Abstract
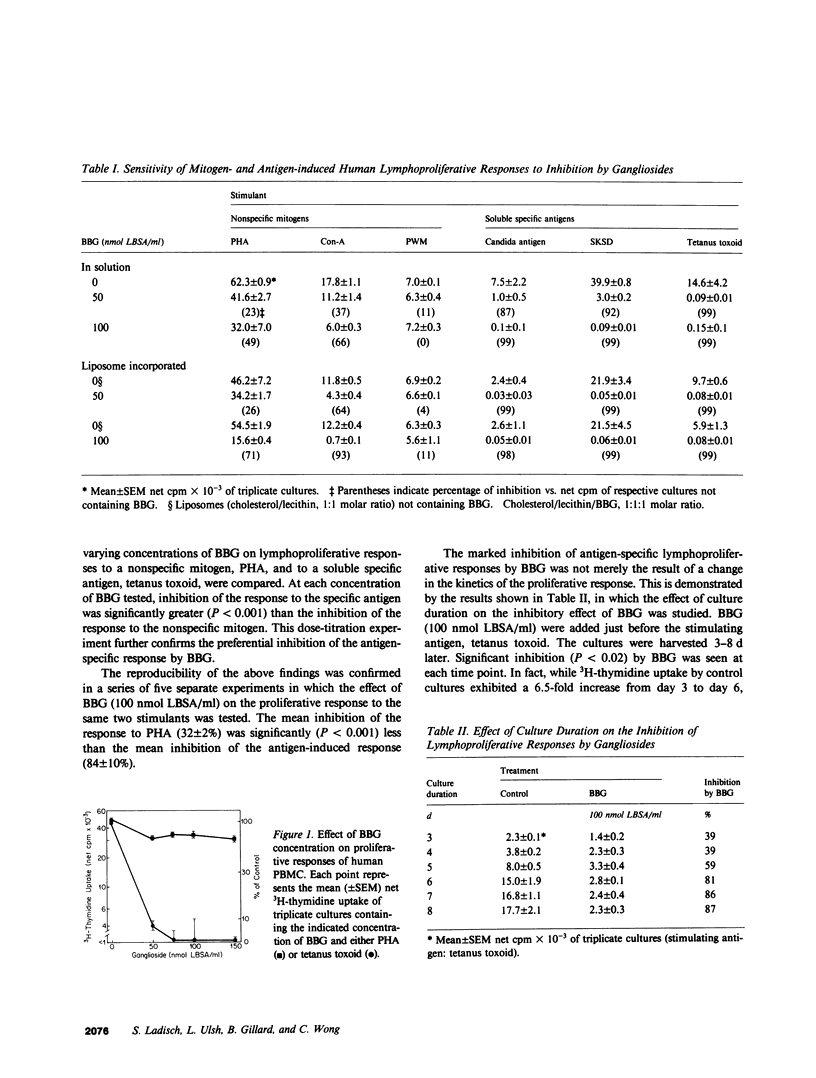
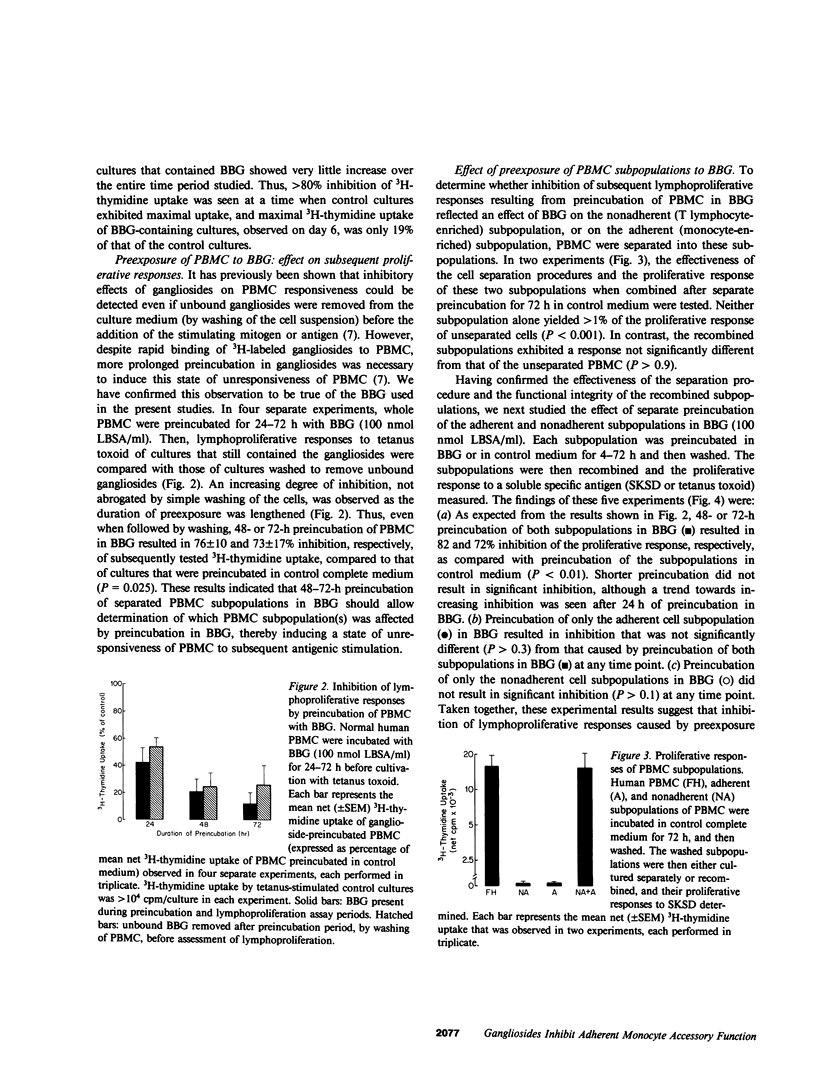
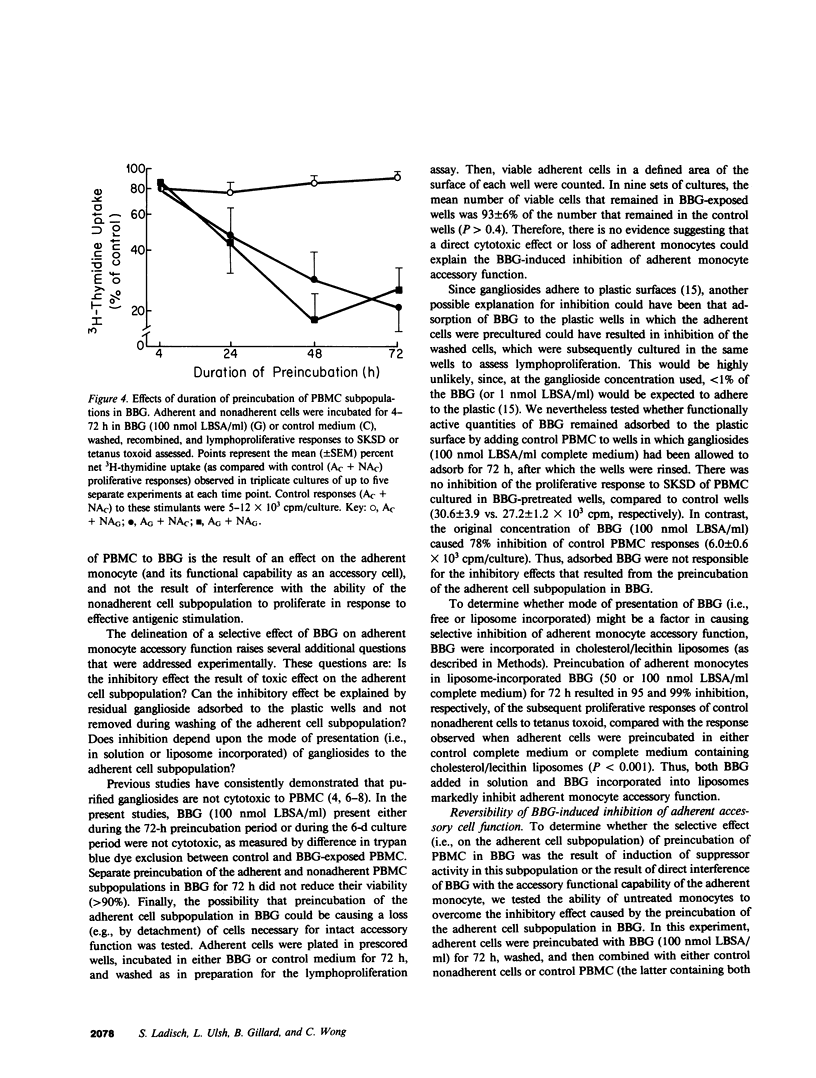
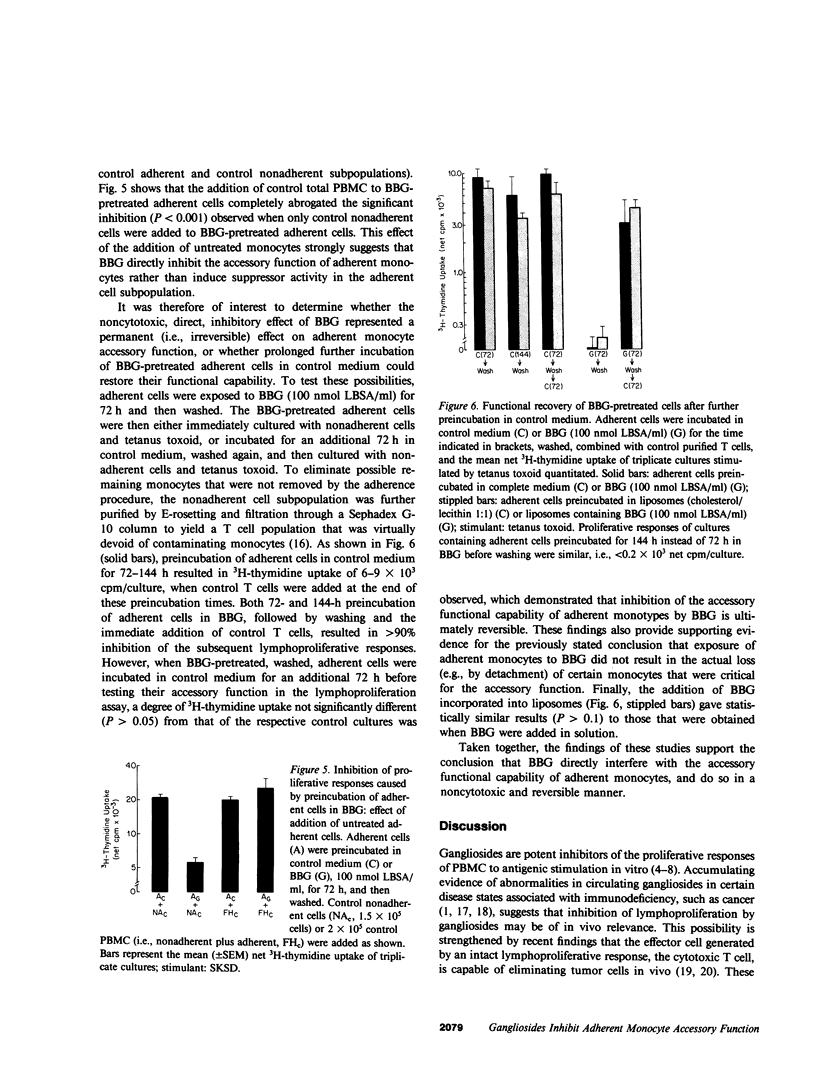
Gangliosides are potent inhibitors of lymphoproliferative responses. Selectively greater inhibitory effects of gangliosides on antigen-induced (vs. mitogen-induced) proliferation have been documented; e.g., 50 nmol of highly purified bovine brain gangliosides (BBG)/ml caused greater than or equal to 87% inhibition of proliferative responses of human peripheral blood mononuclear cells (PBMC) to three soluble specific antigens (Candida, streptokinase-streptodornase, and tetanus toxoid) vs. less than or equal to 37% inhibition of responses to three nonspecific mitogens (phytohemagglutinin, concanavalin A, and pokeweed mitogen). The possibility that BBG interfere with adherent monocyte accessory function, upon which responses to soluble specific antigens are strictly dependent, was therefore considered. PBMC were separated into the adherent and nonadherent subpopulations, exposed to BBG, recombined, and their proliferative responses were measured. Unseparated PBMC preincubated for 48-72 h with 100 nmol BBG/ml and then washed to remove unbound BBG exhibited 73-76% inhibition of subsequent antigen-induced lymphoproliferation. Separate pretreatment of both adherent and nonadherent cell subpopulations in BBG under the same conditions resulted in similar (72-82%) inhibition, which was reproduced by preincubation of only the adherent cells in BBG. Preincubation of only the nonadherent cells in BBG was not inhibitory. Inhibition (a) was independent of whether gangliosides were added in solution or incorporated into liposomes, (b) was abrogated by adding untreated monocytes to cultures containing adherent cells that were preexposed to BBG (excluding the possibility that BBG was inducing suppression mediated by adherent cells), and (c) was reversible by further incubation of BBG-pretreated adherent cells in control medium. Together, these results delineate a mechanism by which gangliosides modulate lymphoproliferative responses--direct, noncytotoxic, and ultimately reversible inhibition of the accessory function of adherent monocytes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Cline M. J., Swett V. C. The interaction of human monocytes and lymphocytes. J Exp Med. 1968 Dec 1;128(6):1309–1325. doi: 10.1084/jem.128.6.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engers H. D., Glasebrook A. L., Sorenson G. D. Allogeneic tumor rejection induced by the intravenous injection of Lyt-2+ cytolytic T lymphocyte clones. J Exp Med. 1982 Oct 1;156(4):1280–1285. doi: 10.1084/jem.156.4.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engers H. D., Sorenson G. D., Terres G., Horvath C., Brunner K. T. Functional activity in vivo of effector T cell populations. I. Antitumor activity exhibited by allogeneic mixed leukocyte culture cells. J Immunol. 1982 Sep;129(3):1292–1298. [PubMed] [Google Scholar]
- Hersh E. M., Harris J. E. Macrophage-lymphocyte interaction in the antigen-induced blastogenic response of human peripheral blood leukocytes. J Immunol. 1968 Jun;100(6):1184–1194. [PubMed] [Google Scholar]
- Holmgren J., Elwing H., Fredman P., Svennerholm L. Polystyrene-adsorbed gangliosides for investigation of the structure of the tetanus-toxin receptor. Eur J Biochem. 1980 May;106(2):371–379. doi: 10.1111/j.1432-1033.1980.tb04583.x. [DOI] [PubMed] [Google Scholar]
- Kloppel T. M., Keenan T. W., Freeman M. J., Morré D. J. Glycolipid-bound sialic acid in serum: increased levels in mice and humans bearing mammary carcinomas. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3011–3013. doi: 10.1073/pnas.74.7.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprowski H., Herlyn M., Steplewski Z., Sears H. F. Specific antigen in serum of patients with colon carcinoma. Science. 1981 Apr 3;212(4490):53–55. doi: 10.1126/science.6163212. [DOI] [PubMed] [Google Scholar]
- Krishnaraj R., Lin J., Kemp R. G. Lectin- and ionophore-stimulated Ca2+ influx in murine lymphocytes: inhibition by disialoganglioside. Cell Immunol. 1983 May;78(1):152–160. doi: 10.1016/0008-8749(83)90268-x. [DOI] [PubMed] [Google Scholar]
- Ladisch S., Gillard B., Wong C., Ulsh L. Shedding and immunoregulatory activity of YAC-1 lymphoma cell gangliosides. Cancer Res. 1983 Aug;43(8):3808–3813. [PubMed] [Google Scholar]
- Ladisch S., Ho W., Matheson D., Pilkington R., Hartman G. Immunologic and clinical effects of repeated blood exchange in familial erythrophagocytic lymphohistiocytosis. Blood. 1982 Oct;60(4):814–821. [PubMed] [Google Scholar]
- Lengle E. E. Increased levels of lipid-bound sialic acid in thymic lymphocytes and plasma from leukemic AKR/J mice. J Natl Cancer Inst. 1979 Jun;62(6):1565–1567. [PubMed] [Google Scholar]
- Lengle E. E., Krishnaraj R., Kemp R. G. Inhibition of the lectin-induced mitogenic response of thymocytes by glycolipids. Cancer Res. 1979 Mar;39(3):817–822. [PubMed] [Google Scholar]
- Miller H. C., Esselman W. J. Modulation of the immune response by antigen-reactive lymphocytes after cultivation with gangliosides. J Immunol. 1975 Sep;115(3):839–843. [PubMed] [Google Scholar]
- Portoukalian J., Zwingelstein G., Abdul-Malak N., Doré J. F. Alteration of gangliosides in plasma and red cells of humans bearing melanoma tumors. Biochem Biophys Res Commun. 1978 Dec 14;85(3):916–920. doi: 10.1016/0006-291x(78)90630-7. [DOI] [PubMed] [Google Scholar]
- SVENNERHOLM L. Quantitative estimation of sialic acids. II. A colorimetric resorcinol-hydrochloric acid method. Biochim Biophys Acta. 1957 Jun;24(3):604–611. doi: 10.1016/0006-3002(57)90254-8. [DOI] [PubMed] [Google Scholar]
- Scala G., Oppenheim J. J. Antigen presentation by human monocytes: evidence for stimulant processing and requirement for interleukin 1. J Immunol. 1983 Sep;131(3):1160–1166. [PubMed] [Google Scholar]
- Skipski V. P., Katopodis N., Prendergast J. S., Stock C. C. Gangliosides in blood serum of normal rats and Morris hepatoma 5123tc-bearing rats. Biochem Biophys Res Commun. 1975 Dec 1;67(3):1122–1127. doi: 10.1016/0006-291x(75)90790-1. [DOI] [PubMed] [Google Scholar]
- Whisler R. L., Yates A. J. Regulation of lymphocyte responses by human gangliosides. I. Characteristics of inhibitory effects and the induction of impaired activation. J Immunol. 1980 Nov;125(5):2106–2111. [PubMed] [Google Scholar]



