Abstract
During submaximal exercise, some otherwise healthy obese women experience breathlessness, or dyspnea on exertion (+DOE), while others have mild or no DOE (−DOE). We investigated whether weight loss could reduce DOE. 29 obese women were grouped based on their Ratings of Perceived Breathlessness (RPB) during constant load 60W cycling: +DOE (n=14, RPB≥4, 34±8yr, 36±3kg/m2) and −DOE (n=15, RPB≤2, 32±8yr, 36±4kg/m2) and then completed a 12-week weight loss program. Both groups lost a moderate amount of weight (+DOE: 6.6±2.4kg, −DOE: 8.4±3.5kg, p<0.001). RPB decreased significantly in the +DOE group (from 4.7±1.1 to 3.1±1.6) and remained low in the −DOE (from 1.5±0.7 to 1.6±1.1) (interaction p<0.002). Most physiological variables measured (i.e. body composition, fat distribution, pulmonary function, oxygen cost of breathing, cardiorespiratory measures) improved with weight loss; however, the decrease in RPB was not correlated with any of these variables (p>0.05). In conclusion, moderate weight loss was effective in reducing breathlessness on exertion in obese women who experienced DOE at baseline.
Keywords: Shortness of Breath, Body Composition, Pulmonary Function, Work of Breathing, Exercise
1. INTRODUCTION
The prevalence of obesity has increased dramatically over the past several decades. Currently, 33% of adults are classified as overweight and another 35% as obese in the United States (Ogden et al., 2014). Obesity is a complex multifactorial condition and is associated with a myriad of medical problems, such as type 2 diabetes, hypertension, stroke and heart attacks, sleep disordered breathing, and respiratory disorders (Azagury and Lautz, 2011; Kenchaiah et al., 2002; Van Gaal et al., 2006).
Dyspnea on exertion (DOE) is also a very common symptom in obesity (Gibson, 2000; O'Donnell et al., 2010; Sin et al., 2002) and a major barrier in the management of obesity. We have repeatedly found approximately one-third of otherwise healthy obese women and men to experience an elevated intensity of dyspnea during submaximal constant load cycling exercise at 60 W or 105 W, respectively (or < 4 METs) (Babb et al., 2008a; Bernhardt and Babb, 2014; Bernhardt et al., 2013b). DOE and breathing discomfort may discourage obese individuals from being physically active.
It is unknown if weight loss alone (i.e. without aerobic exercise training) could decrease DOE in otherwise healthy obese women. This is a clinically important question as the American College of Sports Medicine, the National Institutes of Health, and other agencies recommend a combination of diet and aerobic exercise training for weight loss (1998; Donnelly et al., 2009). Additionally, the mechanism by which weight loss may improve DOE is also unclear; several factors could be involved, such as a decreased oxygen cost of breathing (Babb et al., 2008a), more efficient breathing mechanics and/or breathing pattern (Babb et al., 2002; Babb et al., 2008b), changes in body composition and fat distribution (Babb et al., 2008b), and changes is cardiorespiratory measures and/or exercise capacity (Babb et al., 2008a; Bernhardt and Babb, 2014).
The objectives of this study were to investigate 1) whether weight loss via a 12-week diet and resistance exercise program could reduce DOE in obese women who experienced DOE at baseline and 2) whether changes in body composition, fat distribution, pulmonary function, oxygen cost of breathing, and/or cardiorespiratory measures were associated with the potential reduction in DOE. We hypothesized that weight loss would decrease RPB, i.e. improve exertional dyspnea, during submaximal exercise in those obese women who experienced DOE before entering the weight loss program compared with those obese women who had no or only mild DOE at baseline. Furthermore, we hypothesized that changes in body composition, fat distribution, pulmonary function, oxygen cost of breathing, and/or cardiorespiratory measures during exercise, which could improve with weight loss, would be significantly associated with the decrease in RPB following weight loss.
2. METHODS
2.1 Subjects
Twenty-nine obese women were initially identified based on BMI (≥ 30 ≤ 50 kg/m2), which was confirmed by underwater weighing (body fat ≥ 30 ≤ 55%). Exclusion criteria included history of smoking, asthma, cardiovascular disease, sleep disorders, or musculoskeletal abnormalities that would preclude maximal exercise. Subjects participating in regular vigorous exercise (exercise more than 2×/wk) during the last 6 months were also excluded. Written informed consent was obtained before participation in accordance with the University of Texas Southwestern IRB (STU122010-108).
The present study was designed as a pre-post intervention experiment. Some pre-intervention data have been previously published in manuscript (Bernhardt and Babb, 2014) and abstract form (Bassett et al., 2013; Bernhardt et al., 2013a; Bernhardt et al., 2014; Moran et al., 2013; Pineda et al., 2013).
Participants underwent the same testing procedures before and after a 12-week weight loss program. Testing was performed on four separate visits each before and after the intervention.
2.2. Body composition and pulmonary function (Visit 1)
Standard measures of height and weight were taken. Circumferences measurements were taken at the neck, chest, waist, and hips according to NHANES III guidelines (1994). Hydrostatic weighing was performed as previously described to determine percent body fat, lean body mass, and total body fat mass (Babb et al., 2008a; Babb et al., 2008b). Standard pulmonary function testing including spirometry, lung volume, and diffusing capacity was performed according to ATS/ERS guidelines (model V62W body plethysmograph, SensorMedics) (1995).
2.3 Cycling Exercise Testing (Visit 2)
2.3.1 Submaximal Constant Load Exercise
Before the exercise test, participants were given the following written instructions for Rating of Perceived Breathlessness (RPB): “The number 0 represents no breathlessness. The number 10 represents the strongest or greatest breathlessness that you have ever experienced. Each minute during the exercise test you will be asked to point to a number, which represents your perceived level of breathlessness at the time.” Exercise testing began with subjects seated on an electronically braked cycle ergometer (Lode Corival) with 3 minutes of resting baseline measurements after which a 6-minute constant load exercise cycling test at 60 W was initiated. This exercise work rate was chosen based on prior studies on obese women who obtained ventilatory threshold at approximately 60 W (DeLorey et al., 2005) and has been used previously to establish obese women with strong (i.e. +DOE) or no to mild DOE (i.e. −DOE) (Babb et al., 2008a; Bernhardt et al., 2013b). RPB was collected using the modified Borg scale (Borg, 1982) every two minutes of the test and the last value recorded was used for analysis. Cardiorespiratory responses, including heart rate (HR), blood pressure (SunTech Tango), ventilation (V̇E), and gas exchange (V̇O2 and V̇CO2), were measured at rest and throughout exercise (custom software, DUFIS).
2.3.2 Peak Cardiovascular Exercise Capacity
Peak exercise capacity was determined by graded cycle ergometry to volitional exhaustion or pedal rate ≤ 50 rpm. After resting baseline measurements, subjects started pedaling at 60–65 rpm with an initial work rate of 20 W. Work rate was increased by 20 W each minute until termination of the test; maximal effort was evidenced by achieving predicted peak heart rate > 90%, [lactate] > 7 mmol/L, and respiratory exchange rate > 1.1.
2.4 Oxygen Cost of Breathing (Visit 3)
The oxygen cost of breathing was determined from 6-min measurements of V̇O2 and V̇E at rest and 4-min measurements of V̇O2 and V̇E during eucapnic voluntary hyperpnea at 40L/min and 60L/min as previously described (Babb et al., 2008a). The oxygen cost of breathing was assessed by calculating the slope of the V̇O2 (ml/min) versus V̇E (L/min) relationship at rest and during the two levels of hyperpnea. Linearity of the slope was checked for each subject (r2 range 0.91–1.00).
2.5 Body fat distribution (Visit 4)
Multiple T2-weighted, water-suppressed, Magnetic Resonance Images (MRI) were taken from the sternal notch to the pubic symphysis to estimate fat distribution in the chest, abdominal, subcutaneous, visceral, and peripheral regions as previously described (Babb et al., 2008a; Babb et al., 2011; Bernhardt et al., 2013b). Images were analyzed using custom interactive software (Wafter 1.3).
2.6 Weight Loss Program
Each participant completed a 12-week weight loss program. They received a customized meal plan, weekly shopping lists and breakdown of individual meals and snacks. Additionally, they performed specific resistance exercises under supervision of a personal trainer three times per week. Subjects performed 8 resistive exercises (i.e. lifting weights) with 10 slow (i.e. concentric phase of ~10 s) repetitions each targeting all major muscle groups (i.e. upper body, lower body, core). The resistive exercises were utilized to increase - and/or minimize the loss of - lean body mass and thus increase basal metabolic rate. Aerobic exercise was not performed, so changes after the program could be attributed to weight loss only, not improvements in cardiorespiratory fitness. Participants were encouraged to lose 1–2 lb per week.
2.7 Data Analysis
The 29 obese women were assigned to one of two groups according to their RPB (0 – 10 Borg scale) during minute 6 of the constant load 60 W exercise test. Those with an RPB ≤ 2 were designated as obese women with no or mild dyspnea on exertion (−DOE, n = 15) and those with an RPB ≥ 4 were designated as obese women with strong dyspnea on exertion (+DOE, n = 14). Those women with an RPB = 3 were excluded from the study in order to better delineate differences between the +DOE and −DOE groups. The grouping was based on our previous finding that obese women have an average RPB of 2 ± 1 at ventilatory threshold during incremental exercise (DeLorey et al., 2005) and has been used in previous studies (Babb et al., 2008a; Bernhardt et al., 2013b).
Differences between +DOE and −DOE groups before and after the weight loss program were analyzed using a two-way ANOVA (i.e. weight loss and group) with a repeated measure on one factor (i.e. weight loss). Relationships among variables were determined with Spearman rank correlation. Data was analyzed using SAS 9.2.
3. RESULTS
Weight loss was achieved in all subjects (Figure 1). RPB during 60 W cycling decreased significantly in the +DOE group only (from 4.7±1.1 to 3.1±1.6), and expectedly remained low in the −DOE group (from 1.5±0.7 to 1.6±1.1) (group*WtLoss interaction p=0.002) (Figure 2).
Figure 1.
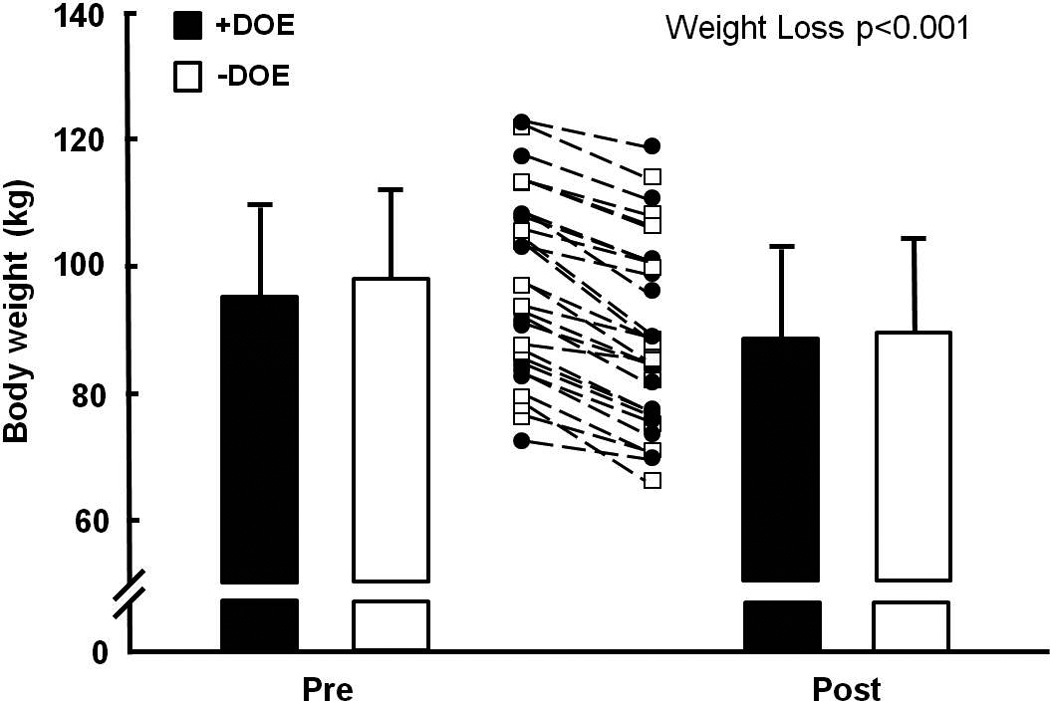
Individual and mean decreases in body weight before and after a 12-week diet and resistance exercise program. +DOE, subjects with strong dyspnea on exertion; −DOE, subjects with no or mild dyspnea on exertion. Values are mean ± SD.
Figure 2.
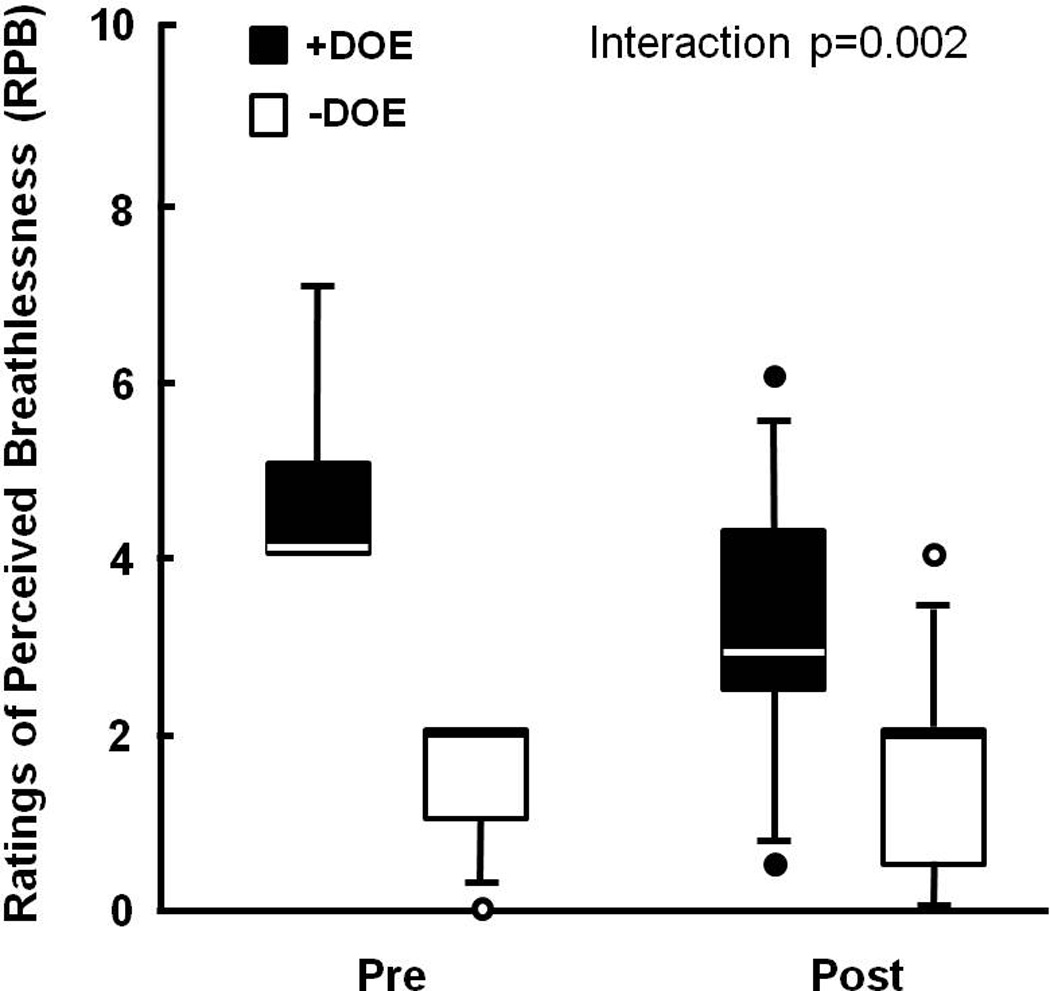
Individual and group averages for Ratings of Perceived Breathlessness (RPB) before and after weight loss. RPB significantly decreased only in the +DOE group (Group × Weight Loss interaction p = 0.002). Box plots define the 25th and 75th percentiles by the ends of the boxes, with a line at the median and error bars defining the 10th and 90th percentiles.
3.1 Changes with weight loss
Table 1 shows the changes in body composition, fat distribution, pulmonary function, oxygen cost of breathing, and cardiorespiratory measures during constant load and peak exercise.
Table 1.
Summary of mean changes in body composition, fat distribution, pulmonary function, oxygen cost of breathing, and cardiorespiratory exercise variables with weight loss (mean ± SD). P-value of interaction only shown when significant. NS, non-significant.
| +DOE | −DOE | P-value Weight Loss |
P-value Group differences |
P-value Interaction |
|
|---|---|---|---|---|---|
| Body composition | |||||
| ΔWeight (kg) | −6.6 ± 2.4 | −8.4 ± 3.5 | <0.001 | NS | |
| ΔBody mass index (kg/m2) | −2.4 ± 1.0 | −3.1 ± 1.5 | <0.001 | NS | |
| ΔBody fat (%) | −2.8 ± 2.2 | −3.9 ± 2.3 | <0.001 | NS | |
| ΔLean body mass (kg) | −1.3 ± 1.7 | −1.3 ± 1.9 | <0.001 | NS | |
| ΔChest circumference (cm) | −3.2 ± 2.7 | −5.0 ± 4.6 | <0.001 | NS | |
| ΔWaist circumference (cm) | −5.7 ± 4.6 | −8.4 ± 8.3 | <0.001 | NS | |
| ΔHip circumference (cm) | −6.1 ± 3.9 | −7.9 ± 4.5 | <0.001 | NS | |
| ΔNeck circumference (cm) | −1.0 ± 1.1 | −1.2 ± 1.0 | <0.001 | NS | |
| ΔWaist:Hip ratio | −0.01 ± 0.04 | −0.01 ± 0.07 | NS | NS | |
| Fat distribution | |||||
| ΔChest Fat (kg) | −0.6 ± 0.4 | −1.0 ± 0.5 | 0.036 | ||
| ΔAbdominal Fat (kg) | −1.3 ± 0.7 | −1.8 ± 1.1 | <0.001 | NS | |
| ΔVisceral Fat (kg) | −0.5 ± 0.3 | −0.7 ± 0.6 | <0.001 | NS | |
| ΔSubcutaneous Fat (kg) | −1.8 ± 1.0 | −2.5 ± 1.1 | <0.001 | NS | |
| ΔPeripheral Fat (kg) | −2.4 ± 2.2 | −3.1 ± 1.4 | <0.001 | NS | |
| Pulmonary function | |||||
| ΔTLC (%pred) | 0 ± 4 | 1 ± 3 | NS | NS | |
| ΔFRC (L) | 0.2 ± 0.2 | 0.2 ± 0.4 | 0.002 | NS | |
| ΔFRC (%TLC) | 4 ± 6 | 4 ± 8 | 0.005 | NS | |
| ΔERV (%TLC) | 4 ± 6 | 5 ± 11 | 0.008 | NS | |
| ΔIC (%pred) | −4 ± 14 | −1 ± 12 | NS | NS | |
| ΔFVC (%pred) | 1 ± 6 | −1 ± 7 | NS | NS | |
| ΔFEV1 (%pred) | 0 ± 5 | −2 ± 6 | NS | NS | |
| ΔFEV1/FVC ratio | −0.1 ± 2.9 | −0.3 ± 2.1 | NS | NS | |
| ΔMVV (%pred) | 9 ± 13 | 5 ± 6 | NS | 0.001 | |
| ΔDLCO (%pred) | 1 ± 8 | 3 ± 8 | NS | NS | |
| Oxygen cost of breathing | |||||
| ΔO2cost Slope | −0.48 ± 0.70 | −0.36 ± 0.67 | 0.003 | NS | |
| Constant load exercise | |||||
| ΔRPB | −1.54 ± 1.45 | 0.10 ± 1.09 | 0.002 | ||
| ΔRPE | −1.71 ± 2.49 | 0.27 ± 1.75 | 0.019 | ||
| ΔV̇O2 (L/min) | −0.07 ± 0.05 | −0.05 ± 0.05 | <0.001 | NS | |
| ΔV̇ O2 (%peak) | −5 ± 5 | −2 ± 6 | 0.034 | 0.002 | |
| ΔV̇E (L/min) | −7.20 ± 5.41 | −2.57 ± 4.80 | 0.021 | ||
| ΔV̇E (%MVV) | −10 ± 5 | −3 ± 3 | 0.001 | ||
| ΔRER | −0.06 ± 0.07 | −0.00 ± 0.08 | 0.044 | ||
| ΔHR (bpm) | −12 ± 11 | −2 ± 7 | <0.001 | 0.013 | |
| ΔHR (%peak) | −6 ± 6 | −1 ± 4 | 0.029 | ||
| Δ[Lactate] (mg/dL) | −1.0 ± 1.0 | −0.4 ± 0.5 | 0.007 | <0.001 | |
| Peak exercise | |||||
| ΔWork rate (W) | 8.6 ± 10.3 | 4.0 ± 13.5 | 0.009 | 0.014 | |
| ΔTime (min) | 0.40 ± 0.46 | −0.10 ± 0.61 | 0.019 | ||
| ΔV̇O2 (L/min) | 0.04 ± 0.10 | −0.03 ± 0.11 | NS | NS | |
| ΔV̇E (L/min) | 1.56 ± 9.75 | −1.10 ± 14.48 | NS | NS | |
| ΔHR (bpm) | −5 ± 7 | −2 ± 4 | 0.003 | NS | |
| Δ[Lactate] (mg/dL) | −0.2 ± 1.1 | −0.4 ± 1.0 | NS | NS | |
TLC, total lung capacity; FRC, functional residual capacity; ERV, expiratory reserve volume; IC, inspiratory capacity; FVC, forced vital capacity, FEV1, forced expiratory volume in 1 s; MVV, maximal voluntary ventilation; DLCO, diffusing capacity of carbon monoxide; RPB, rating of perceived breathlessness; RPE, rating of perceived exertion; V̇O2, oxygen uptake; V̇E, minute ventilation; RER, respiratory exchange rate; HR, heart rate.
Body composition measures, including body mass index, lean body mass, fat mass, and body circumferences, decreased with weight loss in both groups (p < 0.001). Only waist:hip ratio did not change because both waist and hip circumferences decreased similarly.
Fat distribution did not change since fat mass was lost from all body regions, including chest, abdominal, visceral, subcutaneous, and peripheral areas.
Functional residual capacity (FRC) and expiratory reserve volume (ERV) improved by ~4% (as % of total lung capacity (TLC)) with weight loss (p < 0.01), with no significant changes in other lung subdivisions, spirometry, or diffusing capacity. Maximal voluntary ventilation (MVV), as a percent of predicted (%pred), was significantly different between the groups (p < 0.001), but it did not change with weight loss.
Oxygen cost of breathing decreased significantly with weight loss in both groups (p < 0.01) with no significant difference between groups before or after weight loss (p > 0.05).
Cardiorespiratory measures during submaximal constant load exercise at 60 W improved slightly after weight loss mainly in the +DOE group. Absolute V̇O2 (i.e. in L/min) was slightly, yet significantly decreased (p < 0.001) following weight loss with no difference between groups. Relative V̇O2 (i.e. as % of peak) was also decreased following weight loss with a significant difference between groups. Absolute (i.e. in L/min) and relative (i.e. as percent of MVV) V̇E as well as RER and relative HR (as % of peak) showed an interaction effect (interaction weight loss * group, p < 0.05). Absolute HR (in bpm) and blood [lactate] decreased significantly with weight loss and were different between groups.
Cardiorespiratory measures at peak exercise were similar between groups. V̇O2, V̇E, and [lactate] were not different between groups and did not change with weight loss. There was a significant, yet small, increase in the achieved work rate in both groups (by ~8 W in the +DOE group and ~4 W in the −DOE group) and the time to exhaustion increased in the +DOE group by ~24 s and decreased in the −DOE group by ~6 s (interaction group * weight loss, p = 0.019). HR showed a significant, yet small, decrease following weight loss (by ~5 in the +DOE and by ~2 in the −DOE group).
3.2 Associations
Table 2 shows the Spearman rank correlations between the decreased RPB and changes in body composition, fat distribution, pulmonary function, oxygen cost of breathing, and cardiorespiratory measures during constant load and peak exercise in the +DOE group.
Table 2.
Spearman correlations with decreased RPB in the +DOE group.
| Correlation r with ΔRPB |
P-value | |
|---|---|---|
| Body composition | ||
| ΔWeight (kg) | 0.15 | 0.61 |
| ΔBody mass index (kg/m2) | 0.22 | 0.46 |
| ΔBody fat (%) | 0.07 | 0.82 |
| ΔLean body mass (kg) | 0.14 | 0.66 |
| ΔChest circumference (cm) | 0.40 | 0.15 |
| ΔWaist circumference (cm) | −0.38 | 0.18 |
| ΔHip circumference (cm) | 0.24 | 0.41 |
| ΔNeck circumference (cm) | 0.38 | 0.18 |
| ΔWaist:Hip ratio | −0.46 | 0.10 |
| Fat distribution | ||
| ΔChest Fat (kg) | −0.01 | 0.98 |
| ΔAbdominal Fat (kg) | 0.15 | 0.61 |
| ΔVisceral Fat (kg) | 0.17 | 0.57 |
| ΔSubcutaneous Fat (kg) | 0.03 | 0.93 |
| ΔPeripheral Fat (kg) | −0.02 | 0.96 |
| Pulmonary function variable | ||
| ΔTLC (%pred) | 0.17 | 0.56 |
| ΔFRC (%TLC) | −0.35 | 0.23 |
| ΔERV (%TLC) | −0.25 | 0.39 |
| ΔFVC (%pred) | −0.05 | 0.87 |
| ΔFEV1 (%pred) | −0.11 | 0.72 |
| ΔFEV1/FVC ratio | −0.10 | 0.74 |
| ΔMVV (%pred) | 0.25 | 0.39 |
| ΔDLCO (%pred) | −0.07 | 0.81 |
| Oxygen cost of breathing | ||
| ΔO2cost Slope | 0.12 | 0.68 |
| Constant load exercise at 60 W | ||
| ΔRPE | 0.90 | <0.0001 |
| ΔV̇O2 (L/min) | 0.14 | 0.64 |
| ΔV̇O2 (%peak) | 0.36 | 0.20 |
| ΔV̇E (L/min) | 0.40 | 0.16 |
| ΔV̇E (%MVV) | 0.39 | 0.17 |
| ΔRER | −0.05 | 0.86 |
| ΔHR (bpm) | 0.29 | 0.32 |
| Δ [Lactate] (mg/dL) | 0.09 | 0.77 |
| Peak exercise | ||
| ΔWork rate (W) | 0.06 | 0.85 |
| ΔTime (min) | 0.04 | 0.87 |
| ΔV̇O2 (L/min) | −0.18 | 0.54 |
| ΔV̇E (L/min) | −0.07 | 0.81 |
| ΔHR (bpm) | 0.32 | 0.27 |
| Δ [Lactate] (mg/dL) | 0.46 | 0.11 |
For abbreviations see Table 1.
Even though all body composition and fat distribution measures decreased with weight loss, none of these changes were significantly correlated with the decrease in RPB (p > 0.05). Similarly, the changes in pulmonary function and oxygen cost of breathing following weight loss were not associated with the decreased RPB (p > 0.05).
Furthermore, even though there were some significant changes in cardiorespiratory measures during constant load and peak exercise (Table 1), none of these variables were significantly correlated with the decrease in RPB; except for RPE, which showed a weak correlation (r = 0.16, p < 0.001). Because of the changes in cardiorespiratory measures during exercise, we sought to estimate the effects of the most important measures on the change in RPB. We used a backward stepwise multiple regression analysis with ΔRPB as the dependent variable and ΔV̇E (absolute and as a % of MVV), ΔV̇O2 (absolute and as a % of peak V̇O2), ΔHR during 60 W cycling (absolute and as % of predicted), and Δpeak V̇O2 as the independent variables. Only the coefficients of ΔHR (%pred) [Partial R2 = 0.29, F = 6.95, p = 0.015] and absolute ΔV̇E [Partial R2 = 0.14, F = 5.87, p = 0.023] were significantly related to ΔRPB. The coefficient of multiple determination with these two variables was Model R2 = 0.43, indicating that approximately 43% of the variance of the change in ΔRPB could be accounted for by ΔHR (%pred) and absolute ΔV̇E. The regression equation generated yielded: ΔRPB = 0.28 + 0.12 ΔHR (%pred) + 0.12 ΔV̇E.
4. DISCUSSION
We found that 1) moderate weight loss via a 12-week diet and resistance exercise program could reduce ratings of breathlessness during submaximal exercise in obese women who experienced DOE at baseline (the +DOE group), which confirmed our hypothesis. Contrary to our hypothesis we found that 2) changes in body composition, fat distribution, pulmonary function, oxygen cost of breathing, or cardiorespiratory measures during exercise following weight loss were not associated with the reduction in RPB. Thus, although breathlessness during exercise is a common complaint in many obese individuals (Sin et al., 2002), the physiological mechanism(s) driving this symptom remain unclear. However, the significant decrease in dyspnea with only moderate weight loss of ~7 kg is very encouraging for obese individuals who struggle with breathlessness when exercising. Thus, moderate weight loss alone (i.e. without aerobic exercise training) appears to be an effective treatment to reduce exertional dyspnea in otherwise healthy, obese women.
To our knowledge, this is the first study to show the effect of weight loss on dyspnea ratings during exercise in otherwise healthy obese adults. Dyspnea at rest or low physical activity has been studied in obese patients undergoing invasive weight loss surgery. For example, bariatric surgery has been shown to result in significant improvements in self-reported dyspnea during activities of daily living (i.e. climbing two flights of stairs or walking with people of own age) (Karason et al., 2000) and quality of life (El-Gamal et al., 2005). Bariatric surgery results in dramatic weight loss and is a procedure with many associated risks. The present study demonstrates that moderate weight loss of 1–2 lb per week via a reduced calorie diet and resistive exercises three days per week significantly decreased DOE in obese women who had initially rated high breathlessness (i.e. RPB ≥ 4) during moderate cycling exercise at baseline testing. BMI decreased on average by 2.8 ± 1.3 kg/m2 and total body fat decreased by 3.4 ± 2.3%. Only 6 of the 29 subjects (3 +DOE and 3 −DOE) had a drop in BMI into the overweight range (i.e. lowest BMI was 26), while all other subjects were still considered obese following the 12-week weight loss program (i.e. BMI > 30). Additionally, the decrease in DOE was independent not only of the amount of weight lost, but also of the initial body weight (data not shown). We conclude that moderate weight loss achieved via diet and resistive exercises is effective in reducing DOE, which is very encouraging information for obese adults who get very breathlessness while exercising at moderate intensity.
Body composition and fat distribution could affect breathlessness, especially when excess fat is located primarily in the chest and abdomen. Abdominal fat displaces the diaphragm upward and impedes its downward movement during inspiration (DeLorey et al., 2005; Poulain et al., 2006). Fat deposition on the chest wall may impede expansion and excursion of the rib cage during inspiration, either via a direct loading effect or by changing intercostals muscle function. Central obesity, as measured by waist circumference and waist-to-hip ratio, has been shown to be detrimental to lung function (Steele et al., 2009). In the present study, all measures of body composition and fat distribution decreased with weight loss, except for waist-to-hip ratio due to similar decreases in both waist and hip circumferences (Table 1). However, these decreases were not correlated with the decrease in RPB during exercise (Table 2), suggesting that body composition and fat distribution do not have significant effects on breathlessness during exercise.
Obesity alters lung function; most consistently reported are reductions in FRC and ERV (Jones and Nzekwu, 2006; Pelosi et al., 1998). There is overwhelming evidence that significant weight loss improves lung function in both mild-to-moderate and severe obesity (Aaron et al., 2004; De Lorenzo et al., 1999; Emirgil and Sobol, 1973; Hakala et al., 1995; Santesson and Nordenstrom, 1978; Thomas et al., 1989; Wadstrom et al., 1991; Wei et al., 2011). We have previously shown that moderate weight loss improves breathing mechanics during exercise in obese men (Babb et al., 2011). In the present study, we also found improvements in FRC and ERV (Table 1); however, these changes were also not associated with the decreased RPB during exercise (Table 2). Thus, we conclude that pulmonary function also does not contribute significantly to the breathlessness experienced by some obese women during exercise.
In a previous study, we reported that the oxygen cost of breathing was significantly greater in obese women with DOE compared with obese women with no or mild DOE and that it was correlated with the RPB during submaximal exercise (Babb et al., 2008a). However, in the present cohort we failed to find this difference (Bernhardt et al., 2013a). This discrepancy may be due to the smaller numbers of subjects in the previous study (Babb et al., 2008a) and individual variability may have contributed to skewed results. In the present study, there was no significant difference in the oxygen cost of breathing between the two groups (Bernhardt et al., 2013a). Weight loss significantly decreased the oxygen cost of breathing in all subjects (Table 1), but it was not correlated with the decreased in RPB (Table 2), indicating that the work of breathing also did not play a role in the perception of DOE.
Obese individuals have higher oxygen consumption and minute ventilation at all work rates compared with normal weight subjects (Babb et al., 2002; Babb et al., 1991; Ofir et al., 2007; Whipp and Davis, 1984); however, breathlessness ratings at any given V̇O2 or V̇E are similar (Ofir et al., 2007), suggesting that respiratory mechanical factors may not contribute to the DOE. In agreement with our previous studies (Babb et al., 2002; Babb et al., 2008a; Lorenzo and Babb, 2011; Wood et al., 2008) and work by Dempsey (Dempsey et al., 1966), the subjects in the present study had normal ventilatory responses to exercise. There were no differences in the exercise responses between the obese women +DOE and −DOE that would explain why some women experienced much stronger breathlessness, implying that submaximal or maximal exercise variables also do not contribute significantly to breathlessness during exercise.
The lack of associations between the decreased RPB after weight loss and any of the physiological changes measured suggests that psychophysiological mechanisms might contribute to the decreased perception of DOE. Figure 3 shows the potential psychophysiological mechanisms of increased perception of breathlessness in obese individuals with DOE. Respiratory sensations are believed to be consciously perceived via a threshold-gated mechanism (Davenport and Reep, 1995). The idea is that only if ventilation is sufficiently changed or attended to, then this respiratory sensation will be gated through to the higher brain centers and the person becomes aware of his or her breathing (Chan and Davenport, 2008); whereas eucapnic breathing is usually subconscious. It is possible that the gating mechanism is different in those subjects who experience DOE compared with those who do not. Also, weight loss affect the gating of respiratory stimuli, i.e. fewer stimuli could be gated through to the cortex, such that the perception of breathlessness would be reduced following weight loss. There are at least 4 possibilities of why/how the respiratory sensation might be perceived higher in the +DOE subjects and why it might change with weight loss. 1) There could be a difference in the mechanical and/or chemical afferent feedback from the respiratory muscles. 2) There could be an adjustment in corollary discharge from the respiratory control center (el-Manshawi et al., 1986). 3) The gating threshold for sensory transmission might be altered (Davenport et al., 2007). And 4) input from gating modifiers, such as attention, experience, or emotions, may be different (Chan et al., 2012; Tsai et al., 2013; von Leupoldt et al., 2011). The more respiratory sensations are gated through to the sensory cortex, the higher the perception of the intensity of breathlessness would be. Also, the affective dimension of breathlessness could be increased. It is not clear if and how these mechanisms change with weight loss. Future studies are needed to investigate if any of these potential mechanisms play a role in DOE and if and how weight loss can change the gating of respiratory sensation.
Figure 3.
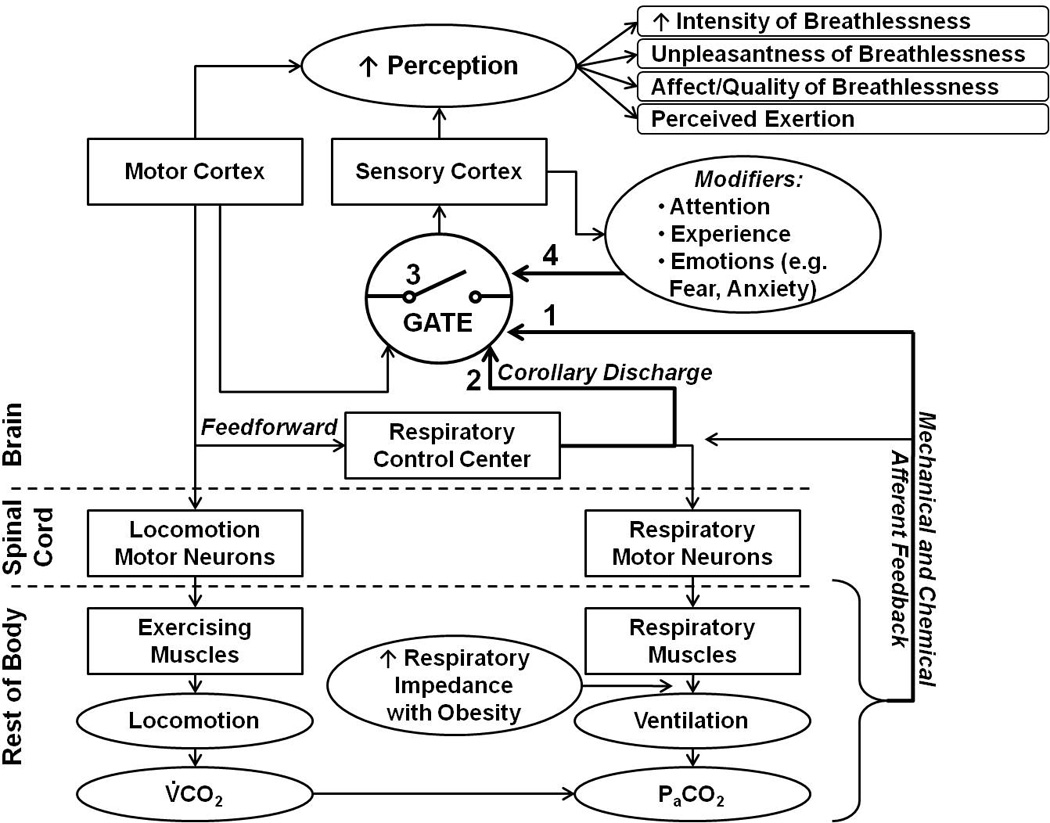
Schematic of potential psychophysiological mechanisms of perception of breathlessness. Respiratory sensations are believed to be consciously perceived via a threshold-gated mechanism; only if ventilation is sufficiently changed or attended to, then this respiratory sensation will be gated through to the higher brain centers and the person becomes aware of his or her breathing. Potential mechanisms that might contribute to differential perception of DOE include: 1) altered afferent feedback, 2) modified corollary discharge from the respiratory control center, 3) changed gating threshold for sensory transmission, or 4) decreased or increased input from gating modifiers. The more respiratory sensations are gated through to the sensory cortex, the higher the perception of the intensity of breathlessness would be.
4.1 Limitations
Subject recruitment and retention for this study was challenging due to various factors, such as intensive time commitment for the multiple testing sessions and the resistive exercise, and the requirement to follow a reduced calorie meal plan for 12 weeks. The limited number of subjects may reduce the generalization of the results, especially to higher obesity levels, older individuals, or patient populations. Nevertheless, a striking decrease in DOE was observed with a moderate weight loss in otherwise healthy obese women.
Future studies are needed to examine the effects of weight loss on DOE in obese men, as well as the effects of aerobic exercise training only (i.e. without weight loss) and a combined weight loss plus aerobic exercise training program on DOE in obese women and men in order to investigate potential mechanism(s) of reduced DOE (i.e. fat mass reduction vs cardiovascular conditioning).
4.2 Conclusions
In conclusion, we found that moderate weight loss was effective in reducing breathlessness on exertion in obese women who experienced DOE at baseline. Thus, health care providers may recommend moderate weight loss as a first step to those otherwise healthy obese patients with strong exertional dyspnea. The physiological mechanism(s) of this reduction in DOE remains unclear since the decrease in RPB was not significantly associated with changes in body composition, fat distribution, pulmonary function, oxygen cost of breathing, or cardiorespiratory measures.
Highlights.
Moderate weight loss effectively reduced dyspnea on exertion (DOE) in obese women
Oxygen cost of breathing decreased following weight loss
Body composition and pulmonary function improved with weight loss
Reduction in DOE was not significantly associated with physiological changes measured
ACKNOWLEDGMENTS
The authors wish to thank Raksa Moran, Todd Bassett, Dr. Santiago Lorenzo, Jessica Pineda, Joseph Genovese, Sarah Haller-Martineau, and Dr. Matthew Spencer for their assistance in various stages of this project, and the staff members of the Texas Health Finley Ewing Cardiovascular & Fitness Center Dallas (Gerry, Susan, Kim, Erika, Mark, Denise, Will, and others) for their expertise in implementing the diet and exercise program. The authors thank Dr. Trey Miller for statistical guidance and Dr. Paul Weatherall for MRI assistance with this project.
Funding for this project was received from National Institutes of Health (HL096782), King Charitable Foundation Trust, Cain Foundation, and Texas Health Presbyterian Hospital Dallas.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Vipa Bernhardt, Email: VipaBernhardt@TexasHealth.org.
Tony G. Babb, Email: TonyBabb@TexasHealth.org.
REFERENCES
- Plan and operation of the Third National Health and Nutrition Examination Survey, 1988–94. Series 1: programs and collection procedures. Vital and health statistics. Ser. 1, Programs and collection procedures. 1994:1–407. [PubMed] [Google Scholar]
- Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: executive summary. Expert Panel on the Identification, Evaluation, and Treatment of Overweight in Adults. Am J Clin Nutr. 1998;68:899–917. doi: 10.1093/ajcn/68.4.899. [DOI] [PubMed] [Google Scholar]
- Aaron SD, Fergusson D, Dent R, Chen Y, Vandemheen KL, Dales RE. Effect of weight reduction on respiratory function and airway reactivity in obese women. Chest. 2004;125:2046–2052. doi: 10.1378/chest.125.6.2046. [DOI] [PubMed] [Google Scholar]
- Azagury DE, Lautz DB. Obesity overview: epidemiology, health and financial impact, and guidelines for qualification for surgical therapy. Gastrointest Endosc Clin N Am. 2011;21:189–201. doi: 10.1016/j.giec.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Babb TG, DeLorey DS, Wyrick BL, Gardner PP. Mild obesity does not limit change in end-expiratory lung volume during cycling in young women. J Appl Physiol. 2002;92:2483–2490. doi: 10.1152/japplphysiol.00235.2001. [DOI] [PubMed] [Google Scholar]
- Babb TG, Korzick D, Meador M, Hodgson JL, Buskirk ER. Ventilatory response of moderately obese women to submaximal exercise. Int J Obes. 1991;15:59–65. [PubMed] [Google Scholar]
- Babb TG, Ranasinghe KG, Comeau LA, Semon TL, Schwartz B. Dyspnea on exertion in obese women: association with an increased oxygen cost of breathing. Am J Respir Crit Care Med. 2008a;178:116–123. doi: 10.1164/rccm.200706-875OC. [DOI] [PubMed] [Google Scholar]
- Babb TG, Wyrick BL, Chase PJ, Delorey DS, Rodder SG, Feng MY, Ranasinghe KG. Weight loss via diet and exercise improves exercise breathing mechanics in obese men. Chest. 2011;140:454–460. doi: 10.1378/chest.10-1088. [DOI] [PubMed] [Google Scholar]
- Babb TG, Wyrick BL, DeLorey DS, Chase PJ, Feng MY. Fat distribution and end-expiratory lung volume in lean and obese men and women. Chest. 2008b;134:704–711. doi: 10.1378/chest.07-1728. [DOI] [PubMed] [Google Scholar]
- Bassett JT, Bernhardt V, Lorenzo S, Moran RB, Haller SF, Pineda JN, Babb TG. Peak oxygen uptake differs between obese women with vs. without dyspnea on exertion. Med Sci Sports Exerc. 2013;45(5S):401. [Google Scholar]
- Bernhardt V, Babb TG. Respiratory symptom perception differs in obese women with strong or mild breathlessness during constant-load exercise. Chest. 2014;145:361–369. doi: 10.1378/chest.12-2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt V, Lorenzo S, Moran RB, Bassett JT, Haller SF, Pineda JN, Babb TG. Oxygen cost of breathing does not differ between women with vs. without dyspnea on exertion. Med Sci Sports Exerc. 2013a;45(5S):292. [Google Scholar]
- Bernhardt V, Moran RB, Bassett JT, Lorenzo S, Pineda JN, Babb TG. Weight loss reduces dyspnea on exertion in obese women independent of improvement in respiratory function. Am J Respir Crit Care Med. 2014;189:A5386. [Google Scholar]
- Bernhardt V, Wood HE, Moran RB, Babb TG. Dyspnea on exertion in obese men. Respir Physiol Neurobiol. 2013b;185:241–248. doi: 10.1016/j.resp.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- Chan PY, Davenport PW. Respiratory-related evoked potential measures of respiratory sensory gating. J Appl Physiol. 2008;105:1106–1113. doi: 10.1152/japplphysiol.90722.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan PY, von Leupoldt A, Bradley MM, Lang PJ, Davenport PW. The effect of anxiety on respiratory sensory gating measured by respiratory-related evoked potentials. Biol Psychol. 2012;91:185–189. doi: 10.1016/j.biopsycho.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport P, Reep R. Cerebral cortex and respiration. In: Dempsey J, Pack A, editors. Regulation of breathing. New York, NY: Dekker; 1995. pp. 362–388. [Google Scholar]
- Davenport PW, Chan PY, Zhang W, Chou YL. Detection threshold for inspiratory resistive loads and respiratory-related evoked potentials. J Appl Physiol. 2007;102:276–285. doi: 10.1152/japplphysiol.01436.2005. (1985) [DOI] [PubMed] [Google Scholar]
- De Lorenzo A, Petrone-De Luca P, Sasso GF, Carbonelli MG, Rossi P, Brancati A. Effects of weight loss on body composition and pulmonary function. Respiration. 1999;66:407–412. doi: 10.1159/000029423. [DOI] [PubMed] [Google Scholar]
- DeLorey DS, Wyrick BL, Babb TG. Mild-to-moderate obesity: implications for respiratory mechanics at rest and during exercise in young men. Int J Obes (Lond) 2005;29:1039–1047. doi: 10.1038/sj.ijo.0803003. [DOI] [PubMed] [Google Scholar]
- Dempsey JA, Reddan W, Rankin J, Balke B. Alveolar-arterial gas exchange during muscular work in obesity. J Appl Physiol. 1966;21:1807–1814. doi: 10.1152/jappl.1966.21.6.1807. [DOI] [PubMed] [Google Scholar]
- Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK American College of Sports, M. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41:459–471. doi: 10.1249/MSS.0b013e3181949333. [DOI] [PubMed] [Google Scholar]
- El-Gamal H, Khayat A, Shikora S, Unterborn JN. Relationship of dyspnea to respiratory drive and pulmonary function tests in obese patients before and after weight loss. Chest. 2005;128:3870–3874. doi: 10.1378/chest.128.6.3870. [DOI] [PubMed] [Google Scholar]
- el-Manshawi A, Killian KJ, Summers E, Jones NL. Breathlessness during exercise with and without resistive loading. J Appl Physiol. 1986;61:896–905. doi: 10.1152/jappl.1986.61.3.896. (1985) [DOI] [PubMed] [Google Scholar]
- Emirgil C, Sobol BJ. The effects of weight reduction on pulmonary function and the sensitivity of the respiratory center in obesity. Am Rev Respir Dis. 1973;108:831–842. doi: 10.1164/arrd.1973.108.4.831. [DOI] [PubMed] [Google Scholar]
- Gibson GJ. Obesity, respiratory function and breathlessness. Thorax. 2000;55(Suppl 1):S41–S44. doi: 10.1136/thorax.55.suppl_1.s41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakala K, Mustajoki P, Aittomaki J, Sovijarvi AR. Effect of weight loss and body position on pulmonary function and gas exchange abnormalities in morbid obesity. Int J Obes Relat Metab Disord. 1995;19:343–346. [PubMed] [Google Scholar]
- Jones RL, Nzekwu MM. The effects of body mass index on lung volumes. Chest. 2006;130:827–833. doi: 10.1378/chest.130.3.827. [DOI] [PubMed] [Google Scholar]
- Karason K, Lindroos AK, Stenlof K, Sjostrom L. Relief of cardiorespiratory symptoms and increased physical activity after surgically induced weight loss: results from the Swedish Obese Subjects study. Arch Intern Med. 2000;160:1797–1802. doi: 10.1001/archinte.160.12.1797. [DOI] [PubMed] [Google Scholar]
- Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, Vasan RS. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- Lorenzo S, Babb TG. Quantification of Cardiorespiratory Fitness in Healthy Nonobese and Obese Men and Women. Chest. 2011 doi: 10.1378/chest.11-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran RB, Bernhardt V, Lorenzo S, Bassett JT, Haller SF, Pineda JN, Babb TG. Pulmonary function does not differ between obese women with and without dyspnea on exertion. Med Sci Sports Exerc. 2013;45(5S):401. [Google Scholar]
- O'Donnell CP, Holguin F, Dixon AE. Pulmonary physiology and pathophysiology in obesity. J Appl Physiol. 2010;108:197–198. doi: 10.1152/japplphysiol.01208.2009. (1985) [DOI] [PubMed] [Google Scholar]
- Ofir D, Laveneziana P, Webb KA, O'Donnell DE. Ventilatory and perceptual responses to cycle exercise in obese women. J Appl Physiol. 2007;102:2217–2226. doi: 10.1152/japplphysiol.00898.2006. (1985) [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelosi P, Croci M, Ravagnan I, Tredici S, Pedoto A, Lissoni A, Gattinoni L. The effects of body mass on lung volumes, respiratory mechanics, and gas exchange during general anesthesia. Anesthesia and analgesia. 1998;87:654–660. doi: 10.1097/00000539-199809000-00031. [DOI] [PubMed] [Google Scholar]
- Pineda JN, Bernhardt V, Lorenzo S, Moran RB, Bassett JT, Haller SF, Babb TG. Similar body composition and fat distribution between obese women with vs. without dyspnea on exertion. Med Sci Sports Exerc. 2013;45(5S):294. [Google Scholar]
- Poulain M, Doucet M, Major GC, Drapeau V, Series F, Boulet LP, Tremblay A, Maltais F. The effect of obesity on chronic respiratory diseases: pathophysiology and therapeutic strategies. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne. 2006;174:1293–1299. doi: 10.1503/cmaj.051299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santesson J, Nordenstrom J. Pulmonary function in extreme obesity. Influence of weight loss following intestinal shunt operation. Acta chirurgica Scandinavica. Supplementum. 1978;482:36–40. [PubMed] [Google Scholar]
- Sin DD, Jones RL, Man SF. Obesity is a risk factor for dyspnea but not for airflow obstruction. Arch Intern Med. 2002;162:1477–1481. doi: 10.1001/archinte.162.13.1477. [DOI] [PubMed] [Google Scholar]
- Steele RM, Finucane FM, Griffin SJ, Wareham NJ, Ekelund U. Obesity is associated with altered lung function independently of physical activity and fitness. Obesity. 2009;17:578–584. doi: 10.1038/oby.2008.584. [DOI] [PubMed] [Google Scholar]
- Thomas PS, Cowen ER, Hulands G, Milledge JS. Respiratory function in the morbidly obese before and after weight loss. Thorax. 1989;44:382–386. doi: 10.1136/thx.44.5.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HW, Chan PY, von Leupoldt A, Davenport PW. The impact of emotion on the perception of graded magnitudes of respiratory resistive loads. Biol Psychol. 2013;93:220–224. doi: 10.1016/j.biopsycho.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–880. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- von Leupoldt A, Vovk A, Bradley MM, Lang PJ, Davenport PW. Habituation in neural processing and subjective perception of respiratory sensations. Psychophysiology. 2011;48:808–812. doi: 10.1111/j.1469-8986.2010.01141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadstrom C, Muller-Suur R, Backman L. Influence of excessive weight loss on respiratory function. A study of obese patients following gastroplasty. The European journal of surgery = Acta chirurgica. 1991;157:341–346. [PubMed] [Google Scholar]
- Wei YF, Tseng WK, Huang CK, Tai CM, Hsuan CF, Wu HD. Surgically induced weight loss, including reduction in waist circumference, is associated with improved pulmonary function in obese patients. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery. 2011;7:599–604. doi: 10.1016/j.soard.2011.04.221. [DOI] [PubMed] [Google Scholar]
- Whipp BJ, Davis JA. The ventilatory stress of exercise in obesity. Am Rev Respir Dis. 1984;129:S90–S92. doi: 10.1164/arrd.1984.129.2P2.S90. [DOI] [PubMed] [Google Scholar]
- Wood HE, Semon TL, Comeau LA, Schwartz B, MacDougall RM, Klocko MN, Babb TG. The ventilatory response to exercise does not differ between obese women with and without dyspnea on exertion. Adv Exp Med Biol. 2008;605:514–518. doi: 10.1007/978-0-387-73693-8_90. [DOI] [PubMed] [Google Scholar]


