Abstract
Introduction
Reolysin®, a proprietary isolate of reovirus Type 3 Dearing, enters and preferentially induces apoptosis of malignant cells. RAS pathway activation has been associated with more efficient reoviral infectivity and enhanced oncolysis. Reovirus is currently in advanced solid tumor phase 1 – 2 trials; no clinical trials have been conducted in patients with hematologic malignancies.
Methodologies
A phase 1 trial treated 12 relapsed myeloma patients at two dose levels. Reolysin was infused daily for 5 days every 28 days. Bone marrow specimens were examined by In situ based hybridization (ISH) for CD138, p38, caspase-3, reoviral RNA and capsid protein at screening and cycle 1 day 8. Junctional adhesion molecule 1 (JAM-1) and cancer up regulated gene 2 (CUG2) were evaluated in patient samples and MM cell lines. Neutralizing Anti-Reovirus Antibody (NARA) assay was performed weekly during cycle 1.
Results
There were no dose limiting toxicities (DLTs), patients reached the 3 x 1010 TCID50 daily on days 1-5 dose level, and grade 3 laboratory toxicities included neutropenia, thrombocytopenia, and hypophosphatemia. In situ hybridization demonstrated reoviral genome confined in MM cells. Reoviral capsid protein and caspase-3 were rarely identified within reoviral RNA positive cells. The longest durations of stable disease were 4, 5 and 8 months.
Conclusions
Treatment with single-agent Reolysin was well tolerated and associated with avid reoviral RNA myeloma cell entry but only minimal intracellular reoviral protein production within MM cells. Our data support that in MM cells, Reolysin-induced oncolysis requires combination therapy, similar to other cancers.
Keywords: Reolysin, Reovirus, Multiple myeloma
INTRODUCTION
Reovirus Serotype 3 – Dearing Strain is a naturally occurring, ubiquitous, non-enveloped human reovirus with a genome that consists of 10 segments of double-stranded RNA(1). Community-acquired reovirus infection in humans is generally mild and limited to the upper respiratory and gastrointestinal tract. Reovirus has been shown to preferentially replicate in malignant cells with transformed RAS pathways (2-6) and mediates antitumor activity via induction of apoptosis, direct cytolysis, and activation of a tumor directed immune response. The specificity of reovirus for cancer cells makes Reolysin, its intravenous form, an attractive drug.
Early phase clinical trials in patients with metastatic solid tumors have shown that single-agent Reolysin is safe and well tolerated (7-9). No dose-limiting toxicities were experienced, and the most severe side effects (grade 3 or 4) included fatigue, flu-like symptoms, neutropenia, lymphopenia and hyponatremia. In these intravenous monotherapy trials, up to 45% of patients had evidence of at least stable disease, but only one patient in the 72 total enrolled achieved a partial response. Phase 1b clinical trials combined chemotherapeutic agents with Reolysin (e.g. gemcitabine, carboplatin, docetaxel and paclitaxel) in melanoma, head and neck, prostate, ovarian, non-small cell lung and pancreatic cancers (10-13). These trials confirmed the safety of Reolysin in combination with other agents and demonstrated an improvement in clinical response with the addition of a chemotherapeutic stressor (supplementary table 1). The mechanisms underlying this improved clinical efficacy are unclear, however, chemotherapy may enhance productive reoviral infection and thereby increase malignant cell apoptosis (14).
Preclinical evidence has demonstrated that Reolysin induces multiple myeloma (MM) cell oncolysis (15, 16) alone and in combination with the proteasome inhibitor (PI) bortezomib, and showed that the combination synergistically up regulated endoplasmic reticulum (ER) stress-related genes and pro-apoptotic genes associated with ER stress (BH3 containing pro-apoptotic bcl-2 family members, NOXA, BIM and PUMA). Thirukkamaran et al (17) showed that the autophagosome marker, LC3-II, was greatly increased following treatment with reovirus; results suggesting that reovirus promoted autophagic cell death via ER-stress induced activation of the Akt-mTOR signaling pathway. Collectively, these studies provided preclinical justification for the use of Reolysin in MM patients.
Reolysin has not previously been infused into patients with relapsed hematologic malignancies. We embarked on a phase 1 trial of Reolysin in relapsed MM patients to demonstrate safety and tolerability. Secondary objectives included response assessment, measurement of neutralizing anti-reoviral antibody (NARA) titers, and staining for productive reoviral infection within patient myeloma cells. In vitro and correlative analyses of MM cell lines and patient samples were undertaken to evaluate potential markers of MM cell sensitivity to reovirus.
MATERIALS AND METHODS
Tissue culture and materials
RPMI-8226 and NCI-H929 MM cell lines were obtained from American Type Cell Culture Collection (ATCC, Manassas, VA, USA). OPM2 cells were a kind gift from Michael Kuehl (NIH). MM cell lines were maintained in RPMI-1640 media supplemented with 10% fetal bovine serum in a humidified incubator 37 °C with 5% CO2. Reolysin used for preclinical studies were a gift from Dr. Matt Coffey (Oncolytics Biotech Inc.)
Immunohistochemistry
The antibody to reovirus capsid protein was a gift from Dr. Matt Coffey (Oncolytics Biotech Inc). The following antibodies were used in this study: antibody to reovirus capsid protein (compliments of Dr. Matt Coffey of Oncolytics Biotech, Inc.), caspase-3 (1:33, antigen retrieval, Abcam), p38 (1:250, antigen retrieval, Abcam), Junctional Adhesion Molecule 1 (JAM-1), and Cancer Upregulating Gene 2 (CUG2). The viral RNA in situ hybridization protocol has been previously published (14, 18, 19). In brief, after digestion in protease, the tissue and reoviral RNA probes (locked nucleic acid modified 5’ digoxigenin tagged, Exiqon) were co-incubated at 60°C for 5 minutes, then hybridized for 2 to 15 hours at 37°C. After a wash in 0.1xSSC and 2% bovine serum albumin at 50°C for 10 minutes, the reoviral RNA-probe complex was visualized via NBT/BCIP (Roche) due to the action of the alkaline phosphatase conjugation to antidigoxigenin antibody. Negative controls included myeloma cases not exposed to reovirus and omission of the probe; myeloma cell lines either infected or sham infected with reovirus served as additional controls.
Optimal detection of junctional adhesion molecule 1 (JAM-1) and CUG2 by immunohistochemistry was determined using the Leica Bond Max (dilution of 1:150 with pretreatment in antigen retrieval solution 2 for 30 minutes at 95°C). Positive controls included malignant cell lines with high sensitivity to reoviral infection. The CUG2 and JAM-1 antibodies were commercially obtained from Abcam.
Detection of neutralizing anti-reovirus antibodies (NARA)
Patient serum was collected at baseline and weekly for 3 weeks during the first cycle of treatment. Dilutions of patient serum were treated with a 1:1000 dilution dose of reovirus (Oncolytics; 2.53x1010 50% tissue culture infective dose (TCID50)/ml) known to cause 80% cell death of L929 mouse cells. The serum and virus mixture were co-incubated for 2 hours to allow any antibodies in the serum to neutralize the virus prior to culturing with L929 cells as previously described (20). Cell survival was measured by MTT assay (ATCC) after 72 hours. Goat serum (Lampire Biological Laboratory) was used as a positive control for the NARA assay. NARA endpoint titer was expressed as the last dilution where any neutralization occurred prior to reovirus-only treated L929 cells (20% survival). NARA titer assay post-test trend analysis was conducted utilizing GraphPad Prism 6 software.
Patients
The Ohio State University Cancer Institutional Review Board approved the phase 1 study, and informed consent was obtained from all enrolled patients (www.clinicaltrials.gov, NCT01533194). Patients with relapsed and refractory myeloma according to the International Myeloma Working Group (IMWG) diagnostic criteria for symptomatic myeloma were enrolled (21). Patients must have received prior lenalidomide and bortezomib therapy, progressed on or within 60 days of the most recent therapy, and had an Eastern Cooperative Oncology Group (ECOG) performance score < 2 or Karnofsky Performance Status > 60%. Prior autologous and allogeneic transplantation were permitted.
Patients were required to have measurable disease defined as serum monoclonal protein > 500 mg/dL, > 200 mg of monoclonal protein in a 24-hour urine sample, or serum immunoglobulin free light chain > 100 mg/L with an abnormal kappa to lambda free light chain ratio. Adequate organ and marrow function was required with absolute neutrophil count > 1000/μL, platelet count ≥ 50,000/μL, Total bilirubin < 1.5 mg/dL, and AST and ALT < 5x the institutional upper limit of normal. There was no serum creatinine requirement. Exclusion criteria included congestive heart failure with a LVEF < 50% at the time of screening.
Study Design
This was an open-label, dose-escalating, single-center phase 1 trial of Reolysin (serotype 3 – replication competent Dearing strain) in which Reolysin was provided by Oncolytics Biotech Inc. and distributed by the Pharmaceutical Management Branch of the National Cancer Institute.
Reolysin was administered intravenously over 60 minutes on days 1 – 5 every 28 days. Bone marrow biopsies were obtained at screening and cycle 1 day 8. Peripheral blood was obtained for correlative analyses on days 1, 8, 15, and between days 22 and 28 of cycle 1. Females of childbearing potential underwent pregnancy testing at the time of screening and patients with a history of congestive heart failure underwent evaluation of cardiac function with an echocardiogram or MUGA scan at screening. The study was designed to enroll an initial cohort of 3 patients at 3 x 109 TCID50/day, and assuming no dose limiting toxicities (DLTs), an additional 9 patients would be treated at 3 x 1010 TCID50/day. These dose levels were chosen based on tolerability in previous trials in patients with advanced solid tumors. Patients were scheduled to continue treatment for up to 12 cycles or until the time of disease progression or intolerance of therapy.
Dose-limiting toxicity
The Common Terminology Criteria for Adverse Events version 3.0 (CTCAE v. 3) was used to grade toxicities. A dose limiting toxicity (DLT) was defined as one of the following occurring during the first cycle of therapy that was determined to be possibly, probably and definitely related to single agent Reolysin: ANC <500/μL lasting 5 days or more or Grade 4 thrombocytopenia (platelet count <25,000/μL) with bleeding; LVEF < 50% in patients that underwent screening echocardiography or development of grade > 2 heart failure in any patient; any grade 3 – 4 non-hematologic adverse event, laboratory abnormalities not correctable within 24 hours, or nausea or vomiting that does not resolve to grade 2 or less within 24 hours and leads to missing > 1 day of study drug; or liver function abnormalities that do not resolve to < grade 1 within 5 days and lead to missing > 1 day of study drug. For grade 3 – 4 non-hematologic toxicities, grade 4 neutropenia or thrombocytopenia, or grade 2 cardiac toxicity, all remaining Reolysin doses in that cycle were held.
Response evaluation
Although response was not the primary endpoint of this trial, patients with measurable disease were assessed by standard IMWG Uniform response criterion (21) with assessment of myeloma proteins once monthly. If a patient had more than one monoclonal protein spike in the serum (or urine) then the monoclonal protein followed for assessing response was the larger one (relative to normal) meeting IMWG criteria for being measurable.
RESULTS
Patients
Twelve refractory patients were enrolled and demographics are summarized in table 1. Eight patients were male, 11 were Caucasian, the median age was 61 (range 48 - 77), and the median ISS stage at the time of enrollment was 2 (range 1 – 3). The median beta-2 microglobulin was 3.2 mg/L (range 1.9 – 28.9) and 5 patients had a baseline creatinine < 1.0 mg/dL. All patients had received prior treatment with lenalidomide and bortezomib. The median number of prior therapies at enrollment was 5 (range 1 – 10) and the median number of lines of therapy was 3.5 (range 1 – 9) as defined by the IMWG.
Table 1.
Patient demographics.
| ID | Age | Gender | Race | ISS Stage at screening | B2M | Cr | Revlimid exposed/refractory | Velcade exposed/refractory | Number of prior therapies | Number of prior lines of therapy | Cytogenetics at screening |
|---|---|---|---|---|---|---|---|---|---|---|---|
| DL1 | |||||||||||
| A | 61 | F | C | 1 | 2.8 | 0.9 | Y/N | Y/N | 9 | 6 | ND |
| B | 77 | M | C | 2 | 3.6 | 1.1 | Y/Y | Y/N | 7 | 7 | 5q+, 19p+, 11q22 tetrasomy |
| C | 48 | M | C | 2 | 2.6 | 1.1 | Y/N | Y/N | 5 | 2 | 13.q14-, 5q+, 19p+, 11q22 tetrasomy, 1q21+ |
| DL2 | |||||||||||
| D | 48 | F | C | 3 | 8.1 | 1.7 | Y/N | Y/N | 7 | 6 | 13.q14-, 13q34-, 1q21+, 1q23+ |
| E | 51 | F | C | 2 | 4.3 | 0.8 | Y/Y | Y/N | 9 | 8 | 17p13-, 13.q14-, 13q34- 1q21+, 1q23+, 1p36- |
| F | 67 | M | C | 1 | 2.6 | 0.9 | Y/Y | Y/N | 5 | 1 | 17p13+, 5q+, 19p+, 11q22+, 1q+, t(11;14) |
| G | 60 | M | C | 3 | 28.9 | 8.9 | Y/N | Y/Y | 10 | 9 | normal |
| H | 55 | F | C | 2 | 1.8 | 0.3 | Y/Y | Y/N | 3 | 1 | normal |
| I | 76 | M | C | 3 | 9.8 | 4.3 | Y/N | Y/N | 5 | 3 | normal |
| J | 48 | M | C | 1 | 1.9 | 0.8 | Y/Y | Y/N | 5 | 1 | 5q+, 19p+ |
| K | 61 | M | C | 2 | 5.3 | 1.9 | Y/N | Y/N | 5 | 4 | 13.q14-, 5q+, 19p+, 11q22+, 12p-, 1q21+, 1q23+, 1p36+ |
| L | 62 | M | AA | 1 | 2.4 | 1.2 | Y/Y | Y/Y | 5 | 3 | 19p+, 11q22+, 12p-, 1q21+, 1q23+, 1p36+* |
Patient demographics of all patients enrolled on the phase 1 single agent Reolysin trial. Patient factors included age, gender, and race; ISS stage, beta-2 microglobulin, cytogenetics and serum creatinine at the time of screening; assessment of prior revlimid and/or velcade exposure, and number of prior therapies and lines of therapy. Abbreviations: B2M = beta-2 microglobulin at screening, Cr = serum creatinine at screening; ISS = International Staging System; F = female, M = male; Y = yes, N = no; C = Caucasian, AA = African American; NA = not applicable, ND = not determined; DL1 = dose level 1 (3 × 109 TCID50/day), DL2 = dose level 2 (3 × 1010 TCID50/day).
Safety and Toxicities
Three patients were treated at dose level 3 x 109 TCID50 and 9 patients were treated at dose level 3 x 1010 TCID50. Treatment was well tolerated and no DLT's were experienced at either dose level. The top ten worst grade adverse events are listed in supplementary figure 1. Grade 3 toxicities were minimal and included 3 patients with neutropenia, 2 with leukopenia and one each with thrombocytopenia and hypophosphatemia. Grade 2 toxicities included leukopenia, anemia, neutropenia, thrombocytopenia and myalgias. Other grade 1 toxicities included increased aspartate aminotransferase and alkaline phosphatase, lymphopenia and nausea. All grade 2 and 3 toxicities occurred in patients treated at dose level 2. Five different patients had evidence of grade 2 and/or 3 hematologic toxicities reported as possibly or probably related to Reolysin. One patient had pancytopenia on day 4 of cycle 1. Resolution was evident by day 11 of cycle 1, and this patient had an associated increase of serum lambda free light chain from 1435 to 3000 mg/L over the course of cycle 1. A second patient had a decrease of the neutrophil and total white blood cell count evident on day 8 of cycle 1. These adverse events were associated with an initial 10% decrease of the m-protein, but the patient rapidly progressed and received only one cycle of therapy. A third patient had evidence of decreased neutrophils, total white blood cell count, and platelets on day 4 of cycle 1. All cytopenias resolved by day 23 of cycle 1 and were associated with a minimal decrease of the serum lambda free light chains during cycle 1. Ultimately, this patient received a total of 2 cycles of treatment. The fourth patient had stable disease over the course of 5 cycles of treatment and did not have evidence of cytopenias during cycle 1, but developed grade 3 decreases of the neutrophil and total white blood cell count during cycle 2. These toxicities were evident on day 4 of cycle 2, and resolved by the start of cycle 3 (see supplementary table 2).
Immunohistochemistry
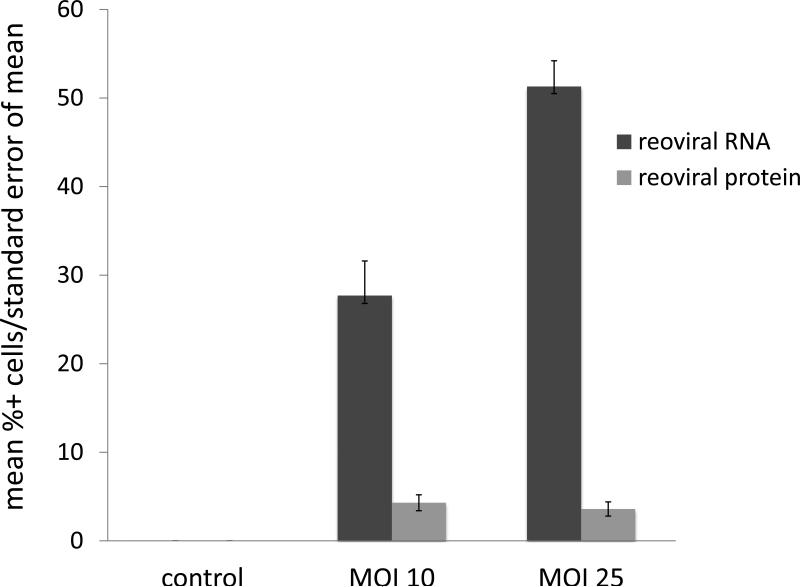
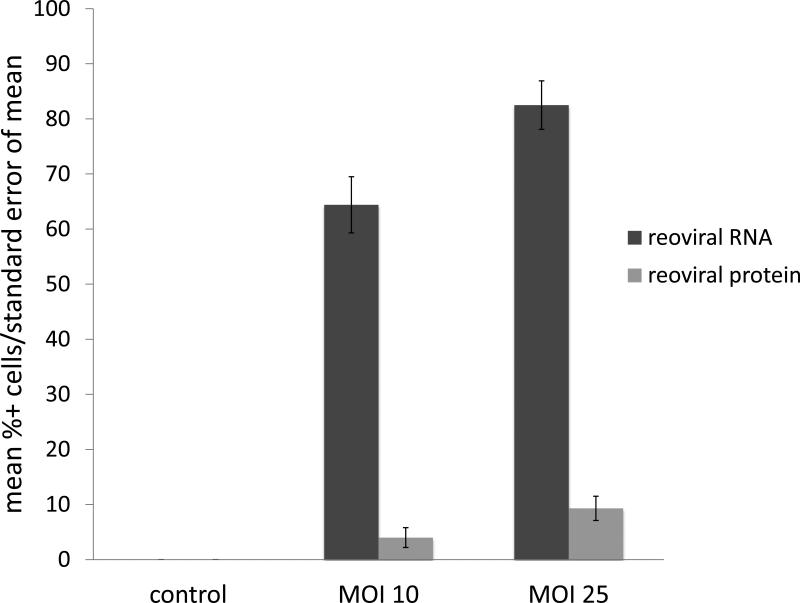
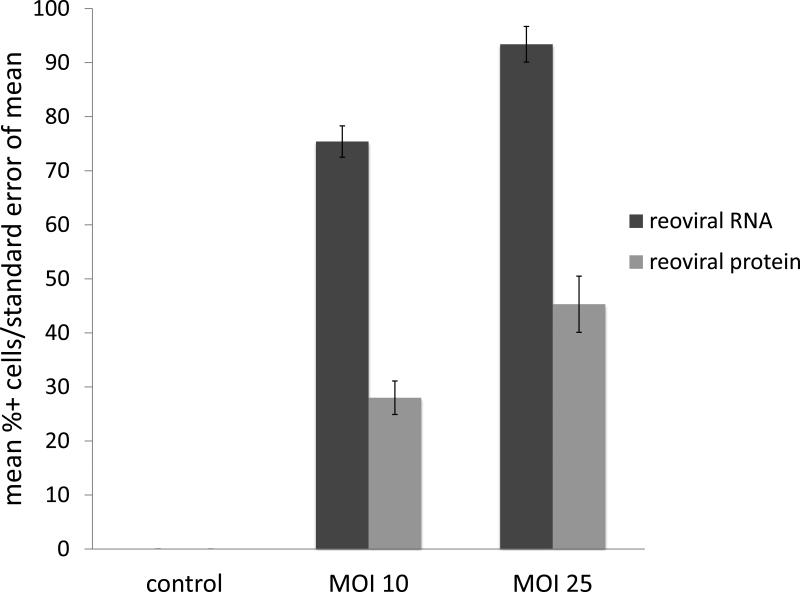
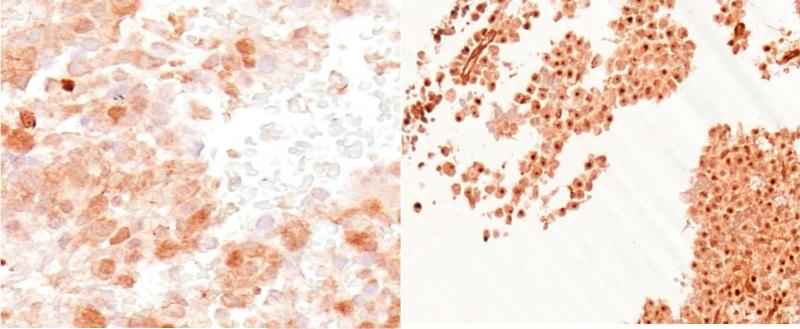
Immunohistochemical (IHC) analysis of sensitive (RPMI-8226), intermediately resistant (H929), and resistant (OPM2) MM cell lines indicated two primary findings; 1) reoviral RNA was present in all cell lines and increased with higher multiplicities of infection (MOI), and 2) viral capsid protein was evident in all cell lines, but to a greater degree in sensitive cells treated at higher MOI (figures 1a – 1c). Further, these findings indicate that resistance to reovirus may be defined by a lack of viral replication or viral capsid protein expression. Immunohistochemical analysis was preferred to real-time quantitative polymerase chain reaction (qRT-PCR) in this study for three primary reasons: 1) IHC specifically identified active viral RNA and/or protein inside of the MM cells, whereas qRT-PCR would be unable to differentiate the location of viral RNA, whether it be on the cell surface or inside the cell, 2) in-situ hybridization (ISH) methods allowed for co-expression analyses, such that we were able to correlate the detection of reovirus based on cell phenotype (e.g. CD138+), and 3) ISH allowed, in part, to determine the relative degree of viral genome and/or proliferation in infected cells.
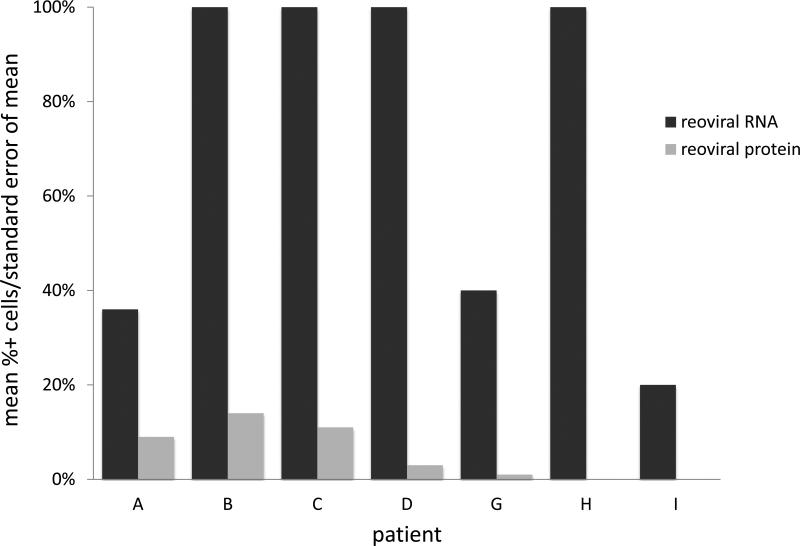
Figure 1. Reoviral RNA and capsid protein expression.
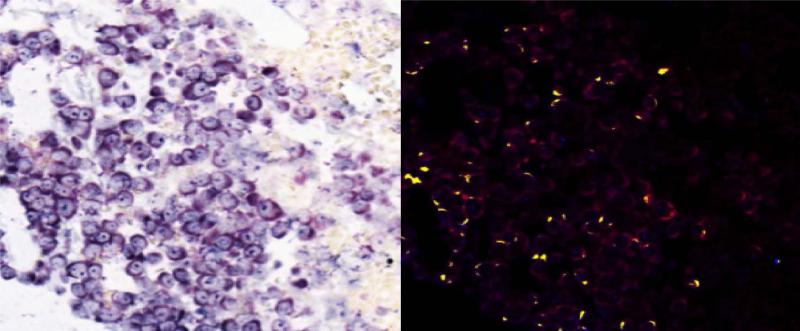
Reoviral RNA was present at multiplicity of infection (MOI) 10 and 25 in resistant (OPM2), intermediately resistant (H929), and sensitive (RPMI-8226) MM cell lines (1a – 1c). Viral RNA and protein increased with increased MOI. Reoviral protein was highest in the sensitive MM cell line RPMI-8226. Seven patients had adequate tissue present after Reolysin treatment for analysis of reoviral RNA and capsid protein expression (1d). Each value represents the percentage of CD138+ cells that were found to have co-localization of reoviral RNA or protein and CD138+. Results indicate that viral RNA was present in a much higher percentage of CD138+ cells than reoviral protein, suggesting that viral entry was present but replication was minimal.
Seven patients had adequate tissue samples for both pre- and post-treatment laboratory evaluation (table 2). Reoviral RNA was found in post-Reolysin bone marrow biopsies in all 7 evaluable patients, and viral protein was found in 5 of the 6 evaluable patient samples. Results confirmed reoviral cell entry as evidenced by positive viral RNA staining in 20 - 100% of myeloma cells (figure 1d). There was no association (p = 0.22) between the percentage of myeloma cells positive for viral RNA and the number of cycles of therapy patients received. Reoviral RNA localized to myeloma cells (figure 2a - 2b). Of the evaluable patient samples, reoviral capsid protein expression and caspase-3 staining was minimal, indicating non-productive infection and limited induction of apoptosis (table 2). Importantly, caspase-3 expression paralleled reoviral capsid protein expression. Expression of p38 was used as an indicator of RAS pathway activation and MM cell permissiveness to reoviral infection (22). Of the evaluable patient samples, p38 expression prior to treatment was evident in a mean of 72% (range 19 – 99%) of myeloma cells (defined by CD138 co-expression), and was 94% and 99% in patients experiencing stable disease for 8 and 4 cycles of treatment, respectively.
Table 2.
Immunohistochemistry
| Patient ID | Reovirus RNA | Reovirus protein | p38 | Caspase-3 | Cycle of treatment |
|---|---|---|---|---|---|
| A | 36% | 9% | 94% | 1% | 8 |
| B | 100% | 14% | 78% | ND | 2 |
| C | 100% | 11% | 98% | 0.3% | 1 |
| D | 100% | 3% | 19% | 1% | 1 |
| G | 40% | 1% | 45% | 0% | 1 |
| H | 100% | 0% | 99% | ND | 4 |
| I | 20% | ND | ND | ND | 5 |
Immunohistochemical analysis of 7 evaluable patient samples; co-expression analyses included CD138+, reoviral RNA, capsid protein, p38, and caspase-3. Abbreviations: Reovirus RNA = reoviral RNA/CD138+ cells post-treatment; Reovirus protein = reoviral protein/CD138+ cells post-treatment; p38/CD138+ = p38 pre-treatment cells; caspase-3 = caspase-3 post-treatment.
Figure 2. Co-expression analyses with immunohistochemistry.
Co-expression analysis for Reoviral RNA (Locked Nuclear Acid in situ hybridization) and CD138 were completed on pre- (2a) and post-treatment (2b) patient samples. The Nuance system was used to convert Reoviral RNA to blue, CD138 to red, and co-expression is yellow. Reoviral RNA positive cells are primarily CD138+ cells indicating that reovirus selectively enters myeloma cells.
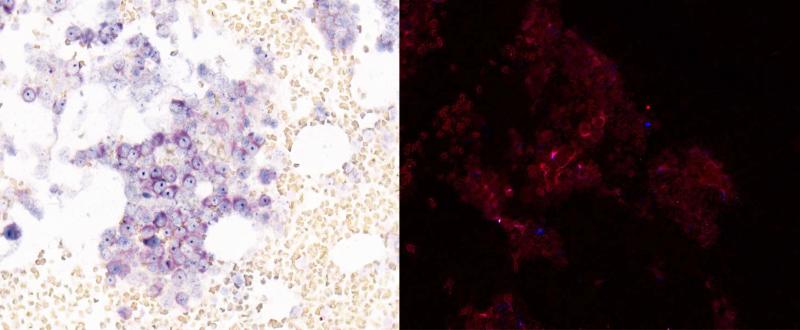
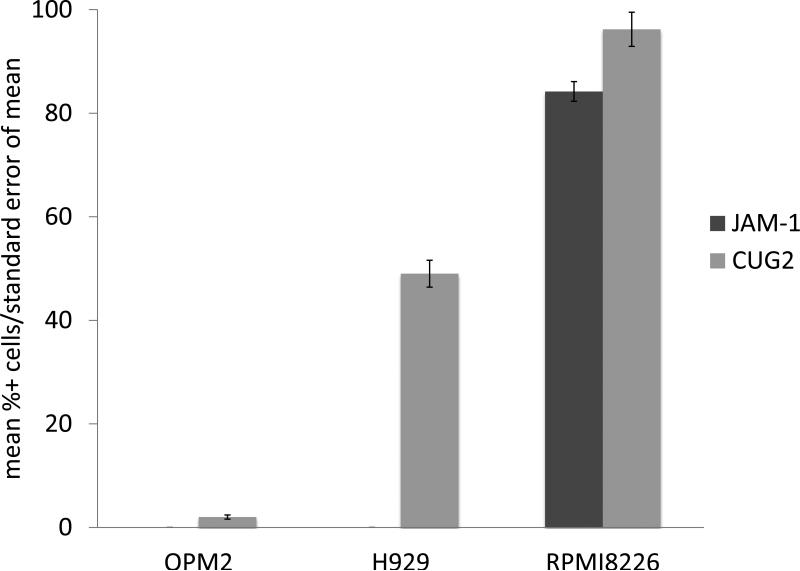
JAM-1 mediates clathrin dependent viral internalization and CUG2 has been shown to inhibit the phosphorylation of PKR, so we analyzed their respective roles in reoviral permissiveness in MM cells. Immunohistochemical analysis of JAM-1 and CUG2 in sensitive (RPMI-8226), intermediately resistant (H929), and resistant (OPM2) MM cell lines indicated that JAM-1 was expressed in sensitive cell lines only while CUG2 was expressed in all cell lines (figure 3a). CUG2 expression was lowest in resistant cell lines and highest in sensitive cell lines. These findings highlighted the possibility that JAM-1 and CUG2 may be markers of reovirus sensitivity in MM cells.
Figure 3. Co-expression analyses of JAM-1 and CUG2.
JAM-1 and CUG2 expression were analyzed in resistant (OPM2), intermediately resistant (H929), and sensitive (RPMI-8226) MM cell lines at MOI 25 (3a). JAM-1 was present in the sensitive cell line only. CUG2 expression was higher in sensitive MM cell lines. Immunohistochemical analysis of CUG2 (3b) utilizing Leica Bond Max shows a representative CUG2 negative (left) sample, and a CUG2 positive patient sample (right). CUG2 expression analysis in two melanoma cell lines (DO4 and CHL) shows that CUG2 expression increases in both cell lines with the addition of paclitaxel (3c).
Patient samples were analyzed for co-expression of JAM-1/CD138 and CUG2/CD138. Low-null JAM-1 expression was evident in all evaluable patient samples, suggesting that decreased tropism of the virus for MM cells is associated with decreased viral efficiency in vivo. CUG2 was evident in two of the evaluable patient samples. One patient had evidence of CUG2 expression in the majority of MM cells pre- and post-treatment (figure 3b). This patient progressed after 2 cycles of treatment and had relatively high p38 expression (98%) on pretreatment sample analysis. Analysis of pre- and post-treatment samples from the patient experiencing the longest duration of stable disease indicated only scattered CUG2 expression. Pretreatment analysis revealed that this patient also had relatively high p38 expression (94%).
NARA response
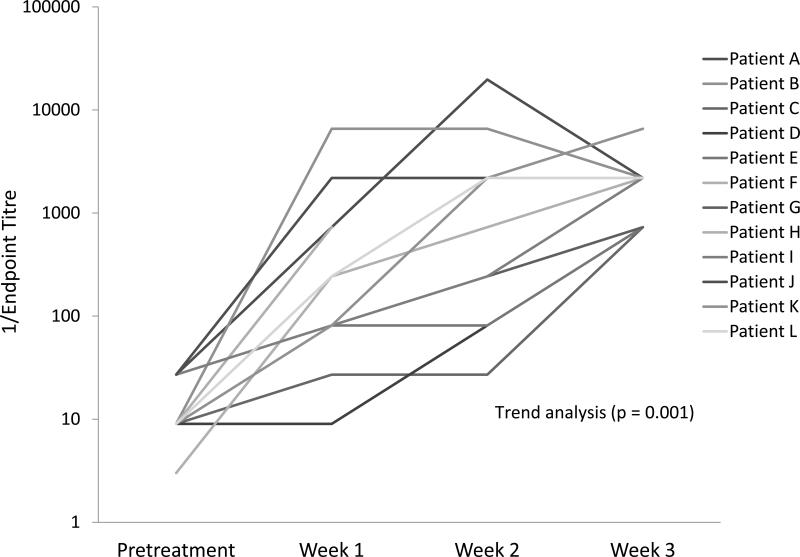
Evaluation of the anti-Reolysin immunologic response revealed that all patients had NARA titers in their serum post-treatment. These data indicate that each patient developed neutralizing antibodies against reovirus (figure 4). Trend analysis indicated a significant increase (p = 0.001) in NARA titers from pretreatment to week 3. Evaluating the 3 patients with the longest duration of stable disease (4, 5, and 8 months) suggested higher week 1 and 2 endpoint titers in the patient receiving 8 cycles of treatment. This data suggests that NARA antibodies may be indicative of a beneficial antitumor immune response mediated by the activation of a cross-reactive T cell population.
Figure 4. NARA titers – pretreatment through week 3.
NARA response pre-treatment and post-treatment weeks 1 – 3 in the 12 enrolled patients. Trend analysis indicates a significant increase in NARA titers over time (p = 0.001).
Response Assessment
All twelve enrolled patients were eligible for response assessment. A total of 6 patients (50%) received more than one cycle of treatment, the median cycles of therapy was 1.5, and the best response was stable disease. One patient received 3 x 109 TCID50 and had minimal evidence of apoptosis (caspase-3) on the post-treatment bone marrow biopsy. This patient completed 8 cycles of treatment and experienced stable disease for 8 months. Significantly, this patient was also noted to have a progressive increase of NARA and a concomitant decrease in the monoclonal protein during cycle 1. Two other patients receiving 3 x 1010 TCID50 had stable disease and were each treated for 4 and 5 cycles, both had stable monoclonal proteins during cycle 1, but caspase-3 staining was not determined on post-treatment bone marrow samples. During cycle 1, a total of 5 patients had decreased myeloma proteins (range 6 – 22%, mean 14%), 3 had minimal increases (6, 7, and 12%), and the remaining 4 had evidence of grossly progressive disease.
DISCUSSION
The purpose of this study was to establish the safety and tolerability of Reolysin in patients with relapsed or refractory multiple myeloma. We have successfully shown that two dose levels of Reolysin (3 x 109 TCID50/day and 3 x 1010 TCID50/day) were well tolerated. No DLTs were experienced and grade 3 toxicities were limited to neutropenia, leukopenia, thrombocytopenia and hypophosphatemia.
Single-agent Reolysin was not associated with any objective responses in relapsed and refractory MM patients. Six of the 12 (50%) patients in this study received more than one cycle of therapy and 3 patients received 4, 5 and 8 cycles of treatment. The longest duration of stable disease was 8 months. This patient was treated with the 3 x 109 TCID50/day dose, had evidence of reoviral capsid protein expression in CD138+ cells, apoptosis on the post-treatment bone marrow biopsy (table 2), and a 13% decrease in the monoclonal protein during cycle 1.
We propose that the lack of objective clinical response in our population resulted from a combination of potential factors, including 1) an inability to overcome inherent viral resistance for productive cytolysis, 2) limited production of a viral mediated antitumor immune response, and/or 3) inadequate viral dosing.
As seen in our patient population (figure 1d), and supported by our in vitro analyses of sensitive, intermediately resistant, and resistant MM cell lines (figure 1a – 1c), the presence of intracellular viral genome but not reoviral capsid protein suggested resistance to active viral proliferation. Of the myriad of factors that could mediate this resistance, we evaluated the roles of cell entry and RAS pathway activation.
Preclinical models suggest that reoviral cell entry is initiated by the engagement of the outer viral capsid filamentous attachment protein σ1 with JAM-1 and a cell-surface carbohydrate. Once attached, ß1-integrin mediated viral internalization occurs via clathrin dependent endocytosis. Following internalization, acid-dependent cysteine proteases in the endocytic compartment remove the outer capsid protein σ3, exposing a viral membrane-penetration protein that is responsible for piercing the endosomal membrane and delivering transcriptionally active virus into the cytoplasm (23).
Whether these fundamental principles accurately define the in vivo mechanisms of cell entry and viral proliferation in MM cells is unknown. To elucidate the importance of JAM-1, we conducted in vitro analyses of sensitive, intermediately resistant, and resistant cell lines, and correlative analyses of patient samples. In vitro analyses confirmed that JAM-1 expression was positively associated with MM cell sensitivity (figure 3a). However, immunohistochemical analysis of patient samples revealed that JAM-1 was not expressed on myeloma cells. Considering that viral RNA and not protein was evident in patient samples, we propose that reovirus may have passively, albeit selectively, entered the myeloma cells by bypassing JAM-1. This hypothesis assumes that reovirus binding to the sialic acid and JAM-1 receptors is necessary for viral uncoating and subsequent replication. Future strategies employing therapeutics that up regulate JAM-1 will likely elucidate whether active clathrin dependent reoviral cell entry is necessary for viral proliferation, viral mediated apoptosis, and subsequent clinical benefit.
Reovirus has been shown to preferentially replicate and induce apoptosis in cells with a constitutively activated RAS pathway (22), potentially via epidermal growth factor receptor (EGFR), N- and/or K-RAS mutations. In MM, the proportion of RAS mutations increases with disease progression, and 46 - 81% of patients with relapsed disease have been found to have N- or K-RAS mutations (25, 26). Recently, a study evaluating the prevalence of RAS mutations in MM patient samples from 133 patients demonstrated N- and K-RAS mutations in similar proportions, and patients with N-RAS mutated tumors, in comparison to those with wild-type tumors, were less sensitive to bortezomib (27). The mechanisms underlying this decreased bortezomib sensitivity of N-RAS mutated tumors require further investigation. Regardless, these findings are instrumental in confirming the equivocal prevalence of N- and K-RAS mutations in MM.
The role of a constitutively activated RAS pathway in MM patients treated with Reolysin was explored in our trial. Numerous studies suggest that reovirus inhibits double-stranded RNA-activated protein kinase (PKR) autophosphorylation in RAS mutated cells, an effect that permits viral replication to occur (2-6). Others suggest that RAS transformation enhances numerous steps in the viral life cycle, including proteolytic disassembly, degree of infectivity, and apoptosis mediated viral release (24). We evaluated p38 mitogen-activated protein kinase (MAPK) as a marker of RAS-pathway activation in our relapsed population (22) and found no obvious association between p38, length of disease stability, or the amount of viral protein present in malignant cells (table 2). These findings suggest that measurement of p38 is not a marker for reoviral sensitivity, intracellular replicative activity, or efficacy in MM patients.
Cancer up regulated gene 2 (CUG2) is a novel oncogene that is overexpressed in numerous malignancies. Like RAS mutations, CUG2 expression is associated with inactivation of PKR and activation of the RAS/p38 MAPK signaling pathway (28). We evaluated the role of CUG2 in vitro and in patient samples. Preclinical analysis of sensitive, intermediately resistant, and resistant MM and melanoma cell lines indicated that CUG2 was more highly expressed in sensitive cell lines (figure 3a). In these models, increased viral load and concomitant exposure to chemotherapy increased CUG2 expression, viral proliferation, and apoptosis (figure 3c). These results suggest that in vitro, CUG2 expression enhances reovirus sensitivity and cytolysis. Analysis of patient samples revealed that two of the 7 evaluable patients had evidence of CUG2 expression. One patient had significant pre- and post-treatment CUG2 expression in MM cells (figure 3b), viral genome in 100% of cells, elevated pretreatment p38 expression (98%), minimal reovirus capsid protein and apoptotic activity, and received 2 cycles of treatment (patient C). In the other patient, CUG2 expression was scattered, 36% of cells contained viral genome, pretreatment p38 expression was high (94%), reoviral capsid protein and apoptosis were minimal, and the patient received 8 cycles of treatment (patient A). Collectively, these results suggest that CUG2 expression may be an indicator of RAS/p38 pathway activation and entry of the virus into MM cells, but not productive infection or clinical response.
NK and T-cell mediated killing has a prominent role in reovirus mediated anti-MM activity (33, 34). Dendritic cells (DC) have the ability to directly respond to reoviral RNA via pattern recognition receptors (PRR) in a viral replication independent manner (29). DC activation by reovirus induces the up regulation of numerous pro-inflammatory cytokines including IFN that function, in part, to increase NK cell activation and recruitment. Extensive cross talk between DCs and NK cells via IFN subsequently acts to generate a tumor reactive cytotoxic T-cell response; one that is likely strengthened by production of viral- and NK cell-mediated tumor associated antigens (TAAs) (33). Numerous patients in our trial had experienced stable disease that was not associated with significant viral proliferation or apoptosis. This finding suggests that disease stability may have resulted from immune mediated cytotoxicity.
Virus clearance may be mediated by neutralizing anti-reovirus antibodies (NARA), but the degree to which its activity limits reovirus-mediated anti-MM activity is unknown. Evidence from the REO 005 trial indicates that antitumor activity persisted despite a 250-fold median increase in NARA titers (20), likely because the virus “hitchhikes” on peripheral blood mononuclear cells (PBMCs) and platelets (35). In our trial, a significant increase in NARA titers was evident over the first cycle of treatment (figure 4). Though our findings are not conclusive, it is unlikely that NARA is able to clear a significant proportion of infused reovirus to preclude antitumor activity in myeloma patients, and in fact, may be instrumental in activating a beneficial antitumor immune response.
Use of viral oncolytics as cytolytic therapy is gaining momentum. Recently, Russel et al. (2014) reported on 2 patients with refractory MM that were treated with 1011 TCID50 MV-NIS, an engineered measles virus (36). Initial infusion resulted in hypotension, tachycardia and high fever in both patients, but each responded to treatment and one achieved complete remission lasting for over 9 months. In fact, as this was a dose escalation trial starting at 106 TCID50, no objective responses were noted until the administration of 1011 TCID50, results suggesting that treatment at or near the MTD is necessary to achieve objective responses. Reolysin as monotherapy or combined with chemotherapy have not reached the MTD with individual doses up to 3 x 1010 TCID50 for 5 consecutive days with cycles repeated every 21 – 28 days (total cycle dose of 1.5 x 1012). Currently, the NCIC Clinical Trials Group is conducting a 4-arm phase 2 trial (www.clinicaltrials.gov - NCT01708993) and this trial is the first to evaluate higher individual doses of Reolysin at 4.5 x 1010 TCID50, days 1 – 3 every 3 weeks, total cycle dose 1.35 x 1011 TCID50. Considering the dose-response relationship evident with other viral oncolytics (37-40), higher individual doses of Reolysin may be necessary to induce clinical benefit in MM patients.
Clearly, further study will be necessary to define a role for Reolysin treatment in MM. Our trial, however, shows that Reolysin is well tolerated and associated with disease stability, though the mechanisms underlying this activity remain unclear. Importantly, our study lays the groundwork for future clinical trials that will further examine the role for Reolysin in treatment refractory MM patients, either as a single agent or in combination.
Supplementary Material
Statement of translational relevance.
Reolysin is associated with clinical efficacy in patients with advanced solid tumors when combined with chemotherapy. This article describes the first use of single agent Reolysin in hematologic malignancy; a phase I trial in relapsed multiple myeloma (MM) patients. Single-agent Reolysin was well tolerated and associated with stable disease in 50% of patients, the longest durations of which was 4, 5 and 8 months. Reoviral RNA and protein were found in bone marrow aspirates cycle 1 day 8 but not at baseline. Ample intracellular reoviral RNA was evident, but reoviral capsid protein expression and caspase-3 staining were minimal, indicating non-lytic infection. Future clinical trials combining Reolysin with a cellular stressor such as a proteasome inhibitor are needed to determine if this combination will induce clinically relevant myeloma cell death.
Acknowledgements
Research reported in this publication was supported by the generous contributions of the Multiple Myeloma Opportunities for Research and Education (MMORE). This research is also supported by the National Cancer Institute of the National Institutes of Health under Award Number U01CA076576 (PI Michael Grever). DWS is supported under Award Number T32CA165998 (PI Miguel Villalona and Steven Devine). The content is solely the responsibility of the authors and does not represent the official views of the National Cancer Institute or the National Institutes of Health.
GJN and GBL receive research funding from Oncolytics Biotech Inc. All other authors are without relevant conflicts of interest. The authors confirm that neither the submitted manuscript nor any similar manuscript, in whole or in part, other than an abstract, is under consideration, in press, or published elsewhere.
Authorship roles: CCH designed and wrote the protocol, enrolled the patients, analyzed the data, wrote the manuscript. DWS analyzed the data, wrote the manuscript. GN, TM, GL analyzed the data. All the authors have contributed substantially to the analysis and interpretation of data, revising the manuscript for important content, and final approval.
References
- 1.Tyler KL, Fields BN. Reoviruses. In: Fields BN, Knipe DM, Chanock RM, et al., editors. Virology. Second Edition. New York: Raven Press, Ltd.. 1990. pp. 1307–28. [Google Scholar]
- 2.Bodkin DK, Nibert ML, Fields BN. Proteolytic digestion of reovirus in the intestinal lumens of neonatal mice. J Virol. 1989;63:4676–81. doi: 10.1128/jvi.63.11.4676-4681.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosen L, Evans HE, Spickard A. Reovirus infections in human volunteers. Am J Hyg. 1963;77:29–37. doi: 10.1093/oxfordjournals.aje.a120293. [DOI] [PubMed] [Google Scholar]
- 4.Rubin DH, Kornstein MJ, Anderson AO. Reovirus serotype 1 intestinal infection: a novel replicative cycle with ileal disease. J Virol. 1985;53:391–8. doi: 10.1128/jvi.53.2.391-398.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strong JE, Coffey MC, Tang D, Sabinin P, Lee PW. The molecular basis of viral oncolysis: usurpation of the Ras signaling pathway by reovirus. EMBO J. 1998;17:3351–62. doi: 10.1093/emboj/17.12.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolf JL, Kauffman RS, Finberg R, Dambrauskas R, Fields BN, Trier JS. Determinants of reovirus interaction with the intestinal M cells and absorptive cells of murine intestine. Gastroenterology. 1983;85:291–300. [PubMed] [Google Scholar]
- 7.Galanis E, Markovic SN, Suman VJ, Nuovo GJ, Vile RG, Kottke TJ, et al. Phase II trial of intravenous administration of Reolysin((R)) (Reovirus Serotype-3-dearing Strain) in patients with metastatic melanoma. Molecular therapy : the journal of the American Society of Gene Therapy. 2012;20:1998–2003. doi: 10.1038/mt.2012.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gollamudi R, Ghalib MH, Desai KK, Chaudhary I, Wong B, Einstein M, et al. Intravenous administration of Reolysin, a live replication competent RNA virus is safe in patients with advanced solid tumors. Investigational new drugs. 2010;28:641–9. doi: 10.1007/s10637-009-9279-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vidal L, Pandha HS, Yap TA, White CL, Twigger K, Vile RG, et al. A phase I study of intravenous oncolytic reovirus type 3 Dearing in patients with advanced cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14:7127–37. doi: 10.1158/1078-0432.CCR-08-0524. [DOI] [PubMed] [Google Scholar]
- 10.Comins C, Spicer J, Protheroe A, Roulstone V, Twigger K, White CM, et al. REO-10: a phase I study of intravenous reovirus and docetaxel in patients with advanced cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16:5564–72. doi: 10.1158/1078-0432.CCR-10-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harrington KJ, Karapanagiotou EM, Roulstone V, Twigger KR, White CL, Vidal L, et al. Two-stage phase I dose-escalation study of intratumoral reovirus type 3 dearing and palliative radiotherapy in patients with advanced cancers. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16:3067–77. doi: 10.1158/1078-0432.CCR-10-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karapanagiotou EM, Roulstone V, Twigger K, Ball M, Tanay M, Nutting C, et al. Phase I/II trial of carboplatin and paclitaxel chemotherapy in combination with intravenous oncolytic reovirus in patients with advanced malignancies. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:2080–9. doi: 10.1158/1078-0432.CCR-11-2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lolkema MP, Arkenau HT, Harrington K, Roxburgh P, Morrison R, Roulstone V, et al. A phase I study of the combination of intravenous reovirus type 3 Dearing and gemcitabine in patients with advanced cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:581–8. doi: 10.1158/1078-0432.CCR-10-2159. [DOI] [PubMed] [Google Scholar]
- 14.Nuovo GJ, Garofalo M, Valeri N, Roulstone V, Volinia S, Cohn DE, et al. Reovirus-associated reduction of microRNA-let-7d is related to the increased apoptotic death of cancer cells in clinical samples. Mod Pathol. 2012;25:1333–44. doi: 10.1038/modpathol.2012.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelly KR, Espitia CM, Mahalingam D, Oyajobi BO, Coffey M, Giles FJ, et al. Reovirus therapy stimulates endoplasmic reticular stress, NOXA induction, and augments bortezomib-mediated apoptosis in multiple myeloma. Oncogene. 2012;31:3023–38. doi: 10.1038/onc.2011.478. [DOI] [PubMed] [Google Scholar]
- 16.Thirukkumaran CM, Shi ZQ, Neri P, Bahlis N, Morris D. Proceedings of the 105th Annual Meeting of the American Association of Cancer Research. AACR; San Diego, CA Philadelphia (PA): Apr 5-9, 2014. Reovirus synergy with proteosome inhibitor carfilzomib and Akt inhibitor perifisone overcomes therapy resistance of multiple myeloma: promising preclinical activity [abstract]. 2014 Abstract nr 1709. [Google Scholar]
- 17.Thirukkumaran CM, Shi ZQ, Luider J, Kopciuk K, Gao H, Bahlis N, et al. Reovirus modulates autophagy during oncolysis of multiple myeloma. Autophagy. 2013;9:413–4. doi: 10.4161/auto.22867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nuovo GJ. In situ detection of microRNAs in paraffin embedded, formalin fixed tissues and the co-localization of their putative targets. Methods. 2010;52:307–15. doi: 10.1016/j.ymeth.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 19.Nuovo GJ, Elton TS, Nana-Sinkam P, Volinia S, Croce CM, Schmittgen TD. A methodology for the combined in situ analyses of the precursor and mature forms of microRNAs and correlation with their putative targets. Nature protocols. 2009;4:107–15. doi: 10.1038/nprot.2008.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White C, Twigger K, Vidal L, De Bono J, Coffey M, Heinemann L, et al. Characterization of the adaptive and innate immune response to intravenous oncolytic reovirus (Dearing type 3) during a phase I clinical trial. Gene therapy. 2008;15:911–20. doi: 10.1038/gt.2008.21. [DOI] [PubMed] [Google Scholar]
- 21.Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K, et al. International uniform response criteria for multiple myeloma. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2006;20:1467–73. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 22.Norman KL, Hirasawa K, Yang A-D, Shields MA, Lee PW. Reovirus oncolysis: the Ras/RalGEF/p38 pathway dictates host cell permissiveness to reovirus infection. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:11099–104. doi: 10.1073/pnas.0404310101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Danthi P, Holm G, Stehle T, Dermody T. Reovirus Receptors, Cell Entry, and Proapoptotic Signaling. In: Pöhlmann S, Simmons G, editors. Viral Entry into Host Cells. Springer; New York: 2013. pp. 42–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marcato P, Shmulevitz M, Pan D, Stoltz D, Lee PW. Ras transformation mediates reovirus oncolysis by enhancing virus uncoating, particle infectivity, and apoptosis-dependent release. Molecular Therapy. 2007;15:1522–30. doi: 10.1038/sj.mt.6300179. [DOI] [PubMed] [Google Scholar]
- 25.Bezieau S, Devilder M-C, Avet-Loiseau H, Mellerin M-P, Puthier D, Pennarun E, et al. High incidence of N and K-Ras activating mutations in multiple myeloma and primary plasma cell leukemia at diagnosis. Human mutation. 2001;18:212–24. doi: 10.1002/humu.1177. [DOI] [PubMed] [Google Scholar]
- 26.Mulligan G, Lichter DI, Di Bacco A, Blakemore SJ, Berger A, Koenig E, et al. Mutation of NRAS but not KRAS significantly reduces myeloma sensitivity to single-agent bortezomib therapy. Blood. 2014;123:632–9. doi: 10.1182/blood-2013-05-504340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mulligan G, Lichter DI, Di Bacco A, Blakemore SJ, Berger A, Koenig E, et al. Mutation of NRAS but not KRAS significantly reduces myeloma sensitivity to single-agent bortezomib therapy. 2014 doi: 10.1182/blood-2013-05-504340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park EH, Park EH, Cho IR, Srisuttee R, Min HJ, Oh MJ, et al. CUG2, a novel oncogene confers reoviral replication through Ras and p38 signaling pathway. Cancer gene therapy. 2010;17:307–14. doi: 10.1038/cgt.2009.83. [DOI] [PubMed] [Google Scholar]
- 29.Errington F, Steele L, Prestwich R, Harrington KJ, Pandha HS, Vidal L, et al. Reovirus activates human dendritic cells to promote innate antitumor immunity. The Journal of Immunology. 2008;180:6018–26. doi: 10.4049/jimmunol.180.9.6018. [DOI] [PubMed] [Google Scholar]
- 30.Errington F, White C, Twigger K, Rose A, Scott K, Steele L, et al. Inflammatory tumour cell killing by oncolytic reovirus for the treatment of melanoma. Gene therapy. 2008;15:1257–70. doi: 10.1038/gt.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gujar SA, Marcato P, Pan D, Lee PW. Reovirus virotherapy overrides tumor antigen presentation evasion and promotes protective antitumor immunity. Molecular cancer therapeutics. 2010;9:2924–33. doi: 10.1158/1535-7163.MCT-10-0590. [DOI] [PubMed] [Google Scholar]
- 32.Steele L, Errington F, Prestwich R, Ilett E, Harrington K, Pandha H, et al. Pro-inflammatory cytokine/chemokine production by reovirus treated melanoma cells is PKR/NF-? B mediated and supports innate and adaptive anti-tumour immune priming. Molecular cancer. 2011;10:20. doi: 10.1186/1476-4598-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prestwich RJ, Ilett EJ, Errington F, Diaz RM, Steele LP, Kottke T, et al. Immune-mediated antitumor activity of reovirus is required for therapy and is independent of direct viral oncolysis and replication. Clinical Cancer Research. 2009;15:4374–81. doi: 10.1158/1078-0432.CCR-09-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prestwich RJ, Errington F, Steele LP, Ilett EJ, Morgan RS, Harrington KJ, et al. Reciprocal human dendritic cell-natural killer cell interactions induce antitumor activity following tumor cell infection by oncolytic reovirus. The Journal of Immunology. 2009;183:4312–21. doi: 10.4049/jimmunol.0901074. [DOI] [PubMed] [Google Scholar]
- 35.Adair RA, Roulstone V, Scott KJ, Morgan R, Nuovo GJ, Fuller M, et al. Cell carriage, delivery, and selective replication of an oncolytic virus in tumor in patients. Science translational medicine. 2012;4:138ra77–ra77. doi: 10.1126/scitranslmed.3003578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Russell SJ, Federspiel MJ, Peng K-W, Tong C, Dingli D, Morice WG, et al. Mayo Clinic Proceedings. Elsevier; 2014. Remission of Disseminated Cancer After Systemic Oncolytic Virotherapy. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bailey K, Kirk A, Naik S, Nace R, Steele MB, Suksanpaisan L, et al. Mathematical model for radial expansion and conflation of intratumoral infectious centers predicts curative oncolytic virotherapy parameters. PloS one. 2013;8:e73759. doi: 10.1371/journal.pone.0073759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berry LJ, Au GG, Barry RD, Shafren DR. Potent oncolytic activity of human enteroviruses against human prostate cancer. The Prostate. 2008;68:577–87. doi: 10.1002/pros.20741. [DOI] [PubMed] [Google Scholar]
- 39.Breitbach CJ, Burke J, Jonker D, Stephenson J, Haas AR, Chow LQ, et al. Intravenous delivery of a multi-mechanistic cancer-targeted oncolytic poxvirus in humans. Nature. 2011;477:99–102. doi: 10.1038/nature10358. [DOI] [PubMed] [Google Scholar]
- 40.Myers RM, Greiner SM, Harvey ME, Griesmann G, Kuffel MJ, Buhrow SA, et al. Preclinical pharmacology and toxicology of intravenous MV-NIS, an oncolytic measles virus administered with or without cyclophosphamide. Clinical pharmacology and therapeutics. 2007;82:700–10. doi: 10.1038/sj.clpt.6100409. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.