Abstract
Central bisegmentectomy (CBS) of the liver is an en bloc hepatic resection of Couiaud segments 4, 5, and 8. The indications for CBS include benign and malignant tumors occupying both the left medial and right anterior segments. However, CBS has rarely been reported. Here, we investigate CBS in patients with suboptimal liver function for whom an extended lobectomy is not an optimal solution. Each case was 1 of 8 patients who underwent CBS for hepatocellular carcinoma (HCC) or colorectal cancer liver metastasis (CRLM) at the Department of Surgery, Jikei University Hospital. Indications for CBS consisted of CRLM in 3 patients and HCC in 5 patients. The median duration of operation was 552 minutes, and median blood loss was 2263 g. No postoperative nor in-hospital mortalities occurred. In this study, 1-, 2-, and 3-year disease-free survival rates were 62.5%, 12.5%, and 12.5%, respectively, and 1-, 2-, and 3-year overall survival rates were 100%, 100%, and 85.7%, respectively. CBS is advocated for central liver tumors in patients with suboptimal liver function for whom extended lobectomy could result in less than optimal remnant liver volume and function.
Key words: Central bisegmentectomy, Colorectal cancer liver metastasis, Hepatocellular carcinoma
Central bisegmentectomy (CBS) of the liver is an en bloc hepatic resection of Couiaud segments 4, 5, and 8.1 McBride and Wallace2 first reported this procedure in 1972. The indications for CBS include benign and malignant tumors occupying both the left medial and right anterior segments. The traditional procedure for such tumors is extended right or left lobectomy or trisegmentectomy. Because of the possibility of conservation of remnant liver parenchymal volume, CBS may be superior to extended lobectomy or trisegmentectomy, especially for patients with low residual liver function due to viral hepatitis or adjuvant chemotherapy. However, CBS has rarely been reported. We herein report our experience with 8 patients who underwent CBS for colorectal cancer liver metastasis (CRLM) or hepatocellular carcinoma (HCC).
Patients and Methods
Between January 2003 and December 2011, 10 patients underwent CBS for HCC or CRLM at the Department of Surgery, Jikei University Hospital, Tokyo, Japan. Of these, 2 patients were excluded: 1 patient who underwent an en bloc CBS and total caudate lobectomy for HCC in the paracaval portion of Couiaud segment 1 with a largest diameter of 34 mm, and 1 patient who underwent CBS and 2 partial hepatic resections with concomitant low anterior resection of the rectum for carcinoid tumor and liver metastases; the remaining 8 patients were included in this retrospective study. Generally, the extent of hepatic resection was determined based on the retention rate of indocyanine green at 15 minutes (ICGR15) before surgery and in reference to the hepatic reserve as described by Miyagawa et al.3
The details of the techniques for CBS were as previously described.4 Our essential points for CBS are as follows: Hepatic parenchymal dissection between the left medial and lateral section is performed by dividing the Glisson branches arising from the right side of the umbilical portion (Fig. 1A), and dissection between the right anterior and posterior sections is performed along the demarcation line by obliterating the Glisson pedicle of the right anterior section (Fig. 1B). For avoiding congestion of the right anterior section, division of the middle hepatic vein is performed after interruption of the inflow vessels to the right anterior section.
Fig. 1.
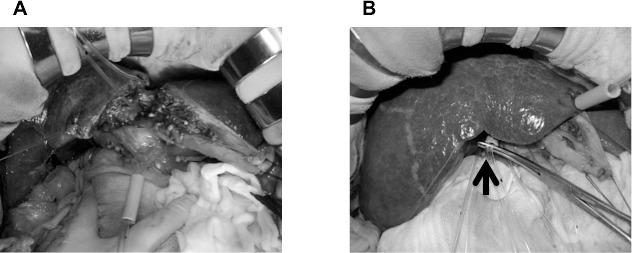
Hepatic parenchymal dissection between the left medial and lateral section is performed by dividing the Glisson branches arising from the right side of the umbilical portion (A), and dissection between the right anterior and posterior sections is performed along the demarcation line by obliterating the Glisson pedicle of the right anterior section (B, arrow).
Recurrence of HCC or CRLM was defined as newly detected hepatic or extrahepatic tumors by ultrasonography, computed tomography, magnetic resonance image, or angiography with or without an increase in serum α-fetoprotein (protein induced by vitamin K absence or antagonist-II) in HCC patients and in serum carcinoembryonic antigen (carbohydrate antigen 19-9) in CRLM patients. For recurrent HCC in the liver, repeated hepatic resection, local ablation therapy, or transarterial chemoembolization was given based on hepatic functional reserve judged mainly by ICGR15. Extrahepatic recurrence was mainly treated conservatively. For recurrent CRLM, repeated hepatic resection, local ablation therapy, or systemic chemotherapy was performed based on hepatic functional reserve judged mainly by number, size, and location of the recurrent liver tumors, ICGR15, and remnant liver volume. For lung metastasis, limited partial lung resection or systemic chemotherapy was carried out. For local recurrence, tumor resection, radiotherapy, or systemic chemotherapy was selected.
This retrospective study was approved by the Ethics Committee of the Jikei University School of Medicine.
Results
Patient characteristics and clinicopathologic variables
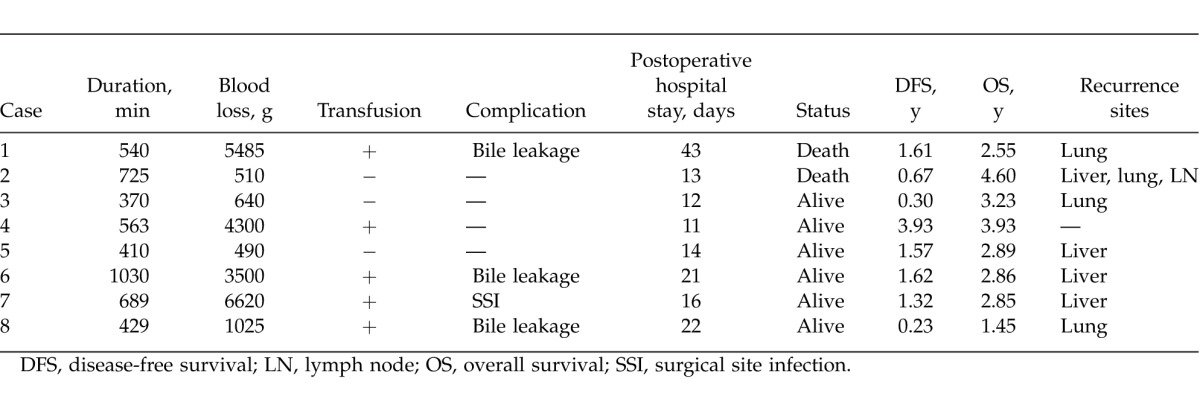
Patient characteristics and clinicopathologic variables are outlined in Table 1 and Table 2. Within the study population, the median age was 68 years (range, 57–80 years), and 2 of them were women. Three patients underwent CBS for CRLM, 5 patients for HCC. Median resected tumor number was 1.5 (range, 1–4), and median largest tumor diameter was 44 mm (range, 15–105 mm). Median duration of operation was 552 min (range, 370–1030 min), and median blood loss was 2263 g (range, 490–6620 g). Five patients received allogeneic blood transfusion. Postoperative complications developed in 4 patients, consisting of bile leakage in 3 patients and surgical site infection in 1 patient. Median postoperative hospital stay was 15 days (range, 11–43 days), and no postoperative or in-hospital mortalities occurred.
Table 1.
Patient characteristics
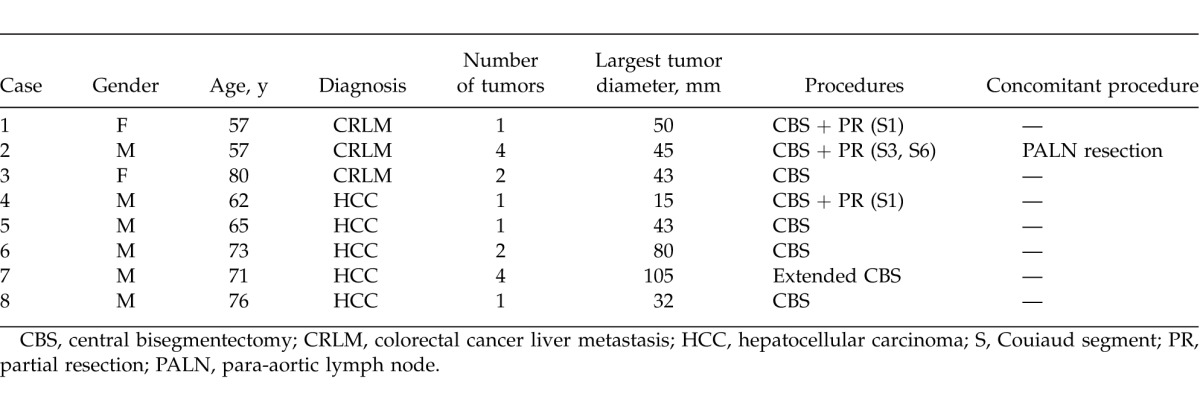
Table 2.
Patient clinicopathologic variables
Postoperative treatment for tumor recurrence and survival
Tumor recurrence developed in all 3 CRLM patients, consisting of lung metastasis in 2 and liver, lung, and lymph node metastases in 1 patient. One patient underwent partial lung resection and is alive and disease free. The other 2 patients received chemotherapy; however, they died due to progression of metastatic tumors, one on postoperative day 931 and the other on postoperative day 1667.
Tumor recurrence developed in 4 HCC patients, consisting of recurrent HCC in the liver in 3 patients and lung metastasis in 1 patient. Partial hepatic resection was performed in 1 patient, partial hepatic resection with concomitant radiofrequency ablation therapy was performed in another patient, and lung resection was performed in a third patient. One patient received transarterial chemotherapy via the hepatic artery. All 5 patients are alive, including 4 disease-free patients. In this study, 1-, 2-, and 3-year disease-free survival rates after CBS for HCC and CRLM were 62.5%, 12.5%, and 12.5% (Fig. 2A), respectively, and 1-, 2-, and 3-year overall survival rates were 100%, 100%, and 85.7% (Fig. 2B), respectively.
Fig. 2.
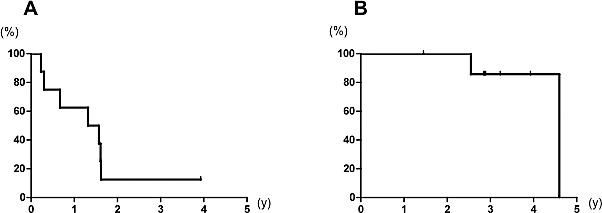
One, two, and three-year disease-free survival rates after CBS were 62.5%, 12.5%, and 12.5%, respectively (A), and overall survival rates were 100%, 100%, and 85.7%, respectively (B).
Discussion
Surgical resection is the only therapeutic strategy that offers a potential cure for patients with either primary or secondary liver tumors, especially those with good performance status and adequate liver function.5,6 Improvements in the understanding of liver anatomy and advancements in image technology have contributed to the development of segmental liver resection.7 The ICGR15 is useful for predicting the safe limit of liver resection in each patient, and liver volumetry using computed tomography is helpful for evaluating whether the remnant liver volume is adequate. For centrally located liver tumors, the conventional operative procedure is extended left or right lobectomy. Because of inadequate remnant liver volume, extended lobectomy bears a considerable risk of postoperative liver failure, particularly in patients with impaired preoperative liver function due to cirrhosis or preoperative chemotherapy. CBS may be superior to extended lobectomy, because more liver parenchyma is conserved.8–10 Preserving more liver parenchyma decreases morbidity and mortality rates11 and increases the possibility of the second hepatic resection in the case of a recurrent intrahepatic tumor.12 However, more extensive dissection of vascular pedicles and larger resection surfaces of liver parenchyma in CBS may result in excessive blood loss, longer duration of operation, or greater incidence of postoperative bile leakage,8,13,14 and CBS has not been frequently used for treatment of centrally located liver tumors. Because of the ability to prevent excessive intraoperative blood loss and postoperative complications using recent surgical techniques, image technologies, and preoperative assessment of liver function and measuring of remnant liver volume, CBS leads to a better therapeutic outcome for centrally located liver tumors, especially in patients with preoperative impaired liver function.
Conclusion
CBS is advocated for central liver tumors in patients with suboptimal liver function, such as when HCC complicates viral hepatitis or cirrhosis, and patients with metastatic liver tumors with associated steatosis due to chemotherapy, for whom extended lobectomy could result in less than optimal remnant liver volume and function.
References
- 1.Couinaud C. Le Foie: Etudes Anatomiques et Chirugicales. Paris: Masson & Cie; 1957. [Google Scholar]
- 2.McBride CM, Wallace S. Cancer of the right lobe of the liver: a variety of operative procedures. Arch Surg. 1972;105(2):289–296. doi: 10.1001/archsurg.1972.04180080139023. [DOI] [PubMed] [Google Scholar]
- 3.Miyagawa S, Makuuchi M, Kawasaki S, Kakazu T. Criteria for safe hepatic resection. Am J Surg. 1995;169(6):589–594. doi: 10.1016/s0002-9610(99)80227-x. [DOI] [PubMed] [Google Scholar]
- 4.Yanaga K. Central bisectionectomy (bisegmentectomy) of the liver (with video) J Hepatobiliary Pancreat Sci. 2012;19(1):44–47. doi: 10.1007/s00534-011-0449-7. [DOI] [PubMed] [Google Scholar]
- 5.Agrawal S, Belghiti J. Oncologic resection for malignant tumors of the liver. Ann Surg. 2011;253(4):656–665. doi: 10.1097/SLA.0b013e3181fc08ca. [DOI] [PubMed] [Google Scholar]
- 6.Wang HQ, Yang JY, Yan LN. Hemihepatic versus total hepatic inflow occlusion during hepatectomy: a systematic review and meta-analysis. World J Gastroenterol. 2011;17(26):3158–3164. doi: 10.3748/wjg.v17.i26.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Billingsley KG, Jarnagin WR, Fong Y, Blumgart LH. Segment-oriented hepatic resection in the management of malignant neoplasms of the liver. J Am Coll Surg. 1998;187(5):471–481. doi: 10.1016/s1072-7515(98)00231-2. [DOI] [PubMed] [Google Scholar]
- 8.Stratopoulos C, Soonawalla Z, Brockmann J, Hoffmann K, Friend PJ. Central hepatectomy: the golden mean for treating central liver tumors? Surg Oncol. 2007;16(2):99–106. doi: 10.1016/j.suronc.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Hu RH, Lee PH, Chang YC, Ho MC, Yu SC. Treatment of centrally located hepatocellular carcinoma with central hepatectomy. Surgery. 2003;133(3):251–256. doi: 10.1067/msy.2003.102. [DOI] [PubMed] [Google Scholar]
- 10.Arkadopoulos N, Kyriazi MA, Theodoraki K, Vassiliou P, Perelas A, Vassiliou I, et al. Central hepatectomy under sequential hemihepatic control. Langenbecks Arch Surg. 2012;397(8):1283–1288. doi: 10.1007/s00423-012-0984-y. [DOI] [PubMed] [Google Scholar]
- 11.Mehrabi A, Mood ZA, Roshanaei N, Fonouni H, Müller SA, Schmied BM, et al. Mesohepatectomy as an option for the treatment of central liver tumors. J Am Coll Surg. 2008;207(4):499–509. doi: 10.1016/j.jamcollsurg.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 12.Chouillard E, Cherqui D, Tayar C, Brunetti F, Fagniez PL. Anatomical bi- and trisegmentectomies as alternatives to extensive liver resections. Ann Surg. 2003;238(1):29–34. doi: 10.1097/01.sla.0000075058.37052.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shirabe K, Kajiyama K, Harimoto N, Tsujita E, Wakiyama S, Maehara Y. Risk factors for massive bleeding during major hepatectomy. World J Surg. 2010;34(7):1555–1562. doi: 10.1007/s00268-010-0495-3. [DOI] [PubMed] [Google Scholar]
- 14.Yamashita Y, Hamatsu T, Rikimaru T, Tanaka S, Shirabe K, Shimada M, et al. Bile leakage after hepatic resection. Ann Surg. 2001;233(1):45–50. doi: 10.1097/00000658-200101000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]


