Abstract
Background
A significant minority of patients receiving cardiac resynchronization therapy (CRT) remain non-responsive to this intervention.
Objective
To determine whether coronary sinus (CS) or baseline peripheral venous (PV) levels of established and emerging heart failure (HF) biomarkers are predictive of CRT outcomes.
Methods
In 73 patients (age 68±12; 83% male; ejection fraction 27 7%) with CS and PV blood drawn simultaneously at the time of CRT implantation, we measured amino-terminal pro-B type natriuretic peptide (NT-proBNP), galectin-3 (gal-3), and soluble (s)ST2 levels. NT-proBNP concentrations>2000 pg/mL, gal-3>25.9 ng/mL, and sST2>35 ng/mL were considered positive, based on established PV cutpoints for identifying “high risk” individuals with HF. CRT response was adjudicated by HF Clinical Composite Score. Major adverse cardiovascular event (MACE) was defined as the composite endpoint of death, cardiac transplant, left ventricular assist device, and HF hospitalization at 2 years.
Results
NT-proBNP concentrations were 20% higher in the CS than periphery, while gal-3 and sST2 were 10% higher in periphery than CS (all p<0.001). There were 45% CRT non-responders at 6 months and 22% MACE. Triple positive CS values yielded the highest specificity of 95% for predicting CRT non-response. Consistently, CS strategies identified patients at higher risk for developing MACE, with over 11-fold adjusted increase for triple positive CS patients compared to triple negative patients (all p≤0.04). PV strategies were not predictive of MACE.
Conclusions
Our findings suggest that coronary sinus sampling of HF biomarkers may be better than peripheral venous blood levels for predicting CRT outcomes. Larger studies are needed to confirm our findings.
Keywords: Biomarker, Coronary sinus, galectin-3, soluble ST2, Cardiac resynchronization therapy
INTRODUCTION
Heart failure (HF) is a leading cause of morbidity and mortality in the US with 50% mortality at 5 years.1 Several candidate HF biomarkers, including the established amino-terminal pro-B type natriuretic peptide (NT-proBNP) and emerging markers of galectin-3 (gal-3) and soluble (s)ST2, have been used in a multi-marker strategy for the assessment of patients with dyspnea and in patients with acute HF for predicting mortality using peripheral venous (PV) samples.2–4
Cardiac resynchronization therapy (CRT) is a device therapy that exerts considerable benefit,5–9 but where approximately one-third of patients are non-responders despite optimal selection and adjustment of pacing parameters.10, 11 Thus, prognostication of these HF patients that would benefit from this effective but nonetheless costly therapy is desirable to provide patients and caregivers with realistic expectations.
There is however a paucity of data examining the role of biomarkers obtained via coronary sinus (CS) blood sampling on CRT response. Of note, the CS can be easily sampled during implantation of the left ventricular pacing lead within the coronary venous tree. In this study of CRT patients, we examined the differences in the CS and PV levels of three HF biomarkers (NT-proBNP, gal-3, and sST2) and evaluated their diagnostic accuracy for predicting CRT non-response and prognostic value for predicting major adverse cardiovascular events (MACE) individually and in multi-marker strategies.
METHODS
Study Population and Protocol
“Biomarkers to Predict CRT Response in Patients With HF” (BIOCRT; Clinical Trials.gov # NCT01949246) is a prospective observational study consisting of New York Heart Association (NYHA) Functional Class II-IV patients undergoing CRT device implantation from a single tertiary hospital, whom blood from the CS and PV were drawn during the time of device implantation. Inclusion and exclusion criteria are detailed in Table 1. We included 73 participants with baseline matched CS and PV samples of all three candidate biomarkers (NT-proBNP, gal-3, and sST2) drawn during CRT implantation between December 2007 and July 2012.
Table 1.
Inclusion and exclusion criteria for BIOCRT study.
Inclusion Criteria
|
Exclusion Criteria
|
CRT denotes cardiac resynchronization therapy; CRT-D, cardiac resynchronization therapy-defibrillator; NYHA, New York Heart Association; EF, ejection fraction; ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker.
Before device implantation, baseline evaluation included medical history, NYHA Class, 12-lead electrocardiography (ECG), and 2D transthoracic echocardiography (Echo) for measurement of LV volumes and diameters, ejection fraction (EF) using the modified biplane Simpson's technique. During device implantation, invasive coronary venography was used to guide left ventricular pacing lead placement. After CRT implantation, study participants return for regular clinic visits at 1, 3, and 6 months and were followed for events through a time horizon of up to 2 years. Echo-guided optimization of the CRT devices was uniformly performed on all patients at 1 month. The Social Security Death Index was performed between April 11, 2013 and May 3, 2013 to search for date of death. Our institutional review board approved the study protocol, and all patients provided written informed consent.
Blood Collection and Storage
At the time of device implantation baseline CS blood was drawn from the CS guiding catheter prior to delivering the CRT lead, and simultaneously PV blood was drawn from one of the upper extremity veins. Blood was collected into tubes containing ethylenediaminetetraacetic acid (EDTA) and tubes without anticoagulant. Samples were immediately centrifuged, and the aliquoted plasma and serum were stored in microcentrifuge tubes at −80 °C until assayed. Anonymized specimens were sent to independent laboratories (Siemens Healthcare Diagnostics Inc., BG Medicine, and Critical Diagnostics) for analysis. In addition to being blinded to the clinical history, the laboratories were blinded to the knowledge of whether the samples were CS or PV. All analyses were performed on a first freeze thaw cycle.
Blood Samples
Serum NT-proBNP measurements (Dimension Vista Flex, Siemens) were performed by a one-step sandwich chemiluminescent immunoassay, with interassay coefficients of variation (CV) of <3% and intraassay CV of <4%. We defined positive levels of NT-proBNP as >2000 pg/mL, as it is a typical median concentration for patients with NYHA Class II-IV HF.12 Plasma gal-3 measurements (BGM Galectin-3, BG Medicine, Inc.) were performed by enzyme linked immunosorbent assay (ELISA), with interassay CV of 2.2%, and intraassay CV of 3.0%. Positive gal-3 was defined as >25.9 ng/mL, based on the manufacturer’s definition of high-risk and as associated with progression of HF.13 Plasma sST2 measurements (Presage® ST2 assay, Critical Diagnostics) were performed using highly sensitive ELISA, with interassay CV of <12% and intraassay CV of 2.3%. Positive sST2 was defined as >35 ng/mL, which is linked to higher risk for events in HF patients.14
End Points
For the definition of a positive response to CRT, patients were classified according to the HF Clinical Composite Score (CCS).15 Responders were defined as those with improved CCS from baseline to 6 month follow-up. Those not meeting this criterion were considered non-responders. MACE was defined as the composite endpoint of death, cardiac transplant, left ventricular assist device (LVAD), and HF hospitalization within 2 years. An outcome panel consisting of two cardiologists, blinded to the biomarker results, determined the clinical response of each subject based on review of the medical record, with disagreement resolved by consensus with a third cardiologist.
Statistical analysis
Descriptive statistics were expressed as mean ± standard deviation (SD) or median with interquartile range for continuous variables and as frequency and percentages for nominal variables, as appropriate. We used Spearman correlation to show the strength in correlation between the CS and PV samples as well as correlation between biomarker concentrations and clinical parameters. We used the Wilcoxon signed rank test to examine the differences between the transcardiac gradients of CS and PV samples. We used Wilcoxon rank-sum test to compare the median biomarker concentrations of two groups. For diagnostic accuracy for the binary biomarker results and CRT non-response, we calculated the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV). Comparisons between sensitivities of two tests and specificities of two tests were performed using McNemar’s test. Cumulative event rates stratified by biomarker results individually or in a multimarker strategy were estimated using the product limit (Kaplan-Meier) methods and compared using the stratified log-rank test. Unadjusted and adjusted Cox proportional hazards models were used to evaluate the association of the biomarkers and MACE. A 2-tailed p-value of <0.05 was considered to indicate statistical significance. All analyses were performed using SAS (Version 9.2, North Carolina).
RESULTS
Patient Characteristics
Table 2 details the baseline patient characteristics for the 73 patients in BIOCRT with simultaneous CS and PV bloods drawn during CRT implantation. These study participants are predominantly older men, with multiple co-morbidities such as diabetes and hypertension. Over half had ischemic cardiomyopathy, left bundle branch block (LBBB), and a prior device (either pacemaker or defibrillator). The majority of subjects were NYHA Class III and were treated with multiple HF medications typical for usual HF care. Notably, mean QRS duration was 168 ms and EF was 27%.
Table 2.
Baseline characteristics of study participants.
| Patient characteristics | |
|---|---|
| Demographics | |
| Age, years | 68±12 |
| Male | 61(84%) |
| BMI, kg/m2 | 28.8±6.1 |
| Diabetes | 25(34%) |
| Hypertension | 54(74%) |
| Ischemic cardiomyopathy | 39(53%) |
| History of atrial fibrillation | 34(47%) |
| Device | 38(52%) |
| PPM | 15(21%) |
| ICD | 26(36%) |
| NYHA | |
| I | 0(0%) |
| II | 9(12%) |
| III | 61(84%) |
| IV | 3(4%) |
| Medications | |
| ACEI/ARB | 57(78%) |
| BB | 64(88%) |
| Spironolactone | 16(22%) |
| Diuretics | 55(75%) |
| ECG parameters | |
| QRS duration, ms | 168±27 |
| LBBB | 39(53%) |
| Paced rhythm | 16(22%) |
| Echocardiography parameters | |
| LVEF | 27±7% |
| LV dimensions, mm | |
| End-diastole (EDD) | 62±9 |
| End-systole (ESD) | 53±10 |
| LV volumes, cm3 | |
| End-diastole (EDV) | 226±73 |
| End-systole (ESV) | 163±60 |
| Laboratory and Biomarker Results | |
| Cr, mg/dL | 1.3±0.4 |
| eGFR, (mL/min/1.73 m2) | 61.0 20.6 |
| NT-proBNP, pg/mL | |
| CS | 1938[761,3353] |
| PV | 1512[693,2786] |
| Transcardiac gradient ( CS-PV) | 237[38,555] |
| Transcardiac ratio (CS/PV) | 1.2[1.1,1.4] |
| gal-3, ng/ml | |
| CS | 16.7[12.5,21.0] |
| PV | 18.1[14.0,23.0] |
| Transcardiac gradient ( CS-PV) | −1.5[−3.9,0.5] |
| Transcardiac ratio (CS/PV) | 0.9[0.8,1.0] |
| sST2, ng/mL | |
| CS | 36.7[24.8,58.8] |
| PV | 41.9[29.4,58.7] |
| Transcardiac gradient (CS-PV) | −4.2 −9.9,1.3] |
| Transcardiac ratio (CS/PV) | 0.9[0.8,1.0] |
Abbreviations as in Table 1. BMI denotes body mass index; CM, cardiomyopathy; AF, atrial fibrillation; PPM, permanent pacemaker; ICD, implantable cardioverter defibrillator; ARB, aldosterone receptor blocker; BB, beta blocker; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; Cr, creatinine; eGFR, estimated glomerular filtration rate; NT-proBNP, amino-terminal pro-B type natriuretic peptide; CS, coronary sinus; PV, peripheral venous; gal-3, galectin-3; and sST2, soluble ST2.
CS and PV Biomarker Levels
In this CRT cohort with relatively preserved renal function, the median baseline concentrations of NT-proBNP, gal-3 and sST2 for the CS and PV samples are presented in Table 2. Results for each biomarker were quite consistent with a population of patients with advanced HF due to LV systolic dysfunction.
There were strong positive correlation between CS and PV samples for each of the 3 biomarkers (NT-proBNP: =0.90, gal-3: =0.73, sST2: =0.89; all p<0.001). Notably, when evaluating the transcardiac gradients between the CS and PV, levels of NT-proBNP were 20% higher in the CS than periphery, while both gal-3 and sST2 were 10% higher in periphery than CS (all p<0.001).
Table 3 details the correlation and comparison between the biomarkers to clinical and Echo parameters relevant in the CRT cohort.
Table 3.
Correlation and comparison between the CS and PV blood samples of the 3 HF biomarkers to clinical and Echo parameters.
| NT-proBNP, pg/mL | gal-3, ng/ml | sST2, ng/mL | ||||
|---|---|---|---|---|---|---|
| CS | PV | CS | PV | CS | PV | |
| Continuous variables, Spearman correlation | ||||||
| Age | 0.50 | 0.61 | 0.30 | 0.39 | −0.04 | −0.02 |
| p<0.001 | p<0.001 | p=0.01 | p<0.001 | p=0.74 | p=0.88 | |
| QRS duration | −0.01 | −0.01 | −0.12 | −0.24 | −0.08 | −0.01 |
| p=0.94 | p=0.93 | p=0.31 | p=0.04 | p=0.48 | p=0.91 | |
| LVEDV | 0.17 | 0.17 | 0.02 | −0.12 | 0.05 | 0.03 |
| p=0.20 | p=0.20 | p=0.89 | p=0.38 | p=0.69 | p=0.82 | |
| LVESV | 0.22 | 0.22 | 0.07 | −0.07 | 0.11 | 0.09 |
| p=0.09 | p=0.09 | p=0.61 | p=0.59 | p=0.39 | p=0.51 | |
| LV EF | −0.30 | −0.37 | −0.07 | 0.01 | −0.15 | −0.09 |
| p=0.01 | p=0.002 | p=0.56 | p=0.95 | p=0.22 | p=0.46 | |
| eGFR | −0.60 | −0.69 | −0.44 | −0.47 | −0.25 | −0.24 |
| p<0.001 | p<0.001 | p<0.001 | p<0.001 | p=0.04 | p=0.04 | |
| Categorical variables, median [25th, 75th %ile] | ||||||
| Male | 1821[806,3560] | 1507[736,2856] | 16.2[12.3,21.0] | 18.1[13.1,23.0] | 39.7[24.7,60.9] | 43.9[29.4,61.4] |
| Female | 2353[331,3144] | 1961[195,2405] | 17.6 [13.4,20.6] | 18.1 [16.0,22.4] | 31.5 [25.7,41.0] | 32.0 [30.1,41.6] |
| p=0.50 | p=0.50 | p=0.19 | p=0.19 | p=0.23 | p=0.01 | |
| Ischemic CM | 2045[836,4613] | 1864[876,4236] | 17.9[12.9,21.0] | 20.0[14.3,23.0] | 35.6[22.2,60.9] | 42.6[26.1,68.7] |
| Nonischemic CM | 1576[709,3065] | 1066[444,2112] | 15.6[11.6,22.2] | 16.3[11.9,23.9] | 36.8[25.3,52.7] | 41.6[31.3,56.5] |
| p=0.72 | p=0.20 | p=0.41 | p=0.20 | p=0.92 | p=0.72 | |
| LBBB | 1202[413,3079] | 991[260,2498] | 15.7[11.8,20.2] | 16.0[12.9,22.8] | 31.3[23.7,59.8] | 37.9[29.4,60.1] |
| Non-LBBB | 2104[1588,4613] | 1802[1080,3306] | 17.6 [13.2,23.2] | 19.2[14.4,24.2] | 42.3[25.2,58.8] | 43.9[28.9,57.7] |
| p=0.13 | p=0.30 | p=0.30 | p=0.13 | p=0.30 | p=0.13 | |
| History AF | 2639[2045,4616] | 2425[1807,4038] | 17.5[14.1,22.2] | 20.8[15.8,28.0] | 41.7[25.3,62.6] | 45.8[33.2,61.4] |
| No history AF | 1074[398,2085] | 870[325,1709] | 15.7[11.6,20.2] | 16.0[11.8,21.3] | 32.3[24.8,55.2] | 38.7[26.6,56.5] |
| p<0.001 | p<0.001 | p=0.91 | p=0.05 | p=0.30 | p=0.13 | |
Using our pre-specified cutpoint for NT-proBNP, 49% of subjects had elevated CS concentrations, while 37% patients had simultaneous elevated PV values. For gal-3, there were 14% and 18% of patients with elevated CS and PV concentrations, respectively. For sST2, there were 53% and 62% of subjects with elevated CS and PV concentrations.
We then considered multi-marker strategies with the 3 biomarkers. Dual-marker positivity was found in 10% of CS samples and 12% of PV samples for NT-proBNP and gal-3; 29% of CS samples and 23% of PV samples for NT-proBNP and sST2; and 11% of CS samples and 15% of PV samples for gal-3 and sST2. In a triple marker strategy, 7% of patients had triple positive CS values and 11% had triple positive PV values.
Clinical Follow-up and Outcomes
The median follow up time was 2.0 years [1.7 years, 2.0 years]. There were 16 (22%) patients with MACE by 2 years, including 15 (21%) patients with a HF hospitalization, 2 (3%) requiring LVAD, 1 (1%) heart transplant, and 6 (8%) deaths.
Diagnostic Accuracy of Biomarker Strategies for CRT Non-response
Table 4 details the diagnostic accuracy using single and multi-marker strategies of NT-proBNP, gal-3, and sST2 with baseline CS and PV samples for predicting 6-month CRT response. Most notably, using a triple marker CS strategy yielded the highest specificity (95%) for predicting CRT non-response, superior in specificity to any single marker strategy (all p<0.01), with the exception of CS gal-3 whose specificity was 90% (p=0.50).
Table 4.
Diagnostic accuracy using single and multi-marker strategy with CS or PV blood for predicting CRT non-response.
| CRT non-response | Sensitivity % (95% CI) | Specificity % (95% CI) | PPV % (95% CI) | NPV % (95% CI) |
|---|---|---|---|---|
| Single Marker Strategy | ||||
| NT-proBNP | ||||
| CS | 64%(45–80) | 63%(46–77) | 58%(41–74) | 68%(50–82) |
| PV | 39%(23–58) | 65%(48–79) | 48%(29–68) | 57%(41–71) |
| gal-3 | ||||
| CS | 18%(7–35) | 90%(76–97) | 60%(26–88) | 57%(44–70) |
| PV | 15%(5–32) | 80%(64–91) | 38%(14–68) | 53%(40–66) |
| sST2 | ||||
| CS | 58%(39–75) | 50%(34–66) | 49%(32–65) | 59%(41–75) |
| PV | 64%(45–80) | 40%(25–57) | 47%(32–62) | 57%(37–76) |
| Dual Marker Strategy | ||||
| NT-proBNP+gal3 | ||||
| CS | 33%(18–52) | 43%(27–59) | 32%(17–51) | 44%(28–60) |
| PV | 58%(39–75) | 43%(27–59) | 45%(30–61) | 55%(36–73) |
| NT-proBNP+sST2 | ||||
| CS | 18%(7–35) | 68%(51–81) | 32%(13–57) | 50%(36–64) |
| PV | 30%(16–49) | 80%(64–91) | 56%(31–78) | 58%(44–71) |
| gal-3+sST2 | ||||
| CS | 36%(20–55) | 50%(34–66) | 38%(21–56) | 49%(33–65) |
| PV | 36%(20–55) | 65%(48–79) | 46%(27–67) | 55%(40–70) |
| Triple Marker Strategy | ||||
| NT-proBNP+gal3+sST2 | ||||
| CS | 9%(2–24) | 95%(83–99) | 60%(15–95) | 56%(43–68) |
| PV | 12%(3–28) | 90%(76–97) | 50%(16–84) | 55%(43–68) |
Prognostic Performance of Biomarker Strategies for MACE
Table 5 show the unadjusted and adjusted risk for developing two-year MACE based on single, dual, or triple-marker approaches with CS blood samples. Consistently, CS strategies with NT-proBNP, irrespective of whether single or multi-marker approach, as well as a dual CS strategy with gal-3 and sST2 appear to be able to identify patients at higher risk for developing MACE. Importantly, neither single marker approach with CS gal-3 or sST2 (Table 5) nor any PV strategies (data not shown, all p=NS) were able to predict future MACE. Moreover, participants with positive triple CS marker results have over 11-fold increase in adjusted hazards for MACE (p≤0.04) as compared to those with triple negative CS results (Table 5).
Table 5.
Risk for 2-year MACE using single and multi-marker strategies with CS blood samples. HR denotes hazard ratio; CI, confidence interval. Abbreviations as in Table 1–3.
| Unadjusted HR (95% CI) | p- value | Age- and sex- Adjusted HR (95% CI) | p- value | EF-Adjusted HR (95% CI) | p- value | eGFR-Adjusted HR (95% CI) | p- value | AF-Adjusted HR (95% CI) | p- value | |
|---|---|---|---|---|---|---|---|---|---|---|
| Single Marker Strategy | ||||||||||
| NT-proBNP positive | 3.4(1.1,10.4) | 0.04 | 4.7(1.4,15.2) | 0.01 | 3.9(1.1,14.1) | 0.04 | 3.0(0.80,11.2) | 0.10 | 4.6(1.3,16.1) | 0.02 |
| gal-3 positive | 2.6(0.85,8.2) | 0.09 | 2.4(0.75,7.6) | 0.14 | 2.8(0.77,10.4) | 0.12 | 2.3(0.73,7.4) | 0.15 | 2.6(0.83,8.3) | 0.10 |
| sST2 positive | 2.1(0.74,6.2) | 0.16 | 2.1(0.71,6.0) | 0.18 | 2.1(0.70,6.1) | 0.19 | 1.9(0.66,5.6) | 0.23 | 2.1(0.73,6.3) | 0.16 |
| Dual Marker Strategy | ||||||||||
| NT-proBNP+gal-3 | ||||||||||
| Both positive | 6.1(1.2,30.4) | 0.03 | 5.4(1.1,26.9) | 0.04 | 9.3(1.8,49.4) | 0.009 | 5.7(1.02,31.7) | 0.05 | 8.5(1.6,46.0) | 0.01 |
| 1 positive | 4.0(1.1,14.4) | 0.04 | 6.4(1.6,25.3) | 0.008 | 2.7(0.72,10.3) | 0.14 | 3.7(0.89,15.2) | 0.07 | 5.4(1.4,21.7) | 0.02 |
| Both negative | 1.0(reference) | - | 1.0(reference) | - | 1.0(reference) | - | 1.0(reference) | - | 1.0(reference) | - |
| NT-proBNP+sST2 | ||||||||||
| Both positive | 8.7(1.1,69.6) | 0.04 | 11.3(1.4,93.1) | 0.03 | 7.6(0.94,61.1) | 0.06 | 7.6(0.82,71.0) | 0.07 | 13.7(1.5,124.4) | 0.02 |
| 1 positive | 4.0(0.50,32.8) | 0.19 | 5.0(0.61,40.9) | 0.13 | 4.0(0.48,33.3) | 0.20 | 3.8(0.44,32.0) | 0.22 | 4.7(0.58,39.0) | 0.15 |
| Both negative | 1.0(reference) | - | 1.0(reference) | - | 1.0(reference) | - | 1.0(reference) | - | 1.0(reference) | - |
| gal-3+sST2 | ||||||||||
| Both positive | 4.6(1.2,17.3) | 0.02 | 4.2(1.1,16.3) | 0.04 | 4.2(0.96,18.3) | 0.06 | 3.9(0.99,14.9) | 0.05 | 4.8(1.2,18.8) | 0.02 |
| 1 positive | 1.4(0.45,4.5) | 0.56 | 1.3(0.42,4.2) | 0.64 | 1.6(0.51,5.2) | 0.41 | 1.3(0.41,4.1) | 0.66 | 1.4(0.45,4.6) | 0.54 |
| Both negative | 1.0(reference) | - | 1.0(reference) | - | 1.0(reference) | - | 1.0(reference) | - | 1.0(reference) | - |
| Triple Marker Strategy | ||||||||||
| NT-proBNP+gal-3+sST2 | ||||||||||
| Triple positive | 16.6(1.7,160.5) | 0.02 | 14.7(1.5,142.7) | 0.02 | 11.1(1.3,93.5) | 0.03 | 13.7(1.2,157.7) | 0.04 | 15.0(1.6,142.1) | 0.02 |
| 1-2 positive | 4.9 (0.63,37.3) | 0.13 | 5.8 (0.74,45.2) | 0.09 | 4.0(0.49,31.8) | 0.19 | 4.4(0.54,35.8) | 0.17 | 5.2(0.65,42.1) | 0.12 |
| Triple negative | 1.0(reference) | - | 1.0(reference) | - | 1.0(reference) | - | 1.0(reference) | - | 1.0(reference) | - |
Figure 1 displays the Kaplan-Meier curves and shows that a triple CS marker strategy (log-rank p=0.01) is predictive of MACE, while a triple PV marker strategy was not (log-rank p=0.56). It is noteworthy to highlight that the probability of early MACE to occur within 6 months for patients with triple positive CS values was remarkably high at 60% (Figure 1), though the number of patients with 6-month MACE were low, occurring in 8 patients (11%) with 7 HF hospitalization, 1 requiring LVAD placement, and 2 deaths.
Figure 1.
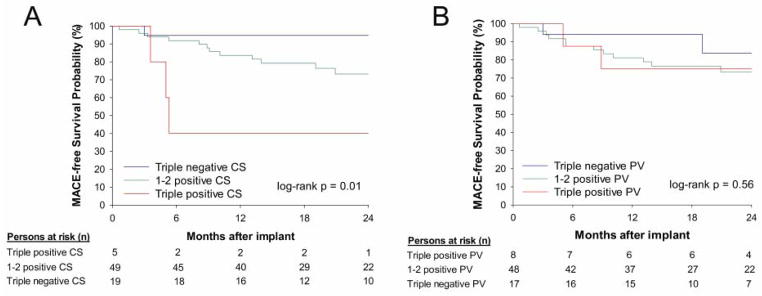
Kaplan-Meier curves for predicting MACE as stratified by triple marker strategy for coronary sinus (A) and peripheral venous (B) samples.
DISCUSSION
The BIOCRT study is the largest CRT cohort reported with simultaneous CS blood and PV blood sampling obtained during device implantation. CS sampling is easily obtainable during CRT implantation. In this prospective observational study which evaluates the role of CS and PV blood sampling in patients undergoing CRT implantation, a triple marker CS strategy with NT-proBNP, gal-3, and sST2 had high specificity of 95% for identifying 6-month CRT non-responders. We found that only CS strategies were predictive of 2-year MACE despite using established PV cutpoints for “high risk” individuals with HF for NT-proBNP,12 gal-3,13 and sST2.14 Most notable was that triple positive CS results yielded the greatest risk with over 11-fold increase in adjusted hazards for developing MACE as compared to participants with triple negative CS results. Interestingly, for these patients with triple positive CS results, there was a 60% probability of developing MACE, and events occurred early within 6 months. Conversely, no single or multi-marker PV strategies were predictive of MACE.
While biomarkers may be utilized individually, there has been a growing trend in using a multi-marker strategy to improve the accuracy of risk prediction of patients. NT-proBNP is an established biomarker of cardiomyocyte stress16 and has a prominent role in clinical practice guidelines for HF diagnosis and prognosis.17 NT-proBNP may be of use to select therapies for HF and identify higher risk patients, while simultaneously providing important information regarding response to therapy.12 Emerging candidate HF biomarkers such as gal-3 (a macrophage marker for fibrosis) and sST2 (a member of the interleukin-1 receptor family and marker of myocyte stress) may provide further prognostic information and enhance risk stratification,4, 18, 19 and both are recently incorporated into HF clinical practice guidelines.17 Gal-3 is associated with activation of fibroblasts and macrophages,20 which are a hallmark of cardiac remodeling and linked to disease progression of HF and poor prognosis.21, 22 The biomarker, sST2, is expressed in isolated cardiac myocytes that are exposed to mechanical strain and is widely considered to be reflective of cardiac remodeling23 and elevated concentrations are additively predictive of mortality relative to NT-proBNP.2, 3, 24, 25 It is of note that our data of transcardiac gradients suggests circulating concentrations of NT-proBNP are primarily cardiac in origin, but a certain percentage of both gal-3 and sST2 may be synthesized peripherally as a peripheral vascular response to the failing heart.
The strength of our study is that this is the largest cohort of matched CS and PV blood samplings of these candidate HF biomarkers in the CRT cohort. Our study supports results from a smaller study of 18 CRT patients with matched CS and PV sampling that found that CS B-type natriuretic peptide (BNP) was associated with HF-related hospitalizations.26 Our study extends to that finding by providing large sample of 73 CS patients and suggests that a multi-marker CS strategy with NT-proBNP, gal-3, and sST2 have prognostic potential for predicting MACE. While prior CRT studies have found that PV NT-proBNP or gal-3 was predictive of mortality and is an independent predictor of MACE,27-29 both the PV NT-proBNP studies had over 800 CRT patients27, 28 and the PV gal-3 study had 260 patients.29 Our discrepant finding that no PV strategy was predictive of MACE may be explained by our small sample size. Remarkably, despite our smaller sample size of 73 patients, abnormal CS results with these 3 biomarkers were significantly associated with worse prognosis, with a gradient effect when using a single CS marker to triple CS marker strategy.
In deciding between single versus multi-marker CS strategy, since CS sampling can only occur when patients undergo CRT implantation, it may be practical to send all three biomarkers at that time. The multi-marker approach is further supported given our results suggesting that non-response is much more likely and the magnitude of MACE risk increases over 11-fold with triple positive CS results. Additionally, as the probability of early MACE within the first 6 months is marked (60%) in those with a triple positive CS marker result, such patients should be monitored more closely.
While risk for MACE following CRT are both complex and may be due to numerous factors, there are several potential clinical implications of our study. First, prognostication provides both patients and caregivers realistic expectations to a therapy that while beneficial for the majority remains ineffective for still a large proportion. Secondly, pro-active risk stratification of this CRT cohort with identification of potential non-responders and those at highest risk for MACE would encourage closer follow up and more individualized care, especially in this current era where multidisciplinary care has been associated with improved clinical outcomes.30 Notably, CS sampling may currently occur too late along the decision making pathway to impact the choice of implanting a CRT device. However, with point-of-care testing for NT-proBNP established,31-33 and future development and availability of point-of-care testing for gal-334 and sST235, rapid measurement of each biomarker may be feasible at the time of implantation. Alternatively, CS sampling can occur before device implantation via the right internal jugular vein or femoral venous approach during right heart catheterization, which are performed routinely in these end-stage HF patients. If a CS sampling strategy such as ours is validated prospectively, one can speculate that this could influence the decision for device therapy, especially in the current era where the delivery of care is becoming progressively more individualized.
Limitations
The study has several notable limitations. Only a small proportion of patients implanted with CRT from a single tertiary center were included in this analysis which may represent a selection and treatment bias and limit its generalizability. While our sample size is relatively small, this is the largest CRT biomarker study with simultaneous CS and PV blood sample for analysis. Despite the small number of patients with triple positive CS results, those who were triple positive had particularly high risk for developing MACE. The large confidence intervals for our hazard ratios are due to small numbers and events, which also limits our ability to perform multivariable adjustments beyond additional one or two variables in this heterogenous group of CRT patients. The biomarkers are not static and are greatly dependent on the patient's HF status on the day it was sampled. However, these patients are chronically in HF and the decision on device implantation is based on the overall progression of the patient over a longitudinal time period where it is felt that without intervention the patient would unlikely improve. Perhaps, serial CS sampling can be an approach pre-operatively during routine right heart catheterizations that can be tested in future studies. Thus, our findings should be considered preliminary and larger studies are needed to confirm our results, as well as determine if implementing a CS biomarker strategy would be cost-effective, especially once point-of-care testing of these biomarkers becomes available or via alternative strategies such as CS sampling during routine right heart catheterization.
CONCLUSION
In patients undergoing CRT, coronary sinus sampling of biomarkers may be more useful than peripheral venous strategies for predicting outcomes, due to differential expression of the 3 HF biomarkers (NT-proBNP, gal-3, and sST2). Combination of the three elevated biomarkers in the CS blood suggests that CS sampling may have an important prognostic role in this highly morbid patient population, where optimizing individualized patient care strategies are becoming increasingly important. Larger studies are needed to validate our initial findings.
Clinical Perspectives.
The coronary sinus (CS) functions as a receptive reservoir of metabolic drainage and circulating biomarkers have differential expression of their CS levels as compared to the periphery depending on the site of production or excretion. Moreover, CS sampling is readily available in patients undergoing cardiac resynchronization therapy (CRT). Given the high residual non-response rate to device therapy, this study from the BIOCRT cohort examines the role of CS and peripheral venous blood sampling of three candidate heart failure biomarkers (NT-proBNP, galectin-3, and soluble ST2) that were drawn at the time of device implantation to predict CRT response and prognosticate patients. Interestingly, patients with triple positive CS levels were likely to be CRT non-responders at 6-months and were at highest risk for having two-year MACE over those who were triple negative, while peripheral venous sampling was not associated with predicting outcome. These preliminary data suggests that there may be a role in coronary sinus sampling of these heart failure biomarkers, especially once point-of-care testing becomes available intra-operatively. Alternative strategies such as coronary sinus sampling during routine right heart catheterization may be a viable pre-procedural option. Future larger studies are need to confirm our findings that patients with triple positive CS levels are at greatest risk of poor outcomes to CRT and such "personalized" approach may ultimately influence the decision-making process on whether or not to implant a CRT device, or proceed to earlier end-stage heart failure interventions, such as cardiac transplantation.
Acknowledgments
Funding Sources: The study was supported by NIH/NHLBI K23HL098370. Dr. Truong also received support from the NIH L30HL093896. Dr. Ajijola is supported by an A.P. Giannini Foundation award. The reagents/assays were provided and performed by Siemens Healthcare Diagnostics Inc., BG Medicine, and Critical Diagnostics.
ABBREVIATIONS
- NT-proBNP
amino-terminal pro-B type natriuretic peptide
- CRT
cardiac resynchronization therapy
- CS
coronary sinus
- gal-3
galectin-3
- HF
heart failure
- MACE
major adverse cardiovascular events
- NYHA
New York Heart Association
- PV
peripheral venous sST2, soluble ST2
Footnotes
Clinical Trial Registration: http://clinicaltrials.gov/ct2/show/NCT01949246 Unique Identifier: NCT01949246
Disclosures: Dr. Truong receives grant support from St. Jude Medical, American College of Radiology Imaging Network, and Duke Clinical Research Institute. Dr. Januzzi is supported in part by the Roman W. Desanctis Distinguished Clinical Scholar Endowment, and receives grant support from Roche Diagnostics, Siemens, BG Medicine, Critical Diagnostics, Singulex, and Thermo Fisher. Dr. Min has served on the medical advisory boards of GE Healthcare, Arineta, Astra Zeneca, and Bristol-Myers Squibb; Speakers' Bureau of GE Healthcare; and received research support from GE Healthcare, Vital Images, and Phillips Healthcare. Dr. Singh receives grant support from St. Jude Medical, Medtronic Inc., Boston Scientific Corp., Sorin Group, Biotronik, BG Medicine and Siemens.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rosamond W, Flegal K, Friday G, et al. Heart disease and stroke statistics--2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 2.Januzzi JL, Jr, Peacock WF, Maisel AS, et al. Measurement of the interleukin family member ST2 in patients with acute dyspnea: results from the PRIDE (Pro-Brain Natriuretic Peptide Investigation of Dyspnea in the Emergency Department) study. J Am Coll Cardiol. 2007;50:607–613. doi: 10.1016/j.jacc.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 3.Rehman SU, Mueller T, Januzzi JL., Jr Characteristics of the novel interleukin family biomarker ST2 in patients with acute heart failure. J Am Coll Cardiol. 2008;52:1458–1465. doi: 10.1016/j.jacc.2008.07.042. [DOI] [PubMed] [Google Scholar]
- 4.van Kimmenade RR, Januzzi JL, Jr, Ellinor PT, Sharma UC, Bakker JA, Low AF, Martinez A, Crijns HJ, MacRae CA, Menheere PP, Pinto YM. Utility of amino-terminal pro-brain natriuretic peptide, galectin-3, and apelin for the evaluation of patients with acute heart failure. J Am Coll Cardiol. 2006;48:1217–1224. doi: 10.1016/j.jacc.2006.03.061. [DOI] [PubMed] [Google Scholar]
- 5.Abraham WT, Fisher WG, Smith AL, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845–1853. doi: 10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 6.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 7.Bristow MR, Saxon LA, Boehmer J, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–2150. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 8.Gregoratos G, Abrams J, Epstein AE, Freedman RA, Hayes DL, Hlatky MA, Kerber RE, Naccarelli GV, Schoenfeld MH, Silka MJ, Winters SL. ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices--summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/NASPE Committee to Update the 1998 Pacemaker Guidelines) J Am Coll Cardiol. 2002;40:1703–1719. doi: 10.1016/s0735-1097(02)02528-7. [DOI] [PubMed] [Google Scholar]
- 9.Vardas PE, Auricchio A, Blanc JJ, et al. Guidelines for cardiac pacing and cardiac resynchronization therapy: the task force for cardiac pacing and cardiac resynchronization therapy of the European Society of Cardiology. Developed in collaboration with the European Heart Rhythm Association. Eur Heart J. 2007;28:2256–2295. doi: 10.1093/eurheartj/ehm305. [DOI] [PubMed] [Google Scholar]
- 10.Bax JJ, Abraham T, Barold SS, et al. Cardiac resynchronization therapy: Part 1--issues before device implantation. J Am Coll Cardiol. 2005;46:2153–2167. doi: 10.1016/j.jacc.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 11.Bax JJ, Abraham T, Barold SS, et al. Cardiac resynchronization therapy: Part 2--issues during and after device implantation and unresolved questions. J Am Coll Cardiol. 2005;46:2168–2182. doi: 10.1016/j.jacc.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 12.Januzzi JL, Jr, Rehman SU, Mohammed AA, et al. Use of amino-terminal pro-B-type natriuretic peptide to guide outpatient therapy of patients with chronic left ventricular systolic dysfunction. J Am Coll Cardiol. 2011;58:1881–1889. doi: 10.1016/j.jacc.2011.03.072. [DOI] [PubMed] [Google Scholar]
- 13.McCullough PA, Olobatoke A, Vanhecke TE. Galectin-3: a novel blood test for the evaluation and management of patients with heart failure. Rev Cardiovasc Med. 2011;12:200–210. doi: 10.3909/ricm0624. [DOI] [PubMed] [Google Scholar]
- 14.Ky B, French B, McCloskey K, et al. High-sensitivity ST2 for prediction of adverse outcomes in chronic heart failure. Circ Heart Fail. 2011;4:180–187. doi: 10.1161/CIRCHEARTFAILURE.110.958223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Packer M. Proposal for a new clinical end point to evaluate the efficacy of drugs and devices in the treatment of chronic heart failure. J Card Fail. 2001;7:176–182. doi: 10.1054/jcaf.2001.25652. [DOI] [PubMed] [Google Scholar]
- 16.Braunwald E. Biomarkers in heart failure. N Engl J Med. 2008;358:2148–2159. doi: 10.1056/NEJMra0800239. [DOI] [PubMed] [Google Scholar]
- 17.Writing Committee M. Yancy CW, Jessup M, et al. 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:e240–e319. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 18.Weinberg EO, Shimpo M, Hurwitz S, Tominaga S, Rouleau JL, Lee RT. Identification of serum soluble ST2 receptor as a novel heart failure biomarker. Circulation. 2003;107:721–726. doi: 10.1161/01.cir.0000047274.66749.fe. [DOI] [PubMed] [Google Scholar]
- 19.Shimpo M, Morrow DA, Weinberg EO, Sabatine MS, Murphy SA, Antman EM, Lee RT. Serum levels of the interleukin-1 receptor family member ST2 predict mortality and clinical outcome in acute myocardial infarction. Circulation. 2004;109:2186–2190. doi: 10.1161/01.CIR.0000127958.21003.5A. [DOI] [PubMed] [Google Scholar]
- 20.Sharma UC, Pokharel S, van Brakel TJ, van Berlo JH, Cleutjens JP, Schroen B, Andre S, Crijns HJ, Gabius HJ, Maessen J, Pinto YM. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation. 2004;110:3121–3128. doi: 10.1161/01.CIR.0000147181.65298.4D. [DOI] [PubMed] [Google Scholar]
- 21.de Boer RA, Lok DJ, Jaarsma T, van der Meer P, Voors AA, Hillege HL, van Veldhuisen DJ. Predictive value of plasma galectin-3 levels in heart failure with reduced and preserved ejection fraction. Annals of medicine. 2011;43:60–68. doi: 10.3109/07853890.2010.538080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah RV, Chen-Tournoux AA, Picard MH, van Kimmenade RR, Januzzi JL. Galectin-3, cardiac structure and function, and long-term mortality in patients with acutely decompensated heart failure. Eur J Heart Fail. 2010;12:826–832. doi: 10.1093/eurjhf/hfq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weinberg EO, Shimpo M, De Keulenaer GW, MacGillivray C, Tominaga S, Solomon SD, Rouleau JL, Lee RT. Expression and regulation of ST2, an interleukin-1 receptor family member, in cardiomyocytes and myocardial infarction. Circulation. 2002;106:2961–2966. doi: 10.1161/01.CIR.0000038705.69871.D9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pascual-Figal DA, Ordonez-Llanos J, Tornel PL, Vazquez R, Puig T, Valdes M, Cinca J, de Luna AB, Bayes-Genis A. Soluble ST2 for predicting sudden cardiac death in patients with chronic heart failure and left ventricular systolic dysfunction. J Am Coll Cardiol. 2009;54:2174–2179. doi: 10.1016/j.jacc.2009.07.041. [DOI] [PubMed] [Google Scholar]
- 25.Pascual-Figal DA, Manzano-Fernandez S, Boronat M, Casas T, Garrido IP, Bonaque JC, Pastor-Perez F, Valdes M, Januzzi JL. Soluble ST2, high-sensitivity troponin T- and N-terminal pro-B-type natriuretic peptide: complementary role for risk stratification in acutely decompensated heart failure. Eur J Heart Fail. 2011;13:718–725. doi: 10.1093/eurjhf/hfr047. [DOI] [PubMed] [Google Scholar]
- 26.Glick A, Michowitz Y, Keren G, George J. Neurohormonal and inflammatory markers as predictors of short-term outcome in patients with heart failure and cardiac resynchronization therapy. Isr Med Assoc J. 2006;8:391–395. [PubMed] [Google Scholar]
- 27.Berger R, Shankar A, Fruhwald F, Fahrleitner-Pammer A, Freemantle N, Tavazzi L, Cleland JG, Pacher R. Relationships between cardiac resynchronization therapy and N-terminal pro-brain natriuretic peptide in patients with heart failure and markers of cardiac dyssynchrony: an analysis from the Cardiac Resynchronization in Heart Failure (CARE-HF) study. Eur Heart J. 2009;30:2109–2116. doi: 10.1093/eurheartj/ehp210. [DOI] [PubMed] [Google Scholar]
- 28.Brenyo A, Barsheshet A, Rao M, Huang DT, Zareba W, McNitt S, Hall WJ, Peterson DR, Solomon SD, Moss AJ, Goldenberg I. Brain natriuretic Peptide and cardiac resynchronization therapy in patients with mildly symptomatic heart failure. Circ Heart Fail. 2013;6:998–1004. doi: 10.1161/CIRCHEARTFAILURE.112.000174. [DOI] [PubMed] [Google Scholar]
- 29.Lopez-Andres N, Rossignol P, Iraqi W, Fay R, Nuee J, Ghio S, Cleland JG, Zannad F, Lacolley P. Association of galectin-3 and fibrosis markers with long-term cardiovascular outcomes in patients with heart failure, left ventricular dysfunction, and dyssynchrony: insights from the CARE-HF (Cardiac Resynchronization in Heart Failure) trial. Eur J Heart Fail. 2012;14:74–81. doi: 10.1093/eurjhf/hfr151. [DOI] [PubMed] [Google Scholar]
- 30.Altman RK, Parks KA, Schlett CL, et al. Multidisciplinary care of patients receiving cardiac resynchronization therapy is associated with improved clinical outcomes. Eur Heart J. 2012;33:2181–2188. doi: 10.1093/eurheartj/ehs107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jorgensen B, Bertsch T, Broker HJ, et al. Multicentre evaluation of a second generation point-of-care assay with an extended range for the determination of N-terminal pro-brain natriuretic peptide. Clin Lab. 2012;58:515–525. [PubMed] [Google Scholar]
- 32.Lepoutre T, Rousseau MF, Ahn SA, Gruson D. Measurement Nt-proBNP circulating concentrations in heart failure patients with a new point-of-care assay. Clin Lab. 2013;59:831–835. doi: 10.7754/clin.lab.2012.120418. [DOI] [PubMed] [Google Scholar]
- 33.Jungbauer CG, Kaess B, Buchner S, Birner C, Lubnow M, Resch M, Debl K, Buesing M, Zerback R, Riegger G, Luchner A. Equal performance of novel N-terminal proBNP (Cardiac proBNP(R)) and established BNP (Triage BNP(R)) point-of-care tests. Biomark Med. 2012;6:789–796. doi: 10.2217/bmm.12.67. [DOI] [PubMed] [Google Scholar]
- 34. [Accessed December 31, 2013]; http://investor.bg-medicine.com/releasedetail.cfm?ReleaseID=535701.
- 35. [Accessed December 31, 2013]; http://www.criticaldiagnostics.com/US/news/news-101513.html.


