Abstract
How the brain takes in information, makes a decision, and acts on this decision is strongly influenced by the ongoing and constant fluctuations of state. Understanding the nature of these brain states and how they are controlled is critical to making sense of how the nervous system operates, both normally and abnormally. While broadly projecting neuromodulatory systems acting through metabotropic pathways have long been appreciated to be critical for determining brain state, more recent investigations have revealed a prominent role for fast acting neurotransmitter pathways for temporally and spatially precise control of neural processing. Corticocortical and thalamocortical glutamatergic projections can rapidly and precisely control brain state by changing both the nature of ongoing activity and by controlling the gain and precision of neural responses.
Introduction
The cerebral cortex is never quiet. From the deepest sleep to solving complex cognitive tasks, cortex displays robust ‘spontaneous activity’ which is not associated with specific sensory or motor content. Far from being intrinsic noise, we now recognize that spontaneous cortical activity reflects dynamic self-organization into various states which biases sensory and motor processing according to internal drives. In the following sections, we provide perspectives about cortical state diversity, mechanisms of modulation, effects on sensory processing and involvement in higher cognitive function. In this review, ‘modulation of cortical state’ refers to both fast (presumably ionotropic-mediated) and slow (metabotropic-mediated) mechanisms, in contrast to ‘neuromodulatory pathways’ which refers to long-range, primarily metabotropic connections.
Diversity of cortical state
Foundational studies of forebrain state [1] provided a highly discrete view of cortical dynamics. Sleep and waking states were unambiguous and distinct, with abrupt transitions between the two. During slow wave sleep, neurons displayed large (10–20 mV) subthreshold oscillations with the spiking phase locked to the depolarized Up state (slow oscillatory state). These dynamics are relatively synchronized throughout the local network, and as a result produce the slow waves seen in the electroencephalogram (EEG)/local field potential (LFP). In the waking state, membrane potential fluctuations to the hyperpolarized phase (Down state) are abolished, neurons are maintained at depolarized potentials and display tonic firing (activated state), the rate of which depends on cell type and layer [2–4]. Reduced amplitude subthreshold oscillations and reduced synchrony across the local network result in the low amplitude EEG/LFP signals (Figure 1A).
Figure 1.
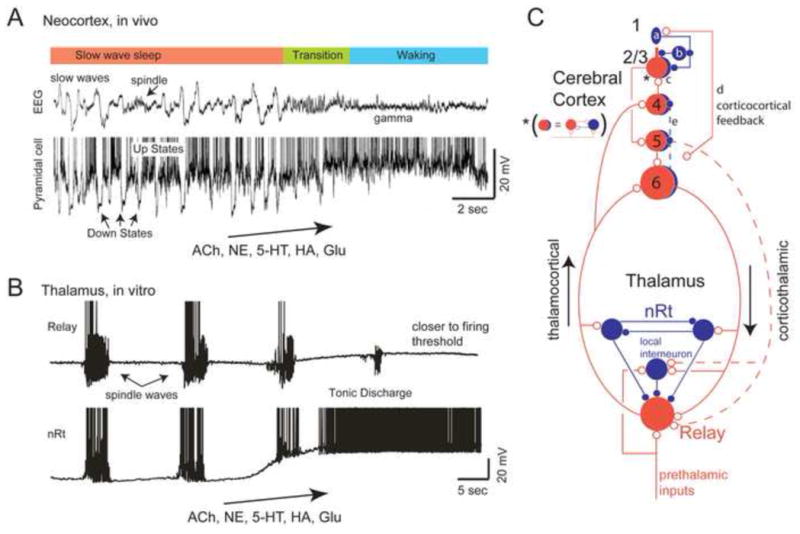
Cortical and thalamocortical networks exhibit state-dependent changes in network activity. A. During slow wave sleep, the EEG and local cortical field potential is dominated by slow waves, which represent the occurrence of Up and Down states in the local network. The transition to waking is associated with the abolition of the Down states, and the enhancement of higher frequency rhythms such as gamma waves. Several neurotransmitters have been implicated in this transition including acetylcholine (ACh), norepinephrine (NE), serotonin (5-HT), histamine (HA), and glutamate (Glu). Illustrated is the local field potential and intracellular recording from a pyramidal cell during the transition from slow wave sleep to waking. B. Thalamic circuits generate sleep spindle waves as a reverberant interaction of the glutamatergic relay cells and the GABAergic inhibitory neurons of the thalamic reticular nucleus (nRt). The combined action of several neurotransmitters, including ACh, NE, 5-HT, HA, and Glu, can depolarize thalamic circuits out of the sleep-like mode into a state of tonic discharge or ready to discharge. One major mechanism of this depolarization is the reduction of K+ conductances that are active at rest. C. Schematic diagram illustrating major intracortical, intrathalamic, and corticothalamic pathways. Neuromodulatory transmitter systems contact all of these elements and can modulate each in unique ways. A common motif in the cortex is the reciprocal connections of excitatory (red neurons) and inhibitory (blue neurons) neurons (indicated by the asterisk). Recent investigations [55–57,82] reveal that VIP interneurons (a) in or near layer 1 can inhibit somatostatin (b) and parvalbumin (c) containing interneurons, resulting in disinhibition of pyramidal cells. Corticocortical connections (d) may specifically engage this disinhibitory circuit. Interlaminar projections within the cortex are not only excitatory, but can also be inhibitory (e), and the activation of this pathway can result in gain modulation [32]. A is from [1]; B is from [50].
Recent intracellular recordings in waking mice have complicated this view. Carl Petersen’s laboratory suggested that cortical state can exhibit slow oscillatory components in waking, but quiescent, mice. Specifically, 3–5 Hz subthreshold oscillations were observed in primary somatosensory cortex of head-fixed, stationary mice, which were eliminated abruptly upon movement (whisking) [5,6] (Figure 2A). Curiously, the subthreshold oscillations observed in stationary mice have a similar structure to oscillations observed during sleep and anesthesia, consisting of large subthreshold fluctuations and phasic firing. Similar cortical activations in mice with movement-related (walking or whisking) state changes have since been observed by other labs and in other cortical regions [7,8**,9**,10**], from which we may generalize that movement correlates with activated cortical dynamics in these animals (Figure 2A, B).
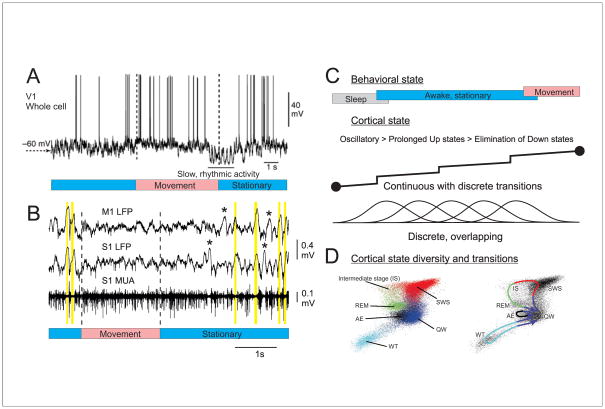
Figure 2.
Characterizing state changes in behaving mice. A. Whole cell recordings from a pyramidal cell in the primary visual cortex of an awake mouse reveal that movement (walking) is associated with a depolarization of the membrane potential and a suppression of low frequency fluctuations. B. Simultaneous local field potential and multiple unit recordings from primary motor and somatosensory cortex of a mouse in the transition from stationary quiescence to movement (whisking). During quiescence, the cortex exhibits synchronized off periods reminiscent of Down states (yellow bars). These putative Down states may occur locally (e.g. asterisks). Whisking is associated with a suppression of these silent periods and the tonic activation of cortical circuits. C. Behavioral and cortical states are often viewed as exhibiting continuous changes delineated by abrupt transitions, although there may also exist multiple overlapping, yet discrete, states and substates. D. Characterization of behavioral state in rodents by principle component analysis of the activity of multiple brain areas reveals the major sleep-waking states seen behaviorally. Note that although the states exist within their own portions of state-space, they are not completely distinct and separate (left). Movement between states follows repeated paths (right). Abbreviations: AE: active exploration; IS: intermediate stage; REM: rapid eye movement sleep; SWS: slow wave sleep; QW: quiet wake; WT: whisker twitching. A from [9]; B unpublished data (EZ, DM); D from [23].
In contrast to the activation associated with movement, we cannot yet fully describe or explain cortical dynamics in stationary mice, particularly across cortical regions. Various laboratories have reported spontaneous activity varying from largely inactive and synchronous, resulting in large “bumps” of synaptic inputs [11,12], to rhythmic barrages of synaptic potentials reminiscent of slow-wave sleep like activity [13,14**] (Figure 3A), to nearly continuously activated [15]. In our recordings from stationary awake mice we find that cortical state varies constantly, ranging from slow oscillatory to activated [8**], and correlates with task engagement (Zagha, McGinley, McCormick unpublished observations). Assessing the baseline dynamics of the cortical waking state is complicated by comparing across species, cortical regions and behavioral tasks. For example, sleep-wake transitions in freely moving mice are frequent and rapid, occurring hundreds of times per day, with the average waking period lasting only a few minutes [16,17] (although the distribution allows for long active periods). This propensity towards rapid and frequent wake to sleep transitions may increase the likelihood for slow oscillatory cortical activity in nominally awake, head fixed mice that are not actively engaged in a task. Indeed, low frequency oscillations, or Down states, increase their prevalence and density with time over the active period in rodents [18*,19]. The equivalent state, if any, in healthy human cortical activity is not yet known. Increased prevalence of Down states in rodents is associated with decreased performance on a learned task [18*]. Therefore, we speculate that the slow oscillatory activity in rodents may be analogous to drowsiness in humans, which is associated with significant performance deficits and enhanced local and global low frequency EEG fluctuations [20–22].
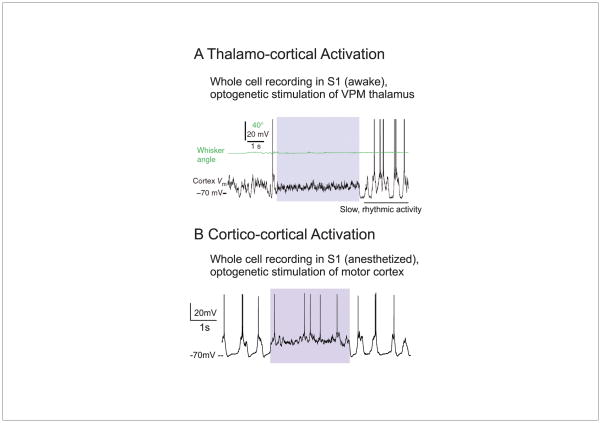
Figure 3.
The stimulation of glutamatergic pathways can result in the rapid activation of cortical networks. A. Whole cell recording from a cortical pyramidal cell during the optogenetic stimulation of thalamus (colored box). During thalamic stimulation, the cortical neuron is rapidly and tonically depolarized and slow fluctuations are suppressed. B. A similar effect is observed upon stimulation of feedback projections from primary motor cortex (M1) to primary somatosensory cortex (S1). Note that both responses exhibit rapid onset and offset kinetics and result in changes in cortical network activity that is similar to arousal, movement, and attention. A is from [14]; B is from [8].
In light of these recent findings, we propose a reassessment of traditional views of cortical state. We consider state to be a recurring set of neural conditions that is stable for a behaviorally significant period of time (Figure 2C, D). Common vernacular presumes a relatively small number of states (e.g. SWS, REM sleep, quiet waking, active waking, attentive) and it is common to treat the transition between these states as global, sudden, and well delineated (Figure 2C). An alternative is that the major states (e.g. waking) actually overlap and flow into one another [23] (Figure 2C, D) with each large state consisting of a number of sub-states, varying for example in either amplitude and/or the degree to which they are generalized throughout cortical and associated networks. Fluctuations of these multiple sub-states in both time and cortical space can result in a highly dynamic and complex control of network responsiveness and processing in relation to behavior. A major task for neuroscience is determining exactly how many sub-states exist and how they organize, interact and influence behavior.
Neurotransmitter systems involved in state control
Ever since the discovery of an ascending reticular activating system by Moruzzi and Magoun [24], a wide range of recording, lesion, stimulation, and pharmacological studies have implicated the broad projecting neuromodulatory systems (e.g. those releasing ACh, NE, 5-HT, DA, HA) in the control of neural and behavioral state (reviewed in [25–28]). These studies have been enormously successful, particularly in explaining the possible mechanisms of state-dependent transitions of thalamic and cortical (neocortex and hippocampus) activities on both a single cell and network level (Figure 1). In addition to these classic studies, more recent investigations have revealed important roles for hypocretin neurons in the hypothalamus [29–31], and fast glutamatergic and GABAergic projections between cortex and other cortical or subcortical regions [8**,14,32**].
A complete cataloging of all of the known neurotransmitter actions that may contribute to state change is beyond the scope of this review. Moreover, despite extensive characterization of the cellular effects of neuromodulators, we lack a basic understanding of the relevant pre- and post-synaptic actions of these neurotransmitters in situ. Especially problematic, for example, is the varying affinities and distances from transmitter release of multiple subtypes of receptors for the same neurotransmitter, the ability of some neurotransmitter systems to activate opposing postsynaptic responses (e.g. opening one K+ current while closing another), the ability of synapses to release more than one transmitter [33], species specific differences in the response of neurons to a given transmitter [34,35], and the dependence of the response of a neuron to a neurotransmitter on the state of activation of other neurotransmitter receptors on that neuron [36]. The recent use of optogenetics to release neurotransmitters from selective terminals will enhance our understanding of the cellular mechanisms of state control. However, optogenetic stimulation in its current form induces highly artificial spatio-temporal patterns of transmitter release, and therefore can only provide suggestive evidence.
Despite the challenges mentioned above, convergent data over the past years provides a useful framework for global state changes. In particular, the closure of specialized K+ currents (e.g. IKleak, IM, IAHP) in cortical and/or thalamic neurons may underlie the shift in cortical networks from non-REM sleep to waking (Figure 1) [25]. Reducing K+ conductances is an effective mechanism for state change, since it depolarizes neurons towards firing threshold, while also causing an increase in excitability through increases in membrane resistance. Acetylcholine, released by brainstem and basal forebrain cholinergic projections to thalamus and cortex, respectively, is likely to play a major role. Notably, acetylcholine induces a muscarinic receptor mediated decrease in K+ conductance in both cortical pyramidal cells and thalamic relay neurons. Additionally, the transition from sleep to waking may also be facilitated by the reduction of these same K+ conductances by the release of NE, HA, 5HT, and other modulators, and the activation of metabotropic glutamate receptors [37,38] in a cell type specific manner. Numerous other neurotransmitter effects, such as cAMP-dependent control of hyperpolarization-activated cation channels, are likely to contribute to state-dependent alterations in forebrain function and communication [25].
Since these classical mechanisms of state control were identified, more recent studies have identified thalamocortical, corticocortical, and corticothalamic pathways that may regulate rapid and spatially specific changes in cortical state [8**,14**,39] (see however [40]) and sensory responsiveness [32**,41]. These pathways use glutamate transmission primarily targeting ionotropic receptors, with possible roles for metabotropic receptors, and have well-defined cortical or thalamic targets. The involvement of ionotropic receptors in these pathways allows them to exhibit especially rapid kinetics, resulting in fast (10s of msec) changes in cortical state (Figure 3) and neural responsiveness (Figure 4). Such fast actions may be particularly beneficial where rapid modulations in local or long range network processing are required, such as changes associated with alterations in context, movement, attentional focus, perception, motivation, or expectation [42,43].
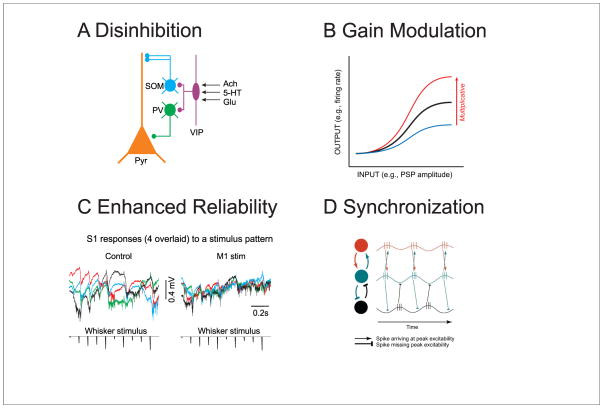
Figure 4.
Circuit effects of rapid and slow modulation of cortical state. A. Recent investigations have revealed that disinhibition may be a significant mechanism modulating the responsiveness of cortical pyramidal cells [55–57,82]. The proposed microcircuit consists of VIP-containing interneurons inhibiting SOM and PV interneurons, resulting in enhanced responsiveness of postsynaptic pyramidal cells. VIP interneurons are modulated by several ionotropic pathways (nicotinic, 5HT3A, glu), which may allow for the rapid modulation of these neurons. B. Multiplicative gain modulation is a major mechanism by which the input-output relationship of cortical neurons may be modulated. Multiplicative gain modulation can be achieved by changes in the mean membrane potential in the presence of membrane potential variance [61]. C. Suppression of ongoing fluctuations in network activity can result in a significant increase in the reliability of cortical responses to sensory stimuli. Illustrated here are the local field potentials evoked in S1 in response to whisker stimulation either with (M1 stim) or without (control) optogenetic stimulation of feedback pathways from M1 to S1. D. The activation of cortical networks may result in selective propagation of neuronal activity by enhancing synchronization, which allows temporal summation of synaptic responses to bring the postsynaptic neuron to firing threshold. B is adapted from [43]; C is from [8]; D is from [75].
While neurotransmitter systems that collectively project to broad areas of the brain are often believed to act on a slow (seconds or longer) time scale and with poor resolution in neural space, this bias is not always the case. Both cholinergic and serotoninergic systems, for example, can activate rapid excitatory postsynaptic potentials in postsynaptic targets through nicotinic and 5HT3A receptors, respectively [44–46] (see also Higley and Picciotto in this issue [83]). In cortical networks, these receptors are often (but not exclusively) located on particular subpopulations of GABAergic interneurons [47,48] (see also Wester and McBain in this issue [84]) and considerable effort has been recently applied to understand the roles of these pathways (see below).
Effects of state on sensory processing
The transition from the slow oscillatory to activated state alters the subthreshold dynamics of most, if not all, cortical and thalamic neurons. In addition to changes in intrinsic conductances, during the activated state cortical neurons maintain depolarized membrane potentials due to continuous and roughly balanced [49] barrages of excitatory and inhibitory synaptic inputs. Thalamic reticular and relay neurons undergo a subthreshold depolarization during the activated state, resulting in a qualitative change in spiking from burst to tonic mode [25,50,51]. The effects of cortical state on pyramidal neuron and parvalbumin interneuron firing rates vary across studies [8–10,52,53*,54], which may reflect differences in cortical area and layer. One relatively consistent and recent finding is that states associated with increased arousal, such as movement or reward, result in activation of a disinhibitory pathway in superficial layers of somatosensory, auditory and visual cortices [55**,56**,57**]. VIP-containing inhibitory interneurons synapse onto somatostatin (apical dendrite-targeting) and parvalbumin (soma-targeting) interneurons. During movement or reinforcement signaling, the VIP neurons become highly active and inhibit somatostatin and parvalbumin interneurons. As a result, the apical dendrites and somata of pyramidal neurons are disinhibited (Figures 1C, 4A). This circuit likely contributes to state-dependent gain effects (see below) related to movement [53*] and active touch [58]. Interestingly, the VIP-containing neurons show rapid depolarization to serotoninergic and cholinergic inputs from subcortical pathways and glutamatergic inputs from higher cortical areas [48,55**,59], positioning these interneurons as effectors of multiple modulatory inputs (Figure 4).
State-dependent changes in the neural elements described above cause specific alterations in sensory processing. First we consider multiplicative changes in neuronal gain, where the input-output relationship of the neuron in different states can be well fit by simple multiplication with a scalar (Figure 4B). Multiplicative gain changes are likely the result of changes in membrane potential in the presence of membrane potential variance [43,60,61]. Thus, changes in membrane potential that result from cortical activation would be expected to result in multiplicative enhancement (or decrement, if hyperpolarized) changes in sensory responsiveness. One particularly striking example of fast neurotransmitter systems controlling neuronal responsiveness is a recently described GABAergic projection from layer 6 to more superficial cortical layers, the activation of which reduces neural gain [32**]. A similar result is obtained in the activation of intrathalamic inhibitory neurons (Figure 1C) by descending corticothalamic projections [32**].
Multiple recent studies have observed increased gain of sensory responses in primary visual cortex with movement or arousal [9**,10**,53*,62,63]. These cortical gain changes appear to be due to a combination of local disinhibition [57**], local network state changes that result in decreased membrane potential variance [10**], and depolarization from neuromodulators [9**]. In mouse somatosensory and auditory cortices, however, sensory responses are reduced in the activated state, both in amplitude and spatial spread [5,54,64–68]. This may result from activation causing greater gain enhancement in interneurons than excitatory neurons [69], increases in tonic inhibition [32**], or alterations in brain state resulting in the suppression of recurrent positive feedback loops, such as those underlying Up states. To generalize, we expect that cortical activation will alter the gain and reliability (see below) of sensory responsiveness in cortical neurons. In animals trained in a behavioral task, the specific pattern of gain modulation may evolve through plasticity to enhance task performance. We speculate that this could be a mechanism to enhance representations of target stimuli while suppressing representations of distracting stimuli, as observed in selective attention tasks [70].
A second mechanism by which cortical state influences sensory processing is by modifying response reliability. In order to be acted upon, representations in sensory cortex must be decoded by higher order cortical regions. As such, spontaneous activity can be a source of noise (variability) when attempting to decode sensory representations [71,72]. Intrinsic slow oscillations limit the coding capacity of cortical circuits to the active Up state, which may have different phase relations on each trial. Accordingly, multiple studies have shown an increased accuracy of sensory coding in the activated compared to the slow oscillatory state [8**,73,74]. Interestingly, these studies were carried out utilizing three different sensory modalities and three different activation mechanisms. The similar effects on sensory coding strongly argue that cortical state, rather than the specific modulator, is the relevant effector. With this conceptual foundation, future studies need to directly probe the relationship between cortical state, trial-to-trial reliability and task performance in behaving animals.
While activation reduces widespread low frequency network oscillations, there is often an increase in locally coherent fluctuations in gamma band frequencies. A third potential mechanism of state-dependent processing is enhancing communication between cortical regions by synchronization of synaptic signals [75]. Synchronization has been particularly well studied in terms of gamma (30–80 Hz) frequency components of cortical activities. A 40 Hz gamma cycle has a 12.5 millisecond active phase, which corresponds roughly to the time constant of pyramidal cells in vivo. Thus, events that occur within a single gamma cycle will appear as functionally synchronous to a post-synaptic neuron. The effectiveness of the synaptic event, however, will depend on the gamma phase of the post-synaptic cell (Figure 4D). Thus, cortical regions that synchronize their active gamma phase will propagate signals more effectively than regions that do not. Extensive work by Pascal Fries and others over the last decade has brought significant experimental evidence to this theory. By recording ECoG signals simultaneously from multiple cortical regions in behaving non-human primates, they observed inter-areal gamma band coherence that was modulated by attention [76]. In the future, higher resolution recording and perturbation methods may enhance our mechanistic understanding of these processes.
Summary and future directions: role of cortical state in higher cognitive function
In summary, research over the past few years has considerably changed our view of cortical state. Instead of reflecting discrete and global changes in cortical dynamics, we now know that cortical state is a complex mixture of overlapping local and global states and sub-states. Through glutamatergic pathways, cortical state modulation can be extremely rapid and spatially targeted. We have also identified multiple mechanisms by which cortical state influences sensory processing, along with circuit elements that may underlie these mechanisms. Ongoing research will continue to hone our understanding of each of these topics. However, the challenges of the future will be in deciphering the roles of cortical state in higher cognitive function. Therefore, we will end with possible insights into this relationship and implications for human intervention.
Perhaps the most promising avenue for studying the roles of cortical state in higher cognitive function is during spatial attention. Spatial attention tasks combined with electrophysiology in non-human primates has proven to be a tractable approach throughout the past 30 years [70]. Furthermore, and as elaborated in detail by Harris and Thiele (2011), there are many similarities between cortical activation and neural changes during attention, including gain of sensory responses, reduced low frequency synchronization and enhanced gamma band synchrony [77]. The identification of cortical feedback pathways as modulators of cortical state [8**] adds a potential mechanism to this hypothesis: attention signals within frontal cortex are manifest in sensory cortex as local cortical activation mediated by direct frontal to parieto-occipital corticocortical pathways. Direct tests of this hypothesis are needed.
Other cognitive processes, including perception, expectation and learning, may rely on targeted cortical state changes to functionally connect distributed cortical networks. Studying such topics will require invasive recordings and cellular manipulations in behaving animals. Moreover, the study of cortical state may be translated to human disease. If cortical state underlies many cognitive and perceptual disorders as some have proposed [78–81], then understanding the precise mechanisms of cortical state will identify specific cellular elements for targeted therapeutic intervention.
Highlights.
Forebrain activity is characterized by rapid transitions between multiple states
Brain state is controlled on different spatial and temporal scales by both classical neuromodulatory systems and point-to-point glutamatergic pathways
Brain state strongly influences sensory responses and behavioral decisions
Glutamatergic feedback and feedforward pathways rapidly control local network state and the gain and reliability of neural responses
Alterations in brain state can enhance neuronal responses through changes in gain, reliability, precision, or synchronization of sensory-motor responses
Acknowledgments
We thank members of the McCormick lab for helpful comments on the manuscript. Supported by NIH 5R01N2026143 (DAM) and F32NS077816 (EZ) and the Kavli Institute of Neuroscience at Yale.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
* of special interest
** of outstanding interest
- 1.Steriade M, Timofeev I, Grenier F. Natural waking and sleep states: a view from inside neocortical neurons. J Neurophysiol. 2001;85:1969–1985. doi: 10.1152/jn.2001.85.5.1969. [DOI] [PubMed] [Google Scholar]
- 2.Beloozerova IN, Sirota MG, Swadlow HA. Activity of different classes of neurons of the motor cortex during locomotion. J Neurosci. 2003;23:1087–1097. doi: 10.1523/JNEUROSCI.23-03-01087.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Connor DH, Peron SP, Huber D, Svoboda K. Neural activity in barrel cortex underlying vibrissa-based object localization in mice. Neuron. 2010;67:1048–1061. doi: 10.1016/j.neuron.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 4.Sakata S, Harris KD. Laminar-dependent effects of cortical state on auditory cortical spontaneous activity. Front Neural Circuits. 2012;6:109. doi: 10.3389/fncir.2012.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crochet S, Petersen CC. Correlating whisker behavior with membrane potential in barrel cortex of awake mice. Nat Neurosci. 2006;9:608–610. doi: 10.1038/nn1690. [DOI] [PubMed] [Google Scholar]
- 6.Poulet JF, Petersen CC. Internal brain state regulates membrane potential synchrony in barrel cortex of behaving mice. Nature. 2008;454:881–885. doi: 10.1038/nature07150. [DOI] [PubMed] [Google Scholar]
- 7.Okun M, Naim A, Lampl I. The subthreshold relation between cortical local field potential and neuronal firing unveiled by intracellular recordings in awake rats. J Neurosci. 2010;30:4440–4448. doi: 10.1523/JNEUROSCI.5062-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8**.Zagha E, Casale AE, Sachdev RN, McGinley MJ, McCormick DA. Motor cortex feedback influences sensory processing by modulating network state. Neuron. 2013;79:567–578. doi: 10.1016/j.neuron.2013.06.008. This study demonstrated that cortico-cortical feedback pathways are robust, rapid and spatially specific modulators of network state. Moreover, this study demonstrated that cortical activation enhances sensory coding by eliminating the intrinsic slow fluctuations that are a major source of sensory response variability. The speed and specificity of cortico-cortical feedback pathways make them ideal substrates for rapid cortical state transitions associated with cognitive processing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9**.Polack PO, Friedman J, Golshani P. Cellular mechanisms of brain state-dependent gain modulation in visual cortex. Nat Neurosci. 2013;16:1331–1339. doi: 10.1038/nn.3464. To understand the subthreshold mechanisms leading to increased visual response gain during locomotion, whole cell recordings were obtained from excitatory and inhibitory visual cortex neurons in awake mice while stationary and running on a treadmill. The authors propose that movement-related membrane potential depolarization and reduced membrane potential variance underlie enhanced visual response gain without a change in orientation tuning. Local application of neuromodulatory antagonists suggests the involvement of norepinephrine in movement-related membrane potential depolarization. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10**.Bennett C, Arroyo S, Hestrin S. Subthreshold mechanisms underlying state-dependent modulation of visual responses. Neuron. 2013;80:350–357. doi: 10.1016/j.neuron.2013.08.007. This study used whole cell recordings of intracellular membrane potential and synaptic currents to determine the subthreshold mechanisms of state-dependent visual sensory responses. They demonstrate that signal-to-noise of sensory responses is enhanced during movement due to larger and more reliable sensory-driven inputs and reduced spontaneous firing as a consequence of reduced membrane potential variance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hromadka T, Zador AM, Deweese MR. Up-states are Rare in Awake Auditory Cortex. J Neurophysiol. 2013 doi: 10.1152/jn.00600.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan AY, Chen Y, Scholl B, Seidemann E, Priebe NJ. Sensory stimulation shifts visual cortex from synchronous to asynchronous states. Nature. 2014;509:226–229. doi: 10.1038/nature13159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamashita T, Pala A, Pedrido L, Kremer Y, Welker E, Petersen CC. Membrane potential dynamics of neocortical projection neurons driving target-specific signals. Neuron. 2013;80:1477–1490. doi: 10.1016/j.neuron.2013.10.059. [DOI] [PubMed] [Google Scholar]
- 14**.Poulet JF, Fernandez LM, Crochet S, Petersen CC. Thalamic control of cortical states. Nat Neurosci. 2012;15:370–372. doi: 10.1038/nn.3035. This study demonstrated that thalamocortical inputs are rapid and robust modulators of network state. Acute thalamic suppression abolished cortical activation with whisking and stimulation of thalamus caused rapid cortical activation. Enhanced thalamic spiking with movement does not require afferent inputs, implying the involvement of a currently unknown central controlling mechanism. [DOI] [PubMed] [Google Scholar]
- 15.Haider B, Hausser M, Carandini M. Inhibition dominates sensory responses in the awake cortex. Nature. 2013;493:97–100. doi: 10.1038/nature11665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Twyver H. Sleep Patterns of Five Rodent Species. Physiology and Behavior. 1969;4:901–905. [Google Scholar]
- 17.Lo CC, Chou T, Penzel T, Scammell TE, Strecker RE, Stanley HE, Ivanov P. Common scale-invariant patterns of sleep-wake transitions across mammalian species. Proc Natl Acad Sci U S A. 2004;101:17545–17548. doi: 10.1073/pnas.0408242101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18*.Vyazovskiy VV, Olcese U, Hanlon EC, Nir Y, Cirelli C, Tononi G. Local sleep in awake rats. Nature. 2011;472:443–447. doi: 10.1038/nature10009. Chronic LFP/MUA recordings in freely moving rats demonstrated global and local changes in cortical states associated with sleep pressure and task performance. In particular, the study reports enhanced slow (2–6 Hz) fluctuations in awake, sleep deprived animals, which can be local or synchronous across cortex, and correlate with reduced performance on a learned task. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curie T, Mongrain V, Dorsaz S, Mang GM, Emmenegger Y, Franken P. Homeostatic and circadian contribution to EEG and molecular state variables of sleep regulation. Sleep. 2013;36:311–323. doi: 10.5665/sleep.2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Torsvall L, Akerstedt T. Sleepiness on the job: continuously measured EEG changes in train drivers. Electroencephalogr Clin Neurophysiol. 1987;66:502–511. doi: 10.1016/0013-4694(87)90096-4. [DOI] [PubMed] [Google Scholar]
- 21.Finelli LA, Baumann H, Borbely AA, Achermann P. Dual electroencephalogram markers of human sleep homeostasis: correlation between theta activity in waking and slow-wave activity in sleep. Neuroscience. 2000;101:523–529. doi: 10.1016/s0306-4522(00)00409-7. [DOI] [PubMed] [Google Scholar]
- 22.Hung CS, Sarasso S, Ferrarelli F, Riedner B, Ghilardi MF, Cirelli C, Tononi G. Local experience-dependent changes in the wake EEG after prolonged wakefulness. Sleep. 2013;36:59–72. doi: 10.5665/sleep.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gervasoni D, Lin SC, Ribeiro S, Soares ES, Pantoja J, Nicolelis MA. Global forebrain dynamics predict rat behavioral states and their transitions. J Neurosci. 2004;24:11137–11147. doi: 10.1523/JNEUROSCI.3524-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moruzzi G, Magoun HW. Brain stem reticular formation and activation of the EEG. Electroencephalogr Clin Neurophysiol. 1949;1:455–473. [PubMed] [Google Scholar]
- 25.McCormick DA, Bal T. Sleep and arousal: thalamocortical mechanisms. Annu Rev Neurosci. 1997;20:185–215. doi: 10.1146/annurev.neuro.20.1.185. [DOI] [PubMed] [Google Scholar]
- 26.Lee SH, Dan Y. Neuromodulation of brain states. Neuron. 2012;76:209–222. doi: 10.1016/j.neuron.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castro-Alamancos MA. Dynamics of sensory thalamocortical synaptic networks during information processing states. Prog Neurobiol. 2004;74:213–247. doi: 10.1016/j.pneurobio.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 28.Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE. Sleep state switching. Neuron. 2010;68:1023–1042. doi: 10.1016/j.neuron.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, 2nd, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richarson JA, Kozlowski GP, Wilson S, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:1–696. doi: 10.1016/s0092-8674(02)09256-5. [DOI] [PubMed] [Google Scholar]
- 31.Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32**.Olsen SR, Bortone DS, Adesnik H, Scanziani M. Gain control by layer six in cortical circuits of vision. Nature. 2012;483:47–52. doi: 10.1038/nature10835. This study identified a cortical circuit mechanism capable of local gain modulation. Stimulation (suppression) of layer 6 neurons caused a decrease (increase) in spontaneous and sensory-evoked activity in more superficial layers, largely due to increased intracortical inhibition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tritsch NX, Ding JB, Sabatini BL. Dopaminergic neurons inhibit striatal output through non-canonical release of GABA. Nature. 2012;490:262–266. doi: 10.1038/nature11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCormick DA, Prince DA. Actions of acetylcholine in the guinea-pig and cat medial and lateral geniculate nuclei, in vitro. J Physiol. 1987;392:147–165. doi: 10.1113/jphysiol.1987.sp016774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monckton JE, McCormick DA. Comparative physiological and serotoninergic properties of pulvinar neurons in the monkey, cat and ferret. Thalamus & Related Systems. 2003;2:239–252. [Google Scholar]
- 36.Marder E. Neuromodulation of neuronal circuits: back to the future. Neuron. 2012;76:1–11. doi: 10.1016/j.neuron.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCormick DA, von Krosigk M. Corticothalamic activation modulates thalamic firing through glutamate “metabotropic” receptors. Proc Natl Acad Sci U S A. 1992;89:2774–2778. doi: 10.1073/pnas.89.7.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Govindaiah G, Venkitaramani DV, Chaki S, Cox CL. Spatially distinct actions of metabotropic glutamate receptor activation in dorsal lateral geniculate nucleus. J Neurophysiol. 2012;107:1157–1163. doi: 10.1152/jn.00401.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hirata A, Castro-Alamancos MA. Neocortex network activation and deactivation states controlled by the thalamus. J Neurophysiol. 2010;103:1147–1157. doi: 10.1152/jn.00955.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fuller PM, Sherman D, Pedersen NP, Saper CB, Lu J. Reassessment of the structural basis of the ascending arousal system. J Comp Neurol. 2011;519:933–956. doi: 10.1002/cne.22559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee S, Carvell GE, Simons DJ. Motor modulation of afferent somatosensory circuits. Nat Neurosci. 2008;11:1430–1438. doi: 10.1038/nn.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bergen JR, Julesz B. Parallel versus serial processing in rapid pattern discrimination. Nature. 1983;303:696–698. doi: 10.1038/303696a0. [DOI] [PubMed] [Google Scholar]
- 43.Haider B, McCormick DA. Rapid neocortical dynamics: cellular and network mechanisms. Neuron. 2009;62:171–189. doi: 10.1016/j.neuron.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiang Z, Prince DA. Heterogeneous actions of serotonin on interneurons in rat visual cortex. J Neurophysiol. 2003;89:1278–1287. doi: 10.1152/jn.00533.2002. [DOI] [PubMed] [Google Scholar]
- 45.Celada P, Puig MV, Artigas F. Serotonin modulation of cortical neurons and networks. Front Integr Neurosci. 2013;7:25. doi: 10.3389/fnint.2013.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brombas A, Fletcher LN, Williams SR. Activity-dependent modulation of layer 1 inhibitory neocortical circuits by acetylcholine. J Neurosci. 2014;34:1932–1941. doi: 10.1523/JNEUROSCI.4470-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rudy B, Fishell G, Lee S, Hjerling-Leffler J. Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Dev Neurobiol. 2011;71:45–61. doi: 10.1002/dneu.20853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alitto HJ, Dan Y. Cell-type-specific modulation of neocortical activity by basal forebrain input. Front Syst Neurosci. 2013;6:79. doi: 10.3389/fnsys.2012.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haider B, Duque A, Hasenstaub AR, McCormick DA. Neocortical network activity in vivo is generated through a dynamic balance of excitation and inhibition. J Neurosci. 2006;26:4535–4545. doi: 10.1523/JNEUROSCI.5297-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee KH, McCormick DA. Modulation of spindle oscillations by acetylcholine, cholecystokinin and 1S,3R-ACPD in the ferret lateral geniculate and perigeniculate nuclei in vitro. Neuroscience. 1997;77:335–350. doi: 10.1016/s0306-4522(96)00481-2. [DOI] [PubMed] [Google Scholar]
- 51.Bezdudnaya T, Cano M, Bereshpolova Y, Stoelzel CR, Alonso JM, Swadlow HA. Thalamic burst mode and inattention in the awake LGNd. Neuron. 2006;49:421–432. doi: 10.1016/j.neuron.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 52.Gentet LJ, Avermann M, Matyas F, Staiger JF, Petersen CC. Membrane potential dynamics of GABAergic neurons in the barrel cortex of behaving mice. Neuron. 2010;65:422–435. doi: 10.1016/j.neuron.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 53*.Niell CM, Stryker MP. Modulation of visual responses by behavioral state in mouse visual cortex. Neuron. 2010;65:472–479. doi: 10.1016/j.neuron.2010.01.033. This study characterized the effects of movement on spontaneous and sensory evoked activity in mouse primary visual cortex. Across broad-spiking (putative excitatory) neurons, the study reports movement-related 2.8 fold increase in sensory responsiveness, without change in spontaneous firing rates or stimulus orientation selectively. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou M, Liang F, Xiong XR, Li L, Li H, Xiao Z, Tao HW, Zhang LI. Scaling down of balanced excitation and inhibition by active behavioral states in auditory cortex. Nat Neurosci. 2014;17:841–850. doi: 10.1038/nn.3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55**.Lee S, Kruglikov I, Huang ZJ, Fishell G, Rudy B. A disinhibitory circuit mediates motor integration in the somatosensory cortex. Nat Neurosci. 2013;16:1662–1670. doi: 10.1038/nn.3544. This study identified a circuit by which motor cortex inputs disinhibit the apical dendrites of pyramidal neurons in primary somatosensory cortex. Specifically, motor cortex neurons preferentially synapse onto VIP-containing interneurons, which inhibit apical dendrite-targeting SOM-containing interneurons. The authors demonstrate that this circuit is engaged during active movement (whisking) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56**.Pi HJ, Hangya B, Kvitsiani D, Sanders JI, Huang ZJ, Kepecs A. Cortical interneurons that specialize in disinhibitory control. Nature. 2013;503:521–524. doi: 10.1038/nature12676. This study characterized a disinhibitory local circuit in auditory sensory and prefrontal cortices of mice. VIP-containing interneurons inhibit apical dendrite-targeting SOM-containing interneurons, which in turn disinhibit pyramidal neurons. This circuit was engaged during learning of an auditory discrimination task, in particular following reinforcement signals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57**.Fu Y, Tucciarone JM, Espinosa JS, Sheng N, Darcy DP, Nicoll RA, Huang ZJ, Stryker MP. A cortical circuit for gain control by behavioral state. Cell. 2014;156:1139–1152. doi: 10.1016/j.cell.2014.01.050. This study characterized the influence of VIP-containing interneurons on sensory responsiveness of visual cortex neurons. Through a series of recording and perturbation experiments, the authors demonstrate that locomotion enhances pyramidal neuron sensory responsiveness by activating VIP-containing interneurons through nicotinic receptor stimulation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu NL, Harnett MT, Williams SR, Huber D, O’Connor DH, Svoboda K, Magee JC. Nonlinear dendritic integration of sensory and motor input during an active sensing task. Nature. 2012;492:247–251. doi: 10.1038/nature11601. [DOI] [PubMed] [Google Scholar]
- 59.Lee S, Hjerling-Leffler J, Zagha E, Fishell G, Rudy B. The largest group of superficial neocortical GABAergic interneurons expresses ionotropic serotonin receptors. J Neurosci. 2010;30:16796–16808. doi: 10.1523/JNEUROSCI.1869-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miller KD, Troyer TW. Neural noise can explain expansive, power-law nonlinearities in neural response functions. J Neurophysiol. 2002;87:653–659. doi: 10.1152/jn.00425.2001. [DOI] [PubMed] [Google Scholar]
- 61.Murphy BK, Miller KD. Multiplicative gain changes are induced by excitation or inhibition alone. J Neurosci. 2003;23:10040–10051. doi: 10.1523/JNEUROSCI.23-31-10040.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ayaz A, Saleem AB, Scholvinck ML, Carandini M. Locomotion controls spatial integration in mouse visual cortex. Curr Biol. 2013;23:890–894. doi: 10.1016/j.cub.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhuang J, Bereshpolova Y, Stoelzel CR, Huff JM, Hei X, Alonso JM, Swadlow HA. Brain state effects on layer 4 of the awake visual cortex. J Neurosci. 2014;34:3888–3900. doi: 10.1523/JNEUROSCI.4969-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fanselow EE, Nicolelis MA. Behavioral modulation of tactile responses in the rat somatosensory system. J Neurosci. 1999;19:7603–7616. doi: 10.1523/JNEUROSCI.19-17-07603.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Castro-Alamancos MA, Oldford E. Cortical sensory suppression during arousal is due to the activity-dependent depression of thalamocortical synapses. J Physiol. 2002;541:319–331. doi: 10.1113/jphysiol.2002.016857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hentschke H, Haiss F, Schwarz C. Central signals rapidly switch tactile processing in rat barrel cortex during whisker movements. Cereb Cortex. 2006;16:1142–1156. doi: 10.1093/cercor/bhj056. [DOI] [PubMed] [Google Scholar]
- 67.Ferezou I, Haiss F, Gentet LJ, Aronoff R, Weber B, Petersen CC. Spatiotemporal dynamics of cortical sensorimotor integration in behaving mice. Neuron. 2007;56:907–923. doi: 10.1016/j.neuron.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 68.Otazu GH, Tai LH, Yang Y, Zador AM. Engaging in an auditory task suppresses responses in auditory cortex. Nat Neurosci. 2009;12:646–654. doi: 10.1038/nn.2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hasenstaub A, Sachdev RN, McCormick DA. State changes rapidly modulate cortical neuronal responsiveness. J Neurosci. 2007;27:9607–9622. doi: 10.1523/JNEUROSCI.2184-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moran J, Desimone R. Selective attention gates visual processing in the extrastriate cortex. Science. 1985;229:782–784. doi: 10.1126/science.4023713. [DOI] [PubMed] [Google Scholar]
- 71.Zohary E, Shadlen MN, Newsome WT. Correlated neuronal discharge rate and its implications for psychophysical performance. Nature. 1994;370:140–143. doi: 10.1038/370140a0. [DOI] [PubMed] [Google Scholar]
- 72.Arieli A, Sterkin A, Grinvald A, Aertsen A. Dynamics of ongoing activity: explanation of the large variability in evoked cortical responses. Science. 1996;273:1868–1871. doi: 10.1126/science.273.5283.1868. [DOI] [PubMed] [Google Scholar]
- 73.Goard M, Dan Y. Basal forebrain activation enhances cortical coding of natural scenes. Nat Neurosci. 2009;12:1444–1449. doi: 10.1038/nn.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marguet SL, Harris KD. State-dependent representation of amplitude-modulated noise stimuli in rat auditory cortex. J Neurosci. 2011;31:6414–6420. doi: 10.1523/JNEUROSCI.5773-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci. 2005;9:474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 76.Bosman CA, Schoffelen JM, Brunet N, Oostenveld R, Bastos AM, Womelsdorf T, Rubehn B, Stieglitz T, De Weerd P, Fries P. Attentional stimulus selection through selective synchronization between monkey visual areas. Neuron. 2012;75:875–888. doi: 10.1016/j.neuron.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Harris KD, Thiele A. Cortical state and attention. Nat Rev Neurosci. 2011;12:509–523. doi: 10.1038/nrn3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Llinas RR, Ribary U, Jeanmonod D, Kronberg E, Mitra PP. Thalamocortical dysrhythmia: A neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc Natl Acad Sci U S A. 1999;96:15222–15227. doi: 10.1073/pnas.96.26.15222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Arnsten AF, Wang MJ, Paspalas CD. Neuromodulation of thought: flexibilities and vulnerabilities in prefrontal cortical network synapses. Neuron. 2012;76:223–239. doi: 10.1016/j.neuron.2012.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gandal MJ, Edgar JC, Klook K, Siegel SJ. Gamma synchrony: towards a translational biomarker for the treatment-resistant symptoms of schizophrenia. Neuropharmacology. 2012;62:1504–1518. doi: 10.1016/j.neuropharm.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Uhlhaas PJ, Singer W. Neuronal dynamics and neuropsychiatric disorders: toward a translational paradigm for dysfunctional large-scale networks. Neuron. 2012;75:963–980. doi: 10.1016/j.neuron.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 82.Pfeffer CK, Xue M, He M, Huang ZJ, Scanziani M. Inhibition of inhibition in visual cortex: the logic of connections between molecularly distinct interneurons. Nat Neurosci. 2013;16:1068–1076. doi: 10.1038/nn.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Higley MJ, Picciotto M. Neuromodulation by Acetylcholine: Examples from Schizophrenia and Depression. Current Opinion in Neurobiology. doi: 10.1016/j.conb.2014.06.004. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wester JC, McBain CJ. Behavioral state-dependent modulation of distinct interneuron subtypes and consequences for circuit function. Current Opinion in Neurobiology. doi: 10.1016/j.conb.2014.07.007. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]