Abstract
Prior investigations have shown that patients with neuronal ceroid lipofuscinosis (NCL) develop neurodegeneration characterized by vision loss, motor dysfunction, seizures, and often early death. Neuropathological analysis of patients with NCL shows accumulation of intracellular autofluorescent storage material, lipopigment, throughout neurons in the central nervous system including in the retina. A recent study of a sibling pair with adult onset NCL and retinal degeneration showed linkage to the region of the progranulin (GRN) locus and a homozygous mutation was demonstrated in GRN. In particular, the sibling pair with a mutation in GRN developed retinal degeneration and optic atrophy. This locus for this form of adult onset neuronal ceroid lipofuscinosis was designated neuronal ceroid lipofuscinosis-11 (CLN11). Based on these clinical observations, we wished to determine whether Grn-null mice develop accumulation of autofluorescent particles and retinal degeneration. Retinas of both wild-type and Progranulin deficient mice were examined by immunostaining and autofluorescence. Accumulation of autofluorescent material was present in Progranulin deficient mice at 12 months. Degeneration of multiple classes of neurons including photoreceptors and retinal ganglion cells was noted in mice at 12 and 18 months. Our data shows that Grn−/− mice develop degenerative pathology similar to features of human CLN11.
Keywords: neurodegeneration, Progranulin, autofluorescent storage material, Neuronal ceroid lipofuscinosis
1. Introduction
Neuronal ceroid lipofuscinoses (NCL) are a group of inherited, neurodegenerative disorders in which there is abnormal lipopigment accumulation in the lysosome (Bartsch et al., 2013; Boustany, 2013). They belong to a broader group of lysosomal storage disorders (Ballabio and Gieselmann, 2009; Cotman et al., 2013). The lysosome is an important cellular organelle involved in the breakdown of macromolecules that can be transported back into the cytoplasm (de Duve, 2005). In NCL, mutations in genes involved in lysosomal function lead to the accumulation of autofluorescent storage material known as lipopigment within lysosomes (Mink et al., 2013). The abnormal accumulation of lipopigment within neurons may lead to eventual neuronal death (Bartsch et al., 2013; Katz et al., 2008).
Clinically, patients with NCL display symptoms of visual loss, motor dysfunction, seizures, and often early death (Boustany et al., 1988; Warrier et al., 2013). Understanding the pathogenesis of lysosomal storage disorders has led to new treatments including Cerezyme for treating Type 1 Gaucher disease, Fabrazyme for treating Fabry disease, and most recently Lumizyme for treating late-onset Pompe disease (Boustany, 2013; Lidove et al., 2010; Schoser et al., 2008); however, there are over 50 inherited diseases of lysosomal dysfunction for which there is no currently available treatment (Hodges and Cheng, 2006; Platt and Lachmann, 2009).
Recent analysis of two siblings with NCL showed linkage to the region of the GRN locus and a novel, homozygous mutation in the progranulin gene (GRN) was identified (Smith et al., 2012). The sibling pair who were Progranulin deficient developed visual loss, seizures, and cerebellar ataxia. Retinal photography of the siblings showed retinal dystrophy (Smith et al., 2012). Optic atrophy was also noted consistent with of a loss of retinal ganglion cells, the retinal cell type that gives rise to the optic nerve. This neurologic disease was termed CLN11 (Smith et al., 2012).
Progranulin is a secreted glycoprotein containing seven and a half granulin repeats (Hu et al., 2010; Zhu et al., 2002). Progranulin binds to neurons through its receptor Sortilin, which mediates endocytosis of Progranulin to the neuronal lysosome (Andersen et al., 2005; Hu et al., 2010). Ablation of Sortilin in Grn+/− mice results in the rescue of Progranulin levels in brain and serum (Hu et al., 2010). Furthermore, reducing Sortilin levels through a small-molecule binder results in an increase in extracellular Progranulin (Lee et al., 2014). Previous studies have shown that Progranulin deficiency leads to lysosomal dysfunction through accumulation of abnormal autofluorescent lipopigments (Ahmed et al., 2010; Petkau et al., 2012); however, the mechanism of Progranulin function within lysosomes is not well understood. Electron microscopy of Progranulin deficient mice showed NCL pathology with evidence of storage granules in a rectilinear complex characteristic of most types of NCL (Smith et al., 2012). A recent study suggested that Progranulin deficiency may lead to lysosomal dysfunction through decreased activation of lysosomal genes through binding to the transcription factor EB (Tanaka et al., 2013); however, no investigation of the retinas in the mice of Grn−/− has been performed.
We examined the retinas of Progranulin deficient mice in order to address whether the accumulation of storage of abnormal autofluorescent lipopigment in the brains of Grn−/− mice is present in the retina and whether this may lead to retinal degeneration. Grn−/− mice were examined for autofluorescence using fluorescence microscopy at age 12 months and retinal degeneration using immunohistochemistry at age 12 and 18 months. Analysis of retinal autofluorescence revealed a significant accumulation of particles in Grn−/− mice retinas compared to controls by 12 months. Further, Grn−/− mice exhibit photoreceptor and retinal ganglion cell degeneration at 12 and 18 months of age. To our knowledge, these studies are the first to show that Grn−/− mice develop age-related retinal degeneration concurrent with accumulation of autofluorescent material in the retina. Given that homozygous mutations in the GRN gene lead to CLN11 in humans, these mice may be of use for further study of NCL pathogenesis.
2. Results
Grn expression in the mature retina
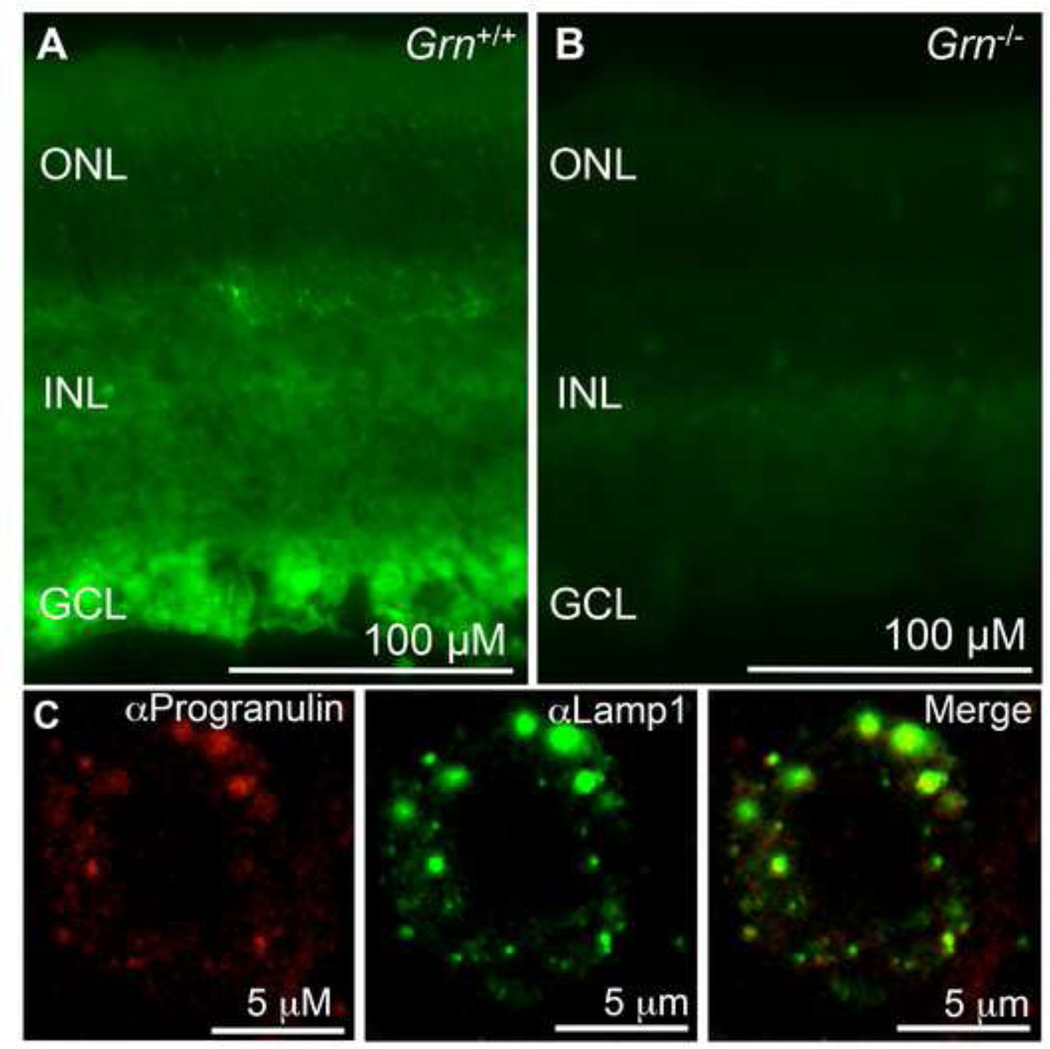
Progranulin was previously reported to be detected in neurons in the mouse brain using immunohistochemistry (Daniel et al., 2000); however, its expression has not been previously reported in the retina. Immunohistochemistry was performed for Progranulin protein in the mature retina. In adult wild-type mice, Progranulin protein was detected in the outer nuclear layer where photoreceptors reside. Progranulin protein was also detected in the inner nuclear layer, and in the ganglion cell layer, where retinal ganglion cells are located (Figure 1A). As expected, Progranulin protein was not detected in Grn−/− mice (Figure 1B).
Figure 1. Progranulin is widely detected throughout the retina and is localized to the lysosome in cortical neurons Figure Legends.
(A) Immunohistochemistry was performed on mature wild-type retinas and expression was detected throughout the retina. (B) Immunohistochemistry was performed on adult Grn−/− retina. As expected, Grn−/− mice did not exhibit expression of Grn. The outer nuclear layer (ONL), inner nuclear layer (INL) are ganglion cell layer (GCL) are labeled. (C) Immunohistochemistry was performed on primary cortical neuronal cultures using anti-Lamp1 (green) and anti-Progranulin antibodies (red).
It has been previously reported that Progranulin co-localizes with a lysosomal marker protein Lamp1 (Almeida et al., 2011; Hu et al., 2010; Tanaka et al., 2013). Given the association of NCL with lysosomal storage defects, we performed immunofluorescence on primary cortical neuronal cultures for Progranulin and Lamp1. As expected, immunostaining showed that intracellular Progranulin co-localized with Lamp1 (Figure 1C), consistent with a portion of Progranulin present in the lysosomes of cortical neurons.
Retinal autofluorescence is detected in the retinas of Progranulin deficient mice
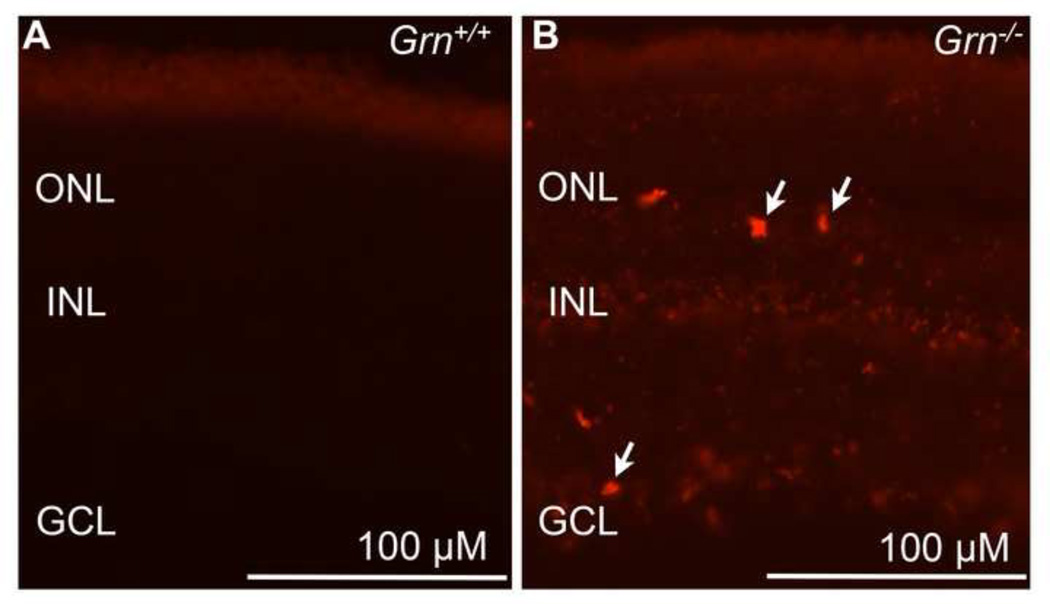
Given that Progranulin deficiency is associated with increased autofluorescent material in the hippocampal neurons in Grn−/− mutant mice, fluorescence microscopy was used to detect autofluorescence in the retinas of Grn−/− mice at 12 months. Autofluorescent particles were visualized at multiple excitation wavelengths including 488 nm and 543 nm. As expected in control adult mice, little to no autofluorescence was observed in retinal neurons, including retinal ganglion cells or photoreceptors (Figure 2A). Interestingly, by 12 months age in Grn−/− mice, substantial accumulation of autofluorescent material was detected throughout the retina using 543 nm excitation (Figure 2B) as well as 488 nm excitation (data not shown). Based on the cell morphology and location, autofluorescence was evident in multiple layers of the retina including the outer nuclear layer where photoreceptors reside and the ganglion cell later where retinal ganglion cells reside. (Figure 2B). These results indicate that Progranulin deficient mice show an increase in autofluorescent particles throughout the retina.
Figure 2. Loss of Progranulin causes accumulation of autofluorescent material in mouse retina.
(A) Retinal autofluorescence using 543 nm excitation was examined in cryostat sections of wild-type mouse retinas and (B) Grn−/− mouse retinas at 12 months age. Areas of autofluorescence corresponding to autofluorescent particles were widely detected throughout the retina in the outer nuclear layer (ONL), inner nuclear layer (INL), and ganglion cell layer (GCL). Arrows indicate areas in the outer nuclear layer and ganglion cell layer that are highly autofluorescent (n=3 mice per group). Exposure times were kept constant for fluorescent microscopy. Scale bar = 100 µm.
Photoreceptor and retinal ganglion cell degeneration in Progranulin deficient mice at 12 and 18 months
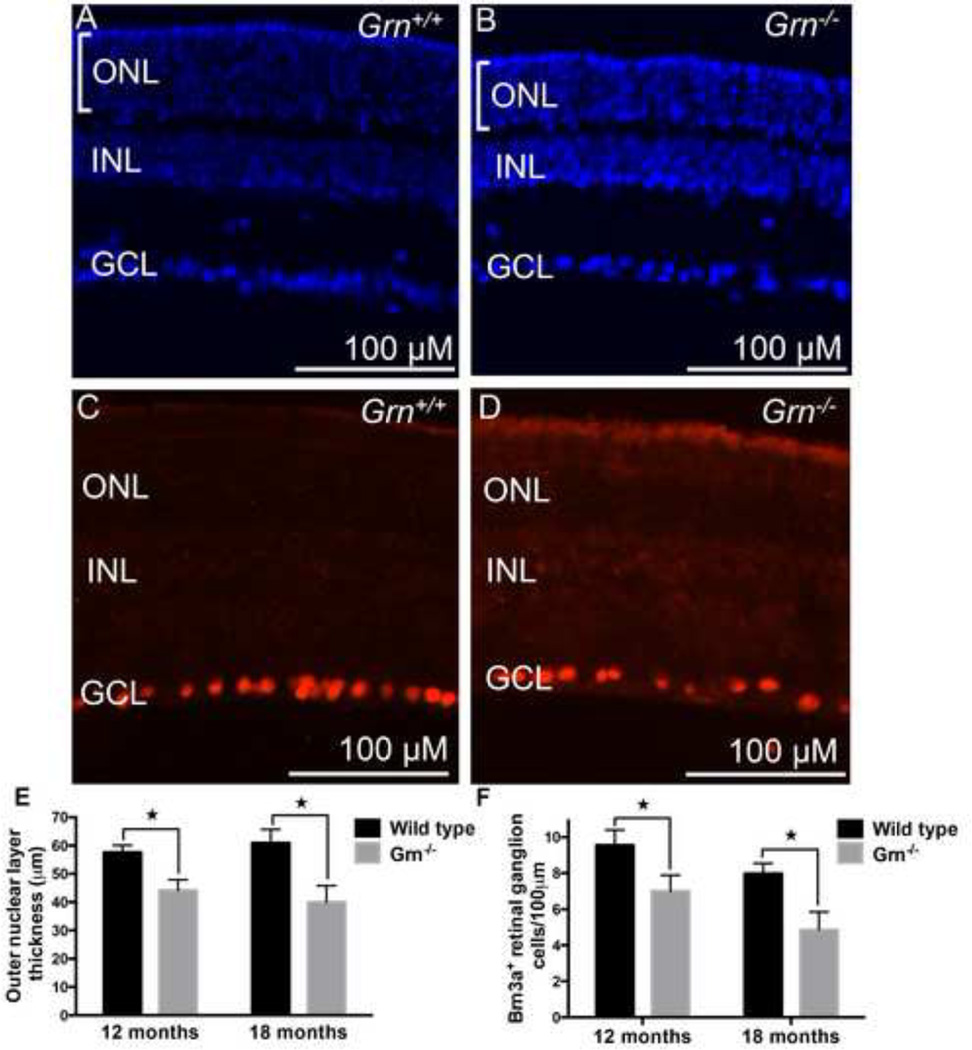
To characterize the functional consequences of Progranulin deficiency, we examined the retinas from Grn−/− and wild-type mice at 12 and 18 months. Analysis of DAPI-stained sections in the central region of the retina showed a progressive decrease in the thickness of the outer nuclear layer (i.e. the photoreceptor cell layer) in Grn−/− mice compared to wild-type controls. At 12 months the photoreceptor cell layer in the Grn−/− mice was reduced by 23% compared to wild-type mice (unpaired Student’s t test P = 0.016; Figure 3E). At 18 months the photoreceptor cell layer in the Grn−/− mice was reduced by 34% compared to wild-type mice (unpaired Student’s t test P = 0.009; Figure 3A, B, E). There was no age-associated decline in the photoreceptor cell layer in the wild-type control mice.
Figure 3. Loss of Progranulin leads to photoreceptor and retinal ganglion cell degeneration at 12 and 18 months of age.
(A) DAPI nuclear staining of a wild-type mouse retina and (B) Grn−/− mouse retina at 18 months age. Bars indicate the thickness of the outer nuclear layer. (C) Immunohistochemistry of wild-type mouse retina for the retinal ganglion cell maker Brn3a, and (D) Grn−/− mouse retina for Brn3a at 18 months. (E) Quantification of outer nuclear thickness was carried out for multiple retinas. The overall thickness of the outer nuclear later is shown for 12 and 18 month wild-type and Grn−/− mouse retinas. (F) Quantification of Brn3a+ cells/100 µm for wild type and Grn−/− mouse retinas at 12 and 18 months. *P<0.05, n=at least 3 mice per group. Error bars = s.d. Measurements were taken in the central regions of the retina.
Immunostaining for Brn3a, a transcription factor expressed in most retinal ganglion cells (Nadal-Nicolas et al., 2009) showed progressive degeneration of Brn3a+ retinal ganglion cells at 12 and 18 months compared to wild-type controls. Sections were quantified from the more central regions of each retina. Mean Brn3a+ retinal ganglion cell density per 100 µm at 12 months was reduced by 27% in Grn−/− mice compared to wildtype controls (unpaired Student’s t test P = 0.048; Figure 3F). Mean Brn3a+ retinal ganglion cell density per 100 µm at 18 months was reduced by 39% in Grn−/− mice compared to wild-type controls (unpaired Student’s t test P = 0.0089; Figure 3C, D, F). There was no significant difference in Brn3a+ retinal ganglion cell density in wild-type controls between 12 and 18 months.
3. Discussion
Humans with neuronal ceroid lipofuscinosis suffer from progressive neurodegeneration with clinical manifestations including loss of vision, seizures, motor dysfunction, and early death (Birch, 1999; Bozorg et al., 2009). Understanding the pathogenesis of abnormal accumulation of lysosomal inclusions has led to enzyme replacement therapies and treatment of patients with diseases such as type I Gaucher disease, Fabry disease, and Pompe disease (Andersson et al., 2005). Despite these advances, there are still over 50 diseases caused by lysosomal dysfunction that, as of present, have no current therapy (Hodges and Cheng, 2006). Understanding the pathogenesis of the genetic defects in patients with NCL is necessary prior to the development of novel therapies.
To gain a better understanding of the pathological process underlying NCL, mice lacking the Grn gene were analyzed. The Grn gene was chosen for the present study as homozygous mutations in the human GRN gene lead to CLN11. Using immunohistochemistry, Grn was identified as being expressed in the outer nuclear layer, where photoreceptors reside. Progranulin protein was also detected in the inner nuclear layer, and the ganglion cell layer, where retinal ganglion cells are located. Confocal microscopy confirmed that Grn also co-localized with the lysosomal marker Lamp1. This is likely to be specific as confirmed by the absence of staining in Progranulin deficient mice. This observation is consistent with those recently reported showing expression of Grn in neurons in the brain and in lysosomes (Ahmed et al., 2010; Almeida et al., 2011).
Next, Grn−/− mice were shown to develop accumulation of autofluorescent material throughout the retina. NCL is characterized by the accumulation of autofluroescent storage material in neurons. Based on its histologic analysis, it consists of an oxidized aggregate of cross-linked proteins, lipids, and sugars that are located within neurons. Usually the macromolecules that develop into abnormal lipopigment are broken down; however, its accumulation occurs during lysosomal dysfunction as in NCL. While the molecular mechanisms underlying certain forms of lysosomal inclusions are known and enzyme replacement therapies available, the majority of diseases in which there is abnormal accumulation of material in lysosomes are not well understood.
Electroretinography was used to analyze retinal function in Grn−/− mice at 18 months; however, the Progranulin deficient mice did not show a significant loss in retinal sensitivity (data not shown). Both scotopic a- and b- wave responses as well as photopic b- waveforms appeared similar between Progranulin deficient mice and age-matched controls at 18 months of age. This suggests that the photoreceptors that remain at late ages are still functional. The preservation of retinal function in Progranulin deficient mice has similarities to the adult variant NCL, which generally displays milder symptoms.
Once lipopigments are generated in wild type neurons, they are slowly eliminated from lysosomal stores within cells (Katz et al., 1999); however, this balance is perturbed in diseases that affect lysosome function. While the mechanism whereby deficiency in Progranulin leads to the accumulation of autofluorescent material is unknown, it may be related to Sortilin’s delivery of Progranulin to lysosomes and its potential role in this organelle’s function (Hu et al., 2010).
Although it is known that autofluorescent lipopigment accumulation within neurons occurs in patients with NCL, a better understanding of the molecular mechanisms underlying its accumulation may lead to new therapies to prevent neuronal death (Shacka, 2012). It is worth noting that while autofluorescent material is evident in Progranulin deficient mice, it cannot be excluded that Progranulin loss itself leads to neuronal death in the retina in a lysosome-independent pathway. Additional studies are ongoing to determine the binding partners of Progranulin in lysosomes. If Progranulin and the lysosomal/proteolytic pathway could be upregulated, it is possible that excess lipopigment accumulation could be reversed in these patients prior to the degeneration of neurons. A recent study showed that Progranulin is a secreted factor of adipose-derived stem cells and shows protective effects against light-induced retinal damage in vivo (Tsuruma et al., 2013), suggesting that Progranulin may have neuroprotective properties.
It is also of interest to note that at 12 and 18 months of age, there was a significant loss of retinal ganglion cells in the more central regions of Progranulin deficient retinas, as assessed by quantification of the retinal ganglion cell marker, Brn3a. This model may have similarities to the optic atrophy, which has been observed in patients with CLN11. Thus, the accumulation of autofluorescent material in Progranulin deficient mice appears to be associated not only with photoreceptor, but also retinal ganglion cell death. Future research is focused on elucidating the mechanism whereby Progranulin loss leads to autofluorescent storage material accumulation, and methods to prevent retinal degeneration.
4. Experimental Procedure
Progranulin deficient mice
Grn−/− mutant mice were obtained from the RIKEN Bioresource Center and were on a C57BL/6J background (Kayasuga et al., 2007). The generation of Grn−/− mice has been described previously (Kayasuga et al., 2007) where the Grn gene containing 13 exons of which exons 2-13 are disrupted using a targeting vector (Kayasuga et al., 2007). Genomic DNA was analyzed for Grn wild-type and mutant alleles using PCR primers described previously (Kayasuga et al., 2007). Age matched C57BL/6J mice were used as wild type controls. Retinas were collected at age 12 and 18 months and tissue sections were prepared as previously described (Hafler et al., 2012). This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Mice were maintained on a 12 hour light-dark schedule and housed in microisolator cages with food and water ad libitum. The protocol was approved by Yale University’s Institutional Animal Care and Use Committee.
Immunohistochemistry and retinal cell count determinations
Mouse retinas were processed for immunostaining as described previously (Hafler et al., 2012). The source of the antibodies were rabbit anti-Brn3a, Santa Cruz H-80 sc-28595 (1:250), sheep anti-mProgranulin, R&D Systems AF2557 (1:500), and rat anti-Lamp1 antibody, Santa Cruz Biotechnology sc-19992 (1:250). These antibodies were applied overnight at 4 degrees Celsius. Secondary antibodies were 568-conjugated donkey antirabbit IgG (1:500; Invitrogen) and Alexa Fluor 488 goat anti-mouse IgG (1:500; Invitrogen), Alexa Fluor 488 goat anti-rat IgG (1:500; Invitrogen), and 568-conjugated donkey anti-sheep IgG (1:500; Invitrogen). Vectashield mounting media with DAPI (Vectorlabs H-1200) was used to stain nuclei. After washing in PBS, fluorescent stains were visualized on Zeiss microscopes with x10 or x20 objectives. The thickness of the photoreceptor layer was measured in 20 µm sections and sections were counted from each retina from the central regions. Within the ganglion cell layer, the number of immunostained Brn3a cells was counted per 100 µm for each retinal section from the central regions. Data was averaged from each retina. At least three independent retinas were quantified for each experiment.
Neuronal Cultures
Cortical neurons were isolated from E17 mice from the C57BL/6J background and were cultured for 14 days as described (Hu et al., 2010). Neurons were stained using rat anti Lamp1 antibody, Santa Cruz Biotechnology sc-19992 (1:500) and sheep anti-mProgranulin R&D Systems AF2557 (1:250) as described above.
Autofluorescence imaging
Mouse retina autofluorescence imaging was performed using a Zeiss Axiophot microscope. Sections of frozen tissue were cut at a thickness of 20µM on a cryostat and examined for autofluorescence. Samples were imaged using a epifluorescence microscope with multiple excitation wavelengths including 488 nm and 543 nm. Retinas were photographed using a 20X Plan objective lens.
Statistics
Standard unpaired Student’s t-tests were used to determine significance across data sets. For all experiments data are expressed as the mean. Levels of significance are shown as for P<0.05. At least three independent retinas were quantified for each experiment.
Highlights.
A mutation in GRN has been linked to Neuronal ceroid lipofuscinosis-11 (CLN11) in humans.
We investigate whether Grn−/− mice develop similar pathology.
Grn−/− mice develop accumulation of autofluorescent storage material in the retina.
Grn−/− mice develop degeneration of photoreceptors and retinal ganglion cells.
This mouse model may be of use for further study of CLN11 pathogenesis.
Acknowledgements
This work was supported by the NIH R01 NS074319, the Yale Department of Ophthalmology and Visual Science, and the Kavli Institute for Neuroscience at Yale University.
Abbreviations
- NCL
Neuronal ceroid lipofuscinosis
- CLN11
Neuronal ceroid lipofuscinosis-11
- ONL
outer nuclear layer
- INL
inner nuclear layer
- GCL
ganglion cell layer
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors’ contributions
BPH and ZAK carried out the experiment, conducted the data analysis and prepared the manuscript. ZJZ participated in the research. SMS participated in the design of the study and helped to draft the manuscript. All the authors have read and approved the final manuscript.
References
- Ahmed Z, Sheng H, Xu YF, Lin WL, Innes AE, Gass J, Yu X, Wuertzer CA, Hou H, Chiba S, Yamanouchi K, Leissring M, Petrucelli L, Nishihara M, Hutton ML, McGowan E, Dickson DW, Lewis J. Accelerated lipofuscinosis and ubiquitination in granulin knockout mice suggest a role for progranulin in successful aging. Am J Pathol. 2010;177:311–324. doi: 10.2353/ajpath.2010.090915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida S, Zhou L, Gao FB. Progranulin, a glycoprotein deficient in frontotemporal dementia, is a novel substrate of several protein disulfide isomerase family proteins. PLoS One. 2011;6:e26454. doi: 10.1371/journal.pone.0026454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen OM, Reiche J, Schmidt V, Gotthardt M, Spoelgen R, Behlke J, von Arnim CA, Breiderhoff T, Jansen P, Wu X, Bales KR, Cappai R, Masters CL, Gliemann J, Mufson EJ, Hyman BT, Paul SM, Nykjaer A, Willnow TE. Neuronal sorting protein-related receptor sorLA/LR11 regulates processing of the amyloid precursor protein. Proc Natl Acad Sci USA. 2005;102:13461–13466. doi: 10.1073/pnas.0503689102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson HC, Charrow J, Kaplan P, Mistry P, Pastores GM, Prakash-Cheng A, Rosenbloom BE, Scott CR, Wappner RS, Weinreb NJ International Collaborative Gaucher Group, U.S.R.C. Individualization of long-term enzyme replacement therapy for Gaucher disease. Genet Med. 2005;7:105–110. doi: 10.1097/01.gim.0000153660.88672.3c. [DOI] [PubMed] [Google Scholar]
- Ballabio A, Gieselmann V. Lysosomal disorders: from storage to cellular damage. Biochim Biophys Acta. 2009;1793:684–696. doi: 10.1016/j.bbamcr.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Bartsch U, Galliciotti G, Jofre GF, Jankowiak W, Hagel C, Braulke T. Apoptotic photoreceptor loss and altered expression of lysosomal proteins in the nclf mouse model of neuronal ceroid lipofuscinosis. Invest Ophthalmol Vis Sci. 2013;54:6952–6959. doi: 10.1167/iovs.13-12945. [DOI] [PubMed] [Google Scholar]
- Birch DG. Retinal degeneration in retinitis pigmentosa and neuronal ceroid lipofuscinosis: An overview. Mol Genet Metab. 1999;66:356–366. doi: 10.1006/mgme.1999.2829. [DOI] [PubMed] [Google Scholar]
- Boustany RM, Alroy J, Kolodny EH. Clinical classification of neuronal ceroid-lipofuscinosis subtypes. Am J Med Genet Suppl. 1988;5:47–58. doi: 10.1002/ajmg.1320310608. [DOI] [PubMed] [Google Scholar]
- Boustany RM. Lysosomal storage diseases--the horizon expands. Nat Rev Neurol. 2013;9:583–598. doi: 10.1038/nrneurol.2013.163. [DOI] [PubMed] [Google Scholar]
- Bozorg S, Ramirez-Montealegre D, Chung M, Pearce DA. Juvenile neuronal ceroid lipofuscinosis (JNCL) and the eye. Surv Ophthalmol. 2009;54:463–471. doi: 10.1016/j.survophthal.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman SL, Karaa A, Staropoli JF, Sims KB. Neuronal ceroid lipofuscinosis: impact of recent genetic advances and expansion of the clinicopathologic spectrum. Curr Neurol Neurosci Rep. 2013;13:366. doi: 10.1007/s11910-013-0366-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel R, He Z, Carmichael KP, Halper J, Bateman A. Cellular localization of gene expression for progranulin. J Histochem Cytochem. 2000;48:999–1009. doi: 10.1177/002215540004800713. [DOI] [PubMed] [Google Scholar]
- de Duve C. The lysosome turns fifty. Nat Cell Biol. 2005;7:847–849. doi: 10.1038/ncb0905-847. [DOI] [PubMed] [Google Scholar]
- Hafler BP, Surzenko N, Beier KT, Punzo C, Trimarchi JM, Kong JH, Cepko CL. Transcription factor Olig2 defines subpopulations of retinal progenitor cells biased toward specific cell fates. Proc Natl Acad Sci USA. 2012;109:7882–7887. doi: 10.1073/pnas.1203138109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges BL, Cheng SH. Cell and gene-based therapies for the lysosomal storage diseases. Curr Gene Ther. 2006;6:227–241. doi: 10.2174/156652306776359522. [DOI] [PubMed] [Google Scholar]
- Hu F, Padukkavidana T, Vaegter CB, Brady OA, Zheng Y, Mackenzie IR, Feldman HH, Nykjaer A, Strittmatter SM. Sortilin-mediated endocytosis determines levels of the frontotemporal dementia protein, progranulin. Neuron. 2010;68:654–667. doi: 10.1016/j.neuron.2010.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz ML, Rice LM, Gao CL. Reversible accumulation of lipofuscin-like inclusions in the retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1999;40:175–181. [PubMed] [Google Scholar]
- Katz ML, Coates JR, Cooper JJ, O'Brien DP, Jeong M, Narfstrom K. Retinal pathology in a canine model of late infantile neuronal ceroid lipofuscinosis. Invest Ophthalmol Vis Sci. 2008;49:2686–2695. doi: 10.1167/iovs.08-1712. [DOI] [PubMed] [Google Scholar]
- Kayasuga Y, Chiba S, Suzuki M, Kikusui T, Matsuwaki T, Yamanouchi K, Kotaki H, Horai R, Iwakura Y, Nishihara M. Alteration of behavioural phenotype in mice by targeted disruption of the progranulin gene. Behav Brain Res. 2007;185:110–118. doi: 10.1016/j.bbr.2007.07.020. [DOI] [PubMed] [Google Scholar]
- Lee WC, Almeida S, Prudencio M, Caulfield TR, Zhang YJ, Tay WM, Bauer PO, Chew J, Sasaguri H, Jansen-West KR, Gendron TF, Stetler CT, Finch N, Mackenzie IR, Rademakers R, Gao FB, Petrucelli L. Targeted manipulation of the sortilin-progranulin axis rescues progranulin haploinsufficiency. Hum Mol Genet. 2014;23:1467–1478. doi: 10.1093/hmg/ddt534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidove O, West ML, Pintos-Morell G, Reisin R, Nicholls K, Figuera LE, Parini R, Carvalho LR, Kampmann C, Pastores GM, Mehta A. Effects of enzyme replacement therapy in Fabry disease--a comprehensive review of the medical literature. Genet Med. 2010;12:668–679. doi: 10.1097/GIM.0b013e3181f13b75. [DOI] [PubMed] [Google Scholar]
- Mink JW, Augustine EF, Adams HR, Marshall FJ, Kwon JM. Classification and natural history of the neuronal ceroid lipofuscinoses. J Child Neurol. 2013;28:1101–1105. doi: 10.1177/0883073813494268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkau TL, Neal SJ, Milnerwood A, Mew A, Hill AM, Orban P, Gregg J, Lu G, Feldman HH, Mackenzie IR, Raymond LA, Leavitt BR. Synaptic dysfunction in progranulin-deficient mice. Neurobiol Dis. 2012;45:711–722. doi: 10.1016/j.nbd.2011.10.016. [DOI] [PubMed] [Google Scholar]
- Platt FM, Lachmann RH. Treating lysosomal storage disorders: current practice and future prospects. Biochim Biophys Acta. 2009;1793:737–745. doi: 10.1016/j.bbamcr.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Schoser B, Hill V, Raben N. Therapeutic approaches in glycogen storage disease type II/Pompe Disease. Neurotherapeutics. 2008;5:569–578. doi: 10.1016/j.nurt.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shacka JJ. Mouse models of neuronal ceroid lipofuscinoses: useful pre-clinical tools to delineate disease pathophysiology and validate therapeutics. Brain Res Bull. 2012;88:43–57. doi: 10.1016/j.brainresbull.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Smith KR, Damiano J, Franceschetti S, Carpenter S, Canafoglia L, Morbin M, Rossi G, Pareyson D, Mole SE, Staropoli JF, Sims KB, Lewis J, Lin WL, Dickson DW, Dahl HH, Bahlo M, Berkovic SF. Strikingly different clinicopathological phenotypes determined by progranulin-mutation dosage. Am J Hum Genet. 2012;90:1102–1107. doi: 10.1016/j.ajhg.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Matsuwaki T, Yamanouchi K, Nishihara M. Increased lysosomal biogenesis in activated microglia and exacerbated neuronal damage after traumatic brain injury in progranulin-deficient mice. Neuroscience. 2013;250:8–19. doi: 10.1016/j.neuroscience.2013.06.049. [DOI] [PubMed] [Google Scholar]
- Tsuruma K, Yamauchi M, Sugitani S, Otsuka T, Ohno Y, Nagahara Y, Ikegame Y, Shimazawa M, Yoshimura S, Iwama T, Hara H. Progranulin, a Major Secreted Protein of Mouse Adipose-Derived Stem Cells, Inhibits Light-Induced Retinal Degeneration. Stem Cells Transl Med. 2013 doi: 10.5966/sctm.2013-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrier V, Vieira M, Mole SE. Genetic basis and phenotypic correlations of the neuronal ceroid lipofusinoses. Biochim Biophys Acta. 2013;1832:1827–1830. doi: 10.1016/j.bbadis.2013.03.017. [DOI] [PubMed] [Google Scholar]
- Zhu J, Nathan C, Jin W, Sim D, Ashcroft GS, Wahl SM, Lacomis L, Erdjument-Bromage H, Tempst P, Wright CD, Ding A. Conversion of proepithelin to epithelins: roles of SLPI and elastase in host defense and wound repair. Cell. 2002;111:867–878. doi: 10.1016/s0092-8674(02)01141-8. [DOI] [PubMed] [Google Scholar]