Abstract
A 78-year-old man was admitted to our hospital with right upper abdominal pain and fever. His general condition was poor. The laboratory data showed severe inflammatory reactions. Computed tomography revealed an irregular tumor in the gallbladder. 18F-fluorodeoxy-glucose positron emission tomography (FDG-PET) showed high uptake by the tumor, with diffuse uptake in the spine. Based on the elevated leukocyte count and FDG-PET findings, a granulocyte-colony stimulating factor (G-CSF)–producing tumor was diagnosed (G-CSF 120 pg/mL). We performed cholecystectomy with central bisegmentectomy of the liver, lymph node dissection and right hemicolectomy. Histologically, the tumor was an adenosquamous cell carcinoma of the gallbladder. Immunohistochemical staining of the tumor cells was positive for G-CSF. Postoperatively, the general condition of the patient was improved. The fever subsided, the leukocyte count and serum G-CSF level normalized, and FDG-PET showed no uptake in the spine postoperatively. The patient showed no signs of recurrence at 27 months after undergoing surgery. FDG-PET is a useful method for diagnosing G-CSF–producing gallbladder carcinoma. Aggressive curative resection for G-CSF–producing gallbladder carcinoma may improve patients' general condition and prognosis.
Key words: Granulocyte-colony stimulating factor, Gallbladder carcinoma, FDG-PET, Immunohistochemistry, Leukocytosis
Granulocyte-colony stimulating factor (G-CSF)–producing tumors were first reported in 1977.1 G-CSF–producing gallbladder carcinomas are rare, with only 22 other reported cases. We herein report a case of G-CSF–producing gallbladder carcinoma and include bibliographic comments.
Case Report
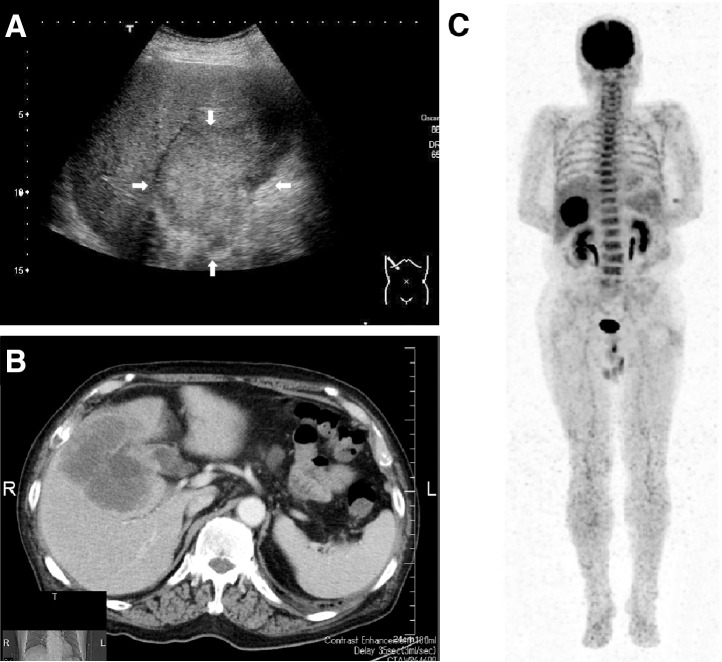
A 78-year-old man was referred to our hospital in May 2010 with right upper abdominal pain and fever. The general condition of the patient was very poor (performance status 3). A physical examination revealed a temperature of 39.0°C, with tenderness and a palpable elastic hard mass in the right upper quadrant of the abdomen. No lymphadenopathy or hepatosplenomegaly was found. The laboratory data showed severe inflammatory reactions, with a leukocyte count of 26,050/μL (92% segmented neutrophils, 2% stab neutrophils, 0% myelocytes, 0% eosinophils, 0% basophils, 2% monocytes, and 4% lymphocytes) and a serum C-reactive protein (CRP) level of 19.3 mg/dL. The serum levels of alkaline phosphatase (ALP) and γ-glutamyl transpeptidase (γ-GTP) were elevated to 1453 U/L (normal range, 115–359 U/L) and 267 U/L (normal range, 11–58 U/L), respectively. The levels of the tumor markers carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) were elevated to 68.5 ng/mL (normal range, <5 ng/mL) and 115.9 U/mL (normal range, <37 U/mL), respectively. Abdominal ultrasonography (US) showed an irregular mass with heterogenous echogenicity (Fig. 1A). Computed tomography (CT) showed an irregular and peripheral enhancing mass (Fig. 1B). 18F-fluorodeoxy-glucose positron emission tomography (FDG-PET) showed markedly high uptake [the maximum standard uptake value (SUVmax) was 16.89] by the tumor, with diffuse uptake in the spine (Fig. 1C). Based on the elevated leukocyte count and the FDG-PET findings, we suspected a diagnosis of a G-CSF–producing tumor. The serum level of G-CSF was elevated at 120 pg/mL (normal range, <39 pg/mL). We diagnosed the patient with a gallbladder carcinoma producing G-CSF and performed a laparotomy. During exploratory laparotomy, a gallbladder tumor was detected showing direct invasion in the liver and transverse colon; however, peritoneal dissemination and liver metastasis were not found. Therefore, we performed cholecystectomy with central bisegmentectomy of the liver, lymph node dissection and right hemicolectomy. The resected specimen measured 12 × 12 cm in size and showed a hard and solid tumor with partially necrotic changes (Fig. 2). A microscopic examination revealed adenosquamous cell carcinoma (Fig. 3A). An immunohistochemical examination using anti–G-CSF monoclonal antibodies (Calbiochem, La Jolla, California) revealed production of G-CSF by the tumor, as the cytoplasm was diffusely positive (Fig. 3B).
Fig. 1.
(A) Abdominal US showing an irregular mass with heterogenous echogenicity (arrows). (B) CT showing an irregular and peripherally enhanced mass. (C) FDG-PET showing markedly high uptake [the maximum standard uptake value (SUVmax) was 16.89] by the gallbladder tumor with diffuse uptake in the spine.
Fig. 2.
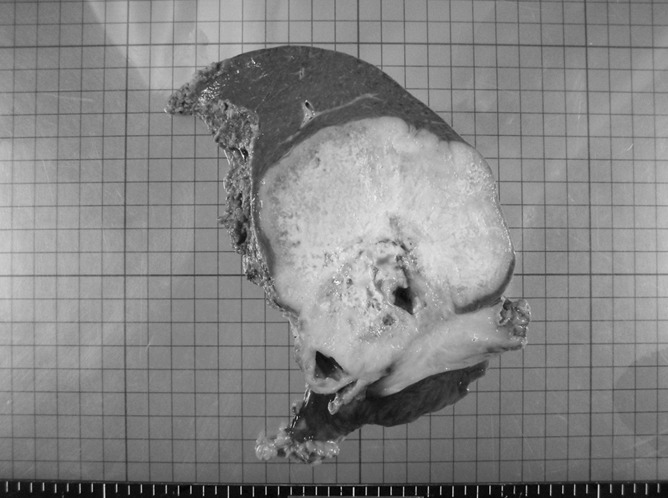
The resected specimen measuring 12 × 12 cm in size and showing a hard and solid tumor with partially necrotic changes.
Fig. 3.
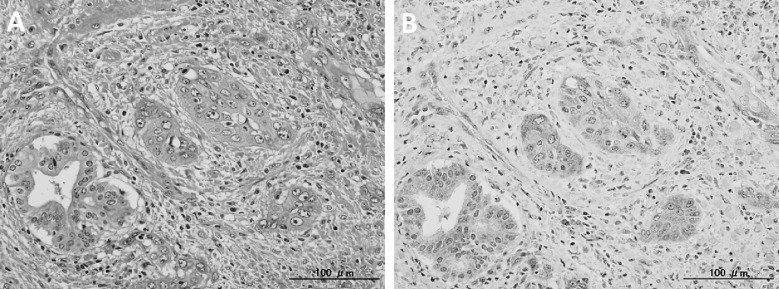
(A) A microscopic examination showing adenosquamous cell carcinoma. H&E (×200). (B) An immunohistochemical examination showing a diffusely positive reaction for G-CSF antibodies in the cytoplasm of the tumor cells (×200).
After surgery, the leukocyte count dramatically decreased to within the normal range, and the fever immediately subsided. The levels of the tumor markers CEA and CA19-9 decreased to 4.1 ng/mL and 11.5 U/mL, respectively. The serum G-CSF level decreased to 28 ng/mL, and FDG-PET showed no diffuse uptake in the spine postoperatively (Fig. 4). The general condition of the patient improved (performance status 0), and he was discharged on the 42nd postoperative day. He has been followed up without the administration of adjuvant chemotherapy, and no evidence of recurrence has been found over 27 months postoperatively.
Fig. 4.
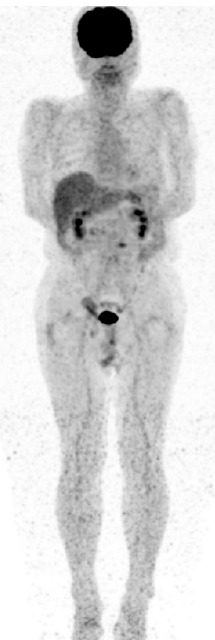
FDG-PET showing no diffuse uptake in the spine postoperatively.
Discussion
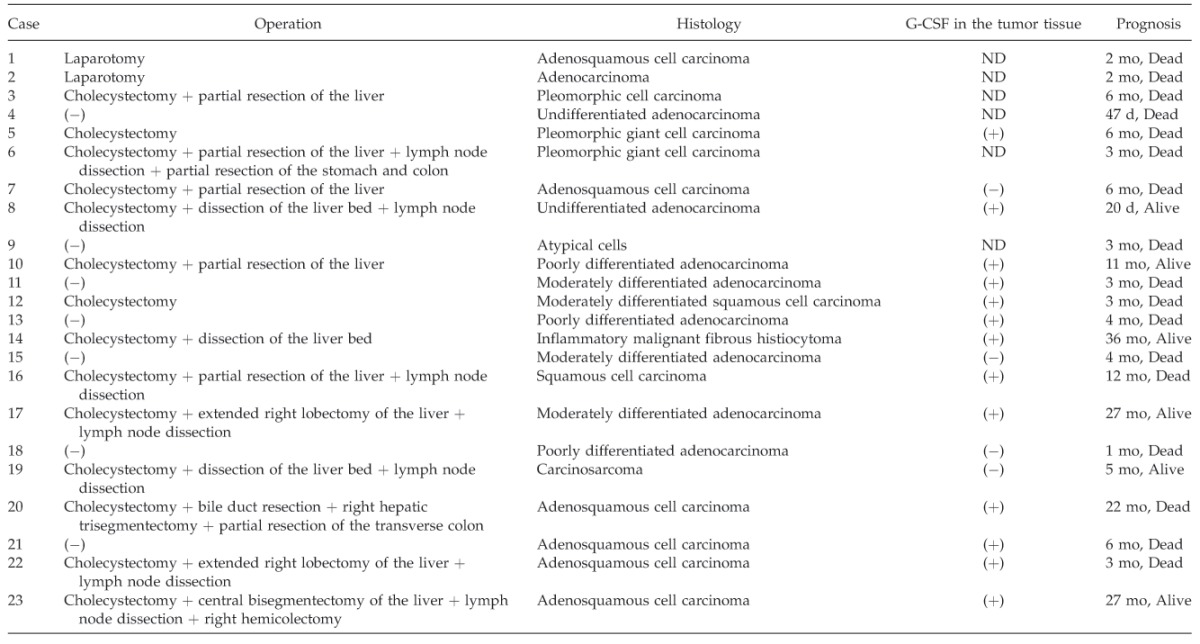
G-CSF, a hematopoietic growth factor, primarily influences the proliferation and differentiation of granulocytic precursors. In 1977, the production of G-CSF by malignant cells was first identified in lung cancer.1 G-CSF–producing tumors often simultaneously produce cytokines such as interleukin-1 (IL-1), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α).2 One report described a G-CSF–producing tumor that simultaneously produced cytokines, including IL-1, IL-6, and TNF-α, causing chronic and progressive inflammation along with tumor growth and exacerbation of symptoms of general wasting with cachexia, which were perceived to reflect worsening of the patient's prognosis.3 There are few reports of G-CSF–producing carcinomas of the gallbladder. Only 23 cases of G-CSF–producing gallbladder carcinoma with leukocytosis have been reported, including our case (Table 1).4–25 In these cases, the tumors affected patients aged 48 to 82 years (average, 68.5 years), including 11 men and 12 women. The most frequent symptoms were abdominal pain and fever. The serum G-CSF levels did not correlate with the leukocyte counts or tumor size. Most of the G-CSF–producing gallbladder carcinomas were adenosquamous cell carcinomas.
Table 1.
Reported cases of G-CSF–producing gallbladder carcinomas
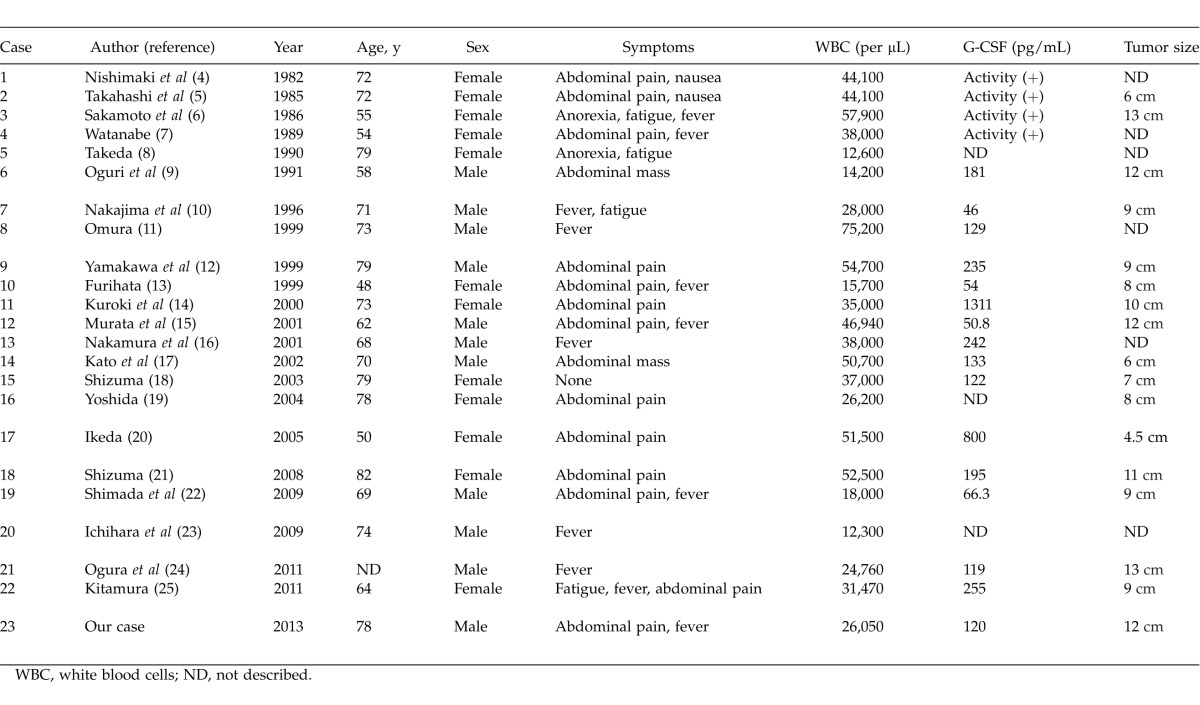
Table 1.
Extended
G-CSF–producing tumors are diagnosed on the basis of elevated serum G-CSF levels and immunohistochemical confirmation of the production of G-CSF in the tumor tissue. In our case, the serum G-CSF level was elevated, and an immunohistochemical examination showed the cytoplasm of the tumor cells to be stained diffusely for G-CSF. Therefore, we diagnosed the patient with a gallbladder carcinoma producing G-CSF.
FDG-PET can be used to assess the nature of a tumor. In our patient, FDG-PET showed markedly high uptake by the gallbladder tumor, with diffuse uptake in the spine. Sugawara et al26 described increased FDG uptake by bone marrow induced by increases in bone marrow metabolism and cellularity in response to G-CSF treatment. In our case, we believe that G-CSF produced by the gallbladder carcinoma increased bone marrow metabolism; hence, the diffuse FDG uptake present in the spine. FDG-PET is a useful method for diagnosing G-CSF–producing tumors.
G-CSF–producing tumors are generally associated with poor prognoses owing to rapid progression and metastasis. The survival period after diagnosis of G-CSF–producing carcinoma is reported to be approximately 3 months.20 Nine of the reported patients died within 3 months; however, 5 of the 12 patients who underwent curative resection survived more than 1 year. Most of the patients with G-CSF–producing tumors were in poor condition as a result of fever. Therefore, the decision to perform curative surgery was delayed. In our case, the general condition of the patient was very poor preoperatively. However, we performed curative resection, and the patient's fever subsided and his general condition dramatically improved postoperatively. He has been well for 27 months with no evidence of recurrence. Aggressive curative resection is the single most effective treatment for G-CSF–producing gallbladder carcinoma, providing the only chance for long-term survival.
In conclusion, FDG-PET is a useful method for diagnosing G-CSF–producing gallbladder carcinoma, and aggressive curative resection for patients in poor condition may improve their general condition and prognosis.
References
- 1.Asano S, Urabe A, Okabe T, Sato N, Kondo Y. Demonstration of granulopoietic factor(s) in the plasma of nude mice transplanted with a human lung cancer and in the tumor tissue. Blood. 1977;49(5):845–852. [PubMed] [Google Scholar]
- 2.Asakawa H, Kobayashi T. The secretion of cytokines and granulocyte colony-stimulating factor by anaplastic and poorly differentiated thyroid carcinoma cell lines. Anticancer Res. 1999;19((1B)):761–764. [PubMed] [Google Scholar]
- 3.Kakinoki K, Takemori Y, Noda Y, Hoso M. An autopsy case of intrahepatic cholangiocarcinoma producing granulocyte-colony stimulating factor. Nihon Shokakibyo Gakkai Zasshi. 2000;97(9):1165–1169. [PubMed] [Google Scholar]
- 4.Nishimaki T, Yoshida K, Takakuwa K, Horita M, Muto T, Takahashi M, et al. A case of carcinoma of the gallbladder producing colony-stimulating factor (CSF) [in Japanese] Nihon Shokakibyo Gakkai Zasshi. 1982;79(6):1336–1340. [PubMed] [Google Scholar]
- 5.Takahashi M, Fujiwara M, Kishi K, Sakai C, Sanada M. MoriyamaY et al. CSF producing gallbladder cancer: case report and characteristics of the CSF produced by tumor cells. Int J Cell Cloning. 1985;3(5):294–303. doi: 10.1002/stem.5530030502. [DOI] [PubMed] [Google Scholar]
- 6.Sakamoto K, Egami H, Yoshimura R, Nakamura S, Ikei S, Mori K, et al. Colony-stimulating factor producing carcinoma of the gallbladder. Jpn J Clin Oncol. 1986;16(1):87–96. [PubMed] [Google Scholar]
- 7.Watanabe Y, Ogino Y, Ubukata E, Sakamoto Y, Matsuzaki O, Shimizu N. A case of a gallbladder cancer with marked hypercalcemia and leukocytosis. Jpn J Med. 1989;28(6):722–726. doi: 10.2169/internalmedicine1962.28.722. [DOI] [PubMed] [Google Scholar]
- 8.Takeda T, Ichiyanagi A, Sano K, Yoshida J, Tsutsumi Y, Miyaji T. A case of gallbladder cancer producing granulocyte colony-stimulating factor. Gastroenterol Jpn. 1990;25(6):762–767. doi: 10.1007/BF02779193. [DOI] [PubMed] [Google Scholar]
- 9.Oguri T, Ikemoto H, Sato H, Abe M, Narahara M, Koyanagi T, et al. A case of gallbladder cancer producing colony stimulating factor with rapid progression [in Japanese] Oitaken Igakukai Zasshi. 1991;10(1):81–84. [Google Scholar]
- 10.Nakajima Y, Takashima T, Naito E, Yoshida J, Senmaru H, Oka M, et al. Case of G-CSF producing gallbladder neoplasm [in Japanese] Nihon Naika Gakkai Zasshi. 1996;85(11):1931–1933. [PubMed] [Google Scholar]
- 11.Omura N, Abe S, Hirai K, Aoki T. A case of granulocyte colony-stimulating factor producing gallbladder cancer. Am J Gastroenterol. 1999;94(1):273–275. doi: 10.1111/j.1572-0241.1999.00817.x. [DOI] [PubMed] [Google Scholar]
- 12.Yamakawa M, Mizuta Y, Mori I, Doi M, Isomoto H, Takeshima F, et al. A case of gallbladder cancer presumed to have produced granulocyte-colony stimulating factor [in Japanese] Rinsyo Kenkyu. 1999;76(9):1779–1782. [Google Scholar]
- 13.Furihata M, Sonobe H, Ohtsuki Y, Enzan H, Tokuoka H, Nakanuma Y. An immunohistochemical study on a case of granulocyte-colony stimulating factor-producing gall-bladder carcinoma. Pathol Int. 1999;49(11):1010–1013. doi: 10.1046/j.1440-1827.1999.00970.x. [DOI] [PubMed] [Google Scholar]
- 14.Kuroki M, Uto H, Ido A, Kuwata G, Nakama T, Ochiai T, et al. A case of gallbladder cancer producing granulocyte-colony stimulating factor and possible parathyroid hormone related protein [in Japanese] Nihon Shokakibyo Gakkai Zasshi. 2000;97(4):478–483. [PubMed] [Google Scholar]
- 15.Murata M, Tateishi H, Nishiyama H, Ito M, Zushi S, Imai Y, et al. A case of granulocyte-colony stimulating factor producing squamous cell carcinoma of the gallbladder [in Japanese] Nihon Shokakibyo Gakkai Zasshi. 2001;98(1):53–57. [PubMed] [Google Scholar]
- 16.Nakamura M, Nishikawa S, Onozawa M, Takagi K, Abo D, Kudoh T, et al. A case of gallbladder cancer producing granulocyte-colony stimulating factor [in Japanese with English abstract] Shiritsu Sapporo Byoin Ishi. 2001;61(1):3–8. [Google Scholar]
- 17.Kato T, Kojima T, Shimizu T, Sasaki H, Abe M, Okushiba S, et al. Inflammatory malignant fibrous histiocytoma of the gallbladder: report of a case. Surg Today. 2002;32(1):81–85. doi: 10.1007/s595-002-8121-z. [DOI] [PubMed] [Google Scholar]
- 18.Shizuma T, Obata H, Hashimoto E, Ikeda I. A case of gallbladder carcinoma accompanied with high levels of serum granulocyte colony-stimulating factor and alpha-fetoprotein [in Japanese with English abstract] Tokyo Joshi Ika Daigaku Zasshi. 2003;73(6):194–199. [Google Scholar]
- 19.Yoshida M, Tabo T, Hayashi H, Onodera H, Imamura Y. A case of squamous cell carcinoma of the gallbladder producing granulocyte-colony stimulating factor [in Japanese with English abstract] Nippon Rinsyo Geka Gakkai Zasshi (J Jpn Soc Clin Surg) 2004;65(9):2459–2463. [Google Scholar]
- 20.Ikeda T, Ohgaki K, Miura M, Aishima S, Shimizu T, Maehara Y. Granulocyte-colony stimulating factor-producing gallbladder cancer without recurrence more than 2 years after resection: report of a case. Surg Today. 2005;35(7):590–593. doi: 10.1007/s00595-004-2981-4. [DOI] [PubMed] [Google Scholar]
- 21.Shizuma T, Nakayama H. A case of gallbladder carcinoma with humoral hypercalcemia of malignancy and leukocytosis [in Japanese] Naika. 2008;102(2):405–407. [Google Scholar]
- 22.Shimada K, Iwase K, Aono T, Nakai S, Takeda S, Fujii M, et al. Carcinosarcoma of the gallbladder producing α-fetoprotein and manifesting as leukocytosis with elevated serum granulocyte colony-stimulating factor: report of a case. Surg Today. 2009;39(3):241–246. doi: 10.1007/s00595-008-3833-4. [DOI] [PubMed] [Google Scholar]
- 23.Ichihara T, Yamashita K, Itoda I, Asada M, Takagi D, Sato K, et al. A resected case of granulocyte colony-stimulating factor producing gallbladder cancer [in Japanese] Tosan Igakkaishi. 2009;31:22–23. [Google Scholar]
- 24.Ogura T, Takii M, Arisaka Y, Masuda D, Kuwabara H, Egashira Y, et al. A case of adenosquamous cell carcinoma of the gallbladder producing G-CSF with diffusely uptake in the spine by FDG-PET [in Japanese with English abstract] Tando. 2011;25(5):759–767. [Google Scholar]
- 25.Kitamura K, Kanemoto H, Furukawa H, Sasaki K, Uesaka K. A case of adenosquamous cell carcinoma of the gallbladder producing granulocyte-colony stimulating factor [in Japanese] Tando. 2011;25(5):815–820. [Google Scholar]
- 26.Sugawara Y, Fisher SJ, Zasodny KR, Kison PV, Baker LH, Wahl RL. Preclinical and clinical studies of bone marrow uptake of fluorine-18-fluorodeoxyglucose with or without granulocyte colony-stimulating factor during chemotherapy. J Clin Oncol. 1998;16(1):173–180. doi: 10.1200/JCO.1998.16.1.173. [DOI] [PubMed] [Google Scholar]