Abstract
Objective
Hypoxic-ischemic white mater brain injury commonly occurs in neonates with hypoplastic left heart syndrome (HLHS). Approximately half of the HLHS survivors exhibit neurobehavioral symptoms believed to be associated with this injury, though the exact timing of the injury is not known.
Methods
Neonates with HLHS were recruited for pre- and post-operative monitoring of cerebral oxygen saturation (ScO2), cerebral oxygen extraction fraction (OEF), and cerebral blood flow (CBF) using two non-invasive optical-based techniques, namely diffuse optical spectroscopy and diffuse correlation spectroscopy. Anatomical magnetic resonance imaging (MRI) scans were performed prior to and approximately one week after surgery in order to quantify the extent and timing of the acquired white matter injury. Risk factors for developing new or worsened white matter injury were assessed using uni- and multi-variate logistic regression.
Results
Thirty-seven neonates with HLHS were studied. In a univariate analysis, neonates who developed a large volume of new, or worsened, postoperative white matter injury had a significantly longer time-to-surgery (p=0.0003). In a multivariate model, longer time between birth and surgery (i.e., time-to-surgery), delayed sternal closure, and higher pre-operative CBF were predictors of post-operative white matter injury. Additionally, longer time-to-surgery and higher pre-operative CBF on morning of surgery were correlated with lower ScO2 (p=0.03 and p=0.05) and higher OEF (p=0.05 and p=0.05).
Conclusions
Longer time-to-surgery is associated with new post-operative white matter injury in otherwise healthy neonates with HLHS. The results suggest that earlier Norwood palliation may decrease the likelihood of acquiring postoperative white matter injury.
Introduction
Approximately 30,000 children each year are born in the United States with congenital heart disease (CHD). Nearly one third of these CHD patients require cardiac surgery in their first year of life.1 With the majority of these patients now reaching school age,2 the focus of research has shifted to addressing the neurodevelopment disabilities seen among survivors of these early heart surgeries. Nearly 50% of the school-age survivors exhibit neurobehavioral symptoms, such as inattention, hyperactivity, and impaired executive function.3–5
Neonatal imaging and neuropathologic studies of patients with complex CHD undergoing infant surgical intervention have revealed a high prevalence of periventricular leukomalacia (PVL).6 PVL is a specific form of hypoxic-ischemic white matter injury that commonly occurs in a vascular watershed zone near the lateral ventricles; it is most often observed in preterm neonates who have neurodevelopmental outcomes remarkably similar to those of term patients with CHD. Studies have shown that PVL is related to neurodevelopmental delays in preterm infants.7–9
To date, clinical investigations in CHD neonates have focused on identifying the pre-, peri-, and post-surgical risk factors linked to PVL in order to mitigate or prevent this injury, but uncertainties about its exact cause and timing remain.10–12 These previous studies have identified possible risk factors for this injury such as brain immaturity, duration of deep hypothermic circulatory arrest, and post-operative cerebral oxygenation.12–14 However, most previous studies have been in mixed populations of neonates with various forms of CHD. Herein we investigate a homogeneous cohort of neonates with hypoplastic left heart syndrome (HLHS). We utilize diffuse optical and diffuse correlation spectroscopies (DOS and DCS, respectively) for non-invasive bedside quantification of pre- and post-operative cerebral hemodynamics15, 16, and we explore the relationship of these parameters and other pre-operative, operative, and post-operative variables to new or worsened PVL seen one week after surgical intervention.
Materials and Methods
Patient Population
All term (37– 42 weeks gestation) newborns with pre- or post-natally diagnosed HLHS admitted to the cardiac intensive care unit (CICU) at The Children’s Hospital of Philadelphia (CHOP) were screened for study inclusion and approached for participation in this prospective study. Exclusion criteria included: birth weight less than 2 kg, a history of neonatal depression (i.e., 5 minute APGAR<5 or cord pH<7.0), perinatal seizures, evidence of end-organ injury, pre-operative cardiac arrest, and/or significant pre-operative intracerebral hemorrhage such as grade 3 or 4 intraventricular hemorrhage.
Study Protocol
All procedures were approved by the Institutional Review Board. Patient demographic data were recorded. A study timeline is presented in Figure 1 and has been described previously17–20. On the morning of surgery, all patients received general endotracheal anesthesia (fentanyl 5–10 μg/kg, pancuronium 0.2mg/kg). Subsequently, they underwent a brain MRI for pre-operative injury assessment. Pre-operative DOS/DCS measurements of cerebral oxygenation (ScO2), oxygen extraction (OEF), and blood flow (CBF) were also made at this time.
Figure 1.
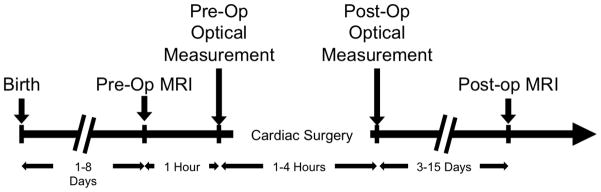
A timeline of the study.
After the MRI, patients underwent cardiopulmonary bypass with deep hypothermic circulatory arrest (DHCA) for their Stage I palliation. Antegrade cerebral perfusion was not used. Operations were performed by one of four cardiac surgeons. pH-stat blood gas management was used. To achieve DHCA, patients underwent core and surface cooling to a nasopharyngeal (NP) temperature of 18°C. Commercial cerebral oximetry was not used to guide intraoperative or post-operative management. Patients received either a Blalock–Taussig shunt or a right ventricle to pulmonary artery shunt (Sano). Surgical strategy included sternal closure where tolerated. Patients born with an intact atrial septum underwent a balloon atrial septostomy soon after birth and prior to surgery.
After surgery, the patients were transported back to the CICU. Postoperative ScO2, OEF and CBF were quantified every 2 hours for the first 12 hours during recovery. Approximately 1 week after surgery, patients underwent a post-operative follow-up MRI scan to assess the development and/or progression of brain injury.
Brain MRI
All images were acquired on a 1.5T Avanto MRI system (Siemens Medical Systems, Malvern, NJ, USA) using a 12-channel head coil. Studies included T1 MPRAGE (Magnetization-Prepared Rapid Acquisition Gradient Echo) and T2 SPACE (Sampling Perfection with Application-optimized Contrasts using different flip angle Evolution) sequences acquired in the axial plane, and the images were later reconstructed in the sagittal and coronal planes. Susceptibility and diffusion weighted sequences were also acquired. The presence of PVL was assessed from T1 sequences in conjunction with diffusion weighted imaging (DWI) in both the pre- and post-operative scans. Manual segmentation of the T1 hyperintense lesions were performed using ITK-SNAP21 and were used to calculate PVL volumes. New or worsened PVL was calculated by the difference in PVL volume between the post and pre-operative scans. Additionally, two independent observers blinded to clinical data evaluated the total brain maturation score (TMS) using axial T1 and T2 images.22, 23
DOS/DCS Measurements
Diffuse optical spectroscopy (DOS) and diffuse correlation spectroscopy (DCS) utilize near-infrared light to noninvasively probe the static and dynamic properties of cortical brain tissue. Our custom-made optical instrument combines these two techniques on a mobile cart that can be used in the MRI suite, the operating room, and at the bedside during recovery.17, 18, 24
DOS (also known as near-infrared spectroscopy (NIRS)) is a widely accepted method to quantify tissue oxygenation. Multi-separation frequency domain DOS, employed in this study, is capable of accurate quantification of cerebral tissue oxygen saturation (ScO2), i.e., in contrast to, commercial oximeters that employ continuous-wave NIRS to monitor trends in saturation. DOS uses photon diffusion theory to relate the measured amplitude attenuation and phase shift of modulated and multiply scattered light detected on the tissue surface to the wavelength-dependent tissue absorption (μa) and scattering (μs′) properties. The wavelength- and time-dependent absorption coefficient, μa(λ, t), depends linearly on oxy- ([HbO2]) and deoxy-hemoglobin ([Hb]) concentration; thus measurements at multiple wavelengths yields these two parameters. From [HbO2] and [Hb], we derive total hemoglobin concentration (THC=[HbO2]+[Hb]) and cerebral tissue oxygen saturation (ScO2=[HbO2]/THC). Cerebral oxygen extraction fraction (OEF) can be calculated from ScO2 and arterial oxygen saturation (SaO2) measured clinically from an arterial blood gas.17 Cerebral blood volume (CBV, mL/100g of tissue) can be calculated from THC.25 The DOS device employed in the present study (Imagent, ISS Inc., Champaign, IL) is amplitude modulated at 110 MHz and employs source lasers at 2 wavelengths, λ=688 and 830 nm.
DCS uses near-infrared light to non-invasively monitor CBF. DCS measures the temporal fluctuations of the reflected light intensity at the tissue surface which are primarily caused by moving red blood cells.15, 26, 27 Correlation diffusion theory is employed to convert these temporal fluctuations into a blood flow index (BFI, measured in units of cm2/s).15 Although this index does not have traditional physiological units of cerebral blood flow (CBF), recent studies have shown that BFI correlates strongly with other gold standard measures of CBF.18, 28 Specifically, Jain et al. validated BFI against CBF measured in the superior sagittal sinus with phase contrast MRI in a similar population of infants with critical CHD.18 Further, BFI can be combined with DOS-measured ScO2 and clinical arterial oxygenation measured clinically from arterial blood samples to give an index of cerebral metabolic rate of oxygen consumption (CMRO2,i).17, 24
DOS and DCS measurements were conducted once pre-operatively, once post-operatively immediately after return to the CICU, and once every 2 hours for the subsequent 12 hours. For statistical analysis of post-operative measurements, we included only the initial value of ScO2 and BFI upon return to the CICU and the lowest measured post-operative value of ScO2. Measurements were made over both the right and left frontal cortex. At each location, four repetitions were acquired in order to account for local inhomogeneities under the optical probe. These 8 repetitions were then averaged together to derive a global measure of ScO2 and BFI. Optical data measured with DOS and DCS, i.e., ScO2, and BFI, were acquired on only a subset of patients due to a change in protocol that used improved optical instrumentation and allowed for these optical measurements partway through enrollment.
Statistical Analysis
Continuous variables were summarized by standard descriptive statistics (mean, standard deviation (SD), and median, interquartile range (IQR) as appropriate), and frequencies and percentages for categorical variables. Because of the highly skewed distribution of PVL volumes in the present dataset, the outcome variable of new or worsened post-operative PVL was dichotomized around its median value.
To test for risk-factors of new or worsened PVL, univariate analysis was performed by employing Wilcoxon rank-sum tests to compare the medians of each explanatory variable between the group with a large volume of PVL and the group with a small volume or no PVL. Furthermore, multivariate logistic regression was performed to evaluate the significance of the explanatory variables to predict the likelihood of developing a large amount (i.e., greater than the median value) of new or worsened post-operative PVL. The final selected model was determined by stepwise selection, which is a combination of backward elimination and forward selections. In stepwise selection, an attempt is made to remove any insignificant variables from the model before adding a significant variable to the model. Each addition or deletion of a variable to or from a model is a separate step and at each step a new model is fitted. The selection criteria was p<0.1. All variables tested in the univariate analysis were included for consideration in the stepwise selection approach.
Results
From October 2008 to March 2013, a total of N=41 neonates were recruited for this study, however post-operative MRIs were not acquired on four patients for the following reasons: two patients were deemed clinically unstable for a postoperative MRI during study period, one subject had a pacemaker placed and was thus unable to undergo an MRI, and one subject was withdrawn from the postoperative MRI due to parental request. Thus, N=37 neonates diagnosed with HLHS (n=30) or HLHS variants (n=7) were included for this analysis as they obtained both pre- and post-operative MRIs. The HLHS variants consisted of unbalanced atrioventricular canal (n=4), double outlet right ventricle (n=2), and mitral valve dysplasia and aortic valve stenosis (n=1), and all were associated with aortic arch hypoplasia. All patients were full term with an average gestational age of 38.9±0.8 weeks, and all but one patient were prenatally diagnosed. Patient demographics and pre-operative and post-operative cerebral hemodynamics are described in Table 1. We note that a subset of this patient population has been described previously.18, 29
Table 1.
Summary of demographic and pre- and post-operative cerebral hemodynamic variables measured for subjects with and without new or worsened PVL > 76.3 mm3, presented by means ± standard deviation for continuous variables and frequencies (%) for categorical variables.
| Variable | All (n=37) | PVL (n=18) | No PVL (n=19) | P |
|---|---|---|---|---|
| Time-to-surgery, days | 4.2±1.9 | 5.3±1.5 | 3.1±1.7 | <0.001* |
| Time between MRI, days | 7.1±2.8 | 7.1±2.9 | 7.0±2.9 | 0.90 |
| Gestational age, wk | 38.8±0.8 | 38.9±0.9 | 38.8±0.7 | 0.59 |
| Birth weight, kg | 3.3±0.5 | 3.3±0.5 | 3.2±0.5 | 0.77 |
| Head circumference, cm | 34.0±1.4 | 34.1±1.5 | 34.0±1.3 | 0.55 |
| Female, n (%) | 19 (51.4) | 10 (55.6) | 9 (47.4) | 0.64 |
| Aortic Stenosis, n (%) | 9 (27.3) | 5 (31.3) | 4 (23.5) | 0.64 |
| Blalock-Taussig shunt, n (%) | 28 (75.7) | 13 (72.2) | 15 (79.0) | 0.65 |
| Intact atrial septum, n (%) | 4 (10.8) | 1 (5.6) | 3 (15.8) | 0.34 |
| TMS | 9.9±0.9 | 10.1±0.9 | 9.8±1.0 | 0.34 |
| Pre-operative SaO2, % | 90.0±5.2 | 90.1±4.0 | 89.9±6.1 | 0.57 |
| Pre-operative cerebral hemodynamics | ||||
| ScO2, % | 48.8±13.3 | 44.3±13.4 | 54.3±11.3 | 0.09# |
| BFI, 10−8 cm2/s | 2.4±1.6 | 3.0±1.9 | 1.9±1.1 | 0.05# |
| CBV, mL/100g | 2.1±0.5 | 2.1±0.6 | 2.1±0.4 | 0.52 |
| OEF | 0.61±0.20 | 0.68±0.22 | 0.54±0.16 | 0.15 |
| CMRO2,I, 10−7 ml/dl × cm2/s | 1.7±1.4 | 2.1±1.9 | 1.4±0.6 | 0.26 |
| Operative variables | ||||
| CPB time, | 92.2±25.3 | 96.8±33.0 | 87.7±14.5 | 0.70 |
| DHCA time, min | 44.8±9.7 | 46.4±12.0 | 43.3±6.7 | 0.74 |
| Lowest NP temperature, | 17.8±0.9 | 17.9±1.0 | 17.6±0.8 | 0.20 |
| Number of bypass runs, n | 1.1±0.4 | 1.2±0.5 | 1.0±0 | 0.14 |
| Delayed sternal closure, n (%) | 5 (13.5) | 4 (22.2) | 1 (5.3) | 0.14 |
| Cardiac arrest prior to post-operative MRI, n (%) | 2 (5.4) | 2 (11.1) | 0 (0) | 0.15 |
| Post-operative cerebral hemodynamics | ||||
| Initial ScO2, % | 40.8±7.2 | 41.3±7.6 | 40.4±7.1 | 0.57 |
| Initial BFI, 10−8 cm2/s | 1.5±0.7 | 1.6±0.9 | 1.3±0.3 | 0.35 |
| Initial CBV, mL/100g | 3.9±0.7 | 3.9±0.7 | 3.8±0.8 | 0.83 |
| Initial OEF | 0.58±0.15 | 0.57±0.14 | 0.59±0.16 | 0.85 |
| Initial CMRO2,i, 10−7 ml/dl × cm2/s | 1.9±1.8 | 2.0±1.4 | 1.7±2.2 | 0.24 |
| Lowest ScO2, % | 35.0±9.4 | 34.2±10.5 | 35.9±8.3 | 0.65 |
| Change from pre- to post-operative | ||||
| ScO2,post-operative- ScO2,pre-operative, % | −8.0 (13.1) | −3.9 (12.5) | −12.4 (12.8) | 0.10 |
| BFIpost-operative/BFIpre-operative, (%) | 1.1 (0.5) | 1.1 (0.6) | 1.0 (0.4) | 0.68 |
A Wilcoxon rank-sum test used to test differences between the two groups. (* significant, # marginally significant). TMS, Total Maturation Score; SaO2, arterial oxygen saturation; ScO2, cerebral tissue oxygen saturation; BFI, Blood Flow Index; CBV, Cerebral Blood Volume; CMRO2,i, Index of Cerebral Metabolic Rate of Oxygen; CPB, Cardiopulmonary Bypass; DHCA, Deep Hypothermic Circulatory Arrest; NP, Nasopharyngeal.
Pre-operative BFI and ScO2 were acquired in n=29/37. Post-operative BFI was acquired in n=29/37, and post-operative ScO2 was acquired in 28/37 subjects due to a change in protocol that used improved optical instrumentation.
Eight (21.6%) patients had pre-operative and 28 (75.7%) had postoperative PVL. An increase in volume from pre- to post-operative PVL was observed in 26/37 (70.3%) of patients, and the acquired volume ranged from 2.5 mm3 to 7577.7 mm3. A highly skewed distribution of PVL volumes existed in this cohort (mean=573.4 mm3; median=76.3 mm3; skewness=3.7), so the volumes were dichotomized around the median value to yield ‘none/small’ volumes and ‘large’ volumes (more clinically significant). Thus, large volumes of new or worsened post-operative PVL occurred in 48.7%. Figure 2 illustrates an example of a large volume (7577.7 mm3) versus a small volume (70.7mm3) of PVL. Table 1 gives the pre- and post-operative and demographic data for those subjects with a large change in post-operative PVL and those without or with a small amount. The group with a large amount of post-operative of PVL had a mean time-to-surgery of 5.3±1.5 days, compared with 3.1±1.7 days for the no or small amount of PVL group (p=0.0003).
Figure 2.
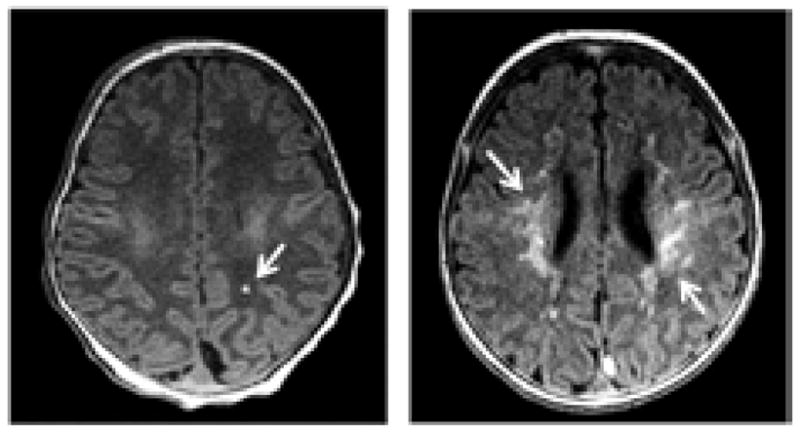
An example of a small volume (left, 70.7 mm3) and a large volume (right, 7577.7 mm3) of PVL.
No clinical indications were identified that resulted in delay of surgery in the group with a large amount of new or worsened PVL. Delays were due to scheduling or availability of the requested surgeon. The day of the week of the infants birth also played a large role in time-to-surgery. For instance, the 7 subjects born on a Wednesday had an average time-to-surgery of 5.1±1.6 days, whereas the 10 subjects born on a Monday had an average time-to-surgery of 3.7±2.2 days.
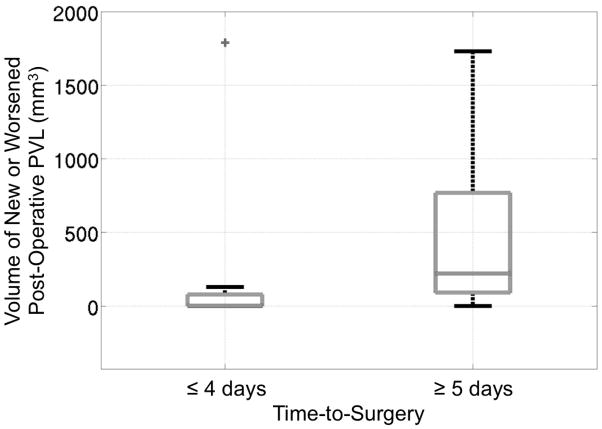
To further illustrate the relationship between time-to-surgery and the likelihood of acquired PVL, Figure 3 shows a boxplot of volume of new or worsened post-operative PVL for those patients with a time-to-surgery of 4 days or less and those with a time-to-surgery of 5 days or more (p=0.0005). The cutoff of 4 days was chosen for this visualization, because it resulted in the most significant difference in volume of acquired PVL between groups born before or after the cut-off. However, a cutoff of 3 or 5 days also resulted in a significant difference in volume of acquired PVL (p=0.005 and 0.02 respectively). Though TMS was included in both the univariate and multivariate analysis, we did not find it to be a significant predictor of new or worsened post-operative PVL.
Figure 3.
Boxplot demonstrating the significant (p=0.0005) difference in volume of new or worsened post-operative PVL for those patients with a time-to-surgery of 4 days or less and those with a time-to-surgery of 5 days or more. Two outliers were removed to improve visualization. One outlier was in the left group and had a PVL volume of 7577.7 mm3, and the other outlier was in the right group and had a PVL volume of 4981.5 mm3. These outliers were only removed from the figure but not from any statistical analysis.
Patients with new or worsened post-operative PVL tended to have higher pre-operative BFI (p=0.05) and lower pre-operative ScO2 (p=0.09). Note, TMS, post-operative ScO2, and DHCA duration were not significantly different between the two groups.
A multivariate logistic regression model resulting from stepwise selection revealed that the probability of acquiring a large amount of PVL was positively associated with time-to-surgery (p=0.005), use of delayed sternal closure (p=0.07) and pre-operative BFI (p=0.08).
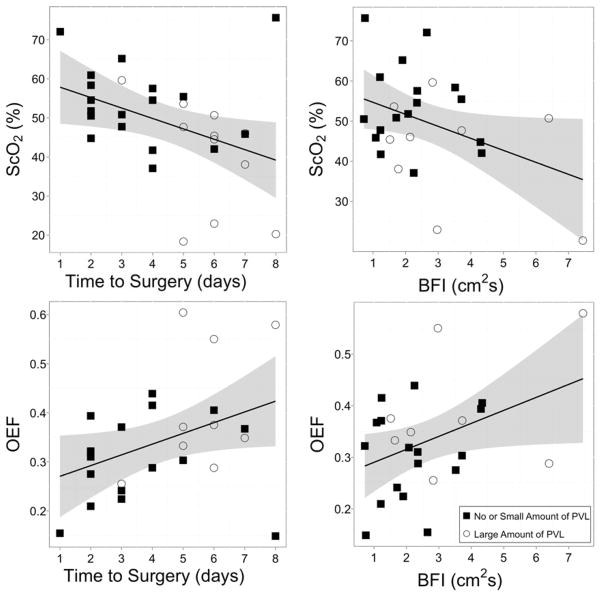
Additionally, we investigated the cross-sectional relationship between pre-operative ScO2 and time-to-surgery between pre-operative ScO2 and pre-operative BFI. As seen in Figure 4, a significant and negative linear correlation (R2=0.17, p=0.03, slope=−2.7±1.2) was observed between time-to-surgery and pre-operative ScO2, and a significant and negative linear correlation (R2=0.15, p=0.05, slope=−3.0±1.4) was observed between pre-operative ScO2 and pre-operative BFI. We also examined the relationship of pre-operative OEF with time-to-surgery and pre-operative BFI (Figure 4). We observed a significant and positive linear correlation (R2=0.15, p=0.05, slope=0.02±0.01) between pre-operative OEF and time-to-surgery and a significant and positive linear correlation (R2=0.16, p=0.05, slope=0.03±0.01) between pre-operative OEF and pre-operative BFI.
Figure 4.
Pre-operative cerebral oxygenation as a function of time-to-surgery (top left) and pre-operative BFI (top right)) and pre-operative oxygen extraction fraction as a function of time-to-surgery (bottom left) and pre-operative BFI (bottom right). The solid line represents the best-fit line to the data (Top Left: R2=0.17, p=0.03, slope=−2.7±1.2; Top Right: R2=0.15, p=0.05, slope=−3.0±1.4; Bottom Left: R2=0.15, p=0.05, slope=0.02±0.01; Bottom Right: R2=0.16, p=0.05, slope=0.03±0.01). The grey ribbon denotes the 95% confidence interval for the mean ScO2 (top) or OEF (bottom). The symbols represent whether or not the subject acquired a large amount of new or worsened post-operative PVL.
Discussion
The major new finding in this study is that acquired post-operative PVL in infants with HLHS was highly correlated (p=0.0003) with longer time between birth and palliative infant heart surgery (time-to-surgery), while peri- and postoperative variables did not predict injury. One possible explanation for the significance of time-to-surgery on the development of post-operative PVL is derived from the negative linear trend observed between pre-operative ScO2 and time-to-surgery (Figure 4), suggesting that cerebral desaturation progresses from birth until surgery. The correlation of pre-operative ScO2 with new or worsened post-operative PVL and time-to-surgery suggest that pre-operative non-invasive optical monitoring of ScO2 could be useful in further understanding the timing and cause for PVL and has the potential to further decrease the risk for injury.
The finding that higher pre-operative BFI is associated with new or worsened post-operative PVL in both a univariate and multivariate analysis was surprising and is currently under further investigation. As seen in Figure 4, higher pre-operative BFI is associated with a lower pre-operative ScO2. This trend is possibly a result of an increasing need for oxygen delivery to meet higher metabolic demand. While this parameter has not been measured in healthy newborn infants, it is likely that CMRO2 increases as infants emerge from the dark, quiet and warm uterine environment into the bright and chaotic world. In infants with severe forms of CHD, this increased oxygen demand may not be well matched with poor and possibly falling cerebral oxygen delivery. This idea is supported by the observed correlation between higher pre-operative OEF and BFI (Figure 4), thus suggesting that the brain is operating on the supply dependent portion of the oxygen consumption/delivery curve.30 Further investigation, including serial measurements of CBF, ScO2, OEF, and CMRO2 from birth to surgery on individual patients with and without severe CHD, is crucial for understanding the role of CBF in injury risk.
We also investigated post-operative ScO2 as a risk-factor for new or worsened post-operative PVL, but we did not find a significant difference between groups in either the initial ScO2 upon return to the CICU or the lowest ScO2 during the first 12 hours of post-operative recovery. Dent et al. reported that post-operative ScO2 less than 45% for at least 180 min was significantly associated with post-operative PVL in infants with HLHS.14 However, in the study by Dent et al., continuous monitoring of ScO2 was used, whereas ScO2 in the present study was only measured once every two hours for the first 12 hours postoperatively. Additionally, this finding by Dent et al. was not replicated later in a mixed population by Andropoulus et al.12
Total duration of DHCA has been previously investigated as a risk factor for the development of new post-operative PVL in a heterogeneous population with multiple forms of CHDs.13 In the present study, however, duration of DHCA was not associated with PVL risk. This finding is likely due to the fact that the population studied herein is homogeneous. Operative variables were assessed as risk factors for new or worsened PVL, but the only variable that rose to significance in the multivariate analysis was whether the sternum was closed primarily or left open due to hemodynamic instability in the immediate postoperative observation period. In the single ventricle reconstruction (SVR) trial, infants who were left with an open sternum post-operatively were at increased risk for both mortality and morbidity.31 This finding held for both delayed sternal closure due to hemodynamic instability following surgery and for centers where the sternum was routinely left open as part of planned post-operative care. The SVR trial did not evaluate post-operative brain MRIs, so there is no known prior association with white matter injury.
Andropoulus et al. reported brain TMS to be the primary risk for both pre- and post-operative PVL in a mixed cohort of neonates with complex CHD.12 In this study, we did not find TMS to be a significant predictor of new or worsened post-operative injury in our homogeneous cohort. However, patients with single ventricle physiology have, in general, a lower average TMS than neonates with other forms of CHD12 and have the highest risk for PVL. In this cohort, we observe a narrow range of TMS. Therefore, the association between TMS and PVL risk could easily be missed in this report. Other patient characteristics such as gender and HLHS subtype (i.e. aortic atresia or aortic stenosis), which have previously been shown to be significant predictors of pre-operative PVL,29 were not significant predictors of acquired post-operative injury in this cohort.
The main limitation to this study is that pre-operative cerebral hemodynamics were only measured on the morning of surgery. Thus all temporal data on time-to-surgery is cross-sectional. To understand the mechanism behind the result that time-to-surgery significantly predicts new or worsened post-operative PVL, longitudinal measures of cerebral hemodynamics from birth until surgery are required. Additionally, we note that our derivation of optical BFI depends on assumptions about the optical properties of the tissue, primarily its reduced scattering coefficient (μs′); the scattering coefficient determination, however, is susceptible to various error sources (e.g., due to flexible probes, imperfect semi-infinite geometry). Recently, Jain et al. demonstrated that optical BFI and CMRO2,i correlated better with blood flow and CMRO2 in the sagittal sinus measured by MRI when the reduced scattering coefficient was assumed to be the same for all subjects.18 In the present study, we measured μs′ for each subject and employed these data in the BFI determination. As a check, we assumed a fixed value for μs′ for all subjects; in this case, our analysis produced a pre-operative BFI that was not significantly different between the groups with and without large post-operative PVL. Therefore, further investigation, including more accurate measures of μs′ and serial measurements of BFI from birth to surgery on individual patients, is crucial for understanding the role of CBF in injury risk.
Additionally, the results reported herein are from a single center; these findings should be confirmed at other centers that manage patients with different operative strategies. However, the high statistical significance of the predictability of new or worsened post-operative PVL by time-to-surgery reported herein strongly suggests that decreasing the time to cardiac surgery in neonates with HLHS will decrease the probability of new or worsened post-operative PVL. Since time-to-surgery was largely based on surgeon availability and the day of the week during which the patient was born, we would like to suggest that these data provide sufficient justification for modifying the operative scheduling of these otherwise healthy patients with HLHS.
Conclusion
We investigated the risk of acquired post-operative PVL in otherwise healthy neonates with HLHS. We observed a large amount of new or worsened postoperative PVL in 48.7% (18/37) of patients in our cohort. The risk for injury in this population significantly increases with longer time to surgical repair. Additionally, a significant correlation exists between time-to-surgery and cerebral oxygen saturation, suggesting that changes in cerebral oxygen metabolism are occurring between birth and surgical repair and may be at the core to this increase risk for injury. Correcting the native, abnormal vascular anatomy as soon as possible may be the most important factor in mitigating brain injury.
Acknowledgments
Funding Sources:
This study was supported by the NIH Grant Nos. NS-072338, NS-60653, HL-007954, HL-007915, P41-EB015893, the Thrasher Research Foundation, and the June and Steve Wolfson Family foundation.
We acknowledge invaluable assistance from Natasha Lavin (respiratory therapist), Justine Wilson (MRI technician), Wesley Baker, Andrew Rouff, the operating room staff from the Children’s Hospital of Philadelphia, and most importantly the patients and their families.
Abbreviations and Acronyms
- BFI
blood flow index
- CBF
cerebral blood flow
- CBV
cerebral blood volume
- CHD
congenital heart disease
- CICU
cardiac intensive care unit
- CHOP
The Children’s Hospital of Philadelphia
- CMRO2
cerebral metabolic rate of oxygen
- CPB
cardio pulmonary bypass
- DCS
diffuse correlation spectroscopy
- DHCA
deep hypothermic circulatory arrest
- DOS
diffuse optical spectroscopy
- DWI
diffusion weighted imaging
- Hb
deoxyhemoglobin
- HbO2
oxyhemoglobin
- HLHS
hypoplastic left heart syndrome
- MRI
magnetic resonance imaging
- NP
nasopharyngeal
- OEF
cerebral oxygen extraction fraction
- PVL
periventricular leukomalacia
- SaO2
arterial oxygen saturation
- ScO2
cerebral tissue oxygen saturation
- TMS
total maturation score
Footnotes
Disclosures
Authors have nothing to disclose with regard to commercial support.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hoffman JIE, Kaplan S. The incidence of congenital heart disease. Journal of the American College of Cardiology. 2002;39:1890–1900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- 2.Graham EM, Zyblewski SC, Phillips JW, Shirali GS, Bradley SM, Forbus GA, Bandisode VM, Atz AM. Comparison of norwood shunt types: Do the outcomes differ 6 years later? Ann Thorac Surg. 2010;90:31–35. doi: 10.1016/j.athoracsur.2010.03.078. [DOI] [PubMed] [Google Scholar]
- 3.Bellinger D, Wypij D, Rivkin M, DeMaso D, Robertson R, Dunbar-Masterson C, Rappaport L, Wernovsky G, Jonas R, Newburger J. Adolescents with d-transposition of the great arteries corrected with the atrial switch procedure. Pediatric Cardiology. 2011;124:1361–1369. doi: 10.1161/CIRCULATIONAHA.111.026963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marino B, Lipkin P, Newburger J, Peacock G, Gerdes M, Gaynor J, Mussatto K, Uzark K, Goldberg C, Johnson W, Li J, Smith S, Bellinger D, Mahle W Committee AHACHD, Young CoCDit, Nursing CoC, Council S. Neurodevelopmental outcomes in children with congenital heart disease: Evaluation and management: A scientific statement from the american heart association. Circulation. 2012;126:1143–1172. doi: 10.1161/CIR.0b013e318265ee8a. [DOI] [PubMed] [Google Scholar]
- 5.Shillingford A, Glanzman M, Ittenbach R, Clancy R, Gaynor J, Wernovsky G. Inattention, hyperactivity, and school performance in a population of school-age children with complex congenital heart disease. Pediatrics. 2008;121:759–767. doi: 10.1542/peds.2007-1066. [DOI] [PubMed] [Google Scholar]
- 6.Mahle W, Tavani F, Zimmerman R, Nicolson S, Galli K, Ganor J, Clancy R, Montenegro L, Spray T, Chiavacci R, Wernovsky G, Kurth C. An mri study on neurological injury before and after congenital heart surgery. Circulation. 2002;106:109–114. [PubMed] [Google Scholar]
- 7.Imamura T, Ariga H, Kaneko M, Watanabe M, Shibukawa Y, Fukuda Y, Nagasawa K, Goto A, Fujiki T. Neurodevelopmental outcomes of children with periventricular leukomalacia. Pediatrics & Neonatology. 2013;54:367–372. doi: 10.1016/j.pedneo.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Fawer CL, Diebold P, Calame A. Periventricular leucomalacia and neurodevelopmental outcome in preterm infants. Archives of Disease in Childhood. 1987;62:30–36. doi: 10.1136/adc.62.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller SP, Ferriero DM, Leonard C, Piecuch R, Glidden DV, Partridge C, Perez M, Mukherjee P, Vigneron DB, Barkovich AJ. Early brain injury in premature newborns detected with magnetic resonance imaging is associated with adverse early neurodevelopmental outcome. Journal of Pediatrics. 2005;147:609–616. doi: 10.1016/j.jpeds.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 10.Bellinger D, Wypij D, du Plessis A, Rappaport L, Riviello J, Jonas R, Newburger J. Developmental and neurologic effects of alpha-stat versus ph-stat strategies for deep hypothermic cardiopulmonary bypass in infants. The Journal of Thoracic and Cardiovascular Surgery. 2001;121:374–383. doi: 10.1067/mtc.2001.111206. [DOI] [PubMed] [Google Scholar]
- 11.Gaynor J, Stopp C, Wypij D, Andropoulus D, Atallah J, Beca J, Duncan K, Ghanayem N, Goldberg C, Hövels-Gúrich H, FI, RJ, BL, Mahle W, McQuillen P, CP, Shekerdemian L, AS, Bellinger D, Newburger J Investigators ICCoN, Investigators PHN. Abstract 12437: Early neurodevelopmental outcomes after cardiac surgery in infancy have not improved; a multi-center retrospective analysis of 1,718 patients. Circulation. 2012;126:A12437. [Google Scholar]
- 12.Andropoulus D, Hunter J, Nelson D, Stayer S, Stark A, McKenzie E, Heinle J, Graves D, FrASER CJ. Brain immaturity is associated with brain injury before and after neonatal cardiac surgery with high-flow bypass and cerebral oxygenation monitoring. The Journal of Thoracic and Cardiovascular Surgery. 2010;139:543–556. doi: 10.1016/j.jtcvs.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beca J, Gunn J, Coleman L, Hope A, Reed PW, Hunt RW, Finucane K, Brizard C, Dance B, Shekerdemian LS. New white matter injury after infant heart surgery is associated with diagnostic group and use of circulatory arrest. Circulation. 2013;127:917–979. doi: 10.1161/CIRCULATIONAHA.112.001089. [DOI] [PubMed] [Google Scholar]
- 14.Dent C, Spaeth J, Jones B, Schwartz S, Glausser T, Hallinan B, Pearl J, Khoury P, Kurth C. Brain magnetic resonance imaging abnormalities after the norwood procedure using regional cerebral perfusion. The Journal of Thoracic and Cardiovascular Surgery. 2005;130:1523–1530. doi: 10.1016/j.jtcvs.2005.07.051. [DOI] [PubMed] [Google Scholar]
- 15.Durduran T, Choe R, Baker WB, Yodh AG. Diffuse optics for tissue monitoring and tomography. Reports on Progress in Physics. 2010;73 doi: 10.1088/0034-4885/73/7/076701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goff DA, Buckley EM, Durduran T, Wang J, Licht DJ. Noninvasive cerebral perfusion imaging in high-risk neonates. Seminars in Perinatology. 2010;34:46–56. doi: 10.1053/j.semperi.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buckley EM, Lynch JM, Goff DA, Schwab PJ, Baker WB, Durduran T, Busch DR, Nicolson SC, Montenegro LM, Naim R, Xiao TL, Spray TL, Yodh AG, Gaynor JW, Licht DJ. Early postoperative changes in cerebral oxygen metabolism following neonatal cardiac surgery: Effects of surgical duration. The Journal of Thoracic and Cardiovascular Surgery. 2012;145:196–205. doi: 10.1016/j.jtcvs.2012.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jain V, Buckley EM, Licht DJ, Lynch JM, Schwab PJ, Naim MY, Lavin N, Nicolson SC, Montenegro LM, Yodh AG, Wehrli FW. Cerebral oxygen metabolism in neonates with congenital heart disease quantified by mri and optics. Journal of Cerebral Blood Flow and Metabolism. doi: 10.1038/jcbfm.2013.214. Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buckley EM, Naim MY, Lynch JM, Goff DA, Schwab PJ, Diaz LK, Nicolson SC, Montenegro LM, Lavin N, Durduran T, Spray TL, Gaynor JW, Putt ME, Yodh AG, Fogel MA, Licht DJ. Sodium bicarbonate causes dose-dependent increases in cerebral blood flow in infants and children with single-ventricle physiology. Pediatric Research. 2013;73:668–673. doi: 10.1038/pr.2013.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lynch JM, Buckley EM, Schwab PJ, Busch DR, Hanna BD, Putt ME, Licht DJ, Yodh AG. Noninvasive optical quantification of cerebral venous oxygen saturation in humans. Academic Radiology. 2014;21:162–167. doi: 10.1016/j.acra.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yushkevich P, Piven J, Hazlett H, Smith R, Ho S, Gee J, Gerig G. User-guided 3d active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage. 2006;31:1116–1128. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 22.Childs AM, Ramenghi LA, Cornete L, Tanner SF, Arthur RJ, Martinez D, Levene MI. Cerebral maturation in premature infants: Quantitative assessment using mr imaging. American Journal of Neuroradiology. 2001;22:1577–1582. [PMC free article] [PubMed] [Google Scholar]
- 23.Licht DJ, Shera DM, Clancy R, Wernovsky G, Montenegro LM, Nicolson SC, Zimmerman RA, Spray TL, Gaynor JW, Vossough A. Brain maturation is delayed in infants with complex congenital heart defects. The Journal of Thoracic and Cardiovascular Surgery. 2009;137:529–536. doi: 10.1016/j.jtcvs.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Durduran T, Zhou C, Buckley EM, Kim MN, Yu G, Choe R, Gaynor JW, Spray TL, Durning SM, Mason Se, Montenegro LM, Nicolson SC, Zimmerman RA, Putt ME, Wang J, Greenberg JH, Detre JA, Yodh AG, Licht DJ. Optical measurement of cerebral hemodynamics and oxygen metabolism in neonates with congenital heart defects. Journal of Biomedical Optics. 2010;15:037004. doi: 10.1117/1.3425884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wyatt JS, Delpy DT, Cope M, Wray S, Reynolds EOR. Quantification of cerebral oxygenation and haemodynamics in sick newborn infants by near infrared spectrophotometry. The Lancet. 1986;328:1063–1066. doi: 10.1016/s0140-6736(86)90467-8. [DOI] [PubMed] [Google Scholar]
- 26.Boas DA, Yodh AG. Spatially varying dynamical properties of turbid media probed with diffusing temporal light correlation. Journal of the Optical Society of America A. 1997;14:192–215. [Google Scholar]
- 27.Pine D, Weitz D, Chaikin P, EH Diffusing wave spectroscopy. Physical Review Letters. 1988;60:1134–1137. doi: 10.1103/PhysRevLett.60.1134. [DOI] [PubMed] [Google Scholar]
- 28.Buckley EM, Cook NM, Durduran T, Kim MN, Zhou C, Choe R, Yu G, Schultz S, Sehgal CM, Licht DJ, Arger PH, Putt ME, Hurt HH, Yodh AG. Cerebral hemodynamics in preterm infants during positional intervention measured with diffuse correlation spectroscopy and transcranial doppler ultrasound. Optics Express. 2009;17:12571–12581. doi: 10.1364/oe.17.012571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goff DA, Shera DM, Tang S, Lavin N, Durning SM, Nicolson SC, Montenegro LM, Rome JJ, Gaynor JW, Spray TL, Vossough A, Licht DJ. Risk factors for preoperative periventricular leukomalacia in term neonates with hypoplastic left heart syndrome are patient related. The Journal of Thoracic and Cardiovascular Surgery. 2013 doi: 10.1016/j.jtcvs.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gutierrez JA, Theodorou AA. Oxygen delivery and oxygen consumption in pediatric critical care. In: Lucking SE, Maffei FA, Tamburro RF, Thomas NJ, editors. Pediatric critical care study guide: Text and review. London: Springer-Verlag; 2012. [Google Scholar]
- 31.Tabbutt S, Ghanayem N, Ravishankar C, Sleeper LA, Cooper DS, Frank DU, Lu M, Pizarro C, Frommelt P, Goldberg CS, Graham EM, Krawczeski CD, Lai WW, Lewis A, Kirsh JA, Mahony L, Ohye RG, Simsic J, Lodge AJ, Spurrier E, Stylianou M, Laussen PC. Risk factors for hospital morbidity and mortality after the norwood procedure: A report from the pediatric heart network single ventricle reconstruction trial. The Journal of Thoracic and Cardiovascular Surgery. 2012;144:882–895. doi: 10.1016/j.jtcvs.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]