Abstract
Thermosensitive liposomes have emerged as a viable strategy for localized delivery and triggered release of chemotherapy. MR-guided focused ultrasound (MRgFUS) has the capability of heating tumors in a controlled manner, and when combined with thermosensitive liposomes can potentially reduce tumor burden in vivo. However, the impact of this drug delivery strategy has rarely been investigated. We have developed a unique liposome formulation modified with p(NIPAAm-co-PAA), a polymer that confers sensitivity to both temperature and pH. These polymer-modified thermosensitive liposomes (PTSL) demonstrated sensitivity to focused ultrasound, and required lower thermal doses and were more cytotoxic than traditional formulations in vitro. A set of acoustic parameters characterizing optimal release from PTSL in vitro was applied in the design of a combined MRgFUS/PTSL delivery platform. This platform more effectively reduced tumor burden in vivo when compared to free drug and traditional formulations. Histological analysis indicated greater tumor penetration, more extensive ECM remodeling, and greater cell destruction in tumors administered PTSL, correlating with improved response to the therapy.
Keywords: drug delivery, thermosensitive liposomes, smart polymers, MR-guided focused ultrasound, NIPAAm, chemotherapy
Introduction
Traditional thermosensitive liposomes (TSL) for triggered release of chemotherapy are comprised of lipids that undergo temperature-dependent phase transitions, becoming more permeable at elevated temperatures [1–4]. More recently, lysolipids have been employed to modify formulations, greatly increasing the rate and amount of drug release while reducing the thermal dose threshold for release [5–7]. However, lysolipids have been shown to desorb from the membrane in the presence of plasma proteins and cellular membrane pools [8, 9], resulting in a reduction in thermosensitivity [9] and premature drug leakage at physiological conditions (up to 80% within 30 min at 37°C) [10–13].
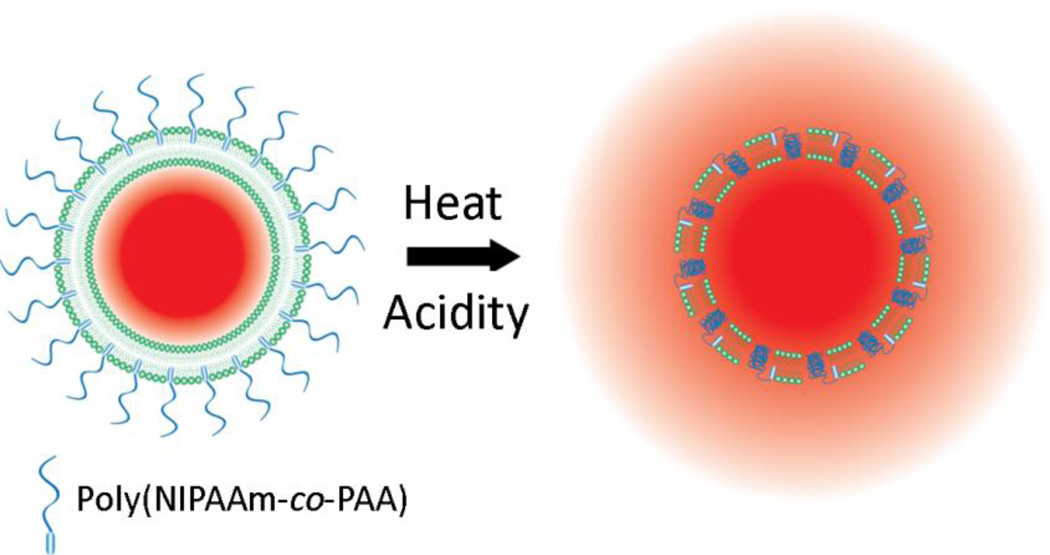
An alternate approach is to incorporate synthetic polymers that are membrane-disruptive and trigger drug release in response to heating. Recently we copolymerized N-isopropylacrylamide (NIPAAm) and propylacrylic acid (PAA) to produce a membrane-disruptive copolymer that is sensitive to both temperature and pH [11] (Fig. 1), the latter component aiming to take advantage of the tumor microenvironment which is known to be mildly acidic [12]. We showed that liposomes modified with NIPAAm-co-PAA stably entrapped drug in serum-containing media and released more than 50% of entrapped doxorubicin rapidly when heated (T≥40°C) or subjected to mild acidity (pH≤6.5) [11]. Thus, these polymer-modified thermosensitive liposomes (PTSL) can be used for localized deposition of chemotherapy in vivo provided that solid tumors can be heated in a localized and controlled manner.
Figure 1.
DOX release from p(NIPAAm-co-PAA)-modified thermosensitive liposomes (PTSL). Release is triggered by both heating and reduction in pH.
Focused ultrasound (FUS) has emerged as a leading modality for non-invasive, heat-triggered drug release from thermosensitive liposomes [14]. Studies with TSL have shown improved tumor response with alternative modalities such as microwave antennas and applicators [15]. These suffer from limited therapeutic depth and are thus only suitable for superficial tumors, and offer less spatial and temporal control of heating compared to FUS [14, 15]. Furthermore, the emergence of MR-compatible FUS transducers has allowed for the adoption of MR thermometry as a tool to noninvasively monitor the spatial distribution of heat deposited by FUS [16–18]. Recent studies have shown that MR-guided focused ultrasound (MRgFUS) can be utilized to deposit heat and trigger local drug release from thermosensitive liposomes within solid tumors [19–22]. However, the impact of this drug delivery strategy on tumor growth has rarely been investigated.
Therefore, the main objective of this study was to evaluate the response of solid tumors to DOX released from PTSL via sustained MRgFUS heating. First, DOX release from PTSL was assessed as a function of acoustic intensity and pulsing scheme. Knowledge gained from these experiments was used to tailor the output from an MRgFUS transducer to sustain a desired elevated temperature in tumors in vivo. PTSL were designed to release DOX in mildly acidic and/or hyperthermic conditions, both of which are achievable in solid tumors. Thus, we conducted in vitro studies to assess the cytotoxicity of DOX released from our dual-sensitive PTSL as a function of temperature and pH. Finally, we evaluated the response of solid tumors to DOX released from PTSL heated with MRgFUS by measuring tumor growth and through histological analysis of tumor remodeling at the cellular level.
Materials and Methods
Materials
N-isopropylacrylamide (NIPAAm), 2,2′-azobis(2-methylpropionitrile) (AIBN), propylacrylic acid (PAA), doxorubicin hydrochloride (DOX), 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), and manganese sulfate (MnSO4) were purchased from Sigma (St. Louis, MO, USA). NIPAAm was recrystallized in hexane and AIBN recrystallized in methanol prior to use. 2-methyl-2-[(dodecylsulfanylthiocarbonyl)sulfanyl]propanoic acid (DMP) was purchased from Strem Chemicals, Inc. (Newburyport, MA, USA). 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), L-a-phosphatidyl-choline(soy-hydrogenated) (HSPC), cholesterol (CHOL), and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (DSPE-PEG-2000) were purchased from Avanti Polar Lipids, Inc. (Alabaster, AL, USA). MTT cell proliferation assay kits, McCoy’s 5A medium, human mammary adenocarcinoma MCF7 cells, and rat mammary adenocarcinoma 13762 MAT B III cells were obtained from American Type Culture Collection (Manassas, VA). Fetal bovine serum (FBS) was purchased from Life Technologies (Grand Island, NY). Dulbecco’s Modified Eagle Medium (DMEM), Penicillin-Streptomycin (PS), and L-Glutamine (L-Glu) were purchased from Corning (Manassas, VA).
Polymer synthesis and characterization
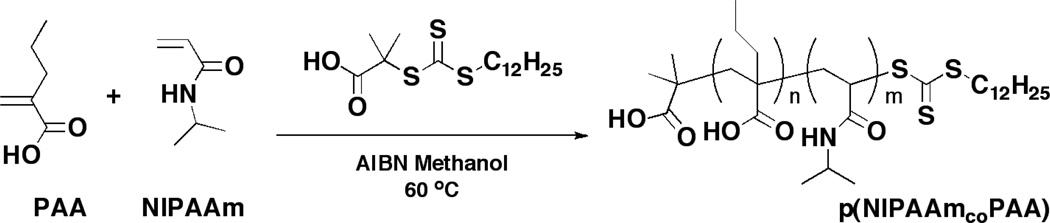
p(NIPAAm-co-PAA) was synthesized using RAFT chemistry with initiator AIBN, chain transfer agent DMP, and monomers NIPAAm and PAA (Scheme 1), as described in [11] with minor modifications. NIPAAm (2.18 g, 19.3 mmol), PAA (0.138 g, 1.21 mmol), DMP (60.8 mg, 167 µmol), and AIBN (15.3 mg, 93.2 µmol) were reacted in degassed methanol at 60°C for 17 hr. Alternatively, DMP and AIBN concentrations were halved to study the effect of degree of polymerization (DP, DP = M0/(CTA0+2·I0), where M0, CTA0, and I0 are initial concentrations of monomer, chain transfer agent, and initiator, respectively). Following precipitation into pentane, dried polymer was dissolved in ethanol and dialyzed against deionized water to remove unreacted monomer and impurities. Samples were then frozen and lyophilized. Polymer molecular weights were determined using a gel permeation chromatograph (Viscotek) with dimethylformamide (DMF) as eluent (1 ml/min) and polystyrene calibration standards. Copolymer composition was determined via 1H-NMR (Varian 400 MHz FT-NMR, deuterated chloroform as solvent) and analysis of peaks as in [11].
Scheme 1.
RAFT copolymerization of N-isopropylacrylamide (NIPAAm) and propylacrylic acid (PAA), originally published in [11].
Preparation and characterization of DOX-loaded liposomes
DOX-loaded liposomes were prepared by the lipid film hydration and extrusion method followed by pH-gradient-driven DOX loading as previously described [11], with slight modifications. Nonthermosensitive liposome (NTSL), traditional thermosensitive liposome (TSL), and polymer-modified thermosensitive liposome (PTSL) were generated, with compositions as follows: NTSL = HSPC:CHOL:DSPE-PEG-2000 at 75:50:3 molar ratio, TSL = DPPC:HSPC:CHOL:DSPE-PEG-2000 at 100:50:30:6, and PTSL with identical composition as TSL but with the addition of copolymer (see below). Chloroform was removed by rotary evaporation (Buchi R-210), leaving a dried film that was then rehydrated in 300 mM MnSO4 (pH 3.5), giving a suspension with lipid concentration of 10 mg/ml. The suspension was extruded [11] after which unentrapped MnSO4 was removed and pH gradient established by dialysis (1 kDa MWCO) against 20 mM HEPES buffer (pH 7.5). DOX was added to the dialyzed suspension at a 1:10 drug-to-lipid mass ratio and the suspension incubated at 38°C for 1 hr and then at room temperature overnight. Unentrapped DOX was removed by dialysis (1 kDa MWCO) against 20 mM HEPES. For PTSL, polymer at 2.5% molar ratio polymer:lipid was added to DOX-loaded liposomes and liposomes were incubated at 30°C for 1 hr and then at room temperature overnight. A final dialysis (50 kDa MWCO) against HEPES removed unincorporated polymer. For the in vitro cytotoxicity and in vivo studies, suspensions were sterile-filtered (0.2 µm) prior to administration. For the in vivo study, a greater concentration of lipid (50 mg/ml) was used to provide more highly concentrated drug suspensions. Liposome size and zeta potentials were measured via dynamic light scattering (Brookhaven Instruments, 90Plus Zeta). DOX:lipid mass ratios were calculated by taking advantage of the self-quenching properties of DOX [11] and quantifying lipid content via total phosphorus assay [23].
Measurement of FUS-triggered release
Drug release from the various formulations was studied as a function of time, temperature, and pH. Two heating modalities were compared: conventional heating with a circulating water bath and heating from focused ultrasound. Drug release from liposomes heated via bath was measured as in [11], and compared to release behavior observed in liposomes exposed to FUS-mediated heating from a 5-MHz transducer (Sonic Concepts).
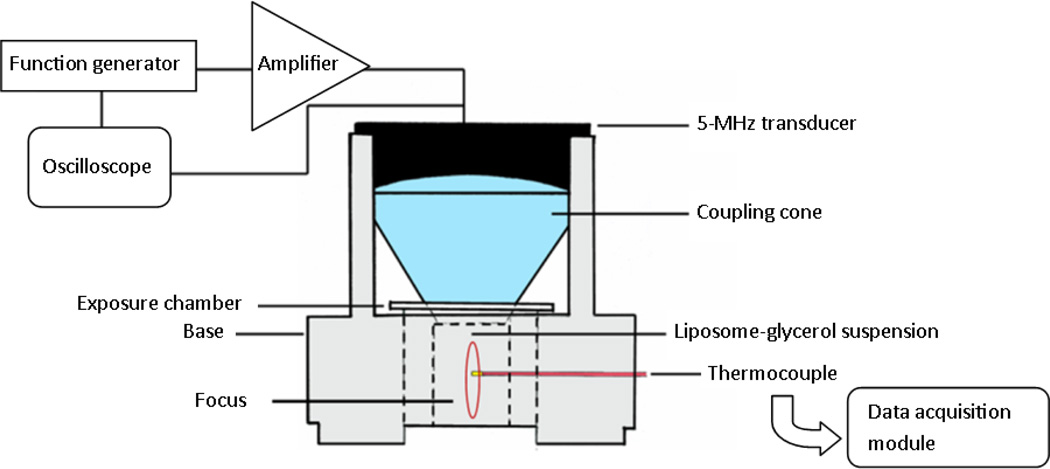
Prior to the release studies, a custom-assembled radiation force balance was used to calibrate the transmitted acoustic intensity (I, source of heating) from the transducer as a function of peak-to-peak excitation voltage (Vpp) (Supporting Information, Fig. S1). The setup illustrated in Fig. 2 was then used to measure temperature elevation as a function of I. A mixture of 50% glycerol in 20 mM HEPES (v/v) was chosen as the ultrasound absorption medium, with a measured attenuation coefficient of 0.795 dB/(cm×MHz) that compares favorably to that of soft tissue [24]. We anticipated the results from this part of the study would define a baseline set of acoustic parameters that could be adjusted if necessary for sustained heating in vivo in the presence of perfusion. The transducer was oriented vertically and attached to a coupling cone filled with degassed water. The exposure chamber (diameter 15 mm, height 20 mm) was filled with the adsorption medium and the transducer-cone allowed to rest on top of the chamber. Spatially, the bottom of the cone correlated with the transducer focus; thus the sample volume was always positioned within the focus. A thin, acoustically transparent Mylar® film separated the cone and the exposure chamber. Insertion of a 0.2-mm needle thermocouple connected to a data acquisition unit (National Instruments, USB-9162) provided online measurements of chamber temperature and allowed feedback control of transducer output to maintain a predetermined temperature elevation. The setup was placed in a shallow water bath at an ambient temperature of 37°C, and the transducer driven at varying Vpp and duty cycle (DC). Ultrasound-mediated heating in the chamber was recorded and plotted as a function of time. By observing the temperature elevation resulting from a given Vpp and DC, and utilizing the relationship between intensity and applied voltage, temperature could be determined as a function of I, and a viable set of acoustic parameters could be chosen.
Figure 2.
Experimental setup for monitoring FUS-mediated thermal elevations and FUS-triggered drug release from liposomes.
The same setup was then used to insonify suspensions containing liposomes. Here the chamber was filled with 3 ml 20 mM HEPES containing 50% v/v glycerol and 20% v/v FBS. The suspension was allowed to equilibrate to 37°C, after which 150 µl DOX-loaded liposome suspension (TSL, PTSL, or NTSL) was added. The transducer was driven at the appropriate acoustic parameters (I and DC to sustain 39°C or 42°C over a 3 min window, determined from the previous calibration studies). A typical exposure consisted of a 16.94 kW/cm2, 50% DC initial exposure followed by a 5.48–11.26 kW/cm2, 25–50% DC exposure to sustain temperatures at 39°C or 42°C. Ultrasound was applied for 3 min, after which the liposome suspension was removed. Drug release was determined by measuring DOX fluorescence as described previously [11]. These results were compared to samples heated with a circulating bath (Neslab EX-7) for 3 min.
In vitro cytotoxicity study
The cytotoxicity of liposomal DOX (PTSL, TSL) and free DOX against MCF-7 cells was determined via cell culture and MTT assay. A low seed density (500 cells/well) combined with a long incubation time after treatment (192 hr) was found to provide the optimal effective drug concentration and sufficient time to maximize observed differences in cytotoxicity between treatment types, respectively.
MCF-7 cells were cultured in monolayers in DMEM growth media (pH 7.5) supplemented with 10% FBS, 1% PS, and 2% L-Glu, and maintained in a humidified incubator at 37°C and 5% CO2 atmosphere. Cells were plated onto flat-bottomed 96-well plates at 500 cells/well and allowed to adhere overnight after which they were exposed for 4 hr to free DOX, DOX-loaded liposomes, or empty liposomes (i.e. non-drug loaded) suspended in growth media. To determine heating effects on cytotoxicity, media (pH 7.5) containing free DOX, DOX-loaded TSL, or DOX-loaded PTSL were heated to 43°C for 5 min using a heated bath and cooled to 37°C prior to 4 hr exposure to cells. Results were compared to cells exposed to identical solutions that were not pre-heated. To determine the impact of mild acidity on cytotoxicity of DOX-loaded PTSL, treatment solutions containing DOX-loaded PTSL at pH 7.5, 7, and 6.5 were exposed to cells for 4 hr. Solutions at pH 7.5, 7, and 6.5 containing equivalent concentrations of empty PTSL were included as negative controls. After the 4 hr treatments, cells in all treatment groups were washed and incubated at 37°C in growth media (pH 7.5) for 192 hr after which an MTT assay was performed per manufacturer’s instruction. Absorbance at 570 nm (SpectraMax M2 plate reader) was measured, and cell viability calculated as the ratio of absorbance of treatment wells to absorbance of wells incubated in drug-free media of the same pH as treatment solutions.
Tumor growth evaluation
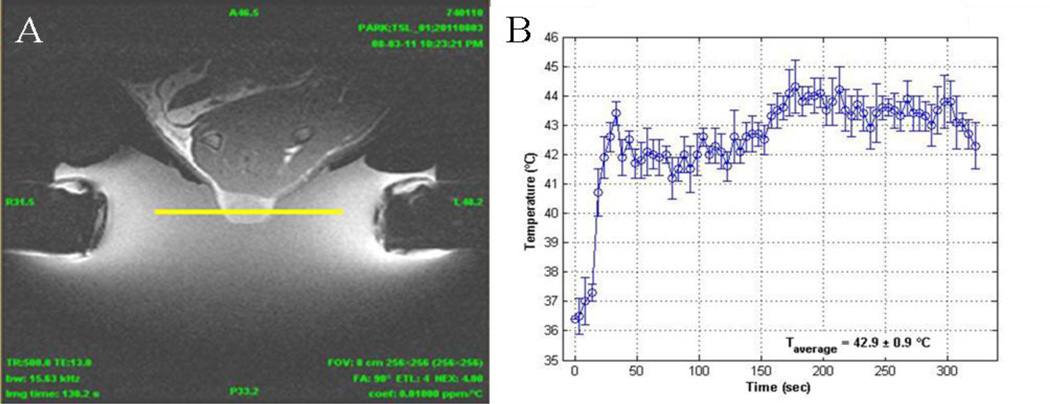
Fischer F344 rats weighing ~200 g were purchased from Charles River Laboratories (Wilmington, MA) and housed in Harvard Medical School’s animal research facility per protocols approved by its Animal Care and Use Committee. 13762 MAT B III cells were cultured in monolayers in McCoy’s 5A growth medium supplemented with 10% FBS and maintained in humidified chamber (37°C, 5% CO2 atmosphere). Approximately 2×106 cells were injected subcutaneously in the hindlimb of rats and tumors allowed to grow to a volume of ~200 mm3 before treatment. Animals were divided into seven treatment groups: (a) untreated; (b) free DOX (5 mg/kg); (c) TSL-DOX (5 mg/kg) + FUS (43°C); (d) PTSL-DOX (3 mg/kg) + FUS (43°C); (e) PTSL-DOX (5 mg/kg); (f) PTSL-DOX (5 mg/kg) + FUS (40°C); and (g) PTSL-DOX (5 mg/kg) + FUS (43°C). In groups receiving FUS, exposures were administered 6 hr after injection, and temperatures monitored and maintained at 40°C or 43°C for 5 min using a custom-built MR-compatible transducer (8 elements sector vortex, f = 1.15 MHz). Transducer output was calibrated using a radiation force balance as described above. MR imaging guided the placement of the transducer focus within the tumor (Fig. 3A), and MR thermometry was used to monitor heating. Desired temperatures ± 1°C were sustained for 5 min (Fig. 3B). After treatment, tumor growth was monitored over time. The long and short axes of the tumor and tumor thickness were measured using a caliper (Mitutoyo Corp., Model CD-6 CS) and tumor volume calculated according to:
| (1) |
If at any point during the experiment the long and short-axes of a tumor both exceeded 15 mm, or if a necrotic tissue region larger than 5 mm in diameter was observed, the animal was euthanized.
Figure 3.
MR-guided FUS (8 elements sector vortex, 1.15 MHz) was used to heat implanted tumors. (A) MR-image of solid tumor during FUS exposure. Yellow line represents plane at which MR thermometry quantifies temperature elevations as a function of time, with trace shown in (B).
Histological evaluation of liposome accumulation and tumor response
Tumor-bearing Fischer rats were treated as described above. Twenty-four hours after liposome injections, tumors were collected to morphologically assess the distribution of liposomes and doxorubicin in tumors related to treatments with (a) TSL-DOX (5 mg/kg) + FUS (40°C); (b) TSL-DOX (5 mg/kg) + FUS (43°C); (c) PTSL-DOX (5 mg/kg) + FUS (40°C); and (d) PTSL-DOX (5 mg/kg) + FUS (43°C). Additionally, 14 days after treatment, tumors were collected to assess the morphological alterations related to FUS treatments at 43°C. Groups included: (a) untreated; (b) free DOX; (c) TSL-DOX (5 mg/kg) + FUS (43°C), and (d) PTSL-DOX (5 mg/kg) + FUS (43°C). Tumors were harvested and flash frozen in OCT with liquid nitrogen. Frozen samples were stored at −20°C until use. For morphological evaluation, 20 µm sections were collected using a cryotome (Microm HM 525), fixed in 4% paraformaldehyde, and stained with haematoxylin and eosin (H&E). Images were captured by a Nikon Eclipse 50i microscope and SPOT camera (Micro Video Instruments, Avon, MA) and histological evaluation was performed. To characterize ECM remodeling as a marker for tumor shrinkage and resolution, quantitative image analyses were performed with fluorescence microscopy using the autofluorescence properties of the ECM. Image thresholding was applied and fluorescent pixels counted and divided by the total number of pixels in each image to calculate percent area. Mean intensities of images were calculated after applying thresholding to create selection masks. Data was represented as averages and standard deviations of percent area and intensity of at least 18 images in each group.
Determination of tumor DOX concentration
For determining tumor DOX concentration, portions of tumors from animals used in the histological evaluation were cut, dried, and weighed. Acidified ethanol (50% EtOH, 0.3 N HCl) was added for 0.05 mg tissue/ml, and the tissue homogenized (Bullet Blender, Next Advance). Following homogenization, samples were stored overnight at 4°C to complete extraction of DOX. Samples were then centrifuged and DOX fluorescence measured (Shimadzu RF-1501, λexcitation = 479 nm, λemission = 558 nm) and compared to standard curves of known DOX concentration to extrapolate the concentration of DOX in the tumor.
Statistical analyses
Measurement of FUS-triggered release
Drug release data was analyzed by one-way analysis of variance (ANOVA). A modified version of Levene’s test was used to test the assumption of equal variance. Provided that equal variance could be assumed, statistically significant differences between pairs of mean values were determined with ANOVA followed by Tukey-Kramer tests. Mean differences with p-values<0.05 were considered statistically significant.
In vitro cytotoxicity
Differences in cell viability between groups were analyzed with the Student’s t test, and mean differences with p-values<0.05 were considered statistically significant.
Tumor growth evaluation
Mean tumor growth of the control groups and treatment groups were analyzed by ANOVA as described above for the FUS-triggered release study.
Results
Polymer synthesis and characterization
The DP for a typical synthesis of p(NIPAAm-co-PAA) was 57.9. A DP of 115.8 (twofold increase) was studied by halving the total concentration of DMP and AIBN. Polymer molecular weights were determined with gel permeation chromatography and found to be linearly proportional to degree of polymerization (Table 1), with DP of 57.9 and 115.8 yielding molecular weights of 21,171 and 40,048 Da, respectively. RAFT yielded polymers with low polydispersity (Mw/Mn of 1.25 and 1.09). Varying the DP had little effect on final copolymer composition.
Table 1.
Poly(N-isopropylacrylamide-co-propylacrylic acid) characteristics
| DP | PAA (mol %) in feed | PAA (mol %) in polymer | Molecular weight (Mn) (Da) | Mw/Mn |
|---|---|---|---|---|
| 57.9 | 6.0 | 4.1a | 21,171b | 1.25 |
| 115.8 | 6.0 | 4.1a | 40,048b | 1.09 |
Estimated from 1H NMR
Determined by GPC in DMF (flow rate 1 ml/min) using polystyrene standards
Liposome synthesis and characterization
Liposomes were of uniform size (~120–130 nm) as determined by dynamic light scattering, with no statistically significant differences observed between groups (Table 2). Mildly negative zeta potentials were observed with NTSL and TSL (−12–13 mV), with no statistically significant difference observed between the formulations. Addition of polymer resulted in a statistically significant reduction in negative zeta potential to −6.5 mV (Table 2). Results of total phosphorus assays were combined with DOX encapsulation calculations (as described in [11]) to determine drug:lipid mass ratios. NTSL, TSL, and PTSL all demonstrated final DOX:lipid mass ratios of approximately 0.06, comparing well with values reported by other groups that tested DPPC-based liposomes loaded at similar initial drug:lipid ratios [10].
Table 2.
Liposome characteristics
| Formulation | Diameter (nm) | Zeta potential (mV) | Drug:Lipid (mg:mg) |
|---|---|---|---|
| NTSL | 129.7 ± 8.7 | −12.0 ± 1.3 | 0.0633 ± 0.005 |
| TSL | 131.7 ± 13.3 | −13.8 ± 5.1 | 0.0669 ± 0.012 |
| PTSL | 122.2 ± 1.1 | −6.5 ± 2.9 | 0.0649 ± 0.018 |
FUS-triggered release in vitro
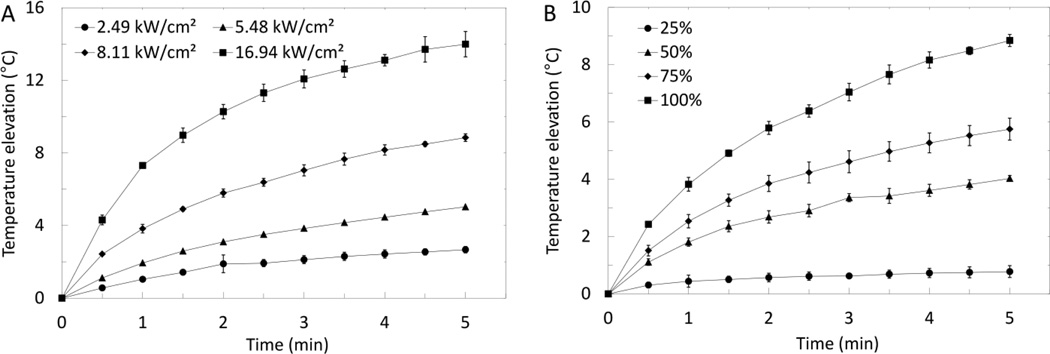
The radiation force generated from a 5-MHz FUS transducer was measured at varying Vpp, and converted to I (see (1) and (2) in Supporting Information). Thermal elevations in a 50% glycerol solution were then measured as a function of time over a range of I and DC (Fig. 4). When the transducer was driven in continuous wave (i.e. 100% DC) and varying I (2.49 – 16.94 kW/cm2), temperature elevations increased exponentially with time (Fig. 4A) in the form ΔT = A·(1−e−t), where A is a constant that varies with intensity, t is time, and ΔT is temperature elevation. Temperature elevations observed at 5 min FUS exposure increased linearly with intensity (R2 = 0.9681, curve not shown). At constant I (8.11 kW/cm2) and varying DC, temperature elevations also increased exponentially with time (Fig. 4B) in the form ΔT = A·(1−e− t), where A is a constant that varies with DC. Temperature elevations observed at 5 min FUS exposure increased linearly with DC (R2 = 0.9874, curve not shown). As I or DC increases, more energy is deposited resulting in an increase in heating rate and a larger peak temperature elevation. Observed temperature elevations plateau, suggesting that the heat deposited is balanced by heat loss. Since PTSL release 50% of encapsulated drug after 5 min incubations at 39.6°C, it was determined that thermal elevations of 3–5°C would be sufficient in subsequent FUS-triggered release studies. Given the exponential relationship between temperature elevation and FUS exposure time, exposures used in these studies consisted of a relatively high-intensity initial exposure to reach desired temperatures, followed by a lower-intensity exposure to maintain temperature elevations. Typical exposures used to achieve and maintain a desired elevated temperature were an initial exposure at 16.94 kW/cm2, 50% DC to reach the temperature of interest, followed by a 5.48–11.26 kW/cm2, 25–50% DC exposure to sustain the desired temperature.
Figure 4.
Temperature elevations in a 50% glycerol/20 mM HEPES (154 mM I) mixture exposed to (A) FUS (f=5 MHz, continuous wave) at varying acoustic intensities, and (B) FUS (f=5 MHz, I=8.11 kW/cm2) at varying DC. Data shown are averages ± SD from n=3 independent experiments.
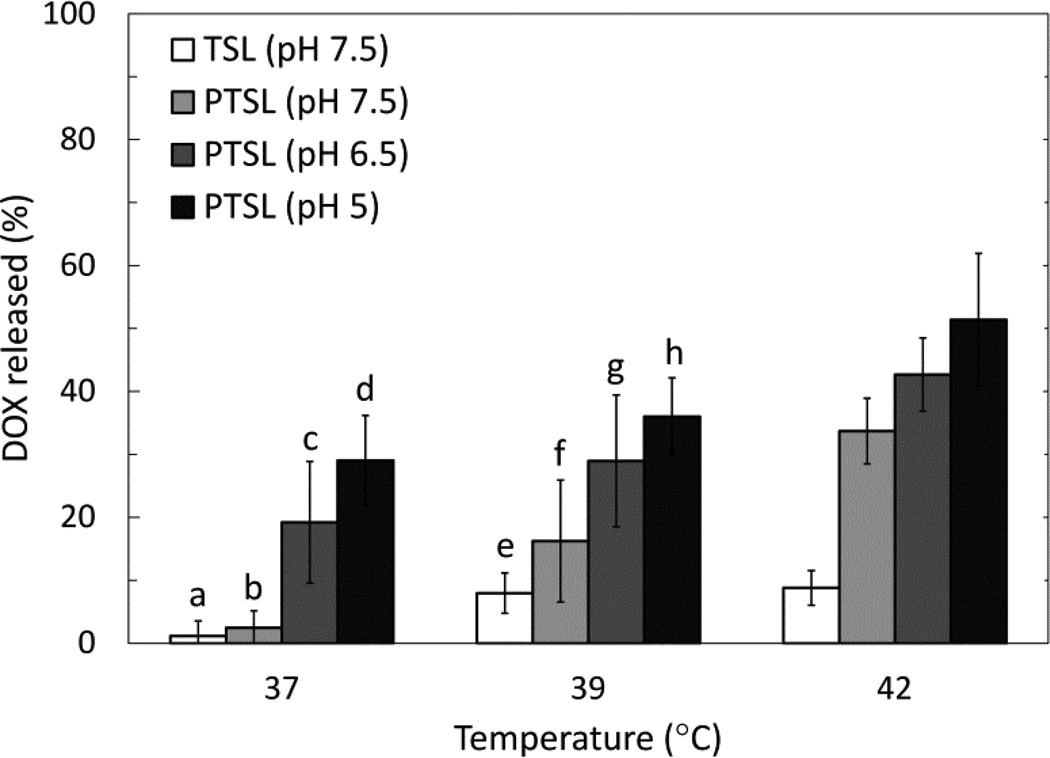
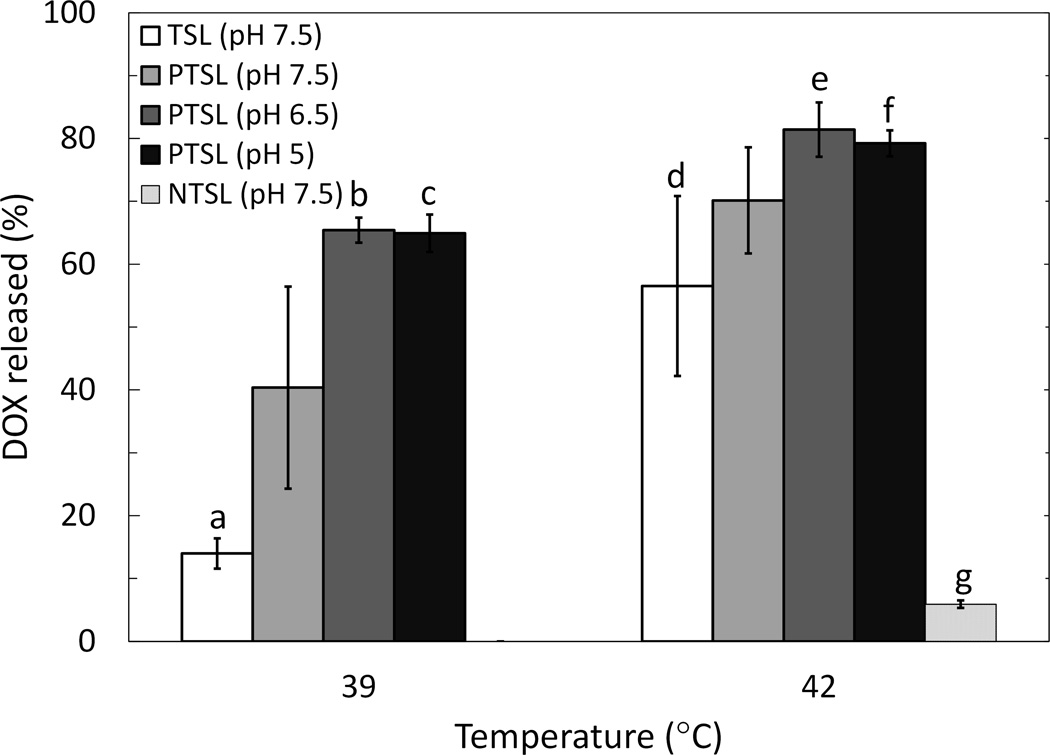
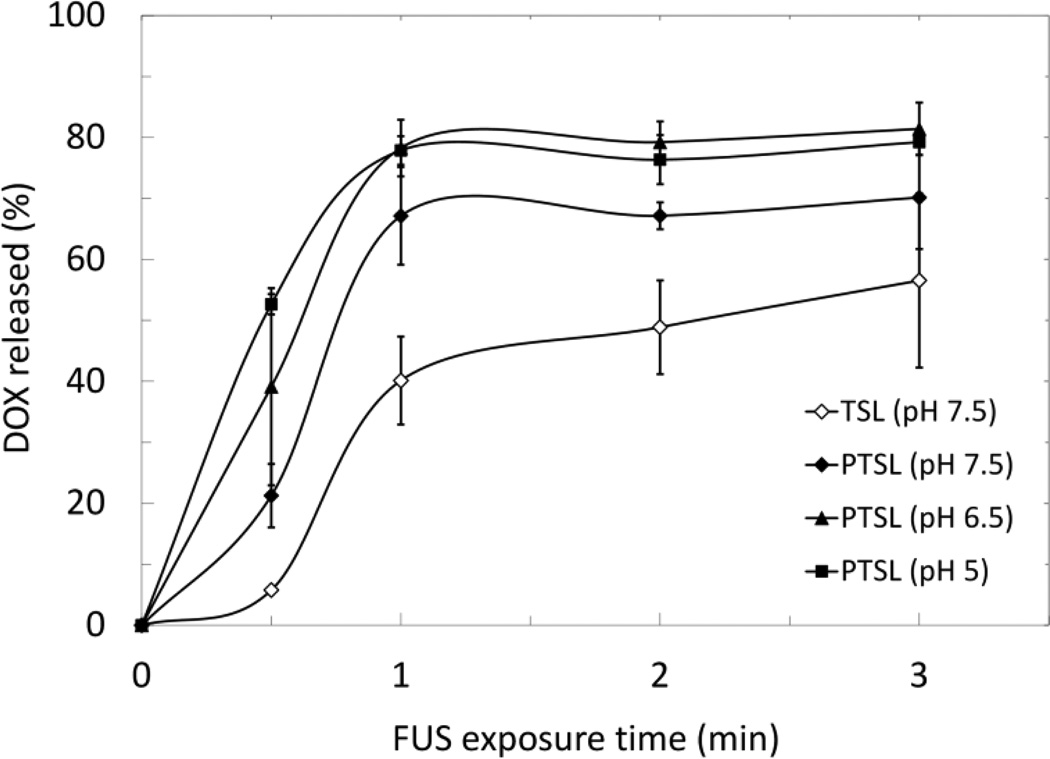
These parameters were used to trigger release of DOX from liposomes using FUS (3 min exposures). PTSL and TSL heated with circulating bath and FUS both demonstrated thermosensitivity at temperatures corresponding to mild hyperthermia (Fig. 5 and 6). Observed drug release was higher in FUS-treated liposomes compared to liposomes heated in a circulating bath irrespective of temperature, formulation, and pH. Aside from the increased overall drug release levels, the same trends were observed with FUS-triggered heating as with bath-triggered heating: PTSL demonstrated greater drug release at a given temperature when compared to TSL, and drug release was sensitive to pH with greater release occurring at lower pH. Minimal drug release was observed with both TSL and PTSL at 37°C (< 5%). NTSL, which must be heated beyond 50°C for triggered DOX release, were sonicated using similar parameters used for heating PTSL and TSL. Observed release from NTSL was also minimal (~5%). Relevant p-values are indicated in Fig. 5 and 6. Lastly, DOX was released from PTSL more rapidly than from TSL when heated by FUS to 42°C, with faster drug release kinetics occurring at lower pH (Fig. 7).
Figure 5.
DOX release in 20% FBS following 3 min incubation in circulating bath. Data shown are averages ± SD from at least n=4 independent experiments. Significant differences: (a, c) p=0.001; (a, d) p=0.00003; (b, c) p=0.009; (b, d) p=0.0002; (e, g) p=0.016; (e, h) p=0.0006; (f, h) p=0.015. Release data at 42°C did not pass Levene’s test of equal variance.
Figure 6.
DOX release in 20% FBS following 3 min FUS exposure (f=5 MHz; 16.94 kW/cm2, 50% DC initial exposure followed by 5.48–11.26 kW/cm2, 25–50% DC to sustain indicated temperatures). Samples were kept at an ambient temperature of 37°C prior to FUS exposure. Data shown are averages ± SD from at least n=4 independent experiments. Significant differences: (a, b) p=0.008; (a, c) p=0.005; (d, e) p=0.0008; (d, f) p=0.002. (g) difference between mean of NTSL drug release at 42°C and means of all other groups, p=0.0001.
Figure 7.
DOX release kinetics in 20% FBS of PTSL and TSL heated with FUS at 42°C (f = 5 MHz: I = 16.94 kW/cm2, 50% DC initial exposure followed by I = 5.48–11.26 kW/cm2, 25–50% DC to sustain 42°C). Data shown are averages ± SD for n=3 independent experiments.
In vitro cytotoxicity study
The cytotoxicity of liposomal DOX (PTSL, TSL) on MCF-7 cancer cells in serum-containing media was compared to that of free DOX. The effects of heating and pH were also studied. MTT assays were performed to measure the viability of cell populations in treatment groups relative to the viability of untreated populations, and results are summarized in Fig. 8–9.
Figure 8.
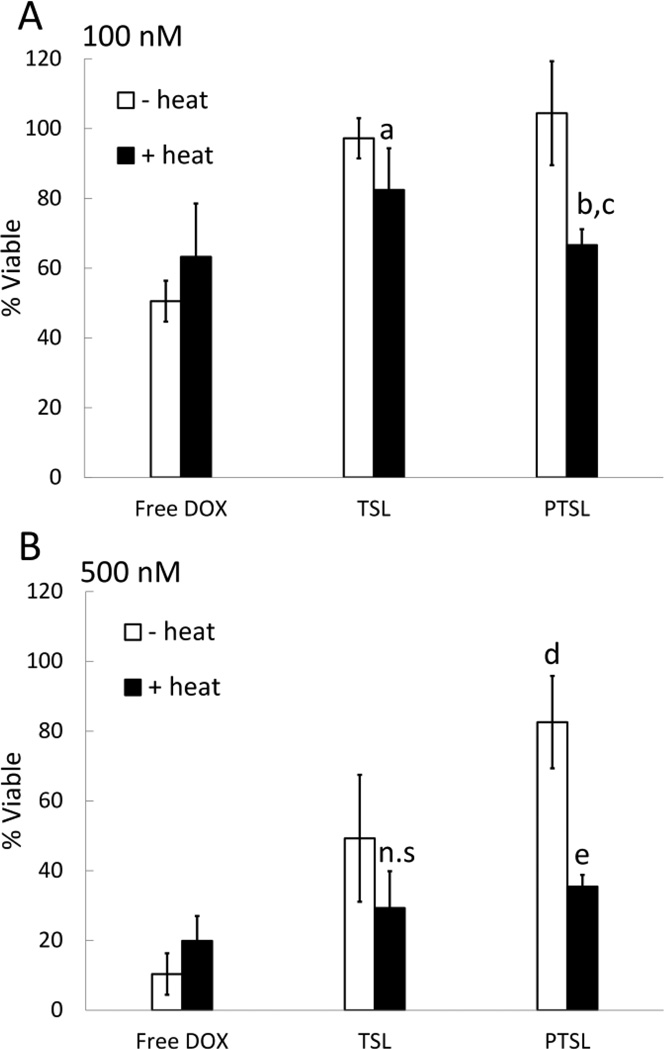
Cytotoxicity of free DOX and DOX-loaded PTSL and TSL at (A) 100 nM and (B) 500 nM DOX. Free DOX or DOX-loaded TSL and PTSL were added to cells for 4 hr. To study effects of heating, equivalent solutions were heated to 43°C for 5 min prior to exposure to cells. Following exposure, solutions were replaced with pH 7.5 media free of liposomes/drug. Cells were incubated for 192 hr after which cell viabilities were determined via MTT assay. Data shown are averages ± SD for n=6 replicates. a p=0.02 between heated/unheated TSL, 100 nM; b p=0.003 between heated/unheated PTSL, 100 nM; c p=0.04 between heated PTSL/heated TSL, 100 nM; d p=0.001 between unheated PTSL/unheated TSL, 500 nM; e p=0.00002 between heated/unheated PTSL, 500 nM; n.s. indicates no significant difference observed between heated/unheated TSL, 500 nM.
Figure 9.
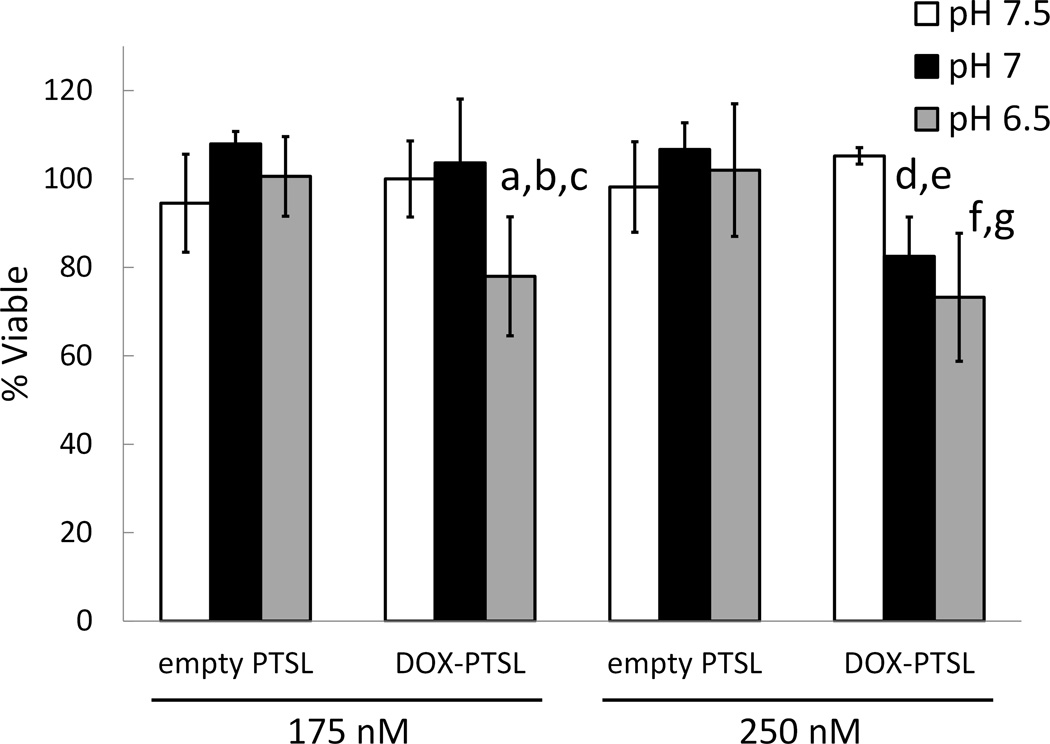
Cytotoxicity of DOX-loaded and empty PTSL at varying pH. DOX-loaded or empty PTSL in growth media at pH 7.5, 7, and 6.5 were added to cells for 4 hr after which solutions were replaced with pH 7.5 media free of liposomes/drug. Cells were incubated for 192 hr after which cell viabilities were determined via MTT assay. Data shown are averages ± SD for n=6 replicates. a p=0.01 between pH 7 and pH 6.5, 175 nM DOX-PTSL; b p=0.001 between pH 7.5 and pH 6.5, 175 nM DOX-PTSL; c p=0.02 between empty PTSL and DOX-PTSL, 175 nM, pH 6.5; d p=0.0002 between pH 7.5 and pH 7, 250 nM DOX-PTSL; e p=0.003 between empty PTSL and DOX-PTSL, 250 nM, pH 7; f p=0.0002 between pH 7.5 and pH 6.5, 250 nM DOX-PTSL; g p=0.016 between empty PTSL and DOX-PTSL, 250 nM, pH 6.5. No significant differences were observed as a function of pH in groups administered the same concentration of empty PTSL.
At 100 nM drug, DOX-loaded TSL and PTSL were not cytotoxic at 37°C whereas free DOX killed roughly 50% of cells (Fig. 8A). When heated to 43°C, however, significant reductions in cell viability were observed with both liposomal formulations, with 80% viability in TSL-treated wells and 65% viability in PTSL- treated wells. When heated, DOX-loaded PTSL were significantly more cytotoxic than DOX-loaded TSL (p=0.04). Heating free DOX to 43°C had no significant effect on its cytotoxicity.
At 500 nM, DOX-loaded TSL and PTSL were again not as cytotoxic as free DOX at 37°C (Fig. 8B). However, treatment with DOX-loaded TSL at this concentration resulted in a significant reduction in viability, even without heat (<50% viable). In contrast, unheated PTSL was significantly less cytotoxic with greater than 80% viability at 37°C (p=0.001). When liposomal formulations were heated, increases in cytotoxicity were once again observed. No significant differences were observed between the cytotoxicity of heated TSL and heated PTSL. However, enough DOX is released from TSL at 37°C that no significant difference in cytotoxicity was observed at 43°C. In contrast, a significant difference was observed between unheated PTSL and heated PTSL (p=0.00002). Heating had no effect on the cytotoxicity of free DOX at 500 nM.
Lastly, the effect of mild acidity on the cytotoxicity of DOX-loaded PTSL was studied in vitro. Cells were incubated with solutions containing either DOX-loaded PTSL or empty PTSL at pH 7.5, 7, and 6.5. To calculate cell viability, absorbance was measured via MTT and compared to the absorbance of cells exposed to buffer of the same pH but in the absence of liposomes. Results of this study are summarized in Fig. 9. At 175 nM, no significant differences in cytotoxicity were observed between cells exposed to DOX-loaded PTSL at pH 7.5 and pH 7. Reducing the pH to 6.5 resulted in significant reduction in viability (<80%, p=0.001). At 250 nM, significant reduction in viability was observed starting at pH 7 (83%, p=0.0002). In contrast, equivalent concentrations of empty PTSL were not cytotoxic at the pH levels studied, and no statistically significant differences in viability were observed as a function of pH. At 175 nM, DOX-loaded PTSL was significantly more cytotoxic than empty PTSL at pH 6.5 (p=0.02). At 250 nM, DOX-loaded PTSL was significantly more cytotoxic than empty PTSL at pH 7 (p=0.003) and at pH 6.5 (p=0.016).
Tumor growth evaluation
The response of tumors to treatment was gauged by measuring tumor growth over a 14-day period and results are summarized in Fig. 10. Tumors in the untreated group grew rapidly, reaching ~5000 mm3 at day 8, leading to the euthanizing of animals due to excessive tumor volume and necrosis. Administration of free DOX at 5 mg/kg resulted in significantly smaller tumor volumes, with tumors reaching ~2000 mm3 at day 8 (p=0.0001) and survival of animals out to day 10.
Figure 10.
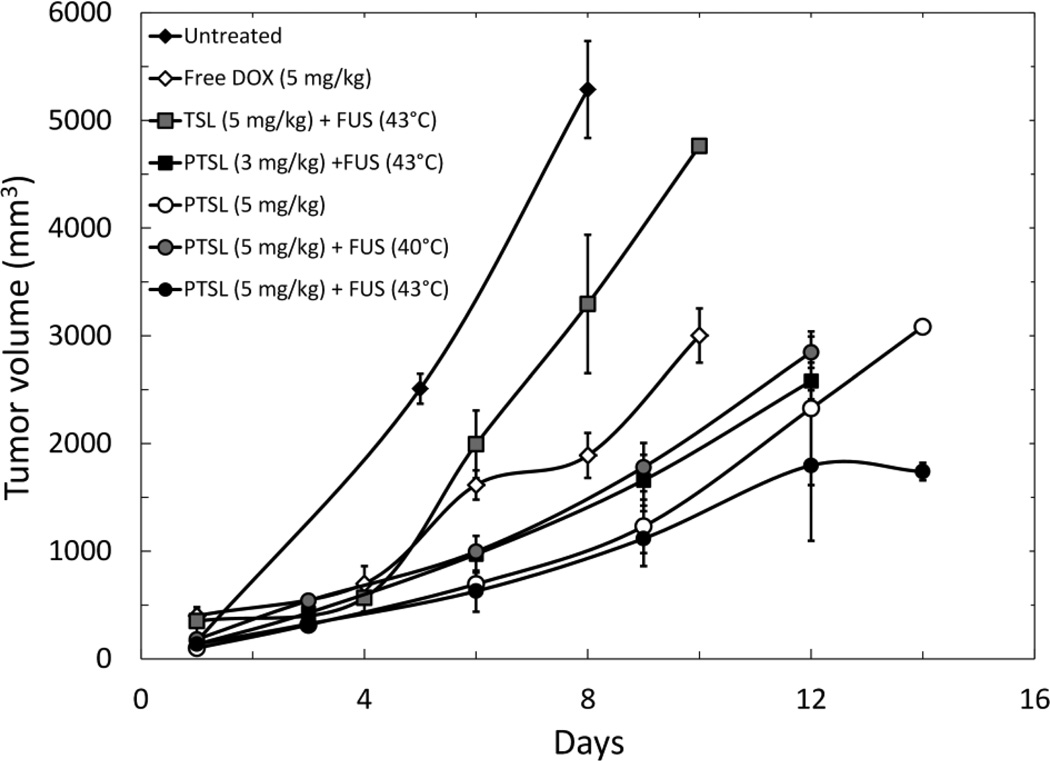
Tumor volume growth curves. Data shown are averages ± SD for a minimum of n=3 replicates (except for the untreated group, for which n=2). All curves that do not have data out to 14 days indicate animals were sacrificed at day of last timepoint due to excessive tumor volume and necrosis.
Administration of DOX-loaded TSL in combination with FUS exposure (43°C) also resulted in significantly smaller tumor volumes and prolonged survival when compared to untreated control, with tumors reaching ~3300 mm3 at day 8 (p=0.0004). However, tumors in the TSL (5 mg/kg) and FUS (43°C) group were significantly larger than those of the free DOX group (p=0.014).
Administration of DOX-loaded PTSL resulted in significant reductions in tumor volume when compared to the other treatment groups. Specifically, at day 6, tumors in rats administered any one of the four PTSL treatment groups were significantly smaller than tumors in untreated rats (p=0.0001), tumors in rats that were administered free DOX (p=0.027 for the least significant difference), and tumors in rats administered TSL-DOX (5 mg/kg) and FUS (43°C) (p=0.0006 for the least significant difference).
The administration of PTSL in combination with FUS (43°C, 5 min) led to the greatest reduction in tumor growth. At day 14, tumors were ~1700 mm3 in volume, compared to tumors in the untreated control group (~5000 mm3 at day 8), TSL (~4500 mm3 at day 10), free DOX (~3000 mm3 at day 10), and PTSL without FUS (~3000 mm3 at day 14). Statistically significant differences between PTSL treatment groups were not detected prior to day 14. At day 14, the difference between PTSL with and without FUS was statistically significant (p=0.002).
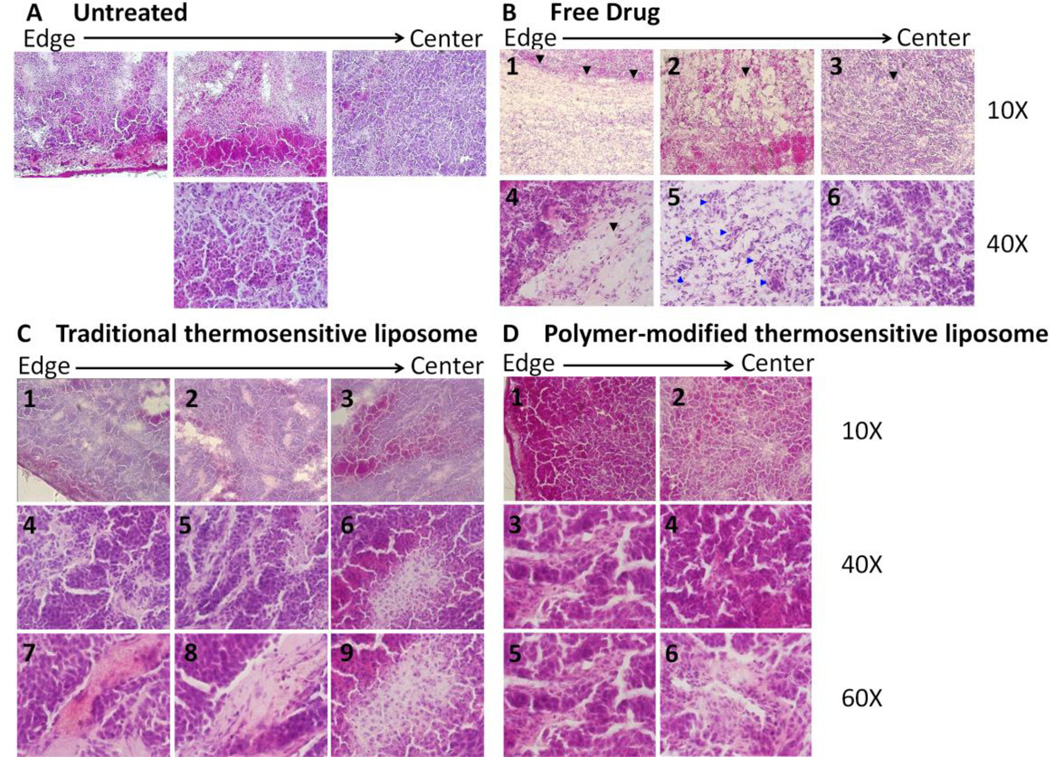
Histological evaluation of DOX effect
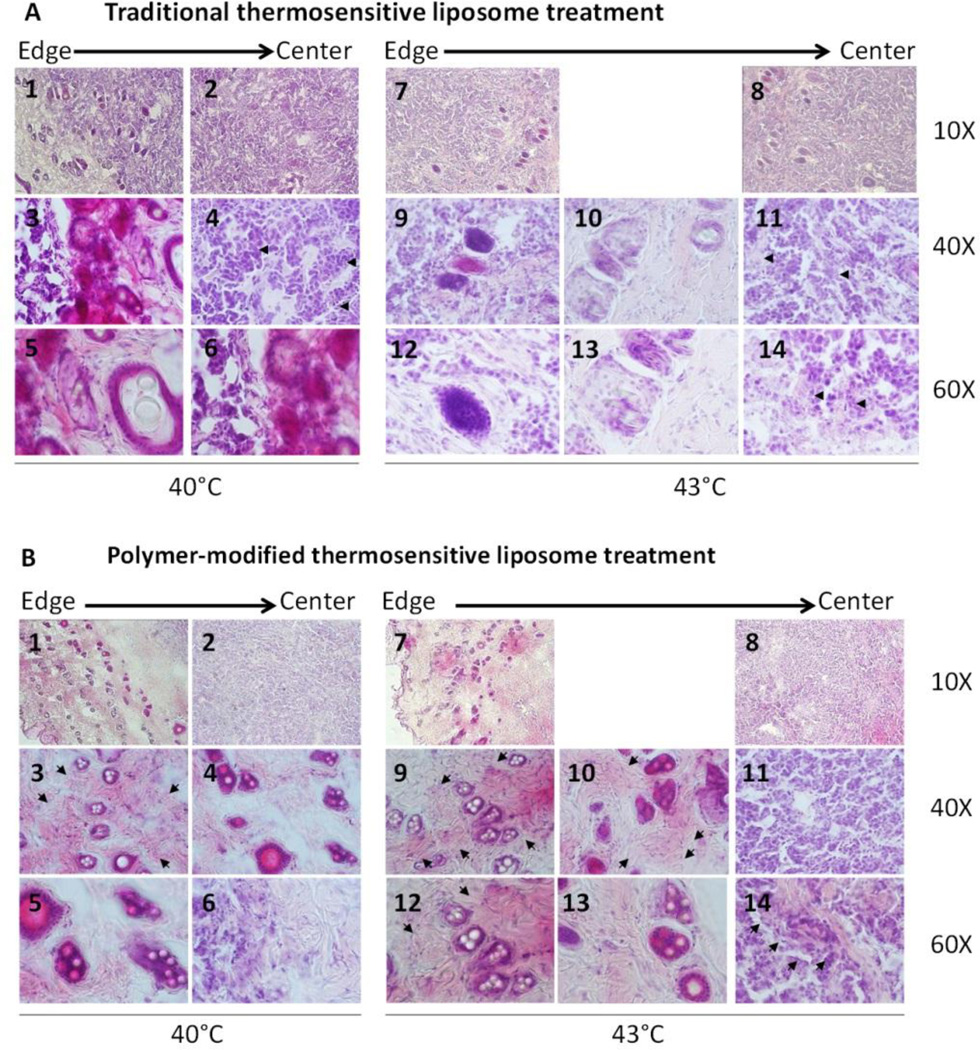
Twenty-four hours after treating with TSL (5 mg/kg) and FUS (40 or 43°C) or PTSL (5 mg/kg) and FUS (40 or 43°C), tumors were removed for histological evaluation to assess morphology and distribution of liposomes within the tumor. Fig. 11 shows representative images of H&E staining. Spherical structures are visible in the sections, which we assume are fused or aggregated liposomes. TSL particles diffuse approximately 20% of the radial distance from the edge towards the center of the tumor with FUS (40°C) and approximately 30% of the radial distance with FUS (43°C). With FUS (43°C) treatment compared to FUS (40°C), the particles are more masked by the surrounding cells as seen in Fig. 11A, panels 9, 10, 12 and 13 compared to panels 3, 5 and 6. ECM deposition is present already at day 1, appearing as pink bundles with both FUS treatments but with a higher level with FUS (43°C) (see arrows, Fig. 11A panels 4, 11 and 14). Also, in these areas, both treatments show degenerative lesions implying cell death.
Figure 11.
Representative images of tumor sections 24 hours after treatment stained with haematoxylin and eosin for morphological evaluation. (A) traditional thermosensitive liposome and (B) polymer-modified thermosensitive liposome.
When the tumor was treated with PTSL (5 mg/kg) and FUS (40 or 43°C) (Fig. 11B), numerous spherical structures were present. These structures diffused approximately 60 % of the radial distance from the edge towards the center of the tumor, a much larger distance than with TSL. PTSL particles are present as droplets with cells around them, but without the masking effect implying thinner cell surroundings than with TSL particles (Fig. 11B, representative panels 3, 4, 5 and 9, 10, 12, 13). In tumors treated with PTSL and FUS, more and very prominent pink collagen bundles are present compared to tumors treated with TSL and FUS. The ECM appears as more numerous bundles with larger area and stronger staining in tumors treated with PTSL and FUS (43°C) compared to PTSL and FUS (40°C), implying that more DOX was released from the liposomes at 43°C and hence stronger tumor remodeling was triggered (see arrows, Fig. 11B panels 3, 9, 10, 12 and 14). Also, tumors treated with PTSL and FUS show more pronounced degenerative lesions than those treated with TSL and FUS.
Next, we evaluated the morphological effects of TSL (5 mg/kg) and PTSL (5 mg/kg) with FUS (43°C) at 14 days after treatment using H&E (Fig. 12). In the untreated group, cell growth was evident (Fig. 12A) whereas free drug treatment slowed tumor progression (Fig. 12B): vacuolization is present throughout the tumor due to the pH change as a result of freed lysosomal enzymes. This effect appears as disorganized homogeneous light pink matter of eosinophilic proteinaceous material (Fig. 12B, representative black arrows in panels 1–4), and is typical in coagulative necrosis with still recognizable tumor architecture. Numerous vessels are also present (Fig. 12B, panel 5, blue arrows) with intact tumor structure (Fig. 12B, panel 6).
Figure 12.
Representative images of tumor sections 14 days after treatment stained with haematoxylin and eosin for morphological evaluation. (A) untreated tumor; (B) free DOX; (C) traditional thermosensitive liposome (43°C); and (D) polymer-modified thermosensitive liposome (43°C)
With liposomal-DOX treatment, both TSL and PTSL carriers induced strong cellular responses (Fig. 12C and 12D) throughout the tumor tissue resulting in extended coagulative necrosis, bubbly appearance (disappearance of stainable nuclei) (Fig. 12C panels 1–3, 8; Fig. 12D panel 6), different sizes of swollen or shrunk nuclei (pyknosis), fragmented nuclei (karyorrhexis), and fading of chromatin material (karyolysis) (Fig. 12C panels 4–6, 9; Fig. 12D panels 1–6). Tumors treated with TSL and FUS still had large ECM bundles (Fig. 12C panels 7 and 8), whereas tumors treated with PTSL and FUS showed shrinked scar formation (Fig. 12D, panels 3–5). Also, the coagulative necrosis was in a noticably earlier stage with TSL treatment, with more patches of eosinophilic proteinaceous material and less advanced focal cell lesions.
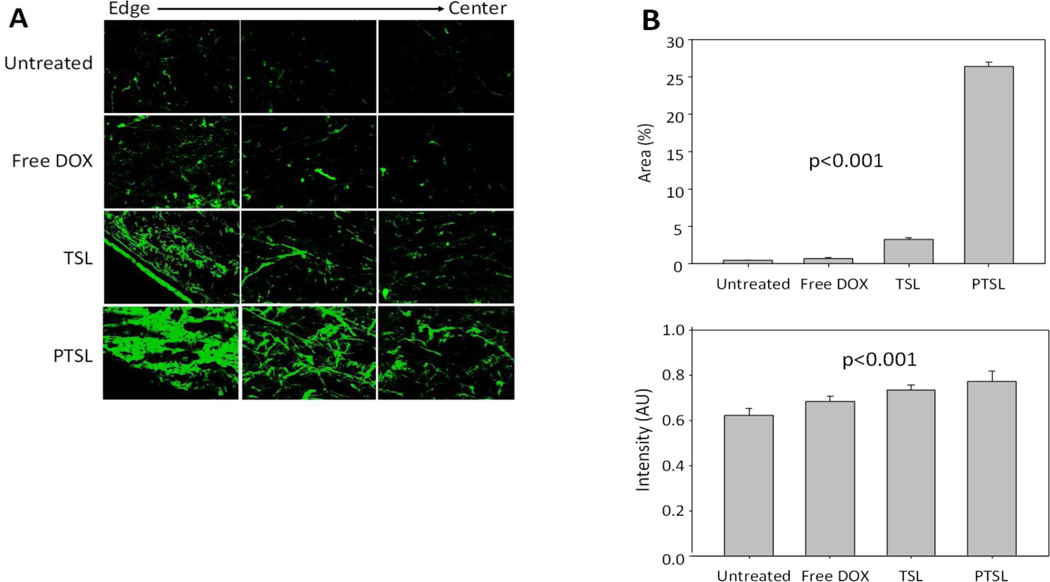
Evaluation of ECM remodeling by fluorescence
We also evaluated the advancement of ECM remodeling as a marker for tumor shrinkage using the auto-fluorescence properties of ECM in the 14-day post-treatment groups. Representative fluorescence images shown in Fig. 13A reveal that very little ECM is present in the untreated tumors, whereas increased levels of fluorescence indicative of ECM remodeling/production is apparent in sections of tumors treated with free drug, TSL, and PTSL. Accordingly, quantitative image analyses of autofluorescence shows significant increase in fluorescence intensity and area of fluorescence (Fig. 13B). These results imply an increased remodeling of ECM due to TSL+FUS treatment and even stronger remodeling induced by PTSL+FUS, which correlate with the suppression of tumor growth measured previously.
Figure 13.
Quantitative evaluation of tumor remodeling across different drug treatments. (A) Representative fluorescence microscopy images of tumor sections 14 days after treatment for morphological evaluation of extracellular matrix (ECM); (B) Percent fluorescence area and intensity in arbitrary units.
Determination of tumor DOX concentration
Homogenized tumor tissue samples in all treatment groups demonstrated measurable levels of DOX, as determined by fluorescence. Animals given PTSL (5 mg/kg) and FUS (43°C) demonstrated higher levels of drug than animals given TSL (5 mg/kg) and FUS (43°C) (7.9 µg DOX/g tissue and 5.8 µg/g, respectively). This difference was not statistically significant.
Discussion
Polymer-modified thermosensitive liposomes (PTSL) were designed to release drug at mildly hyperthermic temperatures (40–42°C) or mildly acidic pH while retaining drug at normal physiological conditions. In this study, we show that FUS exposure can be optimized to control drug release from PTSL, and a combined MRgFUS-PTSL platform effectively slows tumor growth.
Liposomal formulations (NTSL, TSL, PTSL) were synthesized with identical protocols and differed only in lipid composition and/or inclusion of polymer. Size and drug:lipid ratios were almost identical across formulations, an expected finding given the identical preparation and the fact that the bulk lipids DPPC and HSPC differ structurally by only two carbons in their fatty acid chains. A reduction in negative zeta potential was observed with PTSL compared to NTSL and TSL, a finding consistent with the presence of the polymer having an expected effect of shifting the slipping plane further away from the liposome surface. The polymer may also potentially cause an increase in drag and a decrease in mobility, and thereby a reduction in zeta potential. While the stability of the polymer tethering to the liposome surface was not quantified, it can be inferred that the polymer must be fairly stably tethered given that significant improvements were observed with PTSL relative to TSL in vitro and in vivo, and that liposomes were often stored overnight at 4°C prior to testing.
By adjusting acoustic intensity and duty cycle, heating was achieved in a reproducible and predictable manner, demonstrating the high degree of temporal control possible with FUS. Results show that a set of acoustic parameters exists where a pre-determined temperature can be reached and maintained for an extended period. FUS-triggered drug release was examined as a function of temperature, time, and pH, with our results indicating that it is feasible to use FUS to heat and trigger drug release from PTSL, and that PTSL are more responsive to FUS-mediated hyperthermia than TSL. Similar to thermosensitive liposomes containing lysolipids, more than 60% of entrapped DOX was released from PTSL within 1 minute, which is a vast improvement over the responsiveness of traditional TSL and more ideal for clinical applications. The faster release kinetics associated with PTSL suggests that the copolymer assisted in permeating the lipid shell. Furthermore, we noticed that the DOX release rate was accelerated at lower pH levels, a performance attribute for PTSL that could be advantageous in the mildly acidic tumor interstitium. Minimal drug release was observed with PTSL at physiological temperature and pH, indicating that the formulation would be stable in vivo.
At sufficiently high intensities, FUS can cause inertial cavitation – a process where bubbles in liquid rapidly collapse and burst. Liposomes subjected to inertial cavitation would be lysed, releasing their contents into solution [25]. Thus, it was important to differentiate between thermal and mechanical mechanisms of release. To this end, we studied the effects of FUS on nonthermosensitive liposomes (NTSL). NTSL are of the same size as TSL and differ only in the absence of DPPC, and thus any inertial cavitation effects on drug release would presumably affect both formulations equally. Whereas PTSL and TSL both demonstrated sensitivity in response to FUS, only minimal drug release was observed with NTSL, indicating that the FUS-triggered drug release from TSL and PTSL was due solely to a temperature-dependent mechanism (e.g. lipid phase transition or polymer-associated membrane disruption) and not from the mechanical rupture of bubbles during inertial cavitation. The increased overall drug release levels observed with heating from FUS compared to heating from a circulating water bath may have been due to non-uniform heating via FUS, which results in the presence of “hot pockets” and subsequently small regions of enhanced DOX release.
Prior to in vivo studies, we examined the cytotoxicity of the formulation in vitro. When heated at 43°C prior to exposure, PTSL demonstrated enhanced cytotoxicity compared to TSL. This suggests a greater amount of drug is released from PTSL, a finding consistent with our FUS-triggered drug release studies. Moreover, the cytotoxicity of heated PTSL was similar to that of free DOX, indicating near complete release of encapsulated drug. At high concentrations, TSL were cytotoxic in the absence of elevated temperature, suggesting a fair degree of drug leakage at 37°C. Unheated PTSL were significantly less cytotoxic, suggesting the formulation retained drug better than TSL. This increased stability may be due to the presence of hydrated polymer chains providing a steric barrier that reduces interactions between serum proteins and the lipid membrane, a phenomenon that has been observed in other studies [26, 27]. It is important to note that heating free DOX to 43°C prior to exposure had no significant effect on its cytotoxicity, which suggests that the enhanced cytotoxicity observed with heated TSL and PTSL resulted from triggered release of entrapped DOX.
PTSL also demonstrated pH-sensitivity, with greater cytotoxicity observed at reduced pH. These results indicate greater drug release from PTSL at lower pH, a finding consistent with our release studies. Empty PTSL were not cytotoxic, demonstrating that the pH-dependent membrane disruptive properties of the polymer itself did not cause toxicity under low pH conditions. Thus the increases in cytotoxicity at low pH observed with DOX-loaded PTSL were due solely to pH-triggered release of DOX.
Recently, tremendous strides have been made in the application of MRgFUS in combination with thermosensitive liposomes for cancer therapy. Multiple studies have shown MRgFUS can enable real-time monitoring of drug release by measuring the signal arising from release of coencapsulated MR contrast agents [19, 21]. Independent groups have determined that application of MRgFUS in addition to thermosensitive liposomes results in significantly greater drug accumulation at the tumor and also greater drug penetration when compared to liposomes in the absence of ultrasound [20, 22]. Our study builds on these results with the inclusion of in vivo data demonstrating that combining MRgFUS with thermosensitive liposomes significantly reduces tumor burden. Additionally, this is to our group’s knowledge the first such study combining MRgFUS with polymer-modified thermosensitive liposomes, versus previous studies that have focused on conventional TSL or lysolipid-containing TSL.
In vivo we found that PTSL proved to be more effective in slowing tumor growth than free DOX and TSL when administered in combination with MRgFUS. An interesting finding was that tumors in the TSL group were larger than those of the free DOX group. One possible explanation is that the increased accumulation of DOX at the tumor – which presumably occurs via encapsulation of the drug into TSL and exploitation of the EPR effect – is negated by the fairly slow drug release kinetics of the formulation. Results of our in vitro study also indicated that DOX-loaded TSL may release significant amounts of drug after prolonged incubation at physiological temperature, leading to the observation of increased cytotoxicity in the absence of heat. Thus during i.v. administration of DOX-loaded TSL, significant amounts of entrapped drug can leak into circulation. Resulting drug concentrations at the tumor may be lower than what is available with the administration of free DOX, and the drug that does make it to the tumor may be released too slowly, possibly accounting for the poorer response of tumors to TSL therapy compared to treatment with free DOX.
The improved tumor response to PTSL compared to free drug and TSL suggests that drug accumulation at the tumor is improved by delivery via the PTSL formulation, and also that the release rate from PTSL is sufficiently rapid to take advantage of the increased accumulation. When compared with the combined TSL-DOX (5 mg/kg) and FUS (43°C, 5 min) treatment, PTSL-DOX treatments with: (a) identical FUS exposure but reduced dose (3 mg/kg); (b) identical dose (5 mg/kg) but lower thermal dose (40°C, 5 min); and (c) identical dose (5 mg/kg) but no applied FUS; all demonstrated significantly greater reductions in tumor volume. These results suggest that PTSL: (a) releases a greater amount of drug at the tumor in response to heating, thereby reducing the required drug dose; (b) has a lower onset temperature for drug release (Tm), thereby reducing the required thermal dose and improving the safety profile of these formulations; and (c) possesses a pH-sensitivity that responds to the slightly acidic tumor microenvironment, promoting drug release in the absence of externally applied heating. These results compare favorably with our release and in vitro studies, as well as previously published results [11]. Differences within the three PTSL treatment groups mentioned above were difficult to observe. While tumor response to PTSL (5 mg/kg) alone appeared improved compared to PTSL (5 mg/kg) combined with FUS (40°C), this difference was not significant.
Our most important finding was that PTSL in combination with MRgFUS (43°C, 5 min) demonstrated the greatest reduction in tumor growth and outperformed all other treatment methods including free drug, TSL with FUS (43°C), and PTSL with mild FUS (40°C). This suggests that PTSL in combination with the acidic tumor microenvironment and sufficient heating from externally applied FUS provides the highest concentration of drug at the tumor and thus the most effective clinical response. Furthermore these results indicate that a single liposomal injection and application of a single, short (5 min) FUS exposure is able to significantly slow tumor growth. This short term pulse therapy can easily be incorporated into more complex regimens, with multiple administrations of PTSL and/or FUS having the potential to effectively eliminate cancer.
To further characterize the responses between treatment groups, tumors were removed for determination of DOX concentration and histological analysis. Measurable concentrations of DOX were delivered to tumors, with greater concentrations observed in PTSL-treated tumors. This difference was not statistically significant, which was likely due to the nature of the extraction process which detects both released and liposome-encapsulated drug. Histological analyses of tumor sections suggested: a) greater tumor penetration with PTSL compared to TSL, which could be due to less cellular and hence less immunological reaction appearing as less masking by cells (Fig. 11); b) greater cell destruction in PTSL-treated tumors versus TSL-treated tumors (Fig. 12); and c) more extensive ECM remodeling in tumors treated with PTSL versus TSL and free drug (Fig. 13). The ECM remodeling was quantified using the autofluorescent properties of the ECM, and confirmed the greater extent of remodeling observed microscopically. These findings implicate a more effective acute remodeling process that occurs with PTSL-DOX administration, one which halts the growth of tumors by slowing/stopping cell division and promoting cell death and subsequent remodeling and shrinkage of tumor mass.
Conclusion
A novel, dual-sensitive liposome (PTSL) capable of releasing drug in response to both temperature and pH was previously developed. Here we show the formulation is more effective than traditional formulations in vitro, and that it can be optimized for administration with MRgFUS in vivo. PTSL administered in combination with MRgFUS resulted in significantly greater reduction in tumor burden compared to administration of traditional formulations or free drug. Histological analyses of tumors provided further evidence of improved tumor response stemming from the administration of PTSL.
To our knowledge, this is the first study to demonstrate that polymer-modified thermosensitive liposomes administered with MRgFUS can effectively reduce tumor burden in vivo. In addition, this is to our knowledge the only study that combines a thorough characterization of acoustic parameters for FUS-triggered drug release from thermosensitive liposomes with in vivo data demonstrating improved efficacy and supporting histological analyses.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yatvin MB, Weinstein JN, Dennis WH, Blumenthal R. Science. 1978;202:1290–1293. doi: 10.1126/science.364652. [DOI] [PubMed] [Google Scholar]
- 2.Aoki H, Kakinuma K, Morita K, Kato M, Uzuka T, Igor G, Takahashi H, Tanaka R. Int. J. Hyperth. 2004;20:595–605. doi: 10.1080/02656730410001703186. [DOI] [PubMed] [Google Scholar]
- 3.Al-Jamal WT, Al-Ahmady ZS, Kostarelos K. Biomaterials. 2012;33:4608–4617. doi: 10.1016/j.biomaterials.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 4.Gaber MH, Wu NZ, Hong K, Huang SK, Dewhirst MW, Papahadjopoulos D. Int. J. Radiat. Oncol. Biol. Phys. 1996;36:1177–1187. doi: 10.1016/s0360-3016(96)00389-6. [DOI] [PubMed] [Google Scholar]
- 5.Anyarambhatla GR, Needham D. J. Liposome Res. 1999;9:491–506. [Google Scholar]
- 6.Kong G, Anyarambhatla G, Petros WP, Braun RD, Colvin OM, Needham D, Dewhirst MW. Cancer Res. 2000;60:6950–6957. [PubMed] [Google Scholar]
- 7.Needham D, Anyarambhatla G, Kong G, Dewhirst MW. Cancer Res. 2000;60:1197–1201. [PubMed] [Google Scholar]
- 8.Banno B, Ickenstein LM, Chiu GN, Bally MB, Thewalt J, Brief E, Wasan EK. J. Pham. Sci. 2010;99:2295–2308. doi: 10.1002/jps.21988. [DOI] [PubMed] [Google Scholar]
- 9.Sandstrom MC, Ickenstein LM, Mayer LD, Edwards K. J. Control. Release. 2005;107:131–142. doi: 10.1016/j.jconrel.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Chiu GN, Abraham SA, Ickenstein LM, Ng R, Karlsson G, Edwards K, Wasan EK, Bally MB. J. Control. Release. 2005;104:271–288. doi: 10.1016/j.jconrel.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Ta T, Convertine AJ, Reyes CR, Stayton PS, Porter TM. Biomacromolecules. 2010;11:1915–1920. doi: 10.1021/bm1004993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engin K, Leeper DB, Cater JR, Thistlethwaite AJ, Tupchong L, McFarlane JD. Int. J. Hyperth. 1995;11:211–216. doi: 10.3109/02656739509022457. [DOI] [PubMed] [Google Scholar]
- 13.de Smet M, Langereis S, van den Bosch S, Grull H. J. Control. Release. 2010;143:120–127. doi: 10.1016/j.jconrel.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Dromi S, Frenkel V, Luk A, Traughber B, Angstadt M, Bur M, Poff J, Xie J, Libutti SK, Li KC, Wood BJ. Clin. Cancer Res. 2007;13:2722–2727. doi: 10.1158/1078-0432.CCR-06-2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ta T, Porter TM. J. Control. Release. 2013;169:112–125. doi: 10.1016/j.jconrel.2013.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hokland SL, Pedersen M, Salomir R, Quesson B, Stodkilde-Jorgensen H, Moonen CT. IEEE Trans. Med. Imaging. 2006;25:723–731. doi: 10.1109/tmi.2006.873296. [DOI] [PubMed] [Google Scholar]
- 17.Vykhodtseva N, Sorrentino V, Jolesz FA, Bronson RT, Hynynen K. Ultrasound Med. Biol. 2000;26:871–880. doi: 10.1016/s0301-5629(00)00216-7. [DOI] [PubMed] [Google Scholar]
- 18.Holbrook AB, Santos JM, Kaye E, Rieke V, Pauly KB. Magn. Reson. Med. 2010;63:365–373. doi: 10.1002/mrm.22206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Negussie AH, Yarmolenko PS, Partanen A, Ranjan A, Jacobs G, Woods D, Bryant H, Thomasson D, Dewhirst MW, Wood BJ, Dreher MR. Int. J. Hyperth. 2011;27:140–155. doi: 10.3109/02656736.2010.528140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ranjan A, Jacobs GC, Woods DL, Negussie AH, Partanen A, Yarmolenko PS, Gacchina CE, Sharma KV, Frenkel V, Wood BJ, Dreher MR. J. Control. Release. 2012;158:487–494. doi: 10.1016/j.jconrel.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Smet M, Heijman E, Langereis S, Hijnen NM, Grull H. J. Control. Release. 2011;150:102–110. doi: 10.1016/j.jconrel.2010.10.036. [DOI] [PubMed] [Google Scholar]
- 22.Staruch RM, Ganguly M, Tannock IF, Hynynen K, Chopra R. Int. J. Hyperth. 2012;28:776–787. doi: 10.3109/02656736.2012.736670. [DOI] [PubMed] [Google Scholar]
- 23.Chen PS, Toribara TY, Warner H. Anal. Chem. 1956;28:1756–1758. [Google Scholar]
- 24.Culjat MO, Goldenberg D, Tewari P, Singh RS. Ultrasound Med. Biol. 2010;36:861–873. doi: 10.1016/j.ultrasmedbio.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 25.Graham S, Carlisle R, Choi J, Stevenson M, Shah A, Myers R, Fisher K, Peregrino MB, Seymour L, Coussios CC. J. Control. Release. 2014;178:101–107. doi: 10.1016/j.jconrel.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 26.Han HD, Choi MS, Hwang T, Song CK, Seong H, Kim TW, Choi HS, Shin BC. J. Pharm. Sci. 2006;95:1909–1917. doi: 10.1002/jps.20646. [DOI] [PubMed] [Google Scholar]
- 27.Han HD, Shin BC, Choi HS. Eur. J. Pharm. Biopharm. 2006;62:110–116. doi: 10.1016/j.ejpb.2005.07.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.