Abstract
Perioperative blood loss leading to blood transfusion continues to be an issue for total knee arthroplasty (TKA) patients. The US Nationwide Inpatient Sample (NIS) was used to determine annual trends in allogenic blood transfusion rates, and effects of transfusion on in-hospital mortality, length of stay (LOS), costs, discharge disposition, and complications of primary TKA patients. TKA patients between 2000 and 2009 were included (n=4,544,999) and categorized as: (1) those who received a transfusion of allogenic blood, and (2) those who did not. Transfusion rates increased from 7.7% to 12.2%. For both transfused and not transfused groups, mortality rates and mean LOS declined, while total costs increased. Transfused patients were associated with adjusted odds ratios of in-hospital mortality (AOR 1.16; p = 0.184), 0.71 ± 0.01 days longer LOS (p < 0.0001), and incurred ($1,777 ± 36; p < 0.0001) higher total costs per admission.
Introduction
While Total Knee Arthroplasty (TKA) is a common and successful procedure in terms of improving pain and function, it is not without inherent risks. Blood losses commonly ranging from 1,000 to 1,500 mL (and sometimes up to 2,200 mL)1 can often require to autologous blood transfusion, which has been estimated to occur at rates as high as 35% to 53% following TKA2-4. Overall, national transfusion rates have varied throughout the United States over the last 30 years, increasing from 42 units per 1,000 patients in 1979 to 50 units per 1,000 patients in 20015-8. The effectiveness of blood conservation strategies, including lower thresholds for transfusion, postoperative cell salvage, and preoperative autologous blood donation are not clear. There have been few studies that adequately describe the current trends and rates of transfusion in United States hospitals for elective TKA procedures.
Allogenic blood transfusions have potential consequences associated with their use, including infection (viral9 and bacterial10), immunologic responses10,11, intravascular hemolysis10-13, acute lung injury11,14, transfusion-induced coagulopathy, and mistransfusion15. Hemodynamically unstable patients who do not appropriately receive a transfusion are at risk for cerebrovascular accidents and myocardial infarction. Blood management is also an important element in terms of cost, which has increased between 1991 and 2008 and accounts for approximately 2.5% of the overall allocation of hospital cost for primary TKA16.
The primary purpose of our analysis was to determine the rates and trends of allogenic blood transfusions in patients who received a primary TKA in United States hospitals between 2000 and 2009. Secondary objectives were to identify risk factors for transfusion as well as whether allogenic blood transfusion is associated with increased in-hospital mortality, length of stay (LOS), costs, and complications.
Methods
This national cross-sectional study was a review of the Nationwide Inpatient Sample (NIS) database from 2000 to 200917. The NIS database is the largest all-payer inpatient care database available in the United States approximating a sample of 20% of non-federal hospitals. The NIS database has grown since 1988, and currently contains information on almost 8 million hospital stays annually from over 1,045 hospitals in 46 states that participate in the Healthcare Cost and Utilization Project (HCUP), Agency for Healthcare Research and Quality (AHRQ). Data elements available include patient demographics, insurance status, International Classification of Disease, 9th edition (ICD-9-CM) primary and secondary diagnosis and procedure codes, hospital characteristics, admission and discharge status, LOS, and total charges. These data are de-identified, and the study was deemed exempt by our Institutional Review Board.
Patients who received a primary TKA (ICD-9-CCCM 81.54) from January 2000 through December 2009 were included in the study (n=4,544,999) (Figure 1). Exclusion criteria were as follows: age under 18 years, acute infection of lower extremity, previous arthroplasty, metastatic and bone cancer, fracture(s) of the lower limb, and multiple joint replacements within a single admission (Figure 1). The total number of weighted patients following these exclusions was 4,215,449. Patients transfused with autologous blood only (ICD-9-CM codes: 99.00, 99.02; n=285,357) were not included in the analyses, except to compare transfusion trends over time. The remaining n=3,930,092 patients were then categorized into two groups: (1) those who received a transfusion of allogenic blood [ICD-9-CM procedure codes18 99.03 (other transfusion of whole blood), 99.04 (transfusion of packed cells)] alone or in combination with autologous blood (n=467,448), and (2) those who were not transfused with any blood (n=3,462,644).
Fig. 1.
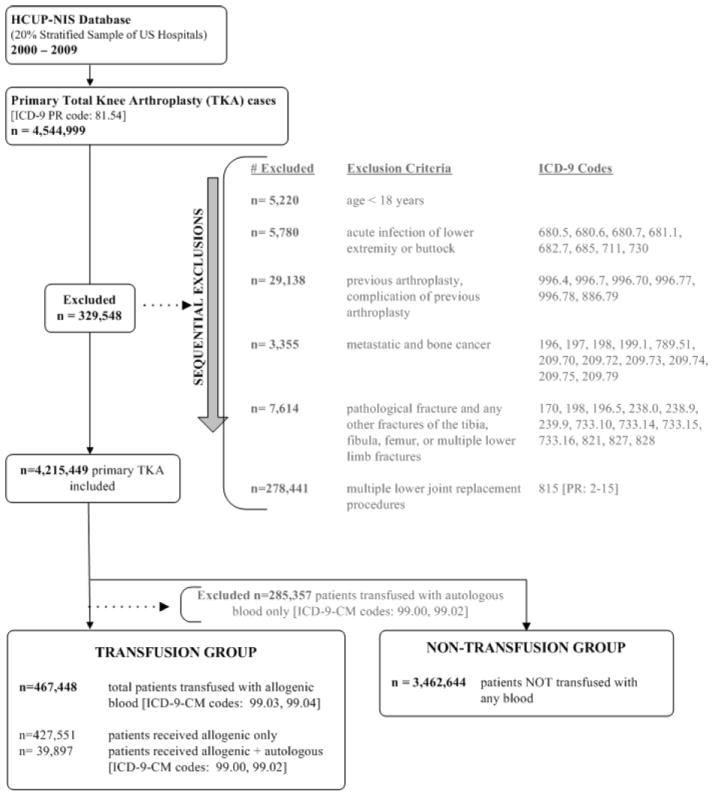
Diagram showing the number of excluded, transfused, and not transfused cases from the total knee arthroplasty cohort (United States 2000 to 2009)
Our primary outcome of interest was annual trends in the rate of allogenic blood transfusion among primary TKA patients. We also examined the effects of blood transfusion among primary TKA patients on in-hospital mortality, hospital LOS, costs, discharge to an extended care facility (rather than home), surgical complications and adverse events. Surgical complications and adverse events were identified using specific ICD-9-CM diagnosis codes, as per previously published algorithms19 (e.g., pulmonary insufficiency, pulmonary embolism, cardiac, venous thrombosis, etc.). In addition, we also examined the effect of blood transfusion following TKA on pulmonary edema/congestion (ICD-9-CM diagnosis codes 514 and 518.4) and superficial (682.5, 682.6, 998.59) and deep (996.66, 996.67) surgical site infections.
Patient demographics (age, gender, race, and insurance status), hospital characteristics [location (rural or urban), region of the United States (northeast, midwest, south and west), teaching status (nonacademic or academic), and bed size (small, medium, or large)]15, and patient comorbidities were included as covariates in the statistical models. Patient comorbidities were analyzed utilizing the Healthcare Cost and Utilization Project (HCUP) Comorbidity software, which uses ICD-9-CM diagnosis codes to flag the presence of 30 different comorbidities16. The covariates were defined as recorded in the discharge summary. Age was grouped into 7 categories representing ten year age brackets (e.g. 40 – 49) for persons aged 40 to 89, with additional groups for persons under 40 and those over 89. Race/Ethnicity was categorized as White, Black, Hispanic, Other, and Missing, the latter of which was created to account for the nearly 25% of records that did not have race/ethnicity recorded – a strategy used by some states as an additional safeguard to protect patient privacy. Insurance status was categorized as Medicare, Medicaid, Private insurance, uninsured, and other.
Statistical Analysis
Distribution of the data and baseline descriptive statistics of the variables were examined, followed by the Pearson Chi-square test and Student’s t-test for examining the association between blood transfusion and the baseline variables. We examined the trends in allogeneic blood transfusion among primary TKA patients from 2000 to 2009. Given the stratified sampling scheme of NIS, weights were used to obtain the national estimates. The trends in in-hospital mortality, LOS, and total costs among the transfused and non-transfused groups were described and compared. Logistic regression models were used to examine the factors associated with allogenic blood transfusion in primary TKA patients, and, the association between allogenic blood transfusion and the outcomes of interest, after adjusting for patient covariates.
We used propensity score matching to estimate the adjusted associations between transfusion and outcomes (i.e., mortality, discharge to in-patient facility, complications and adverse events, LOS, total costs and total charges), while controlling for known confounders. A logistic model was used to estimate the predicted probability of transfusion (i.e. propensity score), using transfusion as the outcome and all study variables as covariates. Each transfusion case was then matched to a control subject using a 1:1 greedy match algorithm based on the propensity score. The associated effect of transfusion was estimated using logistic regression models for binary outcomes or generalized estimating equation models to account for within hospital clustering for continuous outcomes. Costs were calculated by multiplying the total charges by a hospital-specific cost-to-charge ratio provided by HCUP, and were adjusted for inflation based on real GDP in chained 2009 dollars. SAS System for Windows, Version 9.3 was used to perform all analyses.
Results
Of the 4,215,449 weighted national estimate of primary TKA patients, 467,448 received allogenic blood products for an overall transfusion rate of 11.91% (Figure 1). The allogenic transfusion rate was lowest in 2000 at 7.7%, and steadily increased until 2006 where it appears to have plateaued at 12.0% [adjusted odds ratio (AOR); 1.52. 95% confidence interval (CI) 1.46-1.58) and stayed fairly level through 2009 (12.2%; AOR 1.43; CI 1.38 – 1.49). It is worth noting that during the same time period the rate of autologous blood transfusion dropped from 7.9% to 5.0% (Figure 2). From this point forward, autologous transfusions (unless in combination with allogenic transfusion) were not included in the analyses.
Fig. 2.

National rates of allogenic and autologous blood transfusions among primary total knee arthroplasty patients (United States 2000 to 2009)
The distribution of primary TKA patients who did and did not receive allogenic blood products with respect to age, gender, race, and insurance is shown in Table 1. Over 30% of patients over 90 were transfused. In terms of gender, 13.1% of females were transfused compared with 7.6% of males. In terms of race, the highest percentage of transfusion was observed in blacks (18.6%). There were relatively small differences detected between rural (12.0%) and urban (11.0%) hospitals (Table 2). In addition, hospitals in the northeast (13.6%) and of medium bed size (12.1%) had the higher rates of allogenic transfusion.
Table 1.
Demographics of participants who underwent primary TKA (2000-2009 weighted)
| All patients N (column %) | Transfused N (column %) | Not transfused N (column %) | p-value | |
|---|---|---|---|---|
| Total Patients | 4,215,449 | 467,448 (11.1) | 3,462,644 (82.1) | |
| Age (Years) | <.0001 | |||
| < 40 | 24,679 (0.5) | 1,957 (0.4) | 21,454 (0.6) | |
| 41-49 | 206,892 (4.9) | 12,418 (2.7) | 182,901 (5.3) | |
| 50-59 | 817,459 (19.4) | 55,766 (11.9) | 708,086 (20.4) | |
| 60-69 | 1,338,197 (31.8) | 119,142 (25.5) | 1,123,272 (32.4) | |
| 70-79 | 1,345,570 (31.9) | 177,189 (37.9) | 1,072,724 (31.0) | |
| 80-89 | 468,100 (11.1) | 96,601 (20.7) | 344,499 (10.0) | |
| >90 | 14,553 (0.4) | 4,375 (0.9) | 9,708 (0.3) | |
| Gender | <.0001 | |||
| Male | 1,504,993 (35.7) | 113,928 (24.4) | 1,295,373 (37.4) | |
| Female | 2,702,112 (64.1) | 353,110 (75.5) | 2,159,843 (62.4) | |
| Missing | 8,344 (0.2) | 410 (0.1) | 7,429 (0.2) | |
| Race | <.0001 | |||
| White | 2,613,285 (62.0) | 283,183 (60.6) | 2,134,414 (61.7) | |
| Black | 210,856 (5.0) | 39,298 (8.5) | 158,657 (4.6) | |
| Hispanic | 159,826 (3.8) | 24,041 (5.1) | 125,177 (3.6) | |
| Other | 110,928 (2.6) | 17,387 (3.7) | 87,461 (2.5) | |
| Missing | 1,120,554 (26.6) | 103,539(22.1) | 956,935 (27.6) | |
| Insurance | <.0001 | |||
| Medicare | 2,462,793 (58.4) | 335,021 (71.7) | 1,962565 (56.7) | |
| Medicaid | 108,216 (2.6) | 14,666 (3.1) | 88,979 (2.6) | |
| Private/HMO | 1,476,574 (35.0) | 104,062 (22.3) | 1,267,728 (36.6) | |
| Self | 18,174 (0.4) | 2,043 (0.4) | 15,122 (0.4) | |
| Other | 149,692 (3.6) | 11,656 (2.5) | 128,250 (3.7) |
Table 2.
Hospital Characteristics (2000-2009 weighted)
| All patients N (column %) | Transfused N (row %) | Not transfused N (row %) | p-value | |
|---|---|---|---|---|
| Location | 0.024 | |||
| Rural | 562,690 (13.4) | 67,358 (14.2) | 458,974 (13.3) | |
| Urban | 3,642,372(86.4) | 399,297 (85.7) | 2,994,082 (86.5) | |
| Region | <0.001 | |||
| Northeast | 691,695 (16.4) | 94,309 (20.6) | 519,009 (15.0) | |
| Midwest | 1,187,350 (28.2) | 90,554 (20.0) | 1,051,170 (30.4) | |
| South | 1,532,812 (36.4) | 194,875 (41.6) | 1,241,211 (35.9) | |
| West | 803,591 (19.1) | 87,710 (17.8) | 651,255 (18.8) | |
| Teaching Status | 0.268 | |||
| Nonacademic | 2,505,536 (59.4) | 284,172 (60.8) | 2,064,665 (59.6) | |
| Academic | 1,699,526 (40.3) | 182,483 (39.0) | 1,388,391 (40.1) | |
| Bed Size | <0.001 | |||
| Small | 625,925 (14.9) | 70,459 (15.1) | 497,199 (14.4) | |
| Medium | 1,065,491 (25.3) | 128,871 (27.6) | 861,400 (24.9) | |
| Large | 2,513,646 (59.6) | 267,325 (57.2) | 2,094,457 (60.5) |
The most common comorbidities in TKA patients (for both transfused and non-transfused patients) were hypertension (63.1%), uncomplicated diabetes (17.6%), obesity (14.3%), hypothyroidism (13.3%), chronic pulmonary disease (13.0%), deficiency anemia (11.9%), fluid and electrolyte disorders (6.4%) (Figure 3). The prevalence of nearly all comorbidities evaluated was higher in patients who were transfused (p<0.001), with the exception of obesity which was higher for patients who were not transfused (p<0.001). There was no significant difference in the prevalence of solid tumors and peptic ulcer disease, with p-values of 0.155 and 0.885, respectively. The rate of allogenic blood transfusion was highest in patients with weight loss (32.3%), renal failure (27.3%), and deficiency anemia (26.2%).
Fig. 3.
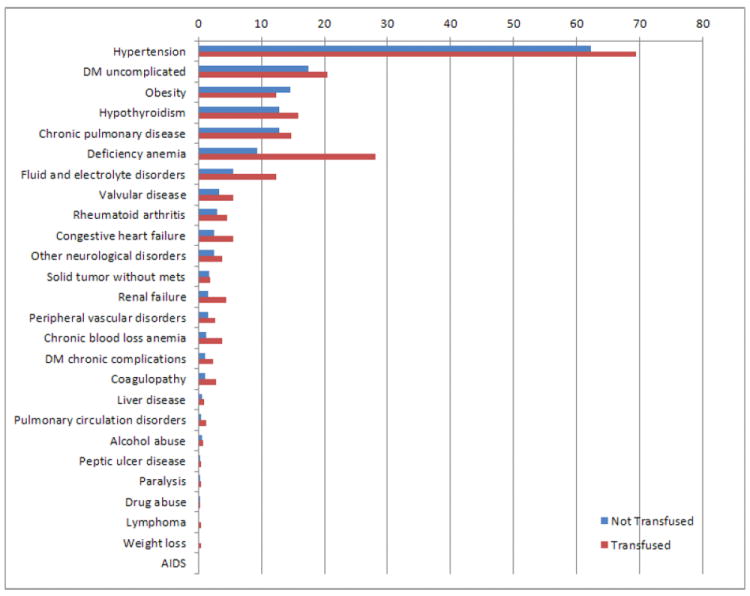
Prevalence of comorbidities in transfused and not transfused total knee arthroplasty patients. DM, diabetes mellitus
Multivariable logistic regression analysis revealed several risk factors for allogenic blood transfusion (c-statistic 0.733; Table 3). Patients above the age of 60 were significantly associated with a greater likelihood for receiving allogenic blood transfusion (p < 0.008). Females were 76% more likely to require a blood transfusion (AOR 1.64, 95% confidence interval 1.73 – 1.79); p < 0.0001). In terms of race, Blacks (AOR 1.82; p < 0.0001) and Hispanics (AOR 1.32; p < 0.0001) both had increased likelihood of transfusion compared with Whites. Patients insured by Medicare (AOR 1.49; p < 0.001), Medicaid (AOR 1.20; p < 0.001), and those who were uninsured (AOR 1.31; p < 0.0001) were also more likely than patients privately insured. Nearly all comorbidities examined were significantly associated (p < 0.0001) with an increased likelihood of transfusion, with the exception of solid tumor (AOR 1.05, p=0.087) and peptic ulcer disease (AOR 0.96, p=0.508).
Table 3.
Predictive Model of Transfusion
| AOR | p-value | |
|---|---|---|
| Age Group | ||
| <40 | Ref | |
| 40 - 49 | 0.81 (0.72-0.90) | 0.0002 |
| 50 - 59 | 0.93 (0.83-1.03) | 0.166 |
| 60 - 69 | 1.16 (1.04-1.29) | 0.008 |
| 70 - 79 | 1.64 (1.47-1.83) | <.0001 |
| 80 - 89 | 2.66 (2.39-2.97) | <.0001 |
| 90+ | 4.13 (3.60-4.74) | <.0001 |
| Sex | ||
| Male | Ref | |
| Female | 1.76 (1.73-1.79) | <.0001 |
| Race | ||
| White | Ref | |
| Black | 1.82 (1.77-1.88) | <.0001 |
| Hispanic | 1.32 (1.28-1.37) | <.0001 |
| Missing | 1.01 (0.99-1.03) | 0.2402 |
| Other | 1.45 (1.40-1.51) | <.0001 |
| Insurance | ||
| Medicare | 1.49 (1.43-1.56) | <.0001 |
| Medicaid | 1.20 (1.17-1.22) | <.0001 |
| Private | Ref | |
| Other | 1.07 (1.03-1.12) | 0.0027 |
| Uninsured | 1.31 (1.17-1.46) | <.0001 |
| Comorbidities | ||
| AIDS | 2.07 (1.27-3.38) | 0.0035 |
| Alcohol abuse | 1.54 (1.41-1.68) | <.0001 |
| Deficiency anemia | 3.26 (3.20-3.32) | <.0001 |
| Rheumatoid arthritis | 1.37 (1.32-1.42) | <.0001 |
| Chronic blood loss anemia | 3.27 (3.14-3.41) | <.0001 |
| Congestive heart failure | 1.52 (1.47-1.58) | <.0001 |
| Chronic pulmonary disease | 1.06 (1.04-1.08) | <.0001 |
| Coagulopathy | 2.04 (1.94-2.15) | <.0001 |
| DM uncomplicated | 1.17 (1.15-1.19) | <.0001 |
| DM chronic complications | 1.64 (1.55-1.73) | <.0001 |
| Drug abuse | 1.37 (1.20-1.57) | <.0001 |
| Hypertension | 1.06 (1.04-1.08) | <.0001 |
| Hypothyroidism | 1.02 (1.00-1.04) | 0.0418 |
| Liver disease | 1.43 (1.31-1.55) | <.0001 |
| Lymphoma | 1.69 (1.49-1.92) | <.0001 |
| Fluid and electrolyte disorders | 1.66 (1.62-1.70) | <.0001 |
| Other neurological disorders | 1.39 (1.33-1.44) | <.0001 |
| Obesity | 0.86 (0.84-0.88) | <.0001 |
| Paralysis | 1.52 (1.36-1.70) | <.0001 |
| Peripheral vascular disorders | 1.29 (1.23-1.35) | <.0001 |
| Pulmonary circulation disorders | 1.48 (1.38-1.60) | <.0001 |
| Renal failure | 1.79 (1.72-1.87) | <.0001 |
| Solid tumor without mets | 1.05 (0.99-1.10) | 0.0866 |
| Peptic ulcer disease | 0.96 (0.86-1.08) | 0.5082 |
| Valvular disease | 1.27 (1.23-1.31) | <.0001 |
| Weight loss | 1.93 (1.68-2.22) | <.0001 |
| Hospital characteristics | ||
| Urban vs. Rural | 0.79 (0.78-0.81) | <.0001 |
| Teaching vs non-teaching | 1.00 (0.99-1.02) | 0.6514 |
| Northeast vs. West | 1.36 (1.33-1.39) | <.0001 |
| Midwest vs. West | 0.58 (0.57-0.59) | <.0001 |
| South vs. West | 1.08 (1.05-1.10) | <.0001 |
| Small bedsize vs. Large | 1.22 (1.20-1.25) | <.0001 |
| Medium bedsize vs. Large | 1.24 (1.22-1.26) | <.0001 |
AOR=adjusted odds ratio; DM=diabetes mellitus. C-statistic = Need to update
The unadjusted mortality rate (per 1,000) in TKA admissions was significantly higher for the transfused group (OR 2.14) than the not transfused group (OR 0.98; p < 0.001). The rate decreased from 2000 to 2009 in both transfused (from 3.34 to 1.63) and not transfused (from 1.25 to 0.52) patients (Figure 4). Mean overall LOS was higher for patients who received a transfusion (4.6 days) when compared to patients who were not transfused (3.7 days; p < 0.001). The unadjusted trends show a decrease from 2000 to 2009 in LOS for both the transfused group (4.9 to 4.1 days) and the not transfused group (4.2 to 3.2 days) (Figure 5). Unadjusted total admission costs were significantly higher for transfused TKA patients ($14,990) than patients not transfused ($12,693; p < 0.001). The trends revealed that total costs increased for both groups between 2000 and 2009 (Figure 6).
Fig. 4.
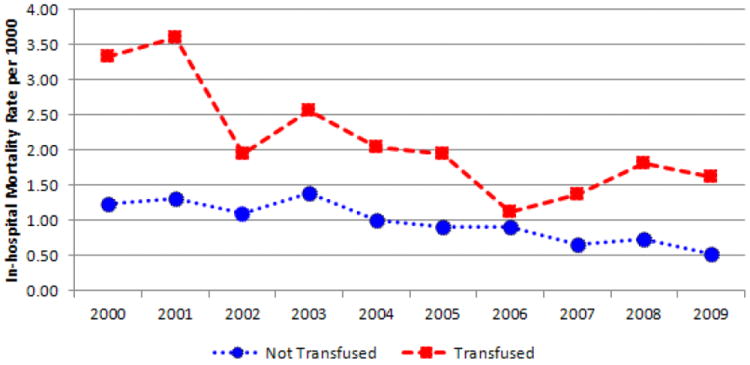
In-hospital mortality rate after total knee arthroplasty in transfused and not transfused patients
Fig. 5.
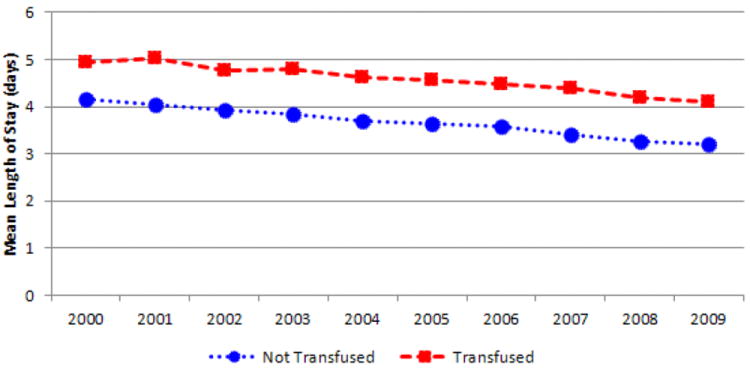
Mean hospital length of stay in transfused and not transfused total knee arthroplasty patients
Fig. 6.
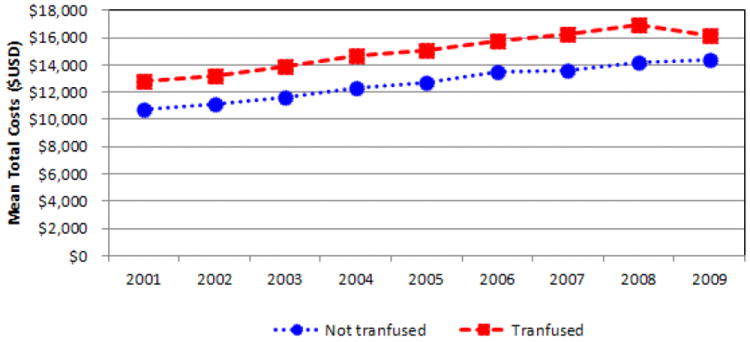
Mean hospitalization costs of transfused and not transfused total knee arthroplasty patients ($USD)
The results of the regression model adjusted for patient characteristics, comorbidities, and hospital variables after propensity matching showed similar results. Patients transfused had a higher rate of in-hospital mortality (AOR 1.16; 95% CI 0.93 – 1.43; p = 0.184), although this was not statistically significant. Transfused patients had 0.718 ± 0.01 days longer LOS (p < 0.0001), and higher total costs per admission ($1,777 ± 36; p < 0.0001). In addition, transfused TKA patients were more likely to be discharged to an extended care facility rather than directly home (AOR 1.35; 95% CI 1.32 – 1.37; p < 0.0001). Allogenic blood transfusion was associated with poorer surgical and medical outcomes following TKA for all outcomes examined (Figure 7). For example, the AOR of developing a postoperative infection was 2.33 (95% CI 1.95 – 2.80; p < 0.0001).
Fig. 7.
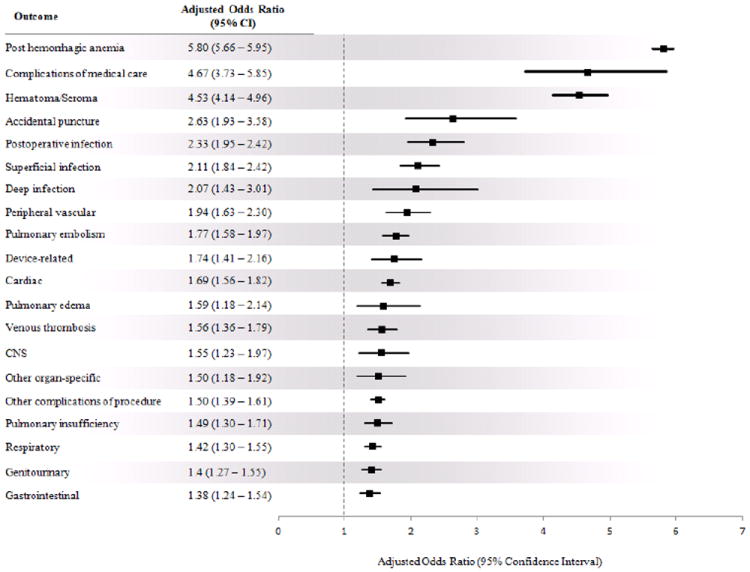
Adjusted effect of allogenic blood transfusion on medical and surgical outcomes after total knee arthroplasty
Discussion
The primary purpose of this study was to describe the trends in the use of allogenic blood transfusion for TKA patients in the United States between 2000 and 2009. The data from the NIS show that the rate of allogenic transfusion has increased in this patient population, which is associated with increased in-hospital mortality, increased LOS, greater total costs, and poorer surgical and medical outcomes. No conclusions regarding autologous transfusion can be made from the current study, as only trend over time was included.
There are no current studies in the literature which utilize nationally representative data to assess transfusion trends in TKA patients. One of largest cohort studies is nearly 15 years old2, which reported results on 4,642 primary unilateral TKA patients from 235 sites in the United States. There were 499 patients who received allogenic blood transfusion, for a rate of 11%, comparable to what is reported in the current study. Segal et al.20 used the NIS data to assess autologous and allogenic blood use for all admissions in the United States in 1996, and reported that only 4.7% of TKA patients received allogenic blood in that year. Recent data from a single-center study reported an allogenic transfusion rate of 11% following 644 primary unilateral TKAs21. In addition, the authors stated that the rates at their institution have trended downward since 2005. It is difficult to discern whether the increased rate of transfusion observed in the present study is real or an artifact of improved coding.
It is not surprising that the comorbidities with the highest odds ratios for receiving transfusion are all related to age and anemia, and included the age groups 80 – 89 (OR 2.66), ages 90 and above (OR 4.13), deficiency anemia (OR 3.26) and chronic blood loss anemia (3.27) (Table 3). Age and anemia as risk factors for blood transfusion following TKA have been well-described in the literature3,21,22.
Blood transfusion has been associated with increased morbidity and mortality throughout the surgical literature22,23. Other studies have reported associations between poorer outcomes following arthroplasty and blood transfusion24. While it is worth noting that these associations are not to be interpreted as causal, it is equally important to recognize these associations and design appropriate studies to address these associations.
Previous reports have stated that arthroplasty patients receiving blood transfusion have an estimated 20% - 25% increase in their hospital LOS25,26, while our data support an adjusted increase of 0.71 days. Postoperative allogenic blood transfusion has been reported to be a cost-effective alternative to autologous blood donation 27. However, the use of transfusion is not without direct and indirect costs, and was shown in this study to results in an adjusted increase of $1,777 total cost per admission, or approximately 18% higher costs than that of a patient who was not transfused. Other reports in the literature have suggested that arthroplasty patients receiving transfusions increase the total hospital admission cost by 10% to 20%, which is comparable to that reported here25,26.
There are limitations inherent to large administrative databases, which one must be mindful of when interpreting the results. The NIS contains only data from inpatient stay records from admission to discharge which cannot be linked with a patient’s past or future encounters. Information regarding readmissions, complications, mortality, etc., post-discharge is therefore not available. Incomplete and/or inaccurate coding is another limitation of these databases which may result in misclassification bias of the predictors and outcomes. One example of this is the obvious undercoding of obesity in this population, with an observed prevalence of only 14.3% in TKA patients between 2000 and 2009, while the literature reports 55% for TKA patients28. In addition, the data made available in the NIS are of limited granularity, with no information on blood loss, perioperative hemoglobin values, use of blood conserving products/techniques, and details regarding the number or units and/or volume of allogenic blood transfused.
Despite blood conservation options and methods to reduce perioperative blood loss, blood transfusion continues to be necessary for many TKA patients. The increased rate of allogenic transfusion and its associations with increased mortality, LOS, cost, and risk of complications is concerning, and emphasizes the need for appropriate risk stratification, conservative transfusion indications, and effective use of blood management strategies and proven technologies. As the use of tranexemic acid and other modern blood conservation strategies continue to rise and show positive results, the potential for change in these trends increases.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sehat KR, Evans RL, Newman JH. Hidden blood loss following hip and knee arthroplasty. Correct management of blood loss should take hidden loss into account. J Bone Joint Surg Br. 2004 May;86(4):561–565. [PubMed] [Google Scholar]
- 2.Bierbaum BE, Callaghan JJ, Galante JO, Rubash HE, Tooms RE, Welch RB. An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg Am. 1999 Jan;81(1):2–10. doi: 10.2106/00004623-199901000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Bong MR, Patel V, Chang E, Issack PS, Hebert R, Di Cesare PE. Risks associated with blood transfusion after total knee arthroplasty. J Arthroplasty. 2004 Apr;19(3):281–287. doi: 10.1016/j.arth.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 4.Rosencher N, Kerkkamp HE, Macheras G, et al. Orthopedic Surgery Transfusion Hemoglobin European Overview (OSTHEO) study: blood management in elective knee and hip arthroplasty in Europe. Transfusion. 2003 Apr;43(4):459–469. doi: 10.1046/j.1537-2995.2003.00348.x. [DOI] [PubMed] [Google Scholar]
- 5.Surgenor DM, Wallace EL, Hao SH, Chapman RH. Collection and transfusion of blood in the United States, 1982-1988. N Engl J Med. 1990 Jun 7;322(23):1646–1651. doi: 10.1056/NEJM199006073222306. [DOI] [PubMed] [Google Scholar]
- 6.Wallace EL, Churchill WH, Surgenor DM, Cho GS, McGurk S. Collection and transfusion of blood and blood components in the United States, 1994. Transfusion. 1998 Jul;38(7):625–636. doi: 10.1046/j.1537-2995.1998.38798346630.x. [DOI] [PubMed] [Google Scholar]
- 7.Sullivan MT, Cotten R, Read EJ, Wallace EL. Blood collection and transfusion in the United States in 2001. Transfusion. 2007 Mar;47(3):385–394. doi: 10.1111/j.1537-2995.2007.01128.x. [DOI] [PubMed] [Google Scholar]
- 8.Wallace EL, Churchill WH, Surgenor DM, et al. Collection and transfusion of blood and blood components in the United States, 1992. Transfusion. 1995 Oct;35(10):802–812. doi: 10.1046/j.1537-2995.1995.351096026360.x. [DOI] [PubMed] [Google Scholar]
- 9.Schreiber GB, Busch MP, Kleinman SH, Korelitz JJ. The risk of transfusiontransmitted viral infections. The Retrovirus Epidemiology Donor Study. N Engl J Med. 1996 Jun 27;334(26):1685–1690. doi: 10.1056/NEJM199606273342601. [DOI] [PubMed] [Google Scholar]
- 10.Sazama K. Reports of 355 transfusion-associated deaths: 1976 through 1985. Transfusion. 1990 Sep;30(7):583–590. doi: 10.1046/j.1537-2995.1990.30790385515.x. [DOI] [PubMed] [Google Scholar]
- 11.Linden JV, Tourault MA, Scribner CL. Decrease in frequency of transfusion fatalities. Transfusion. 1997 Feb;37(2):243–244. doi: 10.1046/j.1537-2995.1997.37297203534.x. [DOI] [PubMed] [Google Scholar]
- 12.Ness PM, Shirey RS, Thoman SK, Buck SA. The differentiation of delayed serologic and delayed hemolytic transfusion reactions: incidence, long-term serologic findings, and clinical significance. Transfusion. 1990 Oct;30(8):688–693. doi: 10.1046/j.1537-2995.1990.30891020325.x. [DOI] [PubMed] [Google Scholar]
- 13.Shulman IA. The risk of an overt hemolytic transfusion reaction following the use of an immediate spin crossmatch. Arch Pathol Lab Med. 1990 Apr;114(4):412–414. [PubMed] [Google Scholar]
- 14.Popovsky MA, Moore SB. Diagnostic and pathogenetic considerations in transfusion-related acute lung injury. Transfusion. 1985 Nov-Dec;25(6):573–577. doi: 10.1046/j.1537-2995.1985.25686071434.x. [DOI] [PubMed] [Google Scholar]
- 15.Madjdpour C, Spahn DR. Allogeneic red blood cell transfusions: efficacy, risks, alternatives and indications. Br J Anaesth. 2005 Jul;95(1):33–42. doi: 10.1093/bja/aeh290. [DOI] [PubMed] [Google Scholar]
- 16.Healy WL, Rana AJ, Iorio R. Hospital economics of primary total knee arthroplasty at a teaching hospital. Clin Orthop Relat Res. 2011 Jan;469(1):87–94. doi: 10.1007/s11999-010-1486-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quality AfHRa. HCUP Nationwide Inpatient Sample (NIS) [January 1, 2013];Healthcare Cost and Utilization Project 2000-2009. www.hcup-us.ahrq.gov/nisoverview.jsp.
- 18.Blanchette CM, Joshi AV, Szpalski M, et al. Burden of blood transfusion in knee and hip surgery in the US and Belgium. J Med Econ. 2009 Sep;12(3):171–179. doi: 10.3111/13696990903172760. [DOI] [PubMed] [Google Scholar]
- 19.Memtsoudis SG, Ma Y, Gonzalez Della Valle A, et al. Perioperative outcomes after unilateral and bilateral total knee arthroplasty. Anesthesiology. 2009 Dec;111(6):1206–1216. doi: 10.1097/ALN.0b013e3181bfab7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Segal JB, Guallar E, Powe NR. Autologous blood transfusion in the United States: clinical and nonclinical determinants of use. Transfusion. 2001 Dec;41(12):1539–1547. doi: 10.1046/j.1537-2995.2001.41121539.x. [DOI] [PubMed] [Google Scholar]
- 21.Noticewala MS, Nyce JD, Wang W, Geller JA, Macaulay W. Predicting need for allogeneic transfusion after total knee arthroplasty. J Arthroplasty. 2012 Jun;27(6):961–967. doi: 10.1016/j.arth.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 22.Spahn DR. Anemia and patient blood management in hip and knee surgery: a systematic review of the literature. Anesthesiology. 2010 Aug;113(2):482–495. doi: 10.1097/ALN.0b013e3181e08e97. [DOI] [PubMed] [Google Scholar]
- 23.Murphy GJ, Reeves BC, Rogers CA, Rizvi SI, Culliford L, Angelini GD. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation. 2007 Nov 27;116(22):2544–2552. doi: 10.1161/CIRCULATIONAHA.107.698977. [DOI] [PubMed] [Google Scholar]
- 24.Pedersen AB, Mehnert F, Overgaard S, Johnsen SP. Allogeneic blood transfusion and prognosis following total hip replacement: a population-based follow up study. BMC Musculoskelet Disord. 2009;10:167. doi: 10.1186/1471-2474-10-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watts CD, Pagnano MW. Minimising blood loss and transfusion in contemporary hip and knee arthroplasty. J Bone Joint Surg Br. 2012 Nov;94(11 Suppl A):8–10. doi: 10.1302/0301-620X.94B11.30618. [DOI] [PubMed] [Google Scholar]
- 26.Report C. MedPAR database based on ICD-9-CM Codes for 100% of Medicare beneficiaries. Data on file. 2008 [Google Scholar]
- 27.Etchason J, Petz L, Keeler E, et al. The cost effectiveness of preoperative autologous blood donations. N Engl J Med. 1995 Mar 16;332(11):719–724. doi: 10.1056/NEJM199503163321106. [DOI] [PubMed] [Google Scholar]
- 28.Namba RS, Inacio MC, Paxton EW. Risk factors associated with deep surgical site infections after primary total knee arthroplasty: an analysis of 56,216 knees. J Bone Joint Surg Am. 2013 May 1;95(9):775–782. doi: 10.2106/JBJS.L.00211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


