Abstract
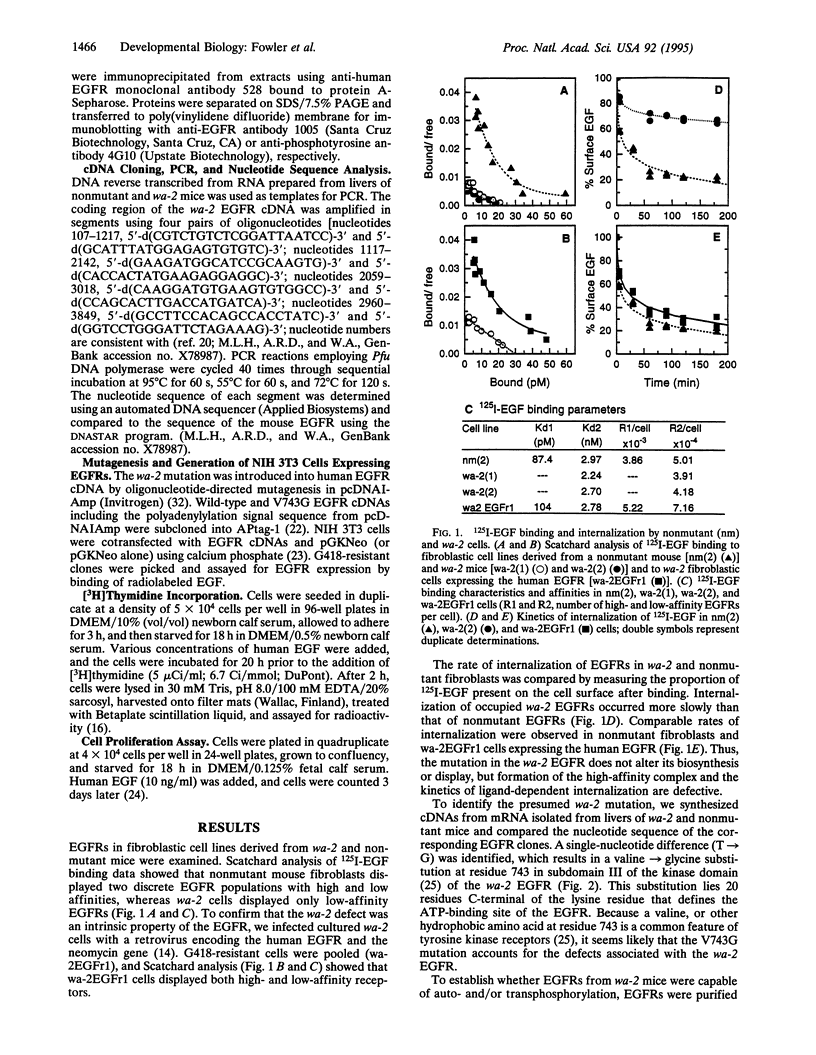
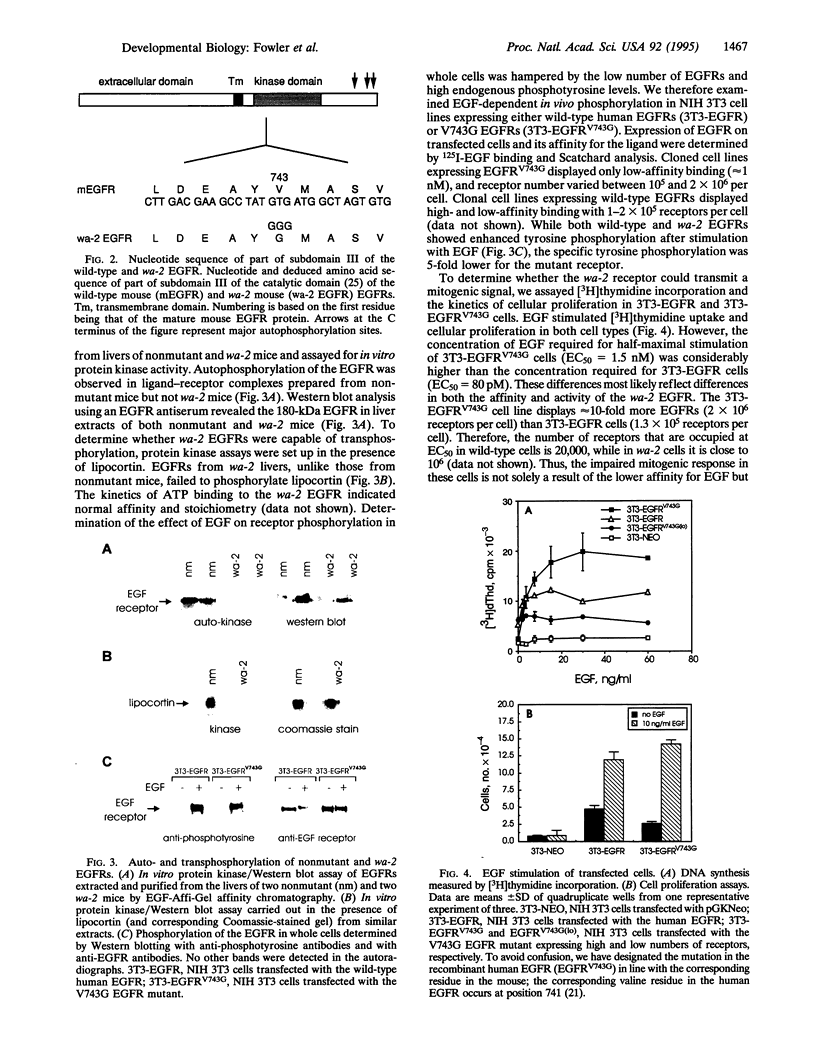
The mutant mouse waved-2 (wa-2) is strikingly similar to transforming growth factor alpha-deficient mice generated by gene targeting in embryonic stem cells. We confirm that wa-2 is a point mutation (T-->G resulting in a valine-->glycine substitution at residue 743) in the gene encoding the epidermal growth factor (EGF) receptor. wa-2 fibroblastic cells lack high-affinity binding sites for EGF, and the rate of internalization of EGF is retarded. Although the tyrosine kinase activity of wa-2 EGF receptors is significantly impaired, NIH 3T3 cells lacking endogenous EGF receptors but overexpressing recombinant wa-2 EGF receptor cDNA are mitogenically responsive to EGF. While young and adult wa-2 mice are healthy and fertile, 35% of wa-2 mice born of homozygous wa-2 mothers die of malnutrition because of impaired maternal lactation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson E. D., Rees A. R. Epidermal growth factor receptors. Mol Cell Biochem. 1981 Feb 11;34(3):129–152. doi: 10.1007/BF02359619. [DOI] [PubMed] [Google Scholar]
- Avivi A., Lax I., Ullrich A., Schlessinger J., Givol D., Morse B. Comparison of EGF receptor sequences as a guide to study the ligand binding site. Oncogene. 1991 Apr;6(4):673–676. [PubMed] [Google Scholar]
- COHEN S. Isolation of a mouse submaxillary gland protein accelerating incisor eruption and eyelid opening in the new-born animal. J Biol Chem. 1962 May;237:1555–1562. [PubMed] [Google Scholar]
- Cohen S., Ushiro H., Stoscheck C., Chinkers M. A native 170,000 epidermal growth factor receptor-kinase complex from shed plasma membrane vesicles. J Biol Chem. 1982 Feb 10;257(3):1523–1531. [PubMed] [Google Scholar]
- Flanagan J. G., Leder P. The kit ligand: a cell surface molecule altered in steel mutant fibroblasts. Cell. 1990 Oct 5;63(1):185–194. doi: 10.1016/0092-8674(90)90299-t. [DOI] [PubMed] [Google Scholar]
- Foster C. S., Smith C. A., Dinsdale E. A., Monaghan P., Neville A. M. Human mammary gland morphogenesis in vitro: the growth and differentiation of normal breast epithelium in collagen gel cultures defined by electron microscopy, monoclonal antibodies, and autoradiography. Dev Biol. 1983 Mar;96(1):197–216. doi: 10.1016/0012-1606(83)90323-8. [DOI] [PubMed] [Google Scholar]
- Fowler K. J. Storage of skin biopsies at -70 degrees C for future fibroblast culture. J Clin Pathol. 1984 Oct;37(10):1191–1193. doi: 10.1136/jcp.37.10.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Haigler H. T., McKanna J. A., Cohen S. Direct visualization of the binding and internalization of a ferritin conjugate of epidermal growth factor in human carcinoma cells A-431. J Cell Biol. 1979 May;81(2):382–395. doi: 10.1083/jcb.81.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luetteke N. C., Phillips H. K., Qiu T. H., Copeland N. G., Earp H. S., Jenkins N. A., Lee D. C. The mouse waved-2 phenotype results from a point mutation in the EGF receptor tyrosine kinase. Genes Dev. 1994 Feb 15;8(4):399–413. doi: 10.1101/gad.8.4.399. [DOI] [PubMed] [Google Scholar]
- Luetteke N. C., Qiu T. H., Peiffer R. L., Oliver P., Smithies O., Lee D. C. TGF alpha deficiency results in hair follicle and eye abnormalities in targeted and waved-1 mice. Cell. 1993 Apr 23;73(2):263–278. doi: 10.1016/0092-8674(93)90228-i. [DOI] [PubMed] [Google Scholar]
- Mann G. B., Fowler K. J., Gabriel A., Nice E. C., Williams R. L., Dunn A. R. Mice with a null mutation of the TGF alpha gene have abnormal skin architecture, wavy hair, and curly whiskers and often develop corneal inflammation. Cell. 1993 Apr 23;73(2):249–261. doi: 10.1016/0092-8674(93)90227-h. [DOI] [PubMed] [Google Scholar]
- Marquardt H., Hunkapiller M. W., Hood L. E., Todaro G. J. Rat transforming growth factor type 1: structure and relation to epidermal growth factor. Science. 1984 Mar 9;223(4640):1079–1082. doi: 10.1126/science.6320373. [DOI] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Okamoto S., Oka T. Evidence for physiological function of epidermal growth factor: pregestational sialoadenectomy of mice decreases milk production and increases offspring mortality during lactation period. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6059–6063. doi: 10.1073/pnas.81.19.6059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paria B. C., Dey S. K. Preimplantation embryo development in vitro: cooperative interactions among embryos and role of growth factors. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4756–4760. doi: 10.1073/pnas.87.12.4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paria B. C., Tsukamura H., Dey S. K. Epidermal growth factor-specific protein tyrosine phosphorylation in preimplantation embryo development. Biol Reprod. 1991 Nov;45(5):711–718. doi: 10.1095/biolreprod45.5.711. [DOI] [PubMed] [Google Scholar]
- Quian X. L., Decker S. J., Greene M. I. p185c-neu and epidermal growth factor receptor associate into a structure composed of activated kinases. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1330–1334. doi: 10.1073/pnas.89.4.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose S. P., Pruss R. M., Herschman H. R. Initiation of 3T3 fibroblast cell division by epidermal growth factor. J Cell Physiol. 1975 Dec;86 (Suppl 2)(3 Pt 2):593–598. doi: 10.1002/jcp.1040860504. [DOI] [PubMed] [Google Scholar]
- Salomon D. S., Kidwell W. R., Kim N., Ciardiello F., Bates S. E., Valverius E., Lippman M. E., Dickson R. B., Stampfer M. Modulation by estrogen and growth factors of transforming growth factor-alpha and epidermal growth factor receptor expression in normal and malignant human mammary epithelial cells. Recent Results Cancer Res. 1989;113:57–69. doi: 10.1007/978-3-642-83638-1_8. [DOI] [PubMed] [Google Scholar]
- Selva E., Raden D. L., Davis R. J. Mitogen-activated protein kinase stimulation by a tyrosine kinase-negative epidermal growth factor receptor. J Biol Chem. 1993 Jan 25;268(3):2250–2254. [PubMed] [Google Scholar]
- Spivak-Kroizman T., Rotin D., Pinchasi D., Ullrich A., Schlessinger J., Lax I. Heterodimerization of c-erbB2 with different epidermal growth factor receptor mutants elicits stimulatory or inhibitory responses. J Biol Chem. 1992 Apr 25;267(12):8056–8063. [PubMed] [Google Scholar]
- Ullrich A., Coussens L., Hayflick J. S., Dull T. J., Gray A., Tam A. W., Lee J., Yarden Y., Libermann T. A., Schlessinger J. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. 1984 May 31-Jun 6Nature. 309(5967):418–425. doi: 10.1038/309418a0. [DOI] [PubMed] [Google Scholar]
- Walker F., Nice E., Fabri L., Moy F. J., Liu J. F., Wu R., Scheraga H. A., Burgess A. W. Resistance to receptor-mediated degradation of a murine epidermal growth factor analogue (EGF-Val-47) potentiates its mitogenic activity. Biochemistry. 1990 Nov 27;29(47):10635–10640. doi: 10.1021/bi00499a009. [DOI] [PubMed] [Google Scholar]
- Wiley L. M., Wu J. X., Harari I., Adamson E. D. Epidermal growth factor receptor mRNA and protein increase after the four-cell preimplantation stage in murine development. Dev Biol. 1992 Feb;149(2):247–260. doi: 10.1016/0012-1606(92)90282-l. [DOI] [PubMed] [Google Scholar]
- Zabel B. U., Fournier R. E., Lalley P. A., Naylor S. L., Sakaguchi A. Y. Cellular homologs of the avian erythroblastosis virus erb-A and erb-B genes are syntenic in mouse but asyntenic in man. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4874–4878. doi: 10.1073/pnas.81.15.4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Rüden T., Wagner E. F. Expression of functional human EGF receptor on murine bone marrow cells. EMBO J. 1988 Sep;7(9):2749–2756. doi: 10.1002/j.1460-2075.1988.tb03129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]






