Abstract
We present a case undergoing successful laparoscopic ligation of the inferior mesenteric artery (IMA) and internal iliac artery (IIA) for the treatment of a symptomatic type II endoleak (T2E) after endovascular aneurysm repair (EVAR). The patient presented with abdominal and back pain 1 year after EVAR. Subsequent enhanced computed tomography scan showed aneurysm sac enlargement from 60 mm to 70 mm, and digital substraction angiography revealed a T2E caused by patent IMA and right IIA. Then the patient underwent successful laparoscopic ligation of the IMA and right IIA. Postprocedural angiogram demonstrated complete resolution of the type II endoleak, and no intraoperative complications occurred. Also, there was no remaining abdominal pain or back pain after the operation.
Key words: Laparoscopic ligation, Endoleak, Endovascular aneurysm repair, Inferior mesenteric artery, Internal iliac artery
Endovascular abdominal aortic aneurysm repair (EVAR) is gaining acceptance as a safe and effective minimally invasive alternative to traditional open surgery in selected patients.1 Type II endoleaks (T2E) from retrograde flow of collateral arterial branch can occur in 20%−30% of patients after EVAR.2 We present a case undergoing successful laparoscopic ligation of the inferior mesenteric artery (IMA) and internal iliac artery (IIA) for the treatment of a symptomatic endoleak from both arteries into the aneurysm sac.
Case Report
A 55-year-old female was incidentally found to have an abdominal aortic aneurysm (AAA) measuring 60 mm in the greatest diameter. She underwent successful EVAR with a Hercules bifurcated stent graft system (Shanghai MicroPort Medical, Shanghai, China). However, the patient presented with abdominal and back pain 1 year later. Enhanced computed tomography (CT) scan showed the diameter of the AAA increased by 10 mm compared to the preoperative images with obscure boundary and an endoleak (Fig. 1A and 1B). Subsequent abdominal digital substraction angiography revealed the T2E was caused by continued retrograde flow originating from the IMA and exiting via the right IIA (Fig. 1C).
Fig. 1.
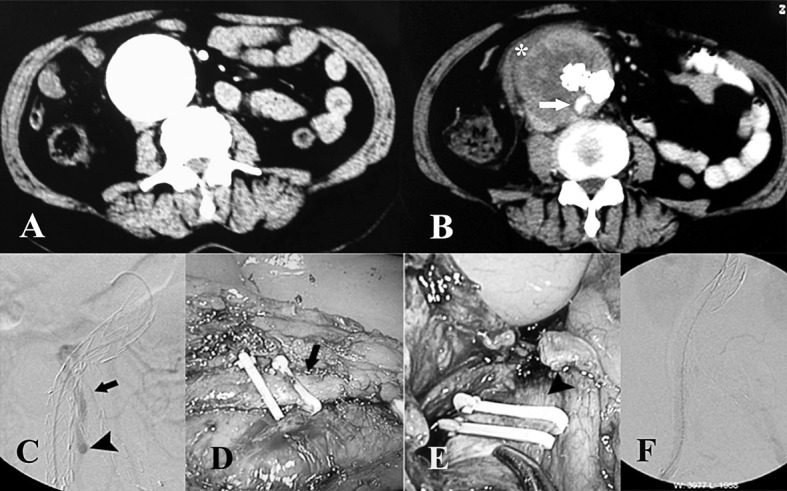
(A) CT scan before EVAR showing a large abdominal aortic aneurysm. (B) CT scan after EVAR showing aneurysm sac enlargement with exudation (asterisk) around the sac and an endoleak (white arrow). (C) An abdominal digital substract angiography showing type II endoleak caused by retrograde flow from the IMA (black arrow) and exiting via the right internal iliac artery (black arrowhead). (D) Laparoscopic ligation of the IMA (black arrow). (E) Laparoscopic ligation of the right internal iliac artery (black arrowhead). (F) Postprocedure angiogram showing resolution of the type II endoleak.
Then the patient underwent laparoscopic ligation of the IMA and right IIA. Under general anesthesia, the patient was positioned supine with the left side slightly elevated on a pillow. The abdomen was prepped in the standard surgical fashion and entered via a Veress needle. Pneumoperitoneum was established at a pressure of 15 mmHg with a CO2 perfusion rate of 6 L/min. A 30° endoscope (Olympus America, USA) attached to a cold light source and Olympus vision system (Olympus America Inc., USA) was placed through a 10 mm trocar in the umbilicus. Two 10 mm trocars were inserted in the right upper and right lower quadrants lateral to the rectus, while another 5 mm trocar was placed in the left upper quadrant. The peritoneum was incised over the aneurysm longitudinally with the harmonic scalpel. The origin of the right IMA was exposed and dissected, then doubly ligated with 10 mm hemoclips (Fig. 1D). The right IIA was identified after vertical incision of peritoneum above the psoas muscle. After opening the proximal part of the right pararectal space, the hypogastric artery was isolated and ligated with hemoclips (Fig. 1E). The laparoscopic operative time was 50 minutes.
Postprocedural angiogram demonstrated complete resolution of the T2E (Fig. 1F). The abdominal pain and back pain were gone after the operation, and no intraoperative complications occurred. The blood loss was about 80 mL. The patient was extubated immediately after the procedure, tolerated a regular diet the following day, and was discharged home on postoperative day 7.
Discussion
Previous studies have shown an incidence of T2E varying from 7.8% to 25% after EVAR.3−5 Although most endoleaks will resolve without therapy, treatment should be performed in case of AAA enlargement occurring after 6 months, persistence of the endoleak after 12 months without AAA sac enlargement, symptomatic or pulsatile sac, enlargement > 5 mm, or when aneurysm sac pressure is > 20% of the systolic blood pressure.6−8 Recurrent as well as persistent T2E are prone to life-threatening complications,9 which should be closely monitored to ensure early and specific treatment.
The uniqueness of our case was the communication between the patent IMA and the right IIA. We believe such connection allowed for the continued retrograde flow into the aneurysm sac originating from the IMA and exiting via the right IIA, leading to an increase in size of the aneurysm by 10 mm over the course of 12 months. This was also supported by studies showing that as the number of feeding vessels into an aneurysm increases, not only does the risk of a T2E increase, but it is also associated with higher intrasac flow velocities, which may prevent spontaneous endoleak seal.
The patent IMA can also be accessed via Riolan arch and embolized by coil, but the IIA was difficult to be accessed. A patent IIA could also cause endoleak even after the IMA was embolized. Therefore, we did not use the transarterial embolized technique in the present case. This endoleak caused severe abdominal and back pain, and CT showed exudation around the sac, suggesting a high risk of aneurysm rupture. As the direct AAA access might lead to aneurysm rupture, this technique was not adopted for this patient. The symptomatic T2E following EVAR was finally treated with laparoscopic ligation of the IMA and IIA, and the post-procedural angiogram showed complete resolution of the T2E. The abdominal pain and back pain were gone after the operation. Compared to traditional open surgery, patients often experience less pain, earlier recovery, and less scarring with laparoscopic surgery.
References
- 1.Zarins CK. The US AneuRx clinical trial: 6-year clinical update 2002. J Vasc Surg. 2003;37(4):904–908. doi: 10.1067/s0741-5214(03)70038-x. [DOI] [PubMed] [Google Scholar]
- 2.Van Marrewijk CJ, Fransen G, Laheij RJ, Harris PL, Buth J, Collaborators EUROSTAR. Is a type II endoleak after EVAR a harbinger of risk? Causes and outcome of open conversion and aneurysm rupture during follow-up. Eur J Vasc Endovasc Surg. 2004;27(2):128–137. doi: 10.1016/j.ejvs.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 3.EVAR trial participants. Endovascular aneurysm repair versus open repair in patients with abdominal aortic aneurysm (EVAR trial 1): randomised controlled trial. Lancet. 2005;365(9478):2179–2186. doi: 10.1016/S0140-6736(05)66627-5. [DOI] [PubMed] [Google Scholar]
- 4.Jones JE, Atkins MD, Brewster DC, Chung TK, Kwolek CJ, LaMuraglia GM, et al. Persistent type 2 endoleak after endovascular repair of abdominal aortic aneurysm is associated with adverse late outcomes. J Vasc Surg. 2007;46(1):1–8. doi: 10.1016/j.jvs.2007.02.073. [DOI] [PubMed] [Google Scholar]
- 5.Abularrage CJ, Crawford RS, Conrad MF, Lee H, Kwolek CJ, Brewster DC, et al. Preoperative variables predict persistent type 2 endoleak after endovascular aneurysm repair. J Vasc Surg. 2010;52(1):19–24. doi: 10.1016/j.jvs.2010.02.023. DOI: 10.1016/j.jvs.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 6.Rhee SJ, Ohki T, Veith FJ, Kurvers H. Current status of management of type II endoleaks after endovascular repair of abdominal aortic aneurysms. Ann Vasc Surg. 2003;17(3):335–344. doi: 10.1007/s10016-003-0002-5. [DOI] [PubMed] [Google Scholar]
- 7.Timaran CH, Ohki T, Rhee SJ, Veith FJ, Garguilo NJ, Toriumi H, et al. Predicting aneurysm enlargement in patients with persistent type II endoleaks. J Vasc Surg. 2004;39(6):1157–1162. doi: 10.1016/j.jvs.2003.12.033. [DOI] [PubMed] [Google Scholar]
- 8.Gelfand DV, White GH, Wilson SE. Clinical significance of type II endoleak after endovascular repair of abdominal aortic aneurysm. Ann Vasc Surg. 2006;20(1):69–74. doi: 10.1007/s10016-005-9382-z. [DOI] [PubMed] [Google Scholar]
- 9.El Batti S, Cochennec F, Roudot-Thoraval F, Becquemin JP. Type II endoleaks after endovascular repair of abdominal aortic aneurysm are not always a benign condition. J Vasc Surg. 2013;57(5):1291–1297. doi: 10.1016/j.jvs.2012.10.118. DOI: 10.1016/j.jvs.2012.10.118. [DOI] [PubMed] [Google Scholar]


