0 | 0.2 | 9.3 | 7.9 |
| #313 | 0.1 | 2 | 2.4 | 42.0 | 8.8 | |
| #314 | 0.1 | 0.4 | 6.4 | 49.3 | 8.9 | |
| #315 | 0 | 0.1 | 5.1 | 49.7 | 3.6 | |
| #316 | 0 | 0.1 | 38.1 | 381.8 | 0.9 | |
Figure 5. HBsAb titers 4 weeks after the 3rd immunization by vaccine group.
Individual subject results, mean +/− SEM are shown. *p<0.05
T-cell immunity
T-cell immunity may help predict the longevity of HBV protection and is likely to be critical to effectiveness of a therapeutic HBV vaccine[36, 37]. We assessed CD4+ T-cell responses to HBsAg a month after the third immunization. One of five (20%) subjects in HBsAg alone group had positive T-cell responses versus 4/6 (67%) and 4/5 (80%) subjects in the 5mg and 10mg Advax groups, respectively (Table 2). Subject #312 in the Advax 10mg adjuvant group who had a borderline seroprotective HBsAb titer (9.3 mIU/mL) conversely had a strong T-cell proliferative response (SI=7.9). Overall, 100% subjects in the Advax groups versus 40% of subjects in the HBsAg alone group achieved either seroprotection and/or a positive T-cell recall response after three immunizations (Table 2).
DISCUSSION
This Phase 1 study provides initial human data on safety, tolerability and immunogenicity of Advax, a novel polysaccharide adjuvant based on microparticles of delta inulin. The study met its primary objective of confirming Advax adjuvant in human subjects was safe and well tolerated at doses up to 10mg. Notably, no systemic AE’s were elicited in subjects that received Advax-adjuvanted vaccine. Physical examination confirmed the absence of injection sites abnormalities such as redness, tenderness, scarring or lumps. Similarly, there was no evidence of any adverse safety signals from analysis of safety blood tests and in particular, there was no evidence of excess inflammatory effects as reflected by changes in ESR, CRP, globulins, complement levels or white blood cell counts post-immunization. This is consistent with the lack of inflammatory side effects of Advax evidenced in non-clinical studies[22]. This distinguishes Advax from other classes of adjuvants including oil emulsions (e.g. Freund’s complete adjuvant, MF59, Montanide), saponins (e.g. Quil A, QS21, Iscoms) and Toll-like receptor (TLR) agonists (e.g. MPL, AS04, flagellin), which are all characterized by high inflammatory activity [38]. Advax’s adjuvant activity appears to be achieved by a mechanism not involving inflammation. This could provide a potential safety advantage over other adjuvants as inflammation may be the mechanism whereby other adjuvants break self-tolerance and induce autoimmune disease. For example, myelin basic protein (MBP) formulated with Freund’s complete adjuvant (FCA) or other inflammatory adjuvants induces experimental allergic encephalomyelitis (EAE) in Lewis rats [39]. However MBP formulated with inulin adjuvant failed to induce EAE in the same model (Peter Cooper, personal communication).
Injection site tolerability can take two forms; immediate injection site pain induced by irritant adjuvants such as Quil A[40] or Iscomatrix® [41] and delayed pain, swelling, heat and tenderness seen 24–48 hours post-immunization with inflammatory adjuvants such as oil emulsions [42]. Neither type of adjuvant-associated injection site reaction was observed in this study, as reflected in the low injection site pain scores and absence of any delayed site reactions post-immunization. This contrasts with HBsAg vaccines formulated with AS04 or oligonucleotide adjuvants that demonstrated increased reactogenicity when compared to alum-based comparators [10, 43].
While group sizes in this Phase 1 study were small, those groups receiving Advax achieved significantly higher rates of seroprotection than those receiving HBsAg alone, consistent with the positive adjuvant action of Advax in pre-clinical studies [23]. Our study used a relatively low 7μg HBsAg dose whereas most commercial alum-based vaccines contain 10–20μg HBsAg. As this was a first-in-man study, doses of Advax adjuvant used were low at only 5–10 times normal murine doses [23]. Importantly, the study confirmed that Advax at doses of 5 and 10mg had measurable adjuvant activity in human subjects. Increased doses of HBsAg and Advax will be tested in future studies to see whether this generates even higher GMTs and seroprotection rates, and could reduce the number of vaccine doses required for seroprotection. Notably, the seroprotection rates achieved with the Advax-adjuvanted vaccine in this study (~80%) were not dissimilar to published rates for three doses of alum-adjuvanted vaccines in adult subjects [24, 44].
A relatively underexplored area is the ability of HBV vaccines to induce T-cell immunity, which may contribute alongside HBsAb to HBV protection[36, 37]. Subjects receiving Advax versus HBsAg alone had higher HBsAg specific CD4 T-cell proliferative responses, consistent with findings in preclinical studies[23]. There was a trend towards greater CD4+ T cell proliferation at the higher Advax dose, suggesting that the maximal T-cell dose may not have yet been reached. Induction of T-cell immunity could represent an advantage of Advax over alum adjuvant in the context of therapeutic HBV vaccines where T cells are needed to clear HBV-infected hepatocytes. Given the good tolerability profile of the Advax 10mg dose, future studies will test whether higher Advax doses can further enhance humoral and cellular immunity.
While alum is the undisputed human adjuvant leader with almost a century of experience, there are nevertheless drivers for development of newer alternatives. In particular, concerns remain of potential of aluminum salts to cause chronic toxicity including macrophagic myofasciitis, a constellation of chronic fatigue type symptoms linked to use of alum-containing vaccines[45] and the broader designation of “autoimmune/inflammatory syndrome induced by adjuvants” (ASIA) linked to use of aluminium hydroxide, squalene, and mineral oil [46]. Questions also remain about the potential for chronic neurotoxicity from aluminum accumulating in the brain[30]. Furthermore, alum-based vaccines induce a marked Th2 bias and thereby increase the risk of vaccine allergy and anaphylaxis[47, 48]. Lastly, alum adjuvants are poor at inducing cellular immunity[49]. Hence, there is a need for additional safe and well-tolerated adjuvants that could act as alum replacements. Whilst formal pharmacokinetic studies are not required for vaccines and hence have not been done for Advax adjuvant, inulin is highly susceptible to hydrolysis [50] leading to increased solubility, and once dissolved inulin polymers are rapidly excreted from the body by glomerular filtration [51]. Hence unlike alum inulin is unlikely to form long-term tissue depots, and has been shown to be safe when administrated intravenously to humans in doses of tens of grams as part of a well established procedure for testing of kidney function [52].
This first-in-man study showed Advax adjuvant to be safe and well tolerated and to enhance both antibody and cellular immunity against co-administered HBsAg. Subsequent human clinical trials have also shown Advax be be safe and well tolerated at doses of 20 to 40mg including as part of a two dose pandemic influenza vaccine [53]. On the basis of these results, future planned HBV vaccine trials will examine the effect of further increasing both the dose of HBsAg and Advax adjuvant with the aim of reducing the required number of immunizations for HBsAb seroconversion from three to two.
HIGHLIGHTS FOR REVIEW.
Phase 1 first-in-man study of HBV vaccine based on Advax, a novel delta inulin-based adjuvant
Advax enhanced both humoral and cellular immune responses to HBsAg
Advax was safe and well tolerated in human subjects
Acknowledgments
We thank Kylie Bragg, Helene Mauboussin and Margaret Menadue for assistance with conduct of the clinical trial and Professor Isaias Raw of the Butantan Institute, Sao Paulo Brazil for the kind gift of the HBsAg antigen. We particularly thank Farukh Khambaty for his support and guidance throughout the development of Advax. We also thank Dr Doug Taupin for invaluable support throughout the course of these studies. P.C. is an Emeritus Visiting Fellow of the Australian National University Medical School and of the John Curtin School of Medical Research, ANU, Canberra, Australia. Development of Advax adjuvant was supported AusIndustry through the Biotechnology Innovation Fund, START, and Commercial Ready programs and the National Institutes of Health through Contracts No. U01AI061142 and HHSN272200800039C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.WHO. World Health Organization Fact Sheet 204. (Revised August 2008). Available at: http://wwwwhoint/mediacentre/factsheets/fs204/en/2008.
- 2.Diminsky D, Schirmbeck R, Reimann J, Barenholz Y. Comparison between hepatitis B surface antigen (HBsAg) particles derived from mammalian cells (CHO) and yeast cells (Hansenula polymorpha): composition, structure and immunogenicity. Vaccine. 1997 Apr-May;15(6–7):637–47. doi: 10.1016/s0264-410x(96)00239-3. [DOI] [PubMed] [Google Scholar]
- 3.Andre FE. Summary of safety and efficacy data on a yeast-derived hepatitis B vaccine. Am J Med. 1989 Sep 4;87(3A):14S–20S. doi: 10.1016/0002-9343(89)90525-1. [DOI] [PubMed] [Google Scholar]
- 4.Keating GM, Noble S. Recombinant hepatitis B vaccine (Engerix-B): a review of its immunogenicity and protective efficacy against hepatitis B. Drugs. 2003;63(10):1021–51. doi: 10.2165/00003495-200363100-00006. [DOI] [PubMed] [Google Scholar]
- 5.Fabrizi F, Dixit V, Messa P, Martin P. Hepatitis B virus vaccine in chronic kidney disease: improved immunogenicity by adjuvants? A meta-analysis of randomized trials. Vaccine. 2012 Mar 16;30(13):2295–300. doi: 10.1016/j.vaccine.2012.01.064. [DOI] [PubMed] [Google Scholar]
- 6.Bertoletti A, Gehring A. Therapeutic vaccination and novel strategies to treat chronic HBV infection. Expert Rev Gastroenterol Hepatol. 2009 Oct;3(5):561–9. doi: 10.1586/egh.09.48. [DOI] [PubMed] [Google Scholar]
- 7.Pan HX, Zeng Y, Song XF, Zhang YJ, Xu K, Liang ZL, et al. Immune response to hepatitis B vaccine with high antigen content in non-responders after standard primary vaccination in Chinese adults. Vaccine. 2014 Jun 17;32(29):3706–12. doi: 10.1016/j.vaccine.2014.02.094. [DOI] [PubMed] [Google Scholar]
- 8.Milich DR, Thornton GB, Neurath AR, Kent SB, Michel ML, Tiollais P, et al. Enhanced immunogenicity of the pre-S region of hepatitis B surface antigen. Science. 1985 Jun 7;228(4704):1195–9. doi: 10.1126/science.2408336. [DOI] [PubMed] [Google Scholar]
- 9.Levie K, Gjorup I, Skinhoj P, Stoffel M. A 2-dose regimen of a recombinant hepatitis B vaccine with the immune stimulant AS04 compared with the standard 3-dose regimen of Engerix-B in healthy young adults. Scand J Infect Dis. 2002;34(8):610–4. doi: 10.1080/00365540110080881. [DOI] [PubMed] [Google Scholar]
- 10.Tong NK, Beran J, Kee SA, Miguel JL, Sanchez C, Bayas JM, et al. Immunogenicity and safety of an adjuvanted hepatitis B vaccine in pre-hemodialysis and hemodialysis patients. Kidney international. 2005 Nov;68(5):2298–303. doi: 10.1111/j.1523-1755.2005.00689.x. [DOI] [PubMed] [Google Scholar]
- 11.Cooper CL, Davis HL, Morris ML, Efler SM, Adhami MA, Krieg AM, et al. CPG 7909, an immunostimulatory TLR9 agonist oligodeoxynucleotide, as adjuvant to Engerix-B HBV vaccine in healthy adults: a double-blind phase I/II study. J Clin Immunol. 2004 Nov;24(6):693–701. doi: 10.1007/s10875-004-6244-3. [DOI] [PubMed] [Google Scholar]
- 12.Heyward WL, Kyle M, Blumenau J, Davis M, Reisinger K, Kabongo ML, et al. Immunogenicity and safety of an investigational hepatitis B vaccine with a Toll- like receptor 9 agonist adjuvant (HBsAg-1018) compared to a licensed hepatitis B vaccine in healthy adults 40–70 years of age. Vaccine. 2013 Nov 4;31(46):5300–5. doi: 10.1016/j.vaccine.2013.05.068. [DOI] [PubMed] [Google Scholar]
- 13.Cooper PD, Petrovsky N. Delta inulin: a novel, immunologically active, stable packing structure comprising beta-D-[2 -> 1] poly(fructo-furanosyl) alpha-D-glucose polymers. Glycobiology. 2011 May;21(5):595–606. doi: 10.1093/glycob/cwq201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooper PD, Barclay TG, Ginic-Markovic M, Petrovsky N. The polysaccharide inulin is characterized by an extensive series of periodic isoforms with varying biological actions. Glycobiology. 2013 Oct;23(10):1164–74. doi: 10.1093/glycob/cwt053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooper PD, Barclay TG, Ginic-Markovic M, Gerson AR, Petrovsky N. Inulin isoforms differ by repeated additions of one crystal unit cell. Carbohydrate polymers. 2014 Mar 15;103:392–7. doi: 10.1016/j.carbpol.2013.12.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Honda-Okubo Y, Saade F, Petrovsky N. Advax, a polysaccharide adjuvant derived from delta inulin, provides improved influenza vaccine protection through broad-based enhancement of adaptive immune responses. Vaccine. 2012 Aug 3;30(36):5373–81. doi: 10.1016/j.vaccine.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Layton RC, Petrovsky N, Gigliotti AP, Pollock Z, Knight J, Donart N, et al. Delta inulin polysaccharide adjuvant enhances the ability of split-virion H5N1 vaccine to protect against lethal challenge in ferrets. Vaccine. 2011 Aug 26;29(37):6242–51. doi: 10.1016/j.vaccine.2011.06.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lobigs M, Pavy M, Hall RA, Lobigs P, Cooper P, Komiya T, et al. An inactivated Vero cell-grown Japanese encephalitis vaccine formulated with Advax, a novel inulin-based adjuvant, induces protective neutralizing antibody against homologous and heterologous flaviviruses. J Gen Virol. 2010 Jun;91(Pt 6):1407–17. doi: 10.1099/vir.0.019190-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larena M, Prow NA, Hall RA, Petrovsky N, Lobigs M. JE-ADVAX Vaccine Protection against Japanese Encephalitis Virus Mediated by Memory B Cells in the Absence of CD8+ T Cells and Pre-Exposure Neutralizing Antibody. Journal of virology. 2013 Apr;87(8):4395–402. doi: 10.1128/JVI.03144-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petrovsky N, Larena M, Siddharthan V, Prow NA, Hall RA, Lobigs M, et al. An inactivated cell culture Japanese encephalitis vaccine (JE-ADVAX) formulated with delta inulin adjuvant provides robust heterologous protection against West Nile encephalitis via cross-protective memory B cells and neutralizing antibody. Journal of virology. 2013 Sep;87(18):10324–33. doi: 10.1128/JVI.00480-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cristillo AD, Ferrari MG, Hudacik L, Lewis B, Galmin L, Bowen B, et al. Induction of mucosal and systemic antibody and T-cell responses following prime- boost immunization with novel adjuvanted human immunodeficiency virus-1-vaccine formulations. J Gen Virol. 2011 Jan;92(Pt 1):128–40. doi: 10.1099/vir.0.023242-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feinen B, Petrovsky N, Verma A, Merkel TJ. Advax-adjuvanted recombinant protective antigen provides protection against inhalational anthrax that is further enhanced by addition of murabutide adjuvant. Clinical and vaccine immunology: CVI. 2014 Apr;21(4):580–6. doi: 10.1128/CVI.00019-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saade F, Honda-Okubo Y, Trec S, Petrovsky N. A novel hepatitis B vaccine containing Advax, a polysaccharide adjuvant derived from delta inulin, induces robust humoral and cellular immunity with minimal reactogenicity in preclinical testing. Vaccine. 2013 Jan 7; doi: 10.1016/j.vaccine.2012.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ioshimoto LM, Rissato ML, Bonilha VS, Miyaki C, Raw II, Granovski N. Safety and immunogenicity of hepatitis B vaccine ButaNG in adults. Revista do Instituto de Medicina Tropical de Sao Paulo. 1999 May;41(3):191–3. doi: 10.1590/s0036-46651999000300011. [DOI] [PubMed] [Google Scholar]
- 25.Lyons AB, Parish CR. Determination of lymphocyte division by flow cytometry. J Immunol Methods. 1994 May 2;171(1):131–7. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 26.Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Int J Surg. 2012;10(1):28–55. doi: 10.1016/j.ijsu.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 27.Marnell L, Mold C, Du Clos TW. C-reactive protein: ligands, receptors and role in inflammation. Clin Immunol. 2005 Nov;117(2):104–11. doi: 10.1016/j.clim.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 28.Gotze O, Muller-Eberhard HJ. The c3-activator system: an alternate pathway of complement activation. J Exp Med. 1971 Sep 1;134(3):90–108. [PMC free article] [PubMed] [Google Scholar]
- 29.Colafrancesco S, Perricone C, Priori R, Valesini G, Shoenfeld Y. Sjogren’s syndrome: another facet of the autoimmune/inflammatory syndrome induced by adjuvants (ASIA) Journal of autoimmunity. 2014 Jun;51:10–6. doi: 10.1016/j.jaut.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 30.Shaw CA, Tomljenovic L. Aluminum in the central nervous system (CNS): toxicity in humans and animals, vaccine adjuvants, and autoimmunity. Immunol Res. 2013 Jul;56(2–3):304–16. doi: 10.1007/s12026-013-8403-1. [DOI] [PubMed] [Google Scholar]
- 31.Cerpa-Cruz S, Paredes-Casillas P, Landeros Navarro E, Bernard-Medina AG, Martinez-Bonilla G, Gutierrez-Urena S. Adverse events following immunization with vaccines containing adjuvants. Immunol Res. 2013 Jul;56(2–3):299–303. doi: 10.1007/s12026-013-8400-4. [DOI] [PubMed] [Google Scholar]
- 32.Ahmed SS, Schur PH, MacDonald NE, Steinman L. Narcolepsy, 2009 A(H1N1) pandemic influenza, and pandemic influenza vaccinations: what is known and unknown about the neurological disorder, the role for autoimmunity, and vaccine adjuvants. Journal of autoimmunity. 2014 May;50:1–11. doi: 10.1016/j.jaut.2014.01.033. [DOI] [PubMed] [Google Scholar]
- 33.Arguedas A, Soley C, Abdelnour A, Sales V, Lindert K, Della Cioppa G, et al. Assessment of the safety, tolerability and kinetics of the immune response to A/H1N1v vaccine formulations with and without adjuvant in healthy pediatric subjects from 3 through 17 years of age. Hum Vaccin. 2011 Jan 1;7(1):58–66. doi: 10.4161/hv.7.1.13411. [DOI] [PubMed] [Google Scholar]
- 34.Vandepapeliere P, Horsmans Y, Moris P, Van Mechelen M, Janssens M, Koutsoukos M, et al. Vaccine adjuvant systems containing monophosphoryl lipid A and QS21 induce strong and persistent humoral and T cell responses against hepatitis B surface antigen in healthy adult volunteers. Vaccine. 2008 Mar 4;26(10):1375–86. doi: 10.1016/j.vaccine.2007.12.038. [DOI] [PubMed] [Google Scholar]
- 35.Langermans JA, Schmidt A, Vervenne RA, Birkett AJ, Calvo-Calle JM, Hensmann M, et al. Effect of adjuvant on reactogenicity and long-term immunogenicity of the malaria Vaccine ICC-1132 in macaques. Vaccine. 2005 Sep 30;23(41):4935–43. doi: 10.1016/j.vaccine.2005.05.036. [DOI] [PubMed] [Google Scholar]
- 36.Wiegand J, Meya S, Schlaphoff V, Manns MP, Mossner J, Wedemeyer H, et al. HBV-specific T-cell responses in healthy seronegative sexual partners of patients with chronic HBV infection. Journal of viral hepatitis. 2010 Sep;17(9):631–9. doi: 10.1111/j.1365-2893.2009.01220.x. [DOI] [PubMed] [Google Scholar]
- 37.Bauer T, Jilg W. Hepatitis B surface antigen-specific T and B cell memory in individuals who had lost protective antibodies after hepatitis B vaccination. Vaccine. 2006 Jan 30;24(5):572–7. doi: 10.1016/j.vaccine.2005.08.058. [DOI] [PubMed] [Google Scholar]
- 38.Petrovsky N, Aguilar JC. Vaccine adjuvants: current state and future trends. Immunol Cell Biol. 2004 Oct;82(5):488–96. doi: 10.1111/j.0818-9641.2004.01272.x. [DOI] [PubMed] [Google Scholar]
- 39.Panitch H, Ciccone C. Induction of recurrent experimental allergic encephalomyelitis with myelin basic protein. Ann Neurol. 1981 May;9(5):433–8. doi: 10.1002/ana.410090504. [DOI] [PubMed] [Google Scholar]
- 40.Sabbatini PJ, Ragupathi G, Hood C, Aghajanian CA, Juretzka M, Iasonos A, et al. Pilot study of a heptavalent vaccine-keyhole limpet hemocyanin conjugate plus QS21 in patients with epithelial ovarian, fallopian tube, or peritoneal cancer. Clin Cancer Res. 2007 Jul 15;13(14):4170–7. doi: 10.1158/1078-0432.CCR-06-2949. [DOI] [PubMed] [Google Scholar]
- 41.Drane D, Maraskovsky E, Gibson R, Mitchell S, Barnden M, Moskwa A, et al. Priming of CD4+ and CD8+ T cell responses using a HCV core ISCOMATRIX vaccine: a phase I study in healthy volunteers. Hum Vaccin. 2009 Mar;5(3):151–7. doi: 10.4161/hv.5.3.6614. [DOI] [PubMed] [Google Scholar]
- 42.Batista-Duharte A, Lindblad EB, Oviedo-Orta E. Progress in understanding adjuvant immunotoxicity mechanisms. Toxicology letters. 2011 Jun 10;203(2):97–105. doi: 10.1016/j.toxlet.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 43.Cooper C, Mackie D. Hepatitis B surface antigen-1018 ISS adjuvant- containing vaccine: a review of HEPLISAV safety and efficacy. Expert Rev Vaccines. 2011 Apr;10(4):417–27. doi: 10.1586/erv.10.162. [DOI] [PubMed] [Google Scholar]
- 44.Rendi-Wagner P, Kundi M, Stemberger H, Wiedermann G, Holzmann H, Hofer M, et al. Antibody-response to three recombinant hepatitis B vaccines: comparative evaluation of multicenter travel-clinic based experience. Vaccine. 2001 Feb 28;19(15–16):2055–60. doi: 10.1016/s0264-410x(00)00410-2. [DOI] [PubMed] [Google Scholar]
- 45.Gherardi RK, Coquet M, Cherin P, Authier FJ, Laforet P, Belec L, et al. Macrophagic myofasciitis: an emerging entity. Groupe d’Etudes et Recherche sur les Maladies Musculaires Acquises et Dysimmunitaires (GERMMAD) de l’Association Francaise contre les Myopathies (AFM) Lancet. 1998 Aug 1;352(9125):347–52. doi: 10.1016/s0140-6736(98)02326-5. [DOI] [PubMed] [Google Scholar]
- 46.Vera-Lastra O, Medina G, del Cruz-Dominguez MP, Jara LJ, Shoenfeld Y. Autoimmune/inflammatory syndrome induced by adjuvants (Shoenfeld’s syndrome): clinical and immunological spectrum. Expert review of clinical immunology. 2013 Apr;9(4):361–73. doi: 10.1586/eci.13.2. [DOI] [PubMed] [Google Scholar]
- 47.Coulson EJ, Stevens H. Quantitative studies in anaphylaxis; effect of the alum adjuvant and route of administration on the sensitizing dose. J Immunol. 1949 Feb;61(2):119–23. [PubMed] [Google Scholar]
- 48.Terhune TD, Deth RC. How aluminum adjuvants could promote and enhance non-target IgE synthesis in a genetically-vulnerable sub-population. Journal of immunotoxicology. 2013 Apr-Jun;10(2):210–22. doi: 10.3109/1547691X.2012.708366. [DOI] [PubMed] [Google Scholar]
- 49.Garcia A, De Sanctis JB. An overview of adjuvant formulations and delivery systems. APMIS: acta pathologica, microbiologica, et immunologica Scandinavica. 2014 Apr;122(4):257–67. doi: 10.1111/apm.12143. [DOI] [PubMed] [Google Scholar]
- 50.Barclay T, Ginic-Markovic M, Johnston MR, Cooper PD, Petrovsky N. Analysis of the hydrolysis of inulin using real time 1H NMR spectroscopy. Carbohydrate research. 2012 May 1;352:117–25. doi: 10.1016/j.carres.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Robson JS, Ferguson MH, et al. The determination of the renal clearance of inulin in man. Q J Exp Physiol. 1949 Jun;35(2):111–34. doi: 10.1113/expphysiol.1949.sp000943. [DOI] [PubMed] [Google Scholar]
- 52.Shannon JA, Smith HW. The Excretion of Inulin, Xylose and Urea by Normal and Phlorizinized Man. J Clin Invest. 1935 Jul;14(4):393–401. doi: 10.1172/JCI100690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gordon DL, Sajkov D, Woodman RJ, Honda-Okubo Y, Cox MM, Heinzel S, et al. Randomized clinical trial of immunogenicity and safety of a recombinant H1N1/2009 pandemic influenza vaccine containing Advax polysaccharide adjuvant. Vaccine. 2012 Aug 3;30(36):5407–16. doi: 10.1016/j.vaccine.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]