Abstract
The present analyses were undertaken to define the mechanisms by which fetuin-A modulates cellular adhesion. FLAG-tagged fetuin-A was expressed in breast carcinoma and HEK-293T cells. We demonstrated by confocal microscopy that fetuin-A co-localizes with histone H2A in the cell nucleus, forms stable complexes with histones such as H2A and H3 in solution, and shuttles histones to exosomes. The rate of cellular adhesion and spreading to either fibronectin or laminin coated wells was accelerated significantly in the presence of either endogenous fetuin-A or serum derived protein. More importantly, the formation of focal adhesion complexes on surfaces coated by laminin or fibronectin was accelerated in the presence of fetuin-A or histone coated exosomes. Cellular adhesion mediated by histone coated exosomes was abrogated by heparin and heparinase III. Heparinase III cleaves heparan sulfate from cell surface heparan sulfate proteoglycans. Lastly, the uptake of histone coated exosomes and subsequent cellular adhesion, was abrogated by heparin. Taken together, the data suggest a mechanism where fetuin-A, either endogenously synthesized or supplied extracellularly can extract histones from the nucleus or elsewhere in the cytosol/membrane and load them on cellular exosomes which then mediate adhesion by interacting with cell surface heparan sulfate proteoglycans via bound histones.
Keywords: Fetuin-A, histones, exosomes, adhesion, tumor cells
Introduction
Fetuin-A is a serum glycoprotein synthesized by the liver and secreted into the blood stream. The widely accepted physiological function of this protein is the inhibition of ectopic calcification [1]. However, it also modulates a number of signaling pathways including those involving TGF-β and insulin [2,3,4,5]. Because of its widespread involvement in so many physiological mechanisms, fetuin-A is now considered a multi-functional protein [6].
Its role in cellular adhesion has been controversial. For many years, the prevailing dogma was that adhesion proteins that co-purify with fetuin-A such as fibronectin, promote the adhesion of cells to extracellular matrices [7,8]. We, however, demonstrated that highly purified fetuin-A was just as effective as crude Pedersen fetuin-A in mediating cellular adhesion [9]. Initially we proposed that annexins particularly AnxA2 and AnxA6 were the putative cell surface receptors for fetuin-A mediated adhesion [10]. However, further mechanistic studies failed to demonstrate the receptor role of annexins. Nevertheless, annexins particularly AnxA6 were deemed essential for fetuin-A endocytic uptake by tumor cells [9]. Interestingly, this fetuin-A uptake was obligatory for strong cellular adhesion and spreading on plastic. Cells in which AnxA6 was knocked down failed to adhere and spread properly on plastic [9].
In order to understand the mechanism(s) by which fetuin-A mediates adhesion, we analyzed the adhesion potential and composition of exosomes secreted in the absence and in the presence of fetuin-A by detached tumor cells in suspension. We had determined in prior studies that detached cells secreted more exosomes than adhered and spread cells [11]. We showed that cellular exosomes that promoted adhesion were those secreted in the presence of fetuin-A, while those secreted in the absence of fetuin-A lacked adhesion promoting properties. Interestingly, the adhesion competent exosomes contained histones and fetuin-A in addition to the usual exosomal marker proteins such as HSP90 [12]. The adhesion incompetent exosomes on the other hand lacked histones, but contained the compendium of exosomal marker proteins [12]. A number of studies have also demonstrated the exosomal mediated adhesion and cellular growth in tumor cell lines in vitro [13,14], while others have implicated these nano-vesicles in the preparation of metastatic niches in vivo [15]. Even though studies have suggested that exosomal associated integrins drive the adhesion process [16,17], we demonstrated that both adhesion incompetent and competent cellular exosomes contain integrins [12], implying that other mechanisms are involved.
Exosomes are nano particles (30-100 nm) that originate from the inward budding of an endosomes's limiting membrane into its lumen, giving rise to endosomes containing multiple intraluminal vesicles known as multivesicular bodies (MVBs). The outer membranes of MVBs can fuse with the plasma membrane and release their intraluminal vesicles to the extracellular milieu as exosomes [18]. Whereas interesting potential physiological roles of exosomes are being unraveled at an ever increasing pace in the literature, the mechanisms that regulate their biogenesis and function particularly in cancer cells are unclear [19].
In the present study, we questioned whether fetuin-A interacted with histones intracellularly and in solution and whether it was responsible for trafficking/shuttling histones from the nucleus to the exosomes and membranes as well as maturation of focal adhesions. A number of plasma proteins such as plasminogen have been shown to interact with histones in solution, mitigating their deleterious effects on cells [20]. Interestingly, plasminogen is capable of attenuating the exosomal mediated adhesion [12], further suggesting that histones are involved in the exosomal mediated adhesion. Even though histones have not been established as bonafide adhesion molecules, their extracellular appearance and suggested roles in this microenvironment have provoked interest in biology [21,22]. For example, a recent report indicated that extracellular histones activated a number of adhesion related signals such as PI3 kinase/Akt in platelets [23].
Materials and methods
Materials
Crude fetuin-A (Pedersen fetuin-A) and histone from calf thymus (lyophilized powder) were purchased from Sigma (St. Louis, MO). Crude fetuin-A was purified according to the procedure detailed in [9]. Antibodies to histone H2A and H3 were purchased from Cell Signaling Technology (Danvers, MA). Monoclonal mouse Anti-FLAG M2, indocarbocyanide (Cy3)-conjugated sheep anti-mouse IgG, FITC-conjugated anti-rabbit IgG and anti-vinculin antibodies were from Sigma. All other antibodies were purchased from Santa Cruz Biotechnology (Dallas, TX) unless stated otherwise. All other reagents were from Sigma unless stated otherwise.
Cells
The breast carcinoma cell line (BT-549) and HEK293T cells were purchased from ATCC (Manassas, VA). A sub-clone of BT-549 forced to express galectin-3 and named BT-549Gal3, was kindly donated by Dr. Avraham Raz (Karmanos Cancer Research Institute, Detroit, MI). Human fetuin A (AHSG) was cloned into the pMZS-3F vector [24] to generate pMZS-3F-fetuin-A.The recombinant or empty vector were then used to transfect BT-549Gal3 cells, selected with increasing concentrations of G418 and the resulting stably transfected clones are herein designated FF94 and EV94 respectively. The parental BT-549 was also stably transfected with the fetuin-A expression vector and selected as above to yield FFBT and the empty vector transfected controls, EVBT. The generated breast carcinoma cell lines were propagated in Dulbecco's modified Eagle's medium/nutrient F-12 (DMEM/F-12) supplemented with 10% heat inactivated fetal bovine serum, 2 mmol/liter L-glutamine, 100 units/ml penicillin, and 50 units/ml streptomycin in a 95% air and 5% CO2 incubator at 37°C. Where indicated, serum free medium (SFM) consisted of DMEM/F-12 in which fetal bovine serum (FBS) was replaced with 0.1% bovine serum albumin (BSA).
Promotion of cellular adhesion and spreading by fetuin-A
The 96-well micro-titer plates were coated with either fibronectin (FN) or laminin (LN) (40 μg/ml) in PBS overnight at 4°C, the wells blocked with 3% (w/v) BSA and an equal number of BT-549Gal3 cells (3 × 104 cells/well) added to the wells in triplicates. The cells were added in the absence (FN; LN) or presence of purified bovine fetuin-A (FetA + FN; FetA + LN). The cells were allowed to adhere for 1 h, 2 h and 8 h at 37°C in a humidified cell incubator. At the end of each incubation period, the wells were washed twice with SFM and the adherent cells fixed in 4% formalin, stained with crystal violet, washed with water and 10% acetic acid added (100 μl/well) to release the stain from the cells. The absorbance was read at 570 nm to indicate the relative number of cells adhered in each well. Each bar is represents average ± S.D. absorbance of 4 wells. The experiment was repeated 3 times with similar data.
Co-localization of fetuin-A and histone H2A in the nucleus of breast carcinoma cells
The G418-selected cells following transfection with FLAG-tagged fetuin-A, were grown on glass coverslips, fixed in cold methanol for 5 min and blocked with 3% BSA for 30 min. This was followed by incubation with mouse anti-FLAG antibodies and subsequently with secondary antibodies labeled with Cy3. After extensive washing, the cells were incubated with rabbit anti-histone H2A antibodies followed by FITC labeled secondary antibodies, washed and visualized by confocal microscopy (Nikon A1r).
Co-immunoprecipitation of nuclear fetuin-A/histone complex
In order to demonstrate that fetuin-A in the nucleus associates with histones, nuclear fractions were prepared from EVBT and FFBT cells as previously described [25]. The clarified nuclear proteins were pre-cleared for one hour at 4°C by incubation with protein A/G agarose (Santa Cruz). Pre-cleared lysates (0.5mg) were then incubated overnight at 4°C with 4 μg of mouse-anti FLAG antibody, and rabbit-anti histone H2A antibodies, followed by a 2 hour incubation with protein A/G beads. The beads were washed five times with RIPA buffer and proteins bound to beads were analyzed by western blotting after elution with 2X SDS-PAGE sample buffer.
Interaction of fetuin-A with histones in solution
To further demonstrate the interaction between fetuin-A and histones in other cells, we transfected HEK-293T cells with the FLAG-tagged fetuin-A vector pMZS-3F-fetuin-A and pMZS-3F empty vector using lipofectamine 2000 (Invitrogen, Grand Island, NY) according to manufacturer's instructions. The FLAG-tagged fetuin-A expressing HEK293T cells were designated FF93T. These cells were selected in increasing concentrations of G418 (300-800 μg/ml) for about 2 weeks. Resistant surviving colonies were screened for expression of FLAG-tagged fetuin-A by western blotting. The FLAG expressing clones were grown to 60-90% confluence and lysed for 30 min on ice in TAP-tag lysis (10% glycerol, 50 mM Hepes-KOH pH 8.0, 100 mM KCl, 2mM EDTA, 0.1% NP-40, 2mM dithiothreitol, 1X protease inhibitors, 10 mM NaF, 0.25 mM Sodium orthovanadate, 50 mM β-glycerolphosphate). The lysate was cleared by centrifugation at 10,000 rpm for 15 min and 1000 μg of lysate incubated with 50 μl of anti-FLAG agarose beads overnight at 4°C with end-over-end rocking. The beads were washed 5 times in TAP-tag lysis buffer and the FLAG-bound proteins eluted in 2x SDS sample buffer. The eluates were resolved in NuPAGE Bis-Tris 4-12% pre-cast gels (Invitrogen, Grand Island NY) and transferred to nitrocellulose membranes and membranes probed with antibodies to fetuin-A, FLAG, histone H2A or H3A. The cells were also transfected with empty vector as control and the cells designated as EV93T.
Gel Overlay experiments
Lyophilized powder of histone from calf thymus was resolved in NuPAGE Bis-Tris gel 4-12% (Invitrogen, Grand Island, NY) and transferred to nitrocellulose membrane. The membrane was blocked with 5% BSA in TBST and then overlaid with either asialofetuin-A or fetuin-A (1 mg/ml). Fetuin-A or asialofetuin-A bound to histones imbedded in the membrane were revealed by probing the membranes with anti-fetuin-A antibodies and visualized by enhanced chemiluminescense (ECL).
Isolation and purification of exosomes from tumor cells in suspension
Exosomes were isolated and purified as described [12]. Briefly, tumor cells growing in 175 cm2 culture flasks were harvested when they were about 80% confluent with 2 mM EDTA in PBS, washed twice in SFM and re-suspended in 0.5-1 ml of SFM containing either BSA or fetuin-A (1 mg/ml). Cells expressing FLAG-tagged fetuin-A as well as their transfection controls were harvested in SFM. Cells that do not express fetuin-A were incubated in the SFM with or without fetuin-A to obtain adhesion competent and incompetent exosomes respectively, for 1 h at 37°C on a rotator. The cells were pelleted and the conditioned medium containing secreted exosomes centrifuged for 20 min at 2000 × g to pellet dead cells and cellular debris. The resultant supernatant was then centrifuged at 20,000 × g to pellet micro vesicles. Exoquick™ (System Biosciences, Mountain View , CA), was added to the supernatant (250 μl/1 ml) mixed and incubated overnight at 4°C and centrifuged for 20 min at 3,000 × g to pellet exosomes. The exosomes were re-suspended in HBS (Hanks buffered saline) and the protein concentration estimated using the Bradford assay (Biorad). To show that the cells remained viable in suspension during the 1 h incubation period, LDH cytotoxicity Assay where the level of LDH in the conditioned medium of suspended cells was performed following Manufacturer's protocol (Roche). Briefly the BT-549Gal3 breast tumor cells (1 × 106 cells/Eppendorf vial) in SFM containing either BSA or fetuin-A (1 mg/ml) were incubated at 37°C for various time points (0-120 min) with end-over-end rocking, after which aliquots of the conditioned medium were used in the assay after pelleting the cells. The level of LDH in each vial was compared and expressed as a percentage of LDH in a vial containing the same number of cells that were partially lysed. The viability of the cells in suspension was also determined by the Trypan blue dye exclusion at the end of incubation periods.
Transmission Electron Microscopy (TEM)
To ascertain that we had isolated pure exosomes, the vesicles (~1 mg/ml) were fixed in 1% (final concentration) of glutaraldehyde in 0.1 M phosphate buffer, pH 7.4. The samples were then allowed to absorb to TEM grids and negatively stained with 1% uranyl acetate. Images were taken from various areas of the grid (15,000X).
Fluorescence Activated Vesicular Sorting (FAVS)
A critical requirement for fetuin-A and or histones to be directly involved in exosomal mediated adhesion, is that they should be located on the extracellular surface of exosomes. To demonstrate this topography, we applied the technique of Fluorescence Activated Vesicular Sorting (FAVS). In this technique, the antibodies to fetuin-A or histone H2A only recognize the molecules if they are on the extracellular surface because they would not be able to cross the vesicular membranes if the molecules were on the luminal surfaces of exosomes. We therefore obtained cellular exosomes from BT-549 transfected with either FLAG-tagged fetuin-A or empty vector control as described above. Exosomes from the fetuin-A transfected cells were named Fet-A exosomes and those from control transfected cells were designated EV exosomes. Twenty-five micrograms of EV or Fet-A exosomes were stained with 1/100 final dilution of anti-fetuin-A (goat poly Ab) for 1 hour at 4°C with constant rotation and in the presence of 10 μg/ml human IVIG (Intravenous Immune Globulin) blocking agent. Exosomes were washed 3 times by pelleting with ultracentrifugation at 200,000 × g for 1 hr. EV and Fet-A exosomes were then counterstained with a 1/100 final dilution of donkey anti-goat secondary conjugated with AlexaFluor 488 for 1 hour at 4°C with constant rotation and in the presence of 10 μg/ml human IVIG blocking agent and washed as before. EV and Fet-A exosomes were then stained with 1/100 final dilution of anti-histone H2A (rabbit poly Ab) for 1 hour at 4°C with constant rotation and in the presence of 10 μg/ml human IVIG blocking agent and then washed as previously indicated. Finally an AlexaFluor 568 conjugated donkey anti-rabbit secondary was used at 1/100 final dilution to stain the histone-H2A primary Ab as indicated above and suspended in PBS supplemented with 25 mM HEPES for FAVS analysis. Appropriate controls with primary only or secondary only were utilized to establish background levels and subsequent gating.
Cell fractionation experiments
BT549 cells transfected with either FLAG-tagged Fetuin-A (FFBT) or the empty vector control (EVBT) were grown to 80% confluence and detached with 2 mM EDTA in PBS. The cells were then washed in serum free medium and re-plated on 145 mm dishes pre-coated with either laminin or fibronectin (40 μg/ml) and incubated for 30 minutes at 37°C in a humidified CO2 incubator. The cells were lysed and fractionated into nuclear chromatin bound, cytosolic and membrane associated proteins as previously described [25]. In brief, cells were re-suspended in hypotonic lysis buffer (10 mM HEPES pH 7.2, 10 mM KCl, 1.5 mM MgCl2, 0.34 M sucrose 10% glycerol, 1mM dithiothreitol, 1mM phenylmethylsulfonyl fluoride, 0.04% Triton X-100); incubated on ice for 15 minutes, then centrifuged at 700 × g for 4 minutes at 4 °C to generate a post nuclear supernatant or PNS fraction. After thoroughly washing the nuclear pellet, chromatin-associated proteins were extracted from the nuclear pellet fraction with RIPA lysis buffer (50mM Tris HCl, pH 7.4, 1% NP-40, 0.5% sodium deoxycholate 150 mM NaCl, 1 mM EDTA) and then clarified at 16,000 × g for 10 min to obtain the soluble chromatin associated proteins or nuclear fraction. Crude membranes and membrane-associated proteins were obtained by centrifugation of the PNS fraction at 100,000 × g for 2 hours. The supernatant (cytosolic fraction) was transferred to new tubes while the crude membrane pellet was solubilized in RIPA buffer and cleared by centrifugation at 16,000 × g for 10 min. Equal amounts of the whole cell lysate, the cytosolic, nuclear and membrane fractions were then analyzed by western blotting.
Modulation of focal adhesion assembly by fetuin-A and adhesion competent exosomes
Glass coverslips were coated with fibronectin (40 μg/ml) in PBS overnight at 4°C. The coverslips were then blocked with 3% BSA for 30 min at 37°C. Breast carcinoma cells (BT-549Gal3) were then added to the coverslips in six well culture plates and incubated for exactly 30 min in SFM containing BSA (1 mg/ml) or purified fetuin-A (1 mg/ml). The cells were also incubated for 30 min in SFM containing 20 μg/ml of either adhesion incompetent (BSA-Exo) or adhesion competent (FetA-Exo) exosomes.
At the end of the incubation period, the medium was aspirated and the cells fixed/permeabilized in 0.5% (w/v) of Triton-X100; 3% (w/v) paraformaldehyde for exactly 2 min. The detergent was removed promptly and the cells washed once with 3% paraformaldehyde and fixed in the same solution for another 30 min. The glass coverslips with the cells were then blocked with 3% BSA in PBS for at least 1 h at 37°C and then incubated with anti-vinculin in PBS for 30 min at 37°C. After washing, the cells were stained with Cy3-secondary antibodies for another 30 min at 37°C. This was followed by another set of washes and the coverslips were mounted with Prolong Gold anti-fade with DAPI and examined on a confocal microscope (Nikon A1r).
Modulation of cellular adhesion by heparin and heparinase III
BT-549Gal3 cells were plated in 96-well micro-titer plates (2 × 104 cells/well) in serum free medium containing exosomes loaded with histones and fetuin-A (20 μg/ml) and in the absence (control) or presence of heparin (40 μg/ml). The cells were incubated for at least 4 hours, the wells washed once with SFM and adherent and spread cells photographed.
The cells were also plated in 96-well micro-titer plates (2 × 104 cells/well) in triplicates and allowed to adhere in the presence of exosomes loaded with histones (20 μg/ml) in the absence (control) and in the presence of heparinase III. This enzymes cleaves the heparin sulfate moieties from the heparan sulfate proteoglycans on the cell surface. After 2 h, non-adherent cells were washed off and adhered cells fixed as above and counted under a microscope (20 × objective).
Uptake of CD63-GFP-labeled exosomes by breast tumor cells
To document the uptake of cellular exosomes, we isolated and purified CD63-GFP-labeled exosomes from BT-CD63 cells as described [11]. Briefly, the BT-CD63 cells (1 × 108) were detached by EDTA and the cells in suspension incubated with purified fetuin-A at 37°C in Eppendorf cuvettes by end-over-end rotation. At the end of the incubation, the cells were pelleted and the supernatant subjected to further centrifugations steps to remove cell debris and microvesicles. Exosomes were precipitated from the final supernatant by ExoQuick™. Breast carcinoma cells, BT-549Gal3 (2 × 104 cells/chamber) were then allowed to attach to glass chamber slides in SFM containing the GFP-labeled exosomes (~20 μg/ml) in the absence and presence of heparin (40 μg/ml). After 30 min incubation period at 37°C, the cells were fixed in 4% formalin and the glass slides prepared for confocal microscopy.
Results
Fetuin-A accelerates the adhesion and cell spreading on fibronectin and laminin
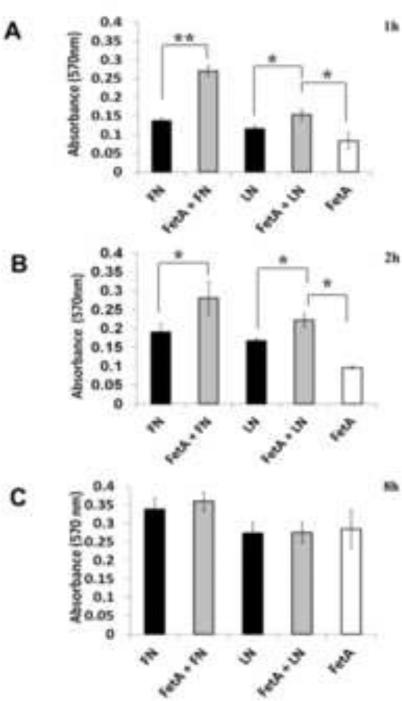
Knowing that purified fetuin-A has the capacity to mediate cellular adhesion to plastic in serum free medium [9], we questioned whether it would also accelerate the adhesion of these cells to fibronectin and collagen coated surfaces in serum free medium. We have shown in three separate experiments that the presence of fetuin-A (0.5-1 mg/ml) accelerates the adhesion and spreading of breast carcinoma cells to both fibronectin and laminin coated surfaces in SFM. Adhesion of cells to either fibronectin (FN) or laminin (LN) was more or less complete within 2 h (Fig. 1A and 1B) in the presence of fetuin-A. In its absence, it took as long as 8 h for cells to fully adhere to either fibronectin or laminin in serum free medium (Fig 1). Cells also adhered, spread and grew on plastic in the presence of fetuin-A alone. However it took a relatively long time (~8 h) for cells to achieve complete adhesion and spreading on plastic in the presence of fetuin-A alone (Fig. 1C). The fetuin-A mediated adhesion to plastic is not attenuated by RGD peptides nor by the absence of Mg2+ or Mn2+ ions both of which are required for integrin driven adhesion. However, calcium ions are required for fetuin-A driven adhesion [10].
Fig. 1. Fetuin-A accelerates the adhesion of tumor cells to fibronectin and laminin coated wells.
The wells of 96-well microtiter plates were coated with either fibronectin or laminin (40 μg/ml) in PBS overnight at 4°C, blocked with 3% BSA and BT-549Gal3 cells added to the wells (3 × 104 cells/well). The cells were incubated in the absence or presence of fetuin-A (1 mg/ml). As controls, the same number of cells were plated on uncoated wells in the presence of fetuin-A (1 mg/ml). The cells were allowed to adhere for 1 h, 2 h and 8 h at 37°C in a humidified incubator. At the end of incubation, the wells were washed 2x with SFM and adherent cells fixed in 4% formalin, stained with crystal violet, washed in water and 10% acetic acid added (100 μl/well) to release the stain from the cells. The absorbance was read at 570 nm to indicate the relative number of cells adhered in each well. Each bar represents average ± S.D. absorbance of 4 wells (* 0.05< P; ** 0.001< P). The experiment was repeated 3 times with similar data.
Fetuin-A interacts with histones in the nucleus of breast carcinoma cells
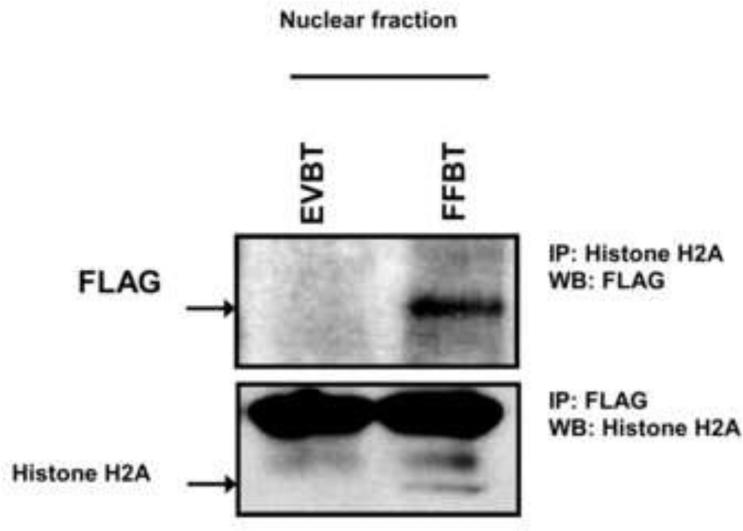
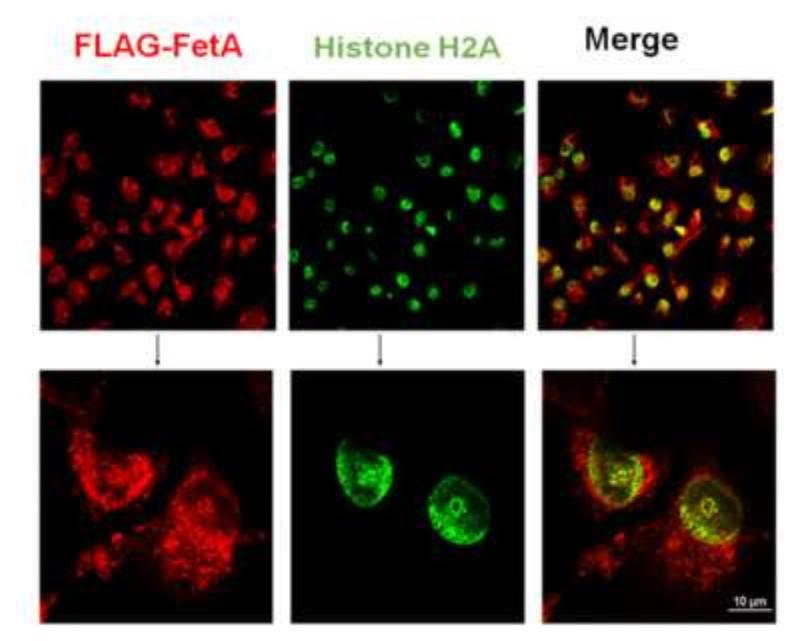
We and others have previously demonstrated the rapid uptake of fetuin-A by tumor and vascular smooth muscle cells from the medium [9,26], and that this uptake was a pre-requisite for fetuin-A mediated cellular adhesion. Subsequent studies from our laboratory demonstrated that intracellular fetuin-A was associated with histones and exosomes. Since histones are normally confined to the cell nucleus, the study suggested that fetuin-A could also be in the nucleus where it potentially interacts with histones. In order for us to follow the trafficking of intracellular fetuin-A more carefully, we expressed human fetuin-A with a FLAG-tag in human breast carcinoma cells and determined that it co-localized with histone H2A as prototype in the cell nucleus (Fig. 2). More importantly when the cells were fractionated, FLAG-tagged fetuin-A was in the nuclear fraction and co-immunoprecipitated with Histone H2A (Fig 3). It should be noted that fetuin-A has the potential to interact with other histone isoforms [12]. The non-specific thick bands that appear above the histone H2A band is most likely the light IgG chain (~25 kDa) which if often observed in immunoprecipitation blots.
Fig. 2. Co-localization of fetuin-A and histone H2A in the cell nucleus.
FLAG-tagged fetuin-A expressing BT-549Gal3 (FF94) cells were plated on glass coverslips and allowed to grow in complete medium until ~ 70% confluent. The cells were fixed in cold methanol for 5 min and blocked with 3% BSA for 30 min. This was followed by incubation with mouse monoclonal anti-FLAG antibodies for 1 h, washed and then incubated with Cy3 secondary for another 1 h. The coverslips were washed and incubated with rabbit anti-histone H2A antibodies for another 1 h. Once again the coverslips were washed and incubated with FITC-labeled secondary antibodies. This was followed by another set of washes and the coverslips were mounted with Prolong Gold anti-fade and examined on a confocal microscope (Nikon Air).
Fig 3. Association of fetuin-A with histones in the cell nucleus.
Nuclear fractions from EVBT and FFBT cells were prepared as described in Materials and Methods. To show the association of fetuin-A and histone H2A as a prototypical histone in the nucleus, after pre-clearance, the chromatin fractions were incubated overnight at 4°C with mouse anti-FLAG and rabbit anti-histone H2A antibodies respectively. This was followed by incubation with protein A/G agarose beads. The beads were washed in RIPA buffer and analyzed by western blot.
Interaction of fetuin-A with histones in solution and in Gel overlay experiments
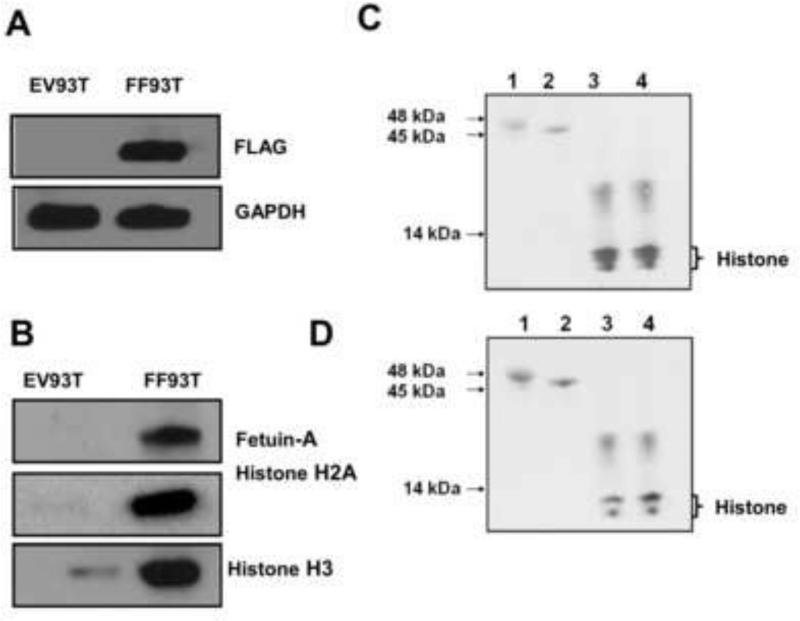
Transfection of FLAG-tagged fetuin-A into HEK 293 cells was quite efficient (Fig. 4A). To further demonstrate the interaction between fetuin-A and histones, anti-FLAG beads pulled down fetuin-A from the cell lysates of fetuin-A transfected (HEK 293T) cells together with the interacting proteins which included histone H2A and histone H3. The study clearly demonstrated that fetuin-A interacted with histones in the cell lysates (Fig 4B), and there was negligible interaction in the lysates of cells transfected with empty vector (EV93T) (Fig. 4B).
Fig 4. Interaction between fetuin-A and histones in solution.
In panel A, cell lysates (100 μg) from FLAG-tagged fetuin-A HEK-93T cells (FF93T) and from those transfected with empty vector (EV93T) were incubated with 50 μl of anti-FLAG beads suspended in agarose. The beads were washed 5x in TAP-tag lysis buffer and then FLAG-fetuin-A bound proteins eluted in 2x sample buffer. The elutes were resolved in NuPAGE Bis-Tris 4-12% pre-cast gels, transferred to nitrocellulose and probed with antibodies to fetuin-A, visualized by ECL, membrane stripped and probed with anti-histone H2A and lastly with anti-histone H3. In panel B, the cell lysates were resolved in the NuPAGE gels, transferred to nitrocellulose and probed with anti-FLAG and with anti-GAPDH for loading control. In panel C, fetuin-A (lane 1) asialofetuin-A (lane 2) histone H2A (lanes 3 and 4) were resolved in the NuPAGE gels, transferred to nitrocellulose paper, blocked with 3% BSA (w/v) and then overlaid with asialofetuin-A. The membrane was washed and probed with anti-fetuin-A antibodies and visualized by ECL. In pane D, the experiment described in panel C was repeated except that instead of overlaying with asialofetuin-A, it was overlaid with fetuin-A.
In order to rule out the possibility that fetuin-A interacted with histones via other proteins, we demonstrated that both asialofetuin-A (Fig. 4C) and fetuin-A (Fig. 4D) bind directly to histone in gel overlay experiments. Furthermore, the experiments suggested that sialic acid residues on fetuin-A are not utilized in this binding interaction since the sialic acid denuded asialofetuin-A interacted with histones to the same extent as fetuin-A.
Presence of fetuin-A and histones on adhesion competent exosomes
It was important to show that during the secretion of exosomes, the suspended cells remained viable. Indeed the suspended cells whether in the presence of BSA or fetuin-A, remained viable for up to 2 h. The cells suspended for various time points excluded Trypan blue dye and the levels of LDH in the conditioned media did not change appreciably from the beginning to the end of the 1 hour incubation period (Table 1).
Table 1.
Incubating tumor cells in suspension during exosome isolation does not compromise their viability. BT549Gal3 cells were incubated with SFM containing either BSA or fetuin-A (1 mg/ml) in Eppendorf tubes (0-120 min) in order to secrete optimal levels of cellular exosomes. At the end of each incubation period, cellular integrity and viability was determined by LDH cytotoxicity assay and Trypan blue dye exclusion.
| Incubation Period (min) | Protein in the incubation medium (1 mg/ml) | Relative Concentration of LDH* in conditioned medium (% ± S.D.) | % Cell viability determined by Trypan blue dye exclusion |
|---|---|---|---|
| 0 min | BSA | 24.2 + 4 | 100 |
| Fetuin-A | 28.7 + 7.8 | 100 | |
| 20 min | BSA | 24.9 + 7.4 | 100 |
| Fetuin-A | 30.7 + 0.07 | 100 | |
| 40 min | BSA | 29.3 + 1.9 | 100 |
| Fetuin-A | 31.3 + 0.2 | 100 | |
| 60 min | BSA | 28.1 + 3.3 | 100 |
| Fetuin-A | 34.5 + 2.5 | 100 | |
| 120 min | BSA | 33.2 + 0.4 | 100 |
| Fetuin-A | 33.4 + 1.7 | 100 |
The relative concentration of lactate dehydrogenase (LDH) in the conditioned medium was determined as % of LDH in the vials containing the same number of cells (1,000,000 cells) that were partially lysed.
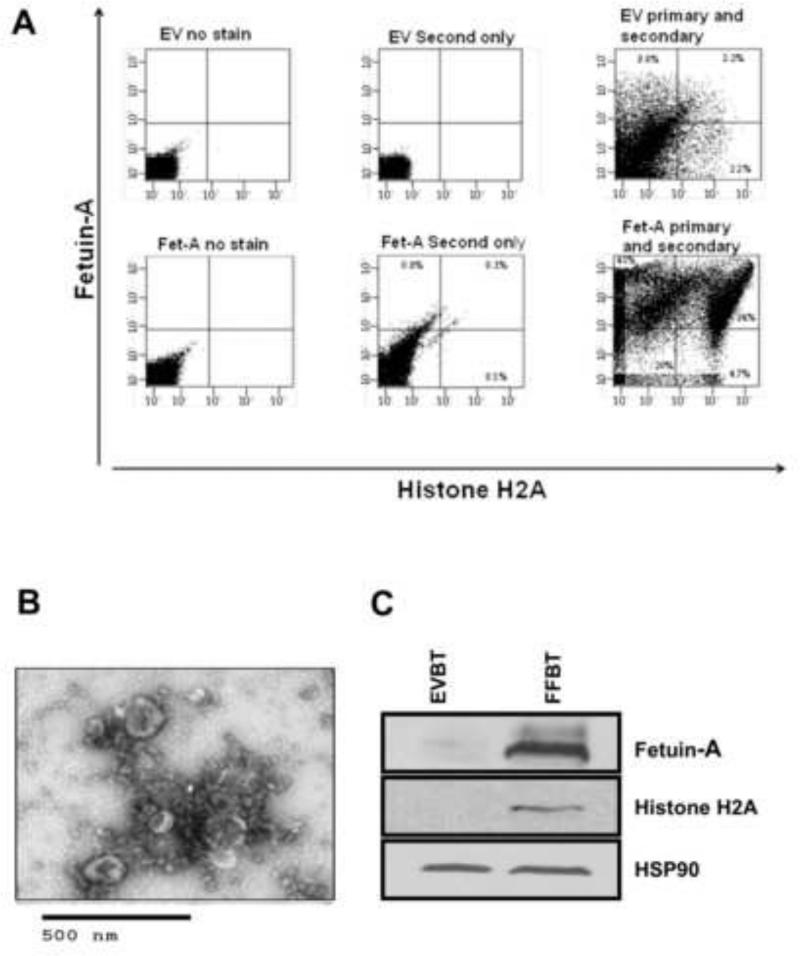
We previously described two forms of cellular exosomes isolated from breast tumor cells. Those that mediated adhesion contained fetuin-A and histones in addition to the other exosomal marker proteins such as HSP90 and annexins. Adhesion incompetent exosomes on the other hand lacked histones and fetuin-A [12]. We therefore questioned whether these proteins could be recognized on the surfaces of adhesion competent exosomes. If either fetuin-A or histones or both were directly involved in the cellular adhesion process, it was critical to show that they are disposed on the exosomal surfaces facing the extracellular milieu and not the lumen of exosomes. To address this problem, we employed the technique of FAVS. It was clear that exosomes isolated from fetuin-A expressing cells had high levels of both fetuin-A and histone H2A on their surfaces (Fig. 5A). Exosomes from cells transfected with empty vector (EV) which were transfection controls, did not contain significant quantities of either fetuin-A or histone H2A. The little fetuin-A and histone H2A detected could be due to residual fetuin-A from culture medium because the antibody used recognized both human and bovine fetuin-A.
Fig. 5. Localization of fetuin-A and histone H2A on the extracellular surfaces of cellular exosomes by FAVS.
In panel A, cellular exosomes (25 μg) from BT-549Gal3 cells transfected with plasmids carrying the fetuin-A (Fet-A) (FF94) or transfected with empty vector (EV94) controls, were stained with anti-fetuin-A antibodies washed and subjected to ultracentrifugation as described in Materials and Methods. This was followed by incubation with secondary antibodies conjugated to Alexa Fluor 488 for 1 h and washed as above. The exosomes (EV and Fet-A) were then stained with anti-histone H2A antibodies followed by secondary antibodies conjugated to AlexaFluor 569, incubated and washed as above. Appropriate controls with secondary antibodies only were utilized to establish background levels and subsequent gating. In panel B, exosomes from FF94 cells were analyzed by TEM that revealed the cup shaped vesicles. In panel C, EVBT (lane 1) and FFBT (lane 2) exosomes were dissolved in SDS-sample buffer, resolved in NuPAGE gels, transferred to nitrocellulose paper and probed with anti-fetuin-A antibodies and visualized by enhanced chemiluminescense (ECL). The blot was stripped and probed in sequential order with anti-Histone H2A antibodies and anti-HSP90 (heat shock protein-90) and again visualized by ECL.
The morphology of cellular exosomes was confirmed by transmission electron microscopy as cup shaped vesicles in the range of 30-100 nm in diameter (Fig. 5B). The presence of histone H2A and fetuin-A in the adhesion competent exosomes was also validated by western blot (Fig. 5C).
Distribution of vinculin, fetuin-A, and histone H2A in the membranes of adhered and well spread cells
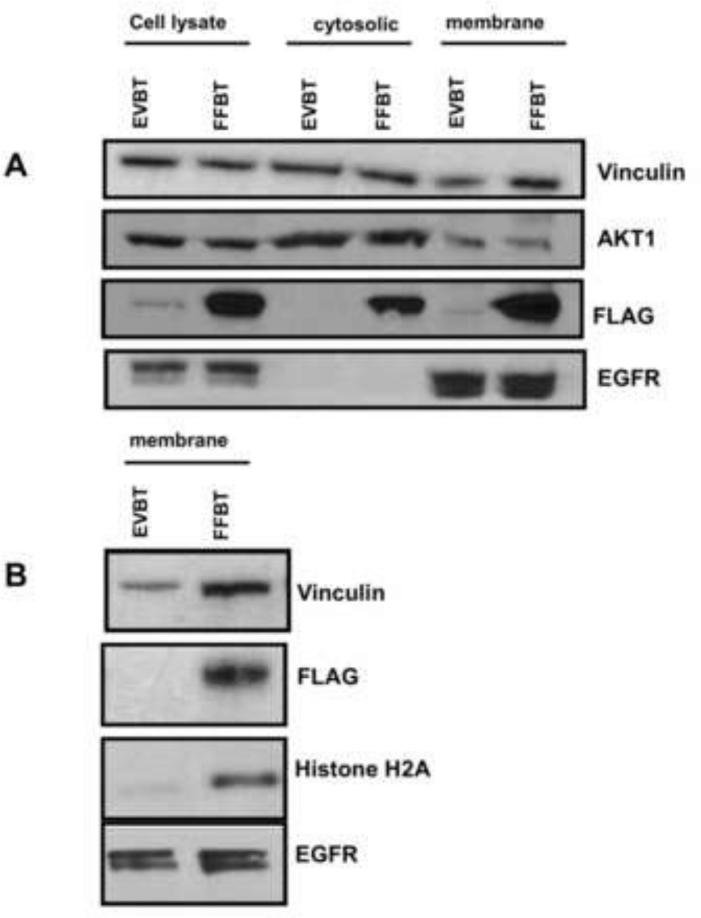
In three separate experiments, we observed that in adhered and spread cells, vinculin, and fetuin-A were in both soluble and plasma membranes fractions (Fig. 6A). In contrast Akt1 was concentrated in the cytosolic fraction relative to the plasma membrane. Histone H2A and epidermal growth factor receptor were concentrated in the plasma membrane fractions relative to the soluble fractions. Epidermal growth factor receptor was used as the loading control for membrane proteins. The presence of histone H2A in the cell membrane of fetuin-A expressing cells was unexpected but again strongly suggested the interaction of fetuin-A with histones in this fraction. The data suggest that during cellular adhesion and spreading, fetuin-A and histones are concentrated on exosomes that interact with components of focal adhesion in the plasma membrane. Interestingly, vinculin, a major cytoskeleton protein and a marker of focal adhesion plaques, was concentrated in the membranes of fetuin-A expressing FFBT cells, particularly when the cells were adhered on fibronectin (Fig. 6B). This suggests that fetuin-A plays a role in the rapid assembly of focal adhesion proteins in the membrane.
Fig. 6. Fetuin-A mediated trafficking of vinculin and histone H2A to the membrane fraction of adhered and spread cells.
Fetuin-A transfected BT-549 cells and empty vector transfection controls were dislodged from culture flasks with EDTA and allowed to adhere to 145 mm petri dishes pre-coated with either laminin or fibronectin (40 μg/ml) and incubated for 30 min at 37°C in a humidified CO2 incubator. The cells were lysed and fractionated into nuclear chromatin bound, cytosolic and membrane associated proteins as described in Materials and Methods. In panel A, the cytosolic and membrane fractions from cells incubated on laminin coated dishes were resolved in NuPAGE gels, transferred to nitrocellulose and probed for vinculin, Akt1, FLAG-fetuin-A, and EGFR. In panel B, the membrane fractions from cells incubated on fibronectin coated dishes were probed for vinculin, FLAG-fetuin-A, histone H2A and EGFR.
Fetuin-A and adhesion competent exosomes accelerate the assembly of focal adhesions in tumor cells
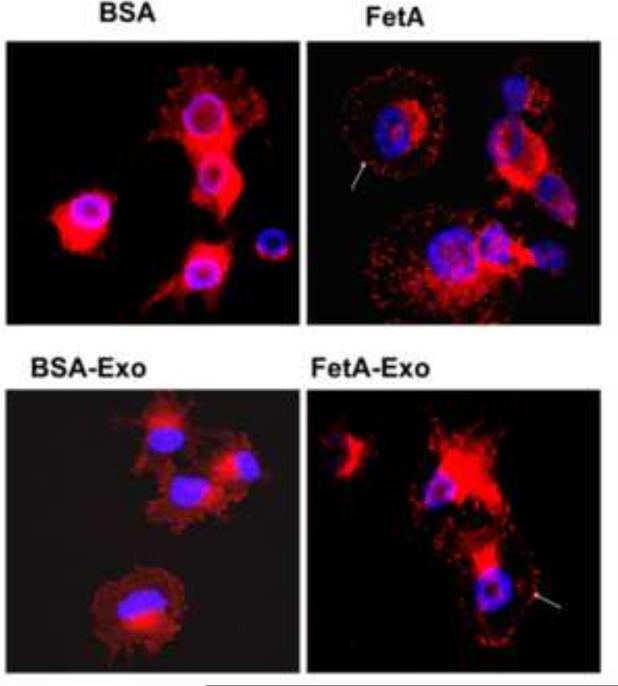
We have consistently demonstrated that cells tend to adhere and spread more rapidly to extracellular matrices either in the presence of adhesion competent exosomes [11,12] or fetuin-A [9]. We show here that focal adhesions, represented by the punctate staining of vinculin at the adhesion plaques, form readily in the presence of either fetuin-A or adhesion competent cellular exosomes (Fig. 7, arrows). Assembly of focal adhesions is complete within 30 min in the presence of either fetuin-A (FetA) or adhesion competent exosomes (FetA-Exo). In their absence, it may take up to 3 h for focal adhesion plaques to properly assemble in these cells (data not shown).
Fig. 7. Fetuin-A/Exosomes accelerate the maturation of focal adhesions.
Glass coverslips were coated with fibronectin (40 μg/ml) in PBS overnight at 4°C, followed by blocking with 3% BSA for 30 min at 37°C. Breast carcinoma cells (BT-549Gal3) were then plated on the coverslips in six-well plates and incubated for exactly 30 min in SFM containing BSA (1 mg/ml) or purified fetuin-A (1 mg/ml). The cells were also incubated for 30 min in SFM containing either adhesion incompetent (BSA-Exo) or adhesion competent (FetA-Exo) exosomes. The medium was aspirated and the cells fixed/permeabilized in 0.5% (w/v) of Triton X-100; 3% (w/v) paraformaldehyde in PBS for exactly 2 min. The detergent was removed and wells containing coverslips washed once with 3% paraformaldehyde/PBS and fixed in the same solution for another 30 min. The glass coverslips with the cells were then blocked with 3% BSA in PBS for 1 h and then incubated with anti-vinculin in PBS for 30 min at 37°C. After washing, the fixed cells were stained with cy3-secondary antibodies for another 30 min at 37°C. This was followed by another set of washes and the coverslips mounted with Prolong Gold anti-fade with DAPI and examined (Nikon A1r).
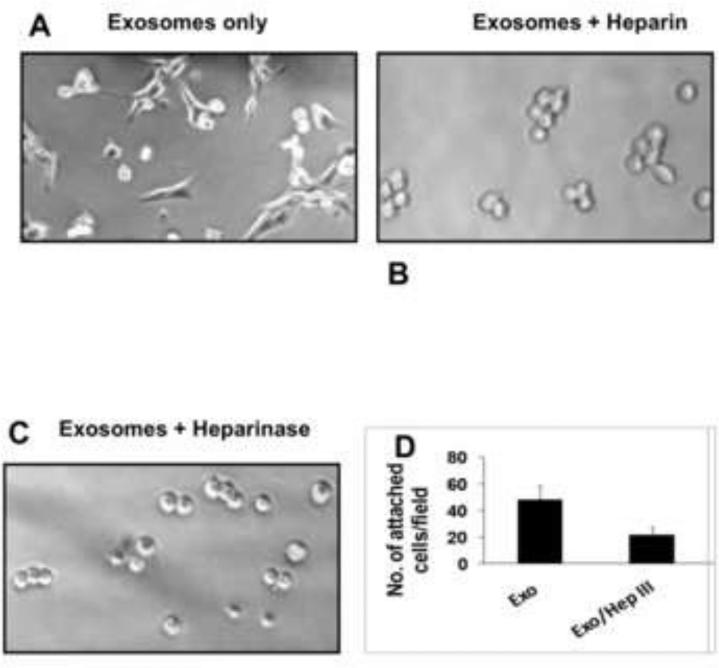
Inhibition of exosomal mediated adhesion by heparin and heparinase III
Exosomes loaded with histones and fetuin-A promote adhesion and cell spreading on plastic (Fig 8A). Here we wanted to confirm that indeed histones are the major mediators of adhesion on exosomes and so proteins that bind to or interact with histones should in theory abrogate exosomal mediated adhesion [12]. Heparin at concentrations ranging from 5-40 μg/ml (data not shown) abrogated exosomal mediated adhesion. At 40 μg/ml, not only was adhesion inhibited, cell spreading was also abolished (Fig. 8B). Interestingly, fetuin-A mediated adhesion of cells to plastic was also abrogated by heparin (data not shown). We reasoned that heparin most likely interacted with the motifs in histones that were responsible for binding to heparan sulfate proteoglycans [27]. We therefore allowed BT-549Gal3 to adhere to plastic in the presence of adhesion competent exosomes and in the absence or presence of heparinase III that specifically cleaves heparin sulfate chains from heparan sulfate proteoglycans. The treatment abrogated cellular adhesion and spreading in the presence of cellular exosomes (Fig. 8C and 8D), again suggesting that histones on exosomes interact with cell surface heparan sulfate proteoglycans. In order to show that it is the cell surface proteoglycans that are involved in the process, we also pre-incubated cells with heparinase III prior to detachment and then following detachment with EDTA, plated cells in 96-well microtiter plates in the presence of adhesion competent exosomes. Similar data as shown in Fig. 8 panel C was obtained (data not shown).
Fig. 8. Inhibition of Exosomal mediated adhesion by heparin and heparinase III.
The BT-549Gal3 cells were plated in 96 well microtiter plates (2 × 104 cells/well) and allowed to adhere in the presence of adhesion competent exosomes (loaded with histones and fetuin-A) (20 μg/ml) in the absence (panel A) and in the presence of heparin (40 μg/ml) (panel B). The same number of cells/well were also allowed to adhere in the presence of adhesion competent exosomes and heparinase III (panel C) and quantified in panel D.
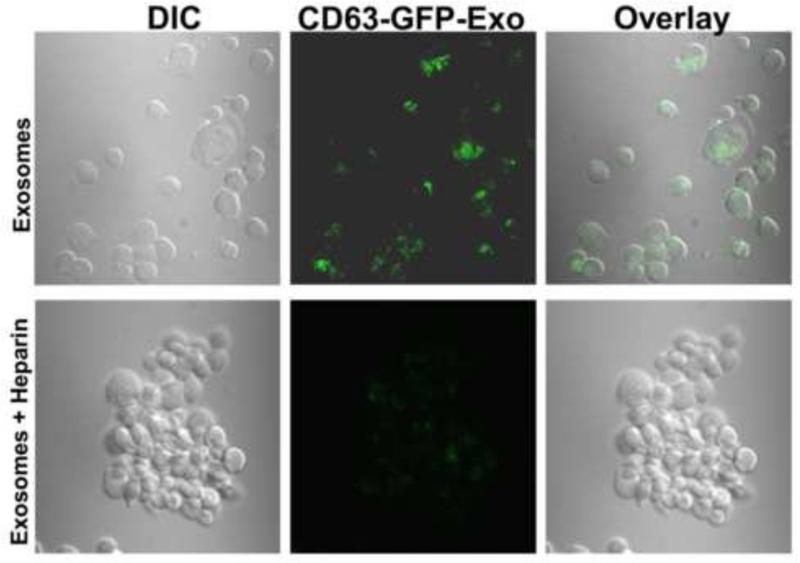
Heparin abrogates the cellular uptake of CD63-GFP-labeled exosomes and exosomal mediated cellular adhesion and spreading
We have provided evidence that the uptake of GFP-labeled exosomes that were also coated with histones (prepared in the presence of fetuin-A) by BT-549Gal3 cells, leads to cellular adhesion and spreading (Fig. 9). This is clearly the thrust of the present work, where we have argued that the role of fetuin-A in adhesion is to drive the secretion of histone coated exosomes whose subsequent uptake by the same exosome secreting or neighboring cells results in cellular adhesion and spreading. Heparin at a concentration of 40 μg/ml completely abrogated the uptake of CD63-GFP-labeled exosomes and concomitantly inhibited the exosomal mediated adhesion (Fig. 9).
Fig 9. Heparin abrogates the uptake of CD63-GFP-labeled exosomes as well as exosomal mediated adhesion and cell spreading.
CD63-GFP-labeled exosomes were purified from BT-CD63 as described in Materials and Methods. BT-549Gal3 cells were then incubated withCD63-GFP-labeled exosomes (~20 μg/ml) from BTCD63 cells for 30 min in glass bottom chambered slides at 37°C in the absence or presence of heparin (40 μg/ml). At the completion of incubation period, the chambers were removed and the slides prepare for confocal microscopy.
DISCUSSION
The impetus for the present studies was our earlier report where we demonstrated that only cellular exosomes carrying histones and fetuin-A, had the potential to mediate adhesion and spreading of tumor cells. These exosomes were secreted by the tumor cells in the presence of fetuin-A [12]. We hereby provide evidence that fetuin-A interacts with histones intracellularly and shuttles them to the exosomal and plasma membrane compartments. The endocytic uptake of histone coated exosomes leads to rapid assembly of focal adhesions, attachment and spreading of tumor cells.
We previously studied the role of fetuin-A in cellular adhesion and growth with the belief that it had specific cell adhesion receptors [9,10]. It is now clear that whereas there are no cell surface receptors for cellular adhesion to fetuin-A, its uptake by cells that respond to it, is required for subsequent adhesion and cell spreading [9]. Cellular adhesion is a complex physiological phenomenon that is not fully understood beyond the identification and characterization of integrins and its ligands in the extracellular matrix [28]. Within the last two decades, the role of other potential players in cellular adhesion such as heparan sulfate proteoglycans and exosomes has attracted considerable attention [29,30]. It is in this context that we propose a new physiological role for fetuin-A in these cellular processes. We show the physical interaction between fetuin-A and cellular histones. The data suggest that fetuin-A interacts with histones intracellularly and is either secreted via exosomes or as a complex with histones and subsequently loads its histone cargo on exosomes extracellularly.
We have demonstrated that the speed of adhesion of tumor cells to extracellular adhesion molecules such as fibronectin and laminin is significantly enhanced in the presence of cellular exosomes that were prepared in the presence of fetuin-A [12]. We now show that fetuin-A similarly accelerates the adhesion of tumor cells to either fibronectin, laminin or plastic and suggest that fetuin-A acts via cellular exosomes. Rapid adhesion of tumor cells particularly those with metastatic potential, to extracellular matrix proteins, is clearly advantageous. Such cells can rapidly turnover their focal adhesions to enable motility [31]. Cells in culture with the ability to adhere rapidly also have a growth and plating advantage over their counterparts that adhere slowly. The ability of fetuin-A to promote the rapid adhesion of cells to extracellular matrix proteins could explain why media containing fetal bovine serum, that contains a high concentration of the protein, is preferred for the culture of tumor cells [7,8]. Furthermore, in vivo studies support the tumor adhesion and growth promoting properties of fetuin-A [4,32].
Whereas we showed that histone H2A co-localizes with fetuin-A in the nucleus in tumor cells forced to express the protein, most cells including breast tumor cells in culture do not synthesize and secrete fetuin-A, relying exclusively on culture media containing fetal bovine serum for their supply. We and others have shown by confocal microscopy that breast tumor cells and smooth muscle cells take up fetuin-A from the medium via a novel endocytic mechanism requiring annexins [9,26]. Since fetuin-A is synthesized and secreted via a classical pathway, it is likely that the cells that were forced to express fetuin-A also secrete and take up fetuin-A in a paracrine or autocrine fashion using the same uptake pathway. The endocytosed fetuin-A enters most of the cellular compartments including the nucleus where it most likely interacts with histones. The fetuin-A uptake mechanisms do not appear to require a receptor [26]. However, once fetuin-A loads its histone cargo on exosomes, the subsequent uptake of histone coated exosomes by the cells requires a receptor, whose preferred ligand appears to be histones on exosomes. For this reason, it was necessary to show by FAVS that fetuin-A and histones are on the extracellular surface of exosomal membranes. The FAVS assay also demonstrated that fetuin-A and histones are not contaminating proteins that were pulled down by the polymer based exosome purification reagent. The protocol involves a number of ultracentrifugation steps that would quickly eliminated proteins that loosely associate with exosomes. Lastly, fetuin-A/ahsg and histones are frequently observed in exosomes isolated from tumor cells [33].
The present studies suggest that the interaction of histone coated exosomes with the putative cell surface receptor signals the assembly of focal adhesion, cell adhesion and spreading. It is established that the assembly/maturation of focal adhesion involves the translocation and concentration of a heterogeneous group of at least 50 different molecules including cytoskeleton proteins and proteoglycans (vinculin, talin, actin, tensin, syndecans, etc.) at the adherent junctions in the plasma membrane [34]. The presence and function of heparan sulfate proteoglycans such as syndercan-4 at these focal adhesion complexes is critical to complete the focal adhesion assembly process [35]. The ligands for the cell surface proteoglycans such as syndecan-4 range from heparin binding domain of fibronectin to growth factors [35]. We hereby suggest histones as part of repertoire of heparan sulfate proteoglycan binding proteins and may act as the ‘eat me’ signal for exosomal uptake. It was demonstrated by modeling studies that proteins containing a high percentage of basic amino acids such as histones have a preferential binding interaction with heparan sulfate proteoglycans [36]. A recent report suggested that the uptake of cellular exosomes via cell surface heparan sulfate proteoglycans receptors promoted cellular motility [37]. This report was of great interest to us because it suggested for the first time the existence of potential ligands for cell surface heparan sulfate proteoglycans on cellular exosomes, needed to speed the assembly of focal adhesion complexes, a process necessary for cellular motility. Exosomes have been shown to promote motility and invasion of tumor cells [38]. The present data support the notion that heparan sulfate proteoglycans are indeed the receptors for internalization of exosomes and suggest that histones that coat these exosomes are the preferred ligands for them. The exosomal mediated motility reported by Christianson et al. [37] was attenuated by heparin which also blunted exosomal uptake by the cells as well as adhesion and cell spreading reported herein. Heparin is a potent inhibitor of histones, potentially disengaging the exosomes from interacting with cell surface heparan sulfate proteoglycans. Interestingly, it has been demonstrated that heparanase, an enzyme that cleaves heparan sulfate from heparan sulfate proteoglycans, can release and increase the secretion of exosomes from adherent cells [39]. Furthermore, heparanase (heparinase III) also attenuated exosomal mediated adhesion and cell spreading reported herein.
In summary, we have provided evidence that fetuin-A in tumor cells is capable of shuttling histones from the cell compartments including nucleus to the exosomes. The uptake of histone coated exosomes leads to cellular adhesion and spreading. This uptake is inhibited by heparin which also attenuates exosomal mediated cellular adhesion. The data suggest that histones on exosomes are ligands for heparan sulfate proteoglycans that mediate focal adhesion assembly signaling which in turn leads to cellular attachment, spreading and motility on various extracellular matrices including plastic.
Fetuin-A associates with histones intracellularly
Fetuin-A shuttles histones to exosomes
Uptake of histone coated exosomes drive focal adhesion assembly
Uptake of histone coated exosomes mediate adhesion and cell spreading
Acknowledgment
This work was supported by Grant 3SC1CA134018-04S1 (J.O.); G12 MD007586 (Meharry Molecular Biology Core); 5SC2-CA170244-01(A.S.); 5U54CA163069-03 (R.K.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Schafer C, Heiss A, Schwarz A, Westenfeld R, Ketteler M, Floege J, Muller-Esterl W, Schinke T, Jahnen-Dechent W. The serum protein alpha 2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J Clin Invest. 2003;112:357–366. doi: 10.1172/JCI17202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Demetriou M, Binkert C, Sukhu B, Tenenbaum HC, Dennis JW. Fetuin/alpha2-HS glycoprotein is a transforming growth factor-beta type II receptor mimic and cytokine antagonist. J Biol Chem. 1996;271:12755–12761. doi: 10.1074/jbc.271.22.12755. [DOI] [PubMed] [Google Scholar]
- 3.Swallow CJ, Partridge EA, Macmillan JC, Tajirian T, DiGuglielmo GM, Hay K, Szweras M, Jahnen-Dechent W, Wrana JL, Redston M, Gallinger S, Dennis JW. alpha2HS-glycoprotein, an antagonist of transforming growth factor beta in vivo, inhibits intestinal tumor progression. Cancer Res. 2004;64:6402–6409. doi: 10.1158/0008-5472.CAN-04-1117. [DOI] [PubMed] [Google Scholar]
- 4.Guillory B, Sakwe AM, Saria M, Thompson P, Adhiambo C, Koumangoye R, Ballard B, Binhazim A, Cone C, Jahanen-Dechent W, Ochieng J. Lack of fetuin-A (alpha2-HS-glycoprotein) reduces mammary tumor incidence and prolongs tumor latency via the transforming growth factor-beta signaling pathway in a mouse model of breast cancer. Am J Pathol. 2010;177:2635–2644. doi: 10.2353/ajpath.2010.100177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goustin AS, Derar N, Abou-Samra AB. Ahsg-fetuin blocks the metabolic arm of insulin action through its interaction with the 95-kD beta-subunit of the insulin receptor. Cell Signal. 2013;25:981–988. doi: 10.1016/j.cellsig.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 6.Mori K, Emoto M, Inaba M. Fetuin-A: a multifunctional protein. Recent Pat Endocr Metab Immune Drug Discov. 2011;5:124–146. doi: 10.2174/187221411799015372. [DOI] [PubMed] [Google Scholar]
- 7.Nie Z. Fetuin: its enigmatic property of growth promotion. Am J Physiol. 1992;263:C551–562. doi: 10.1152/ajpcell.1992.263.3.C551. [DOI] [PubMed] [Google Scholar]
- 8.Brown WM, Saunders NR, Mollgard K, Dziegielewska KM. Fetuin--an old friend revisited. Bioessays. 1992;14:749–755. doi: 10.1002/bies.950141105. [DOI] [PubMed] [Google Scholar]
- 9.Sakwe AM, Koumangoye R, Goodwin SJ, Ochieng J. Fetuin-A ({alpha}2HS-glycoprotein) is a major serum adhesive protein that mediates growth signaling in breast tumor cells. J Biol Chem. 2010;285:41827–41835. doi: 10.1074/jbc.M110.128926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kundranda MN, Ray S, Saria M, Friedman D, Matrisian LM, Lukyanov P, Ochieng J. Annexins expressed on the cell surface serve as receptors for adhesion to immobilized fetuin-A. Biochim Biophys Acta. 2004;1693:111–123. doi: 10.1016/j.bbamcr.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Koumangoye RB, Sakwe AM, Goodwin JS, Patel T, Ochieng J. Detachment of breast tumor cells induces rapid secretion of exosomes which subsequently mediate cellular adhesion and spreading. PLoS One. 2011;6:e24234. doi: 10.1371/journal.pone.0024234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watson K, Koumangoye R, Thompson P, Sakwe AM, Patel T, Pratap S, Ochieng J. Fetuin-A triggers the secretion of a novel set of exosomes in detached tumor cells that mediate their adhesion and spreading. FEBS Lett. 2012;586:3458–3463. doi: 10.1016/j.febslet.2012.07.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT, Jr., Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ochieng J, Pratap S, Khatua AK, Sakwe AM. Anchorage-independent growth of breast carcinoma cells is mediated by serum exosomes. Exp Cell Res. 2009;315:1875–1888. doi: 10.1016/j.yexcr.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sceneay J, Smyth MJ, Moller A. The pre-metastatic niche: finding common ground. Cancer Metastasis Rev. 2013;32:449–464. doi: 10.1007/s10555-013-9420-1. [DOI] [PubMed] [Google Scholar]
- 16.Clayton A, Turkes A, Dewitt S, Steadman R, Mason MD, Hallett MB. Adhesion and signaling by B cell-derived exosomes: the role of integrins. FASEB J. 2004;18:977–979. doi: 10.1096/fj.03-1094fje. [DOI] [PubMed] [Google Scholar]
- 17.Ngora H, Galli UM, Miyazaki K, Zoller M. Membrane-bound and exosomal metastasis-associated C4.4A promotes migration by associating with the alpha(6)beta(4) integrin and MT1-MMP. Neoplasia. 2012;14:95–107. doi: 10.1593/neo.111450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 19.Soekmadji C, Russell PJ, Nelson CC. Exosomes in prostate cancer: putting together the pieces of a puzzle. Cancers (Basel) 2013;5:1522–1544. doi: 10.3390/cancers5041522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pemberton AD, Brown JK, Inglis NF. Proteomic identification of interactions between histones and plasma proteins: implications for cytoprotection. Proteomics. 2010;10:1484–1493. doi: 10.1002/pmic.200900818. [DOI] [PubMed] [Google Scholar]
- 21.Xu J, Zhang X, Monestier M, Esmon NL, Esmon CT. Extracellular histones are mediators of death through TLR2 and TLR4 in mouse fatal liver injury. J Immunol. 2011;187:2626–2631. doi: 10.4049/jimmunol.1003930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Semeraro N, Ammollo CT, Semeraro F, Colucci M. Sepsis, thrombosis and organ dysfunction. Thromb Res. 2012;129:290–295. doi: 10.1016/j.thromres.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 23.Carestia A, Rivadeneyra L, Romaniuk MA, Fondevila C, Negrotto S, Schattner M. Functional responses and molecular mechanisms involved in histone-mediated platelet activation. Thromb Haemost. 2013;110:1035–1045. doi: 10.1160/TH13-02-0174. [DOI] [PubMed] [Google Scholar]
- 24.Babu M, Butland G, Pogoutse O, Li J, Greenblatt JF, Emili A. Sequential peptide affinity purification system for the systematic isolation and identification of protein complexes from Escherichia coli. Methods Mol Biol. 2009;564:373–400. doi: 10.1007/978-1-60761-157-8_22. [DOI] [PubMed] [Google Scholar]
- 25.Sakwe AM, Nguyen T, Athanasopoulos V, Shire K, Frappier L. Identification and characterization of a novel component of the human minichromosome maintenance complex. Mol Cell Biol. 2007;27:3044–3055. doi: 10.1128/MCB.02384-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen NX, O'Neill KD, Chen X, Duan D, Wang E, Sturek MS, Edwards JM, Moe SM. Fetuin-A uptake in bovine vascular smooth muscle cells is calcium dependent and mediated by annexins. Am J Physiol Renal Physiol. 2007;292:F599–606. doi: 10.1152/ajprenal.00303.2006. [DOI] [PubMed] [Google Scholar]
- 27.Henriquez JP, Casar JC, Fuentealba L, Carey DJ, Brandan E. Extracellular matrix histone H1 binds to perlecan, is present in regenerating skeletal muscle and stimulates myoblast proliferation. J Cell Sci. 2002;115:2041–2051. doi: 10.1242/jcs.115.10.2041. [DOI] [PubMed] [Google Scholar]
- 28.Winograd-Katz SE, Fassler R, Geiger B, Legate KR. The integrin adhesome: from genes and proteins to human disease. Nat Rev Mol Cell Biol. 2014;15:273–288. doi: 10.1038/nrm3769. [DOI] [PubMed] [Google Scholar]
- 29.Jacquemet G, Humphries MJ, Caswell PT. Role of adhesion receptor trafficking in 3D cell migration. Curr Opin Cell Biol. 2013;25:627–632. doi: 10.1016/j.ceb.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Kilsdonk JW, van Kempen LC, van Muijen GN, Ruiter DJ, Swart GW. Soluble adhesion molecules in human cancers: sources and fates. Eur J Cell Biol. 2010;89:415–427. doi: 10.1016/j.ejcb.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 31.Kuo JC. Mechanotransduction at focal adhesions: integrating cytoskeletal mechanics in migrating cells. J Cell Mol Med. 2013;17:704–712. doi: 10.1111/jcmm.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kundranda MN, Henderson M, Carter KJ, Gorden L, Binhazim A, Ray S, Baptiste T, Shokrani M, Leite-Browning ML, Jahnen-Dechent W, Matrisian LM, Ochieng J. The serum glycoprotein fetuin-A promotes Lewis lung carcinoma tumorigenesis via adhesive-dependent and adhesive-independent mechanisms. Cancer Res. 2005;65:499–506. [PubMed] [Google Scholar]
- 33.Li CC, Eaton SA, Young PE, Lee M, Shuttleworth R, Humphreys DT, Grau GE, Combes V, Bebawy M, Gong J, Brammah S, Buckland ME, Suter CM. Glioma microvesicles carry selectively packaged coding and non-coding RNAs which alter gene expression in recipient cells. RNA Biol. 2013;10:1333–1344. doi: 10.4161/rna.25281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schlie-Wolter S, Ngezahayo A, Chichkov BN. The selective role of ECM components on cell adhesion, morphology, proliferation and communication in vitro. Exp Cell Res. 2013;319:1553–1561. doi: 10.1016/j.yexcr.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 35.Bass MD, Morgan MR, Humphries MJ. Integrins and syndecan-4 make distinct, but critical, contributions to adhesion contact formation. Soft Matter. 2007;3:372–376. doi: 10.1039/b614610d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cardin AD, Weintraub HJ. Molecular modeling of protein-glycosaminoglycan interactions. Arteriosclerosis. 1989;9:21–32. doi: 10.1161/01.atv.9.1.21. [DOI] [PubMed] [Google Scholar]
- 37.Christianson HC, Svensson KJ, van Kuppevelt TH, Li JP, Belting M. Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. Proc Natl Acad Sci U S A. 2013;110:17380–17385. doi: 10.1073/pnas.1304266110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Higginbotham JN, Demory Beckler M, Gephart JD, Franklin JL, Bogatcheva G, Kremers GJ, Piston DW, Ayers GD, McConnell RE, Tyska MJ, Coffey RJ. Amphiregulin exosomes increase cancer cell invasion. Curr Biol. 2011;21:779–786. doi: 10.1016/j.cub.2011.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thompson CA, Purushothaman A, Ramani VC, Vlodavsky I, Sanderson RD. Heparanase regulates secretion, composition, and function of tumor cell-derived exosomes. J Biol Chem. 2013;288:10093–10099. doi: 10.1074/jbc.C112.444562. [DOI] [PMC free article] [PubMed] [Google Scholar]