Abstract
Vesicouterine fistula (VUF) is a rare type of genitourinary fistula. Lower-segment cesarean section is the leading cause of VUF. Patients mostly present with the classical triad of menouria, amenorrhea, and urinary incontinence, with the history of a previous cesarean section. Conservative management with catheterization and open, laparoscopic, and robotic surgeries are the prescribed treatment options. We present the case of a 35-year-old woman who presented with cyclical menouria and urinary incontinence. After diagnosis of VUF by cystoscopy, the laparoscopic approach was chosen. During the procedure, we used anterior abdominal wall peritoneum and adjacent adipose tissue interposition for the first time, instead of omental interposition, because of the unavailability of omentum. The postoperative period was uneventful, and the procedure was successful. In conclusion, the laparoscopic approach is feasible and the anterior abdominal wall peritoneal flap can be used instead of omentum for tissue interposition when the omentum is not available.
Key words: Vesicouterine fistula, Laparoscopy, Abdominal flap, Interposition, Youssef's Syndrome
Vesicouterine fistula (VUF) is a rare type of genitourinary fistula that accounts for 1% to 4% of all reported urogenital fistulas.1 Lower-segment cesarean section is the leading cause of VUF. The VUF prevalence increased with the elevated cesarean section ratios in recent decades. In the literature, 18 cases of VUF were reported between 1908 and 1946, whereas the number of reported cases increased to 74 between 1947 and 1986. Finally, Yip and Leung2 reported 64 new cases in just 11 years, between 1986 and 1997. The management of VUF becomes more important when the prevalence for an 11-year time interval approximately equals the prevalence for the prior 38 years.2
Inadequate bladder mobilization, accidental suturing of the bladder wall, and direct trauma are the possible mechanisms causing VUF. These patients mostly present with the classic triad of menouria, amenorrhea, and urinary incontinence, with a history of previous cesarean section.
The diagnosis depends on clinical suspicion, especially for delayed cases. It is easier to diagnose when the patient presents with menouria after genitourinary surgery or when the patient has a history involving, for example, vacuum/forceps application. However, some patients present with normal vaginal menses and absence of menouria. These patients may have symptoms of irritation of the urinary tract. Vaginal examination, cysroscopy, cystography, hysterography, hysteroscopy, ultrasonography, a computed tomography (CT) scan, or magnetic resonance imaging would be used for diagnosis. Herein we report the laparoscopic management of a patient with VUF using the anterior abdominal wall flap for interposition.
Case
A 35-year-old woman (gravida 2, para 2) with 2 previous cesarean sections presented with recurrent cyclical suprapubic pain, menouria, and cyclical urinary incontinence. The last cesarean section was carried out 6 years ago, and the first one was carried out 10 years ago. According to the patient's medical history, the first cesarean section had been uneventful, whereas massive hematuria had been observed after the second cesarean section. The patient reported that the bladder drainage had been applied for only 2 days. The hematuria had continued after removal of the bladder catheter for about 2 weeks; however, no intervention had been carried out for hematuria. The incontinence was observed only after micturition and during coitus. Suprapubic pain and urinary incontinence had been evident since her last cesarean section. Her complaints showed a cyclical pattern and were observed during menstruation.
The Incontinence Impact Questionnaire-7 (IIQ) and Urinary Distress Inventory-6 (UDI) were applied before and after treatment. The IIQ and UDI short forms are widely used, valid, reliable quality-of-life assessment tools for patients with urinary symptoms.3,4 Moreover, both have validation studies in Turkish.5 Thus, these are the most-used quality-of-life assessment questionnaires in scientific papers investigating urinary system disorders. The IIQ and UDI were designed by Shumaker et al.6 The original form of the IIQ has 30 self-administrated questions covering 4 domains: physical activity, social relationships, travel, and emotional health. The original UDI, which complements the IIQ, has 19 questions. Both forms were shortened for ease of application, with high reliability (the IIQ short form has 7 questions and its highest score is 28, and the UDI has 6 questions and its highest score is 24). High scores are related to impaired quality of life. The pretreatment IIQ score was 15, and the UDI score was 11. The visual analog score (VAS) is a measurement instrument that is generally used to assess the perception of pain. A horizontal line 10 cm in length is drawn and anchored by word descriptors at each end such as no pain and very severe pain.7 The patient marks on the line the point that they feel represents their perception of pain. The measurement in centimeters represents the score. Pretreatment VAS was 7. Physical examination of the abdomen and vagina were unremarkable. Ultrasonography of the kidneys, ureters, and bladder were normal. Ultrasonography of the uterus showed a thin hypoechogenic line between the full bladder and the uterine cavity at the site of the uterine scar. Renal function tests and other routine blood tests were within normal limits. A urine test revealed 15 erythrocytes during premenstrual period. The cyclical pattern of symptoms made us consider urinary tract endometriosis or VUF as a possible diagnosis. After we excluded urinary infection, cystoscopy was performed to inspect the bladder during menstruation. We observed an orifice of fistula at the bladder's dome and a jet of menstrual blood from the orifice (Fig. 1A). The combination of transvaginal ultrasonography and cystoscopy, which was performed during menstruation, led us to the diagnosis; thus, we did not perform any further diagnostic procedures, such as a renal CT or micturiting cystourethrogram.
Fig. 1.
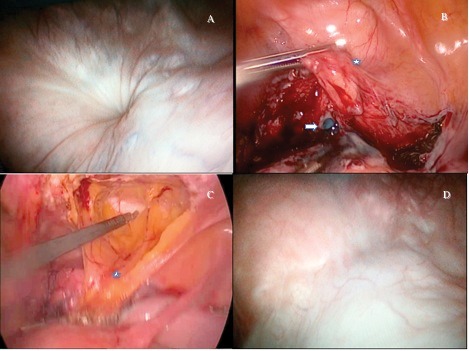
Laparoscopic and cystoscopic images. (A) The cystoscopic view of the 0.5-mm orifice of fistula at the bladder dome. (B) Methylene blue leakage from the bladder after excision of the fistula tract (white arrow). The asterisk shows bladder. (C) The mobilization of the peritoneum of the anterior abdominal wall with a transverse incision. The asterisk shows the mobilized peritoneal flap. (D) Postoperative control cystoscopy revealed well-healed scar tissue at the bladder dome.
Surgical Technique
We chose the laparoscopic approach to treat the VUF. After placement of 4 ports (a 10-mm intraumblical port, a 5-mm pararectal port on either side, and a left flank port), we observed dense adhesion of the bladder and uterus. The adhesion between the uterus and the bladder dissected sharply. After isolation of the fistula tract, it was excised. The defect on the posterior wall of the bladder (Fig. 1B) was closed with “figure eight” sutures of 3-0 polyglactin. After the reparation, the bladder was gently filled with methylene blue to rule out any leak. Then cyctoscopy was performed, and no hemorrhage was seen. The uterine wound was closed with 2 interrupted sutures of 2-0 polyglactin. Omental tacking was not done because of shortage of the omentum. Instead, the preperitoneal adipose tissue from the anterior abdominal wall was tacked onto the posterior wall of the bladder as a barrier between the uterus and the bladder. A transverse incision was made in the anterior abdominal wall peritoneum (similar to the startup incision of a laparoscopic burch operation) 4 cm above the bladder reflection between the medial umbilical folds using sharp dissection with electrosurgical scissors, and the adipose tissue was mobilized with gentle, blunt dissection (Fig. 1C). Mobilized peritoneal tissue with adjacent adipose tissue was fixed between the uterus and bladder with interrupted sutures of 3-0 polyglactin. A tube drain was introduced through the right flank port.
The postoperative period was uneventful, and the patient was discharged from the hospital on the second postoperative day with a Foley urethral catheter, which was removed after 10 days. The drain tube was removed on the second day of operation when we did not observe any blood or urine leakage. We used broad-spectrum antibiotics to prevent any possible infection due to the operation or the prolonged 10-day catheterization. Ten days later, a CT cystogram was performed to check for a urine leak. There was no leakage, so the Foley urethral catheter was removed. We repeated cystoscopy 6 weeks later and observed scar tissue with no recurrence (Fig. 1D). The IIQ score dropped to 0, and the UDI score dropped to 0. VAS dropped to 1.
Discussion
Cesarean section is the leading cause of VUF (more than 75% of cases).8 Lower-segment cesarean section is a major risk factor.9 It can be reasonably predicted that the diagnosis of VUF will be more common in the future than it is today, considering the elevated cesarean section rates. VUF should be kept in mind in patients with massive or moderate hematuria during the early postpartum period. The patient can present with menouria, amenorrhea, urinary incontinence, or a combination of these symptoms. The urinary incontinence in vesico-vaginal fistulas is usually continuous, and the pressure of the vaginal side of the fistula is usually lower than that of the vesical side. However, in cases of VUF, the pressure in the uterus is usually higher than the intravesical pressure. Thus, the incontinence may be present just during coitus or menstruation.
Continuous bladder drainage is the conservative management option for patients who are in the early postpartum phase with a small fistula. Those who perform the second cesarean section might choose to leave the urinary catheter in for a longer period of time to observe hematuria. Moreover, this intervention can be beneficial for the recovery of the fistula tract. Supplementation of bladder drainage with medical therapy such as oral contraceptive pills or gonadotropin-releasing hormone analogs is beneficial if the menouria is present. In this way, blood drainage through the fistula tract can be prevented. However, the success rate of continuous bladder drainage is less than 5%.10 Thus, surgical management is the mainstay. The surgical principles are liberal excision of scar tissues around the fistula, tension-free closure of the wound, and the interposition of an omental flap. Interrupted, absorbable sutures should be used to avoid necrosis. The repaired region must have a good blood supply. A surgical drain should be placed at the site of the repair.
The presented patient had dramatic pain relief and improvement in quality-of-life assessments after the operation. We think that a minimally invasive laparoscopic approach has a role in quick convalescence.
Open surgical management has good results.10,11 On the other hand, the laparoscopic technique has advantages, such as quicker convalescence, a shorter hospital stay, and better cosmetic results, with success rates similar to those of open surgery.12 Laparoscopy provides better visualization through magnification. However, this technique requires advanced laparoscopic-suturing ability. Different from other described techniques, we used the anterior abdominal wall peritoneal flap with adjacent adipose tissue interposition as a barrier instead of omentum. To the best of our knowledge, it is the first report describing preperitoneal adipose tissue mobilization and interposition instead of omentum or epiploic appendices of the sigmoid colon. In the literature, omentum is the major flap that is used for tacking, because it is mobile and has a good blood supply.2 Myouterine flaps13 or appendices epiploica flaps would be the second choice after omentum, where it was sometimes of insufficient length to be used or formerly excised. We think that preparation of an anterior abdominal wall flap would be easier than myouterine flaps when using minimally invasive techniques such as laparoscopy or robotic surgery. The appendices epiploica flap requires repositioning of the colon between the bladder and uterus, but it seems less physiological than the anterior abdominal flap.
In conclusion, the laparoscopic approach is feasible, and the anterior abdominal wall peritoneal flap can be used instead of omentum for tissue interposition in cases where the omentum is not available.
References
- 1.Iloabachie GC, Njoku O. Vesico-uterine fistula. Br J Urol. 1985;57(4):438–439. doi: 10.1111/j.1464-410x.1985.tb06305.x. [DOI] [PubMed] [Google Scholar]
- 2.Yip SK, Leung TY. Vesicouterine fistula: an updated review. Int Urogynecol J Pelvic Floor Dysfunct. 1998;9(5):252–256. doi: 10.1007/BF01901500. [DOI] [PubMed] [Google Scholar]
- 3.Yu HJ, Wong WY, Chen J, Chie WC. Quality of life impact and treatment seeking of Chinese women with urinary incontinence. Qual Life Res. 2003;12(3):327–333. doi: 10.1023/a:1023250632395. [DOI] [PubMed] [Google Scholar]
- 4.Uebersax JS, Wyman JF, Shumaker SA, McClish DK, Fantl JA. Short forms to assess life quality and symptom distress for urinary incontinence in women: the Incontinence Impact Questionnaire and the Urogenital Distress Inventory. Continence Program for Women Research Group. Neurourol Urodyn. 1995;14(2):131–139. doi: 10.1002/nau.1930140206. [DOI] [PubMed] [Google Scholar]
- 5.Cam C, Sakalli M, Ay P, Cam M, Karateke A. Validation of the short forms of the incontinence impact questionnaire (IIQ-7) and the urogenital distress inventory (UDI-6) in a Turkish population. Neurourol Urodyn. 2007;26(1):129–133. doi: 10.1002/nau.20292. [DOI] [PubMed] [Google Scholar]
- 6.Shumaker SA, Wyman JF, Uebersax JS, McClish D, Fantl JA. Health-related quality of life measures for women with urinary incontinence: the Incontinence Impact Questionnaire and the Urogenital Distress Inventory. Continence Program in Women (CPW) Research Group. Qual Life Res. 1994;3(5):291–306. doi: 10.1007/BF00451721. [DOI] [PubMed] [Google Scholar]
- 7.Wewers ME, Lowe NK. A critical review of visual analogue scales in the measurement of clinical phenomena. Res Nurs Health. 1990;13(4):227–236. doi: 10.1002/nur.4770130405. [DOI] [PubMed] [Google Scholar]
- 8.Rao MP, Dwivedi US, Datta B, Vyas N, Nandy PR, Trivedi S, et al. Post caesarean vesicouterine fistulae—Youssef syndrome: our experience and review of published work. ANZ J Surg. 2006;76(4):243–245. doi: 10.1111/j.1445-2197.2006.03591.x. [DOI] [PubMed] [Google Scholar]
- 9.Ikechebelu JI, Ugboaja JO, Okeke CF. Post-cesarean vesicouterine fistula (Youssef syndrome): report of two cases. J Obstet Gynaecol Res. 2011;37(7):912–915. doi: 10.1111/j.1447-0756.2010.01428.x. [DOI] [PubMed] [Google Scholar]
- 10.Hadzi-Djokic JB, Pejcic TP, Colovic VC. Vesico-uterine fistula: report of 14 cases. BJU Int. 2007;100(6):1361–1363. doi: 10.1111/j.1464-410X.2007.07067.x. [DOI] [PubMed] [Google Scholar]
- 11.Drissi M, Karmouni T, Tazi K, El Khader K, Koutani A. Ibn Attya A et al. Vesicouterine fistulas: an experience of 17 years [in French] Prog Urol. 2008;18(3):173–176. doi: 10.1016/j.purol.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 12.Chibber PJ, Shah HN, Jain P. Laparoscopic O'Conor's repair for vesico-vaginal and vesico-uterine fistulae. BJU Int. 2005;96(1):183–186. doi: 10.1111/j.1464-410X.2005.05592.x. [DOI] [PubMed] [Google Scholar]
- 13.Char D, Krasnokutsky S, Frischer Z, Shah SM, Bayshtok J, Khan SA. Surgically correcting a vesicouterine fistula with a myouterine flap. A case report. J Reprod Med. 1997;42(6):372–374. [PubMed] [Google Scholar]


