Abstract
In this study, we serologically and pathologically examined the clinical significance of fibroblast growth factor (FGF) expression in patients with colorectal cancer. Serum basic FGF (bFGF) levels in 92 surgical colorectal cancer patients and 31 controls were measured, and the relationship between those levels and clinicopathological factors were examined. Immunohistochemical study was also conducted on specimens from 51 cancer patients, and the association between bFGF staining and serum levels were investigated. An examination of clinicopathological factors revealed significant differences in bFGF levels between stage 0-IIIb and stage IV cancers (P = 0.013). Lymphatic invasion was one factor that differed significantly. Patients with a tumor 30 mm or smaller had a bFGF level of 7.65 ± 1.11 pg/ml while patients with a tumor 31 mm or larger had a bFGF level of 8.53 ± 3.22 pg/ml; significant differences in these bFGF levels were noted (P < 0.05). Patients with a tumor that had no lymphatic invasion (ly0) had a bFGF level of 7.25 ± 0.66 pg/ml, those with a tumor that had minimal lymphatic invasion (ly1) had a bFGF level of 7.99 ± 1.68 pg/ml, and those with a tumor that had moderate lymphatic invasion (ly2) had a bFGF level of 9.17 ± 4.23 pg/ml. bFGF levels differed significantly for tumors with no/minimal lymphatic invasion (ly0-ly1) and those with moderate lymphatic invasion (ly2) (P < 0.0001). Serological examination of bFGF levels during the proliferation of colorectal cancer revealed that moderate lymphatic invasion can be readily distinguished.
Among the mechanisms responsible for the progression of cancer, cell motility is an important factor that allows cancer cells to detach from the primary tumor and invade nearby tissue. Growth factors that facilitate this motility include epidermal growth factor (EGF), transforming growth factor β (TGF-β), and hepatocyte growth factor (HGF). Fibroblast growth factors (FGFs) have significant interaction with cell growth, differentiation, and functioning, and they play a vital role in processes such as maintaining tissue and repairing damage. Recent reports have indicated that FGFs are involved in pathologies associated with excessive cell growth and angiogenesis, such as tumor formation. The current study examined FGF expression serologically and pathologically in patients undergoing surgery for colorectal cancer, and this study also examined the clinical significance of that expression.
Subjects
Serologic investigation
There were 92 patients with colorectal cancer who underwent surgery in the Second Department of Surgery II at Tokyo Women's Medical University Hospital, from July 2000 to March 2003. There were 31 control subjects without cancer. Serum bFGF levels of both groups were measured and compared. The relationship between serum levels and clinicopathologic factors and prognosis was studied in patients with colorectal cancer.
Patients with colorectal cancer (50 males and 42 females) had a mean age of 63.9 ± 11.1 years. Cancer was located in the colon (C-S, Rs) in 64 patients and in the rectum (Ra, Rb, P) in 28 patients. The cancer was a well-differentiated adenocarcinoma in 46 patients, a moderately differentiated adenocarcinoma in 40 patients, a poorly differentiated adenocarcinoma in 6 patients, and a mucinous carcinoma in 6 patients. The pathologic depth of invasion was m in 5 patients, sm in 7 patients, mp in 20 patients, ss (a1) in 46 patients, se (a2) in 12 patients, and si (ai) in 2 patients. The clinical stage of the cancer was stage 0 in 3 patients, stage I in 5 patients, stage II in 26 patients, stage IIIa in 20 patients, stage IIIb in 12 patients, and stage IV in 9 patients. The 31 patients without cancer broke down into 16 males and 15 females with a mean age of 57.3 ± 13.4 years. Ten of these patients had an inguinal hernia, 4 patients had cholelithiasis, 1 patient had an incisional hernia, and 1 patient had hemorrhoids. No serious complications were noted, and blood was collected while the patient's condition was not acute.
Immunohistologic investigation
Of the 92 patients with colorectal cancer, 51 underwent serologic investigation. Sections of the innermost portion of surgical specimens were immunohistochemically stained with anti-bFGF antibody, and the relationship between bFGF expression and pathologic factors was examined.
Clinicopathologic findings were denoted in accordance with the Japanese Classification of Colorectal Carcinoma.
Methods
Serologic investigation
Peripheral venous blood was collected preoperatively from patients with colorectal cancer and patients without cancer. After centrifugation, serum was kept frozen at −80°C, and serum was thawed before measurement. Measurement was done with an hFGFb ELISA Kit (BioSource International, Camarillo, CA), and serum FGF levels were measured using 1-step sandwich ELISA. Specifically, 100 μL of buffer was added to each well of an antibody plate and 100 μL of the sample was added. Plates were covered and allowed to stand at room temperature for 2 hours. The reaction mixture was then removed and plates were washed 4 times with wash solution. Two hundred μL of FGF labeled antibody was added to each well and plates were covered and allowed to stand at room temperature for 2 hours. After the reaction mixture was removed, plates were washed 4 times with wash solution and 200 μL of chromophore solution was added to each well. Plates were allowed to stand at room temperature for 30 minutes. and then a stop solution was added in increments of 50 μL. Absorbance was measured with a microplate absorbance spectrophotometer, and antibody concentrations were determined based on a standard curve.
Immunohistologic investigation
The tissue specimens used were formalin-fixed paraffin-embedded sections. Specimens were stained with peroxidase (HRP)-labeled antibody using anti-human bFGF antibody (Upstate cell signaling solution). Specifically, 4-μm sections of the innermost portion of the tumor were prepared. After deparaffinization, sections were immersed in 3% H2O2 in methanol for 10 minutes. and endogenous peroxidase activity was blocked. Specimens were autoclaved at 120°C for 10 minutes and nonspecific binding was blocked with skim milk for 10 minutes. Specimens were then immunohistochemically stained using the ABC technique. After sections were allowed to react with diluted normal serum at room temperature for 10 minutes, they were allowed to react with 20-fold diluted anti-human bFGF antibody as the primary antibody for 60 minutes. They were then allowed to react with biotin-labeled anti-mouse IgG antibody as the secondary antibody for 30 minutes. They were then allowed to react with ABC reagent for 60 minutes. Color was developed with DAB solution. After nuclear staining with hematoxylin, sections were dehydrated and embedded and then examined microscopically.
Statistical analysis
Results were subjected to a t-test. Statistical analysis between groups was performed with a χ2 test (Fisher's exact test), and P < 0.05 was deemed to indicate a significant difference.
Results
Serologic investigation
Patients with colorectal cancer had a serum FGF level of 8.16 ± 2.57 pg/mL while patients without cancer had a serum FGF level of 7.69 ± 1.72 pg/mL. Significant differences in serum FGF levels were not noted (Fig. 1).
Fig. 1.
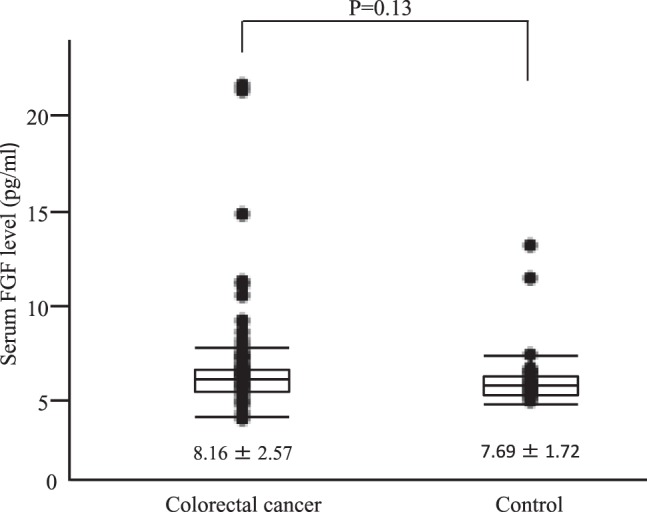
Serum bFGF levels between Colorectal cancer group and Control group.
Statistically significant differences in terms of sex and tumor location were not noted in patients with colorectal cancer.
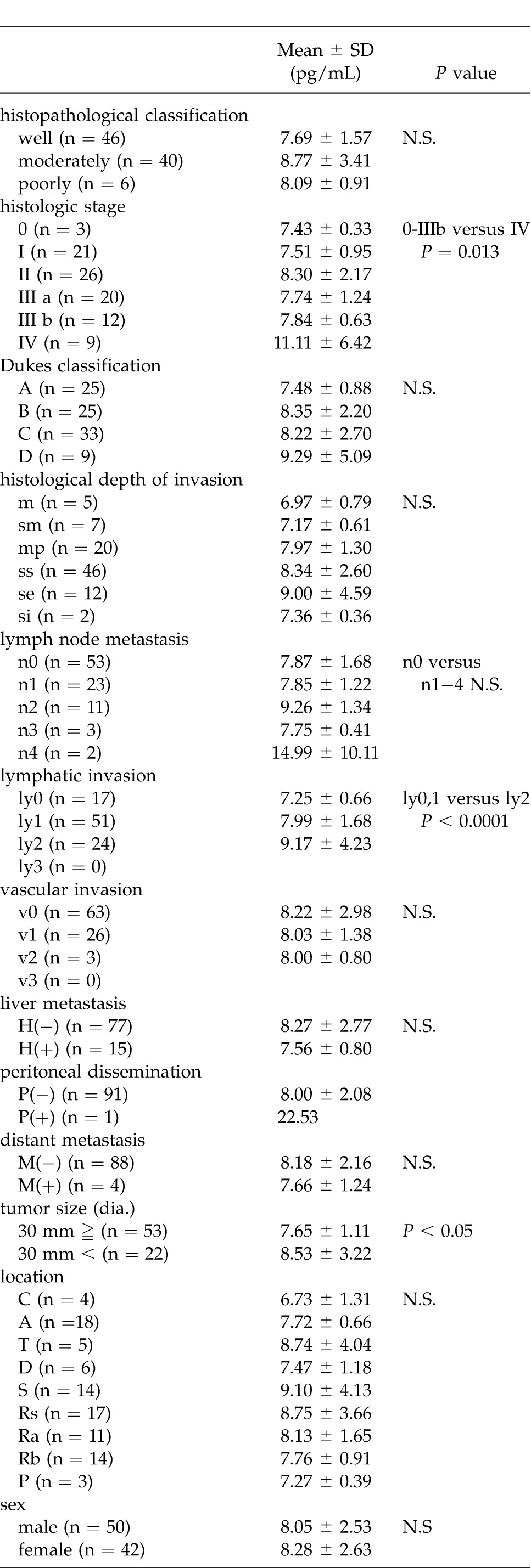
An examination of clinicopathologic factors revealed no statistically significant differences in terms of histopathologic classification, Dukes classification, venous invasion, liver metastasis, or distant metastasis. In terms of tumor size, staging, histologic depth of invasion, lymph node metastasis, lymphatic invasion, and peritoneal dissemination; however, serum bFGF levels tended to be higher as the cancer progressed. In terms of histologic staging, significant differences (P = 0.0013) were noted between cancers that were stages 0-IIIb and those that were stage IV. Patients with a tumor 30 mm or smaller had a serum bFGF level of 7.65 ± 1.11 pg/mL while patients with a tumor 31 mm or larger had a serum bFGF level of 8.53 ± 3.22 pg/mL; significant differences (P < 0.05) in serum bFGF levels were noted. Significant differences in terms of the histologic depth of invasion were not noted, but serum bFGF levels tended to be higher as cancer penetrated further through the bowel wall. Significant differences in terms of histological lymph node metastasis were not noted, but serum bFGF levels tended to be higher with more extensive lymph node metastasis. Significant differences (P < 0.0001) were noted between minimal lymphatic invasion (ly0 and ly1) and moderate lymphatic invasion (ly2). Significant differences in peritoneal dissemination were not noted, but 1 patient with peritoneal dissemination had an extremely high level of serum bFGF 22.53 pg/mL (Table 1).
Table 1.
The relationship between clinicopathologic factors and serum FGF levels
Immunohistologic investigation
FGF staining was performed on specimens from 51 of the 92 patients whose serum FGF levels were measured.
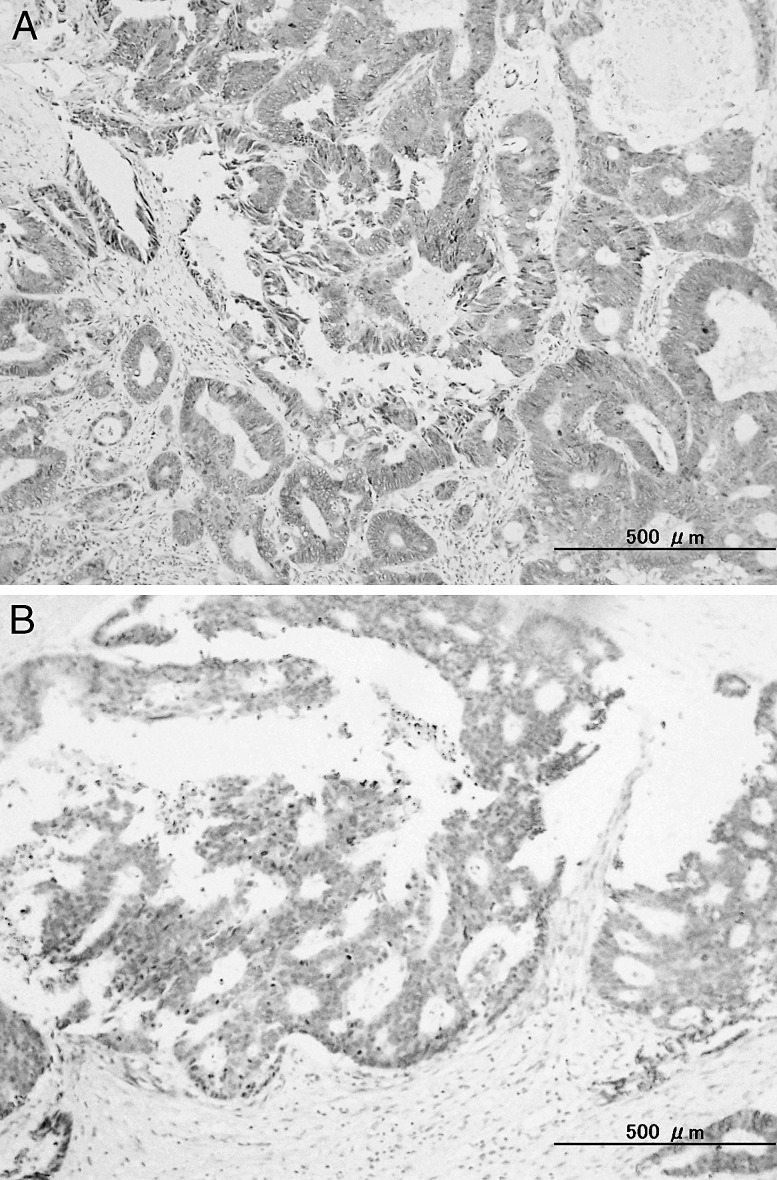
Patients in whom 30% or more cancer cells had a stained cytoplasm were deemed to be FGF-positive while patients in whom fewer than 30% of cancer cells had a stained cytoplasm were deemed to be FGF-negative (Fig. 2).
Fig. 2.
FGF expression in colorectal cancer, positive (A). FGF expression in colorectal cancer, negative (B).
Of the 51 patients, 40 (78.4%) had specimens with positive staining while 11 (21.6%) had specimens with negative staining.
Patients who had specimens that stained positive for FGF had a serum FGF level of 8.87 ± 3.59 pg/mL while patients who had specimens that stained negative for FGF had a serum FGF level of 7.13 ± 1.03 pg/mL. FGF levels tended to be higher in patients who had specimens that stained positive for FGF than those in patients who had specimens that stained negative for FGF, but significant differences were not noted (Fig. 3).
Fig. 3.
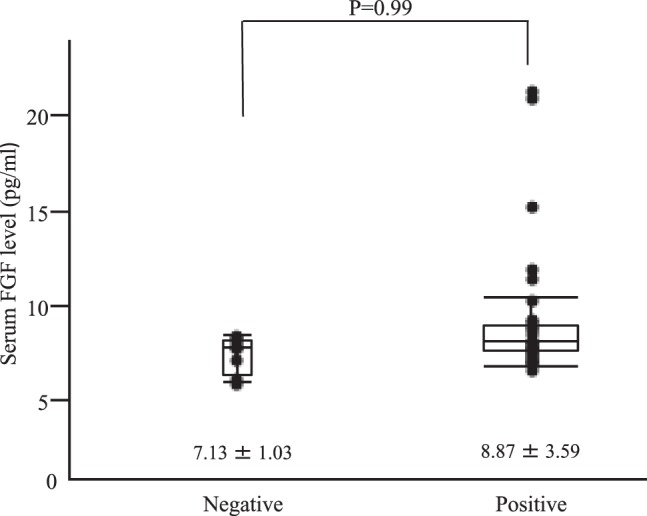
Relationship between serum FGF levels and tissue FGF expression.
Discussion
FGFs are proteins that were discovered in the pituitary of cattle that cause fibroblasts to proliferate.1 FGFs were subsequently found to fall into one of 2 groups, acidic FGFs (FGF-1) and basic FGFs (bFGF and FGF-2), because of differences in their isoelectric point. At the present time, 22 members of the FGF family have been identified in humans.2 bFGF is ubiquitous in the body and has strong affinity for glycosaminoglycans like heparan sulfate. bFGF is stored by binding with glycosaminoglycans present on the cell surface or in extracellular matrix.3 When bFGF is actually needed, cells secrete various enzymes like heparinase that free bFGF from glycosaminoglycans so that it can reach target cells. Levels of bFGF in the blood are extremely low, and bFGF administered in the blood quickly disappears. bFGF is an extremely unstable substance, and it often ceases activity while it is being measured. Blood must be promptly centrifuged after it is collected and serum must be collected. Repeated freezing and thawing is unacceptable.3
FGFs are not merely fibroblast growth factors but instead act on various cells. When they are produced normally, FGFs are involved in cell growth, differentiation, and functioning in processes such as maintaining tissue and repairing damage. FGFs are also reportedly involved in pathologies like tumors. Cancer cells need a supply of nutrients and oxygen via new blood vessels to grow and develop. Angiogenesis is controlled by a balance of promoters and inhibitors. Vascular endothelial growth factor (VEGF) and angiopoietin (Ang) play a key role in the balance as angiogenic growth factors. bFGF has also received attention as yet another angiogenic growth factor.4–6 The detailed mechanisms of the association between FGF signaling and the onset of malignant tumors remain unclear. At the present time, however, mutations in FGF receptors are assumed to constantly activate FGF signaling and ultimately promote the growth and metastasis of tumor cells.7
Patients with malignant tumors of the breast, lungs, thyroid, colon, and kidneys have reportedly higher serum bFGF levels than do healthy individuals.8−12 Granato et al8 reported that patients with breast cancer had significantly higher serum bFGF levels, rather than serum VEGF levels, in comparison to healthy individuals, and they proposed that serum bFGF levels might serve as a tool to diagnose breast cancer. Ueno et al9 measured serum bFGF levels in patients with lung cancer in the form of adenocarcinoma (AD), squamous cell carcinoma (SQ), or small cell carcinoma (SCC). They found that patients with AD had a serum bFGF level of 7.6 [0.5–32.5, median(range)] pg/m, those with SQ had a serum bFGF level of 7.4 (0.5–36.7) pg/m, and those with SC had a serum bFGF level of 7.1 (0.5–34.8) pg/mL. These levels were significantly (P < 0.05) higher than the serum bFGF level of 3.0 (1.5–6.0) pg/m they noted in healthy individuals. Moreover, Ueno et al also reported that bFGF levels in SC were associated with prognosis. Veselý et al10 reported that patients with thyroid cancer had a serum bFGF level of 5.69 ± 5.58 ng/mL, and this was significantly higher (P < 0.01) than the serum bFGF level of 1.47 ± 1.77 ng/mL they noted healthy individuals. Landriscina et al11 reported that patients with colon cancer had a serum bFGF level of 14.3 ± 12 pg/mL, which was significantly higher (P < 0.04) than the serum bFGF level of 6.1±3 pg/mL they noted healthy individuals, but they also reported that serum bFGF levels were not associated with the stage of cancer.
Takei et al13 compared serum bFGF levels before and after surgery for breast cancer and found them to significantly lower postoperatively compared to the levels preoperatively. Takei et al also noted the potential presence of bFGF secreted by tumor cells. Landriscina et al11 studied colon cancer by measuring bFGF levels in normal mucosa, in the tumor, and in mucosa around the tumor. In contrast to Takei et al, Landriscina et al reported lower bFGF levels in the tumor and mucosa around the tumor than in normal mucosa. Blood in the mesentery has high levels of bFGF levels, and bFGF may possibly be released at irregular intervals. Immunohistochemical evidence of bFGF has been reported in lung cancer. Comparison of patients who tested positive for bFGF and those who tested negative revealed significant differences in their 5-year survival rates, suggested an association between that bFGF is associated with prognosis.14 The current study examined the association between immunohistologic evidence of bFGF and serum bFGF levels in colon cancer cells but failed to find an association between the two. A study on the relationship to the extent of progression reported that a higher proportion of patients had high bFGF levels when they had renal cell carcinoma in a more advanced histological stage.15 The study reported that a higher proportion of patients had high serum bFGF levels (>30 pg/mL) particularly when they had worse pathologic parameters of T (tumor size) and V (vascular invasion) and cancer with a higher grade.15 Another study reported finding no differences in bFGF levels in different clinical stages of non-small cell lung cancer, but in patients with small cell cancer it found that bFGF levels may be associated with the clinical stage as well as the therapeutic efficacy of chemotherapy and radiotherapy and prognosis.9
The current study found no significant differences in the serum bFGF levels of patients with colon cancer and patients without cancer. If, however, patients with a serum bFGF level higher than the mean + 2SD of a normal individual (11.13 pg/mL) are deemed to be patients with elevated bFGF levels (Table 2), then 2 patients with stage IV cancer in particular had markedly elevated bFGF. All 6 patients with elevated bFGF levels had cancer that tested positive for lymphatic invasion.
Table 2.
Six patients with elevated serum FGF levels
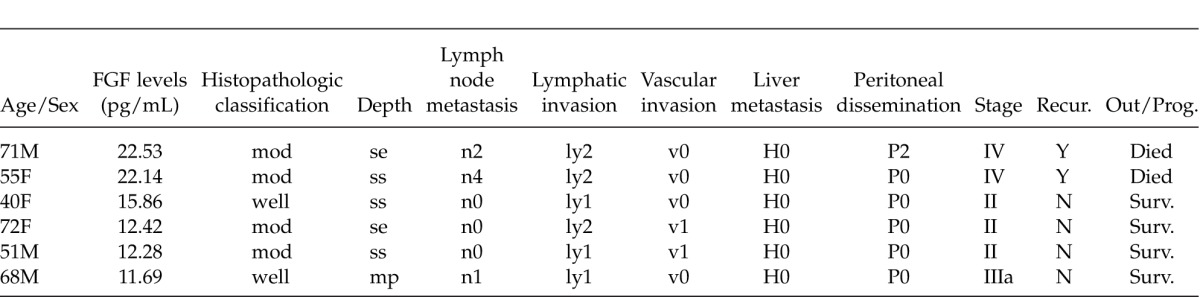
Looking at a breakdown of the patients with stage IV cancer indicates that 5 (mean: 7.5 ± 1.12 pg/mL) had liver metastasis, 4 (mean: 7.66 ± 1.24 pg/mL) had other distant metastases, 1 (22.53 pg/mL) had peritoneal dissemination, and 2 (mean: 14.99 ± 10.1 pg/mL) had n4 metastasis. Although few patients had peritoneal dissemination or n4 metastasis, they tended to have higher serum FGF levels.
The current study measured FGFs serologically and it immunohistochemically stained specimens from patients with colon cancer in order to explore the clinical significance of FGFs. FGF levels tend to be higher in patients with cancer that penetrated further through the bowel wall, a larger tumor, and more severe lymph node metastasis. Patients with stage IV cancer and extensive lymphatic invasion in particular had significantly higher FGF levels.
In the mouse cornea, bFGF reportedly promotes lymphangiogenesis via signaling from VEGF receptor 3 (VEGFR-3), which is expressed by lymphatic endothelial cells. Clinically, bFGF may reportedly be associated with lymph node metastasis in lung carcinoma.18 The current study noted significant differences (P < 0.0001) between minimal lymphatic invasion (ly0 and ly1) and moderate lymphatic invasion (ly2). This study only found 2 patients who had lymph node metastasis (n4), but they tended to have higher bFGF levels. These findings suggest that bFGF is somehow involved in lymphangiogenesis.
Acknowledgments
The authors would like to thank Mrs. Tabe (SPL Laboratory, Tokyo, Japan) for kindly providing instrumentation. The authors are also grateful to Mr. Sakurada (Department of Surgical Pathology, Tokyo Women's Medical University, Tokyo, Japan) for excellent technical advice. The authors declare that they have no conflict of interest.
References
- 1.Burgess WH, Maciag T. The heparin-binding (fibroblast) growth factor family of proteins. Annu Rev Biochem. 1989;58:575–606. doi: 10.1146/annurev.bi.58.070189.003043. [DOI] [PubMed] [Google Scholar]
- 2.Itoh N, Ornitz D. Evolution of the Fgf and Fgfr gene families. Trends Genet. 2004;20(11):563–569. doi: 10.1016/j.tig.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 3.Tsuji W, Yamashiro H, Toi M. Basic fibroblast growth factor (FGF-2) Rinsho N, editor. H Y. 2010;68(7):118–120. In. ed. [PubMed] [Google Scholar]
- 4.Beenken A, Mohammadi M. The FGF family: biology, pathophysiology and therapy. Nat Rev Drug Discov. 2009;8(3):235–253. doi: 10.1038/nrd2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer. 2010;10(2):116–129. doi: 10.1038/nrc2780. [DOI] [PubMed] [Google Scholar]
- 6.Chaffer C, Dopheide B, Savagner P, Thompson E, Williams E. Aberrant fibroblast growth factor receptor signaling in bladder and other cancers. Differentiation. 2007;75(9):831–842. doi: 10.1111/j.1432-0436.2007.00210.x. [DOI] [PubMed] [Google Scholar]
- 7.Greulich H, Pollock P. Targeting mutant fibroblast growth factor receptors in cancer. Trends Mol Med. 2011;17(5):283–292. doi: 10.1016/j.molmed.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Granato A, Nanni O, Falcini F, Folli S, Mosconi G, De Paola F, et al. Basic fibroblast growth factor and vascular endothelial growth factor serum levels in breast cancer patients and healthy women: useful as diagnostic tools? Breast Cancer Res. 2004;6(1):R38–R45. doi: 10.1186/bcr745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ueno K, Inoue Y, Kawaguchi T, Hosoe S, Kawahara M. Increased serum levels of basic fibroblast growth factor in lung cancer patients: relevance to response of therapy and prognosis. Lung Cancer. 2001;31((2–3)):213–219. doi: 10.1016/s0169-5002(00)00187-2. [DOI] [PubMed] [Google Scholar]
- 10.Veselý D, Astl J, Matucha P, Sterzl I, Betka J. Serum levels of angiogenic growth factors in patients with thyroid gland tumors and parathyroid adenoma. Neuro Endocrinol Lett. 2003;24(6):417–419. [PubMed] [Google Scholar]
- 11.Landriscina M, Cassano A, Ratto C, Longo R, Ippoliti M, Palazzotti B, et al. Quantitative analysis of basic fibroblast growth factor and vascular endothelial growth factor in human colorectal cancer. Br J Cancer. 1998;78(6):765–770. doi: 10.1038/bjc.1998.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurobe M, Takei Y, Ezawa H, Hayashi K. Increased level of basic fibroblast growth factor (bFGF) in sera of patients with malignant tumors. Horm Metab Res. 1993;25(7):395–396. doi: 10.1055/s-2007-1002129. [DOI] [PubMed] [Google Scholar]
- 13.Takei Y, Kurobe M, Uchida A, Hayashi K. Serum concentrations of basic fibroblast growth factor in breast cancer. Clin Chem. 1994;40(10):1980–1981. [PubMed] [Google Scholar]
- 14.Takanami I, Imamura T, Hashizume T, Kikuchi K, Yamamoto Y, Yamamoto T, et al. Immunohistochemical detection of basic fibroblast growth factor as a prognostic indicator in pulmonary adenocarcinoma. Jpn J Clin Oncol. 1996;26(5):293–297. doi: 10.1093/oxfordjournals.jjco.a023235. [DOI] [PubMed] [Google Scholar]
- 15.Fujimoto K, Ichimori Y, Kakizoe T, Okajima E, Sakamoto H, Sugimura T, et al. Increased serum levels of basic fibroblast growth factor in patients with renal cell carcinoma. Biochem Biophys Res Commun. 1991;180(1):386–392. doi: 10.1016/s0006-291x(05)81305-1. [DOI] [PubMed] [Google Scholar]
- 16.Kubo H, Cao R, Brakenhielm E, Mäkinen T, Cao Y, Alitalo K. Blockade of vascular endothelial growth factor receptor-3 signaling inhibits fibroblast growth factor-2-induced lymphangiogenesis in mouse cornea. Proc Natl Acad Sci U S A. 2002;99(13):8868–8873. doi: 10.1073/pnas.062040199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao R, Ji H, Feng N, Zhang Y, Yang X, Andersson P, et al. Collaborative interplay between FGF-2 and VEGF-C promotes lymphangiogenesis and metastasis. Proc Natl Acad Sci U S A. 2012;109(39):15894–15899. doi: 10.1073/pnas.1208324109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Volm M, Koomägi R, Mattern J. PD-ECGF. bFGF, and VEGF expression in non-small cell lung carcinomas and their association with lymph node metastasis. Anticancer Res. 1999;19((1B)):651–655. [PubMed] [Google Scholar]